Abstract
Pregnancy reduces maternal risk of breast cancer in the long-term, but the biological determinants of the protection are unknown. Animal experiments suggest that estrogens and progesterone could be involved, but direct human evidence is scant. A case-control study (536 cases, 1,049 controls) was nested within the Finnish Maternity Cohort. Eligible were primiparous women, who delivered at term a singleton offspring before age 40. For each case, two individually matched controls by age (±6 months) and date of sampling (±3 months) were selected. Estradiol, estrone, and progesterone in first-trimester serum were measured by High Performance Liquid Chromatography Tandem Mass Spectrometry and sex-hormone binding globulin (SHBG) by immunoassay. Odds ratios (OR) and 95% confidence intervals (CI) were estimated through conditional logistic regression. In the whole study population, there was no association of breast cancer with any of the studied hormones. In analyses stratified by age at diagnosis, however, estradiol concentrations were positively associated with risk of breast cancer before age 40 (upper quartile OR, 1.81; CI, 1.08-3.06), but inversely associated with risk in women who were diagnosed ≥age 40 (upper quartile OR, 0.64; CI, 0.40-1.04), pinteraction 0.004. Risk estimates for estrone mirrored those for estradiol, but were less pronounced. Progesterone was not associated with risk of subsequent breast cancer. Our results provide initial evidence that concentrations of estrogens during the early parts of a primiparous pregnancy are associated with maternal risk of breast cancer and suggest that the effect may differ for tumors diagnosed before and after age 40.
Keywords: breast cancer, pregnancy, estrogens, progesterone, nested case-control study
INTRODUCTION
Maternal elevations in sex-steroid hormones during gestation are likely to be involved in the molecular changes that underlie the effect of pregnancy on risk of breast cancer.1, 2 In several rodent species, treatment of young virgin animals prior to carcinogen exposure with high concentrations of estradiol and progesterone of duration equivalent to that of a normal pregnancy successfully reproduces the protection against mammary carcinogenesis that a natural pregnancy confers.2, 3 Treatment with these hormones sharply curtails overall incidence, the number of tumors per animal, and prolongs latency.2, 3 However, in humans, the association of pregnancy, and likely of sex-steroids, with maternal risk is complex: early age at first completed pregnancy is crucial for the establishment of protection4, childbirth is followed by a transient increase in risk5, 6 and the protective effect becomes apparent only after age 40-457-10. Recent epidemiological evidence strongly suggests that the well-established inverse associations of parity and early age at first birth with breast cancer are driven by associations with estrogen receptor (ER)-positive breast cancer (which accounts for about 80% of all diagnosed tumors), while they are not associated with risk of hormone-receptor negative disease 11, 12 and may be related to increased risk of triple negative tumors 11. So far, direct human data on the association of sex-steroids during pregnancy with breast cancer are limited to a single nested case-control study (194 cases) in which third trimester progesterone tended to be inversely associated with risk, but the associations with estrogens were less clear.13
The present study was designed to address the hypothesis that maternal breast cancer risk is reduced in women who experience comparatively elevated concentrations of estradiol, estrone, progesterone and sex hormone binding globulin (SHBG, the major protein carrier of estradiol in the circulation) during their first full-term primiparous pregnancy (FTP) as suggested by experimental studies3. We hypothesized that the protective association would be stronger the younger the age of the mother at pregnancy in line with the established direct association of breast cancer with age at first completed pregnancy. Additionally, we wanted to explore possible effect modification by age at breast cancer diagnosis (as pregnancy protects against breast cancer only after age 40, when the proportion of hormone receptor-positive disease increases and that of hormone receptor-negative disease decreases) and time to cancer diagnosis.
MATERIALS AND METHODS
Study population
The Finnish Maternity Cohort (FMC) stores at −25°C more than 1.3 million serum samples collected during the latter part of the first, or the early weeks of the second trimester.14 The biobank contains serum samples from more than 98% of all pregnancies in the country since 1983.
Selection of cases and controls
A case-control study was nested within the FMC. Eligible were FMC members with samples donated during 6 to 14 gestational week of a primiparous, singleton, full-term pregnancy (defined as pregnancy lasting ≥37 and <44 weeks), who were less than age 40 at blood collection with no history of in-situ breast or any invasive cancer (except non-melanoma skin cancer). To maximize study efficiency to explore effect modification by age at first pregnancy within a limited budget, all cases in the category of age at first FTP with smallest number of cases (< 25 years, n=263) were included and the same number of cases (263) from each of the other 3 age at first FTP categories (25-29, 30-34, 35-39) were selected at random (total 1,052 cases).
Incident invasive breast cancers diagnosed >6 months after blood donation among FMC members were identified through a linkage of a restricted FMC file (containing limited information on parity and gestational age) with the Finnish Cancer Registry (Figure 1). The smallest number of cases were observed in the youngest maternal age group (n=263) and an equal number of cases in each of the 3 older age groups were selected at random. Up to 12 potentially eligible controls per each case matched on age at sampling (±6 months), date of sampling (±3 months) and who were alive at the time of the diagnosis of the index case were identified.
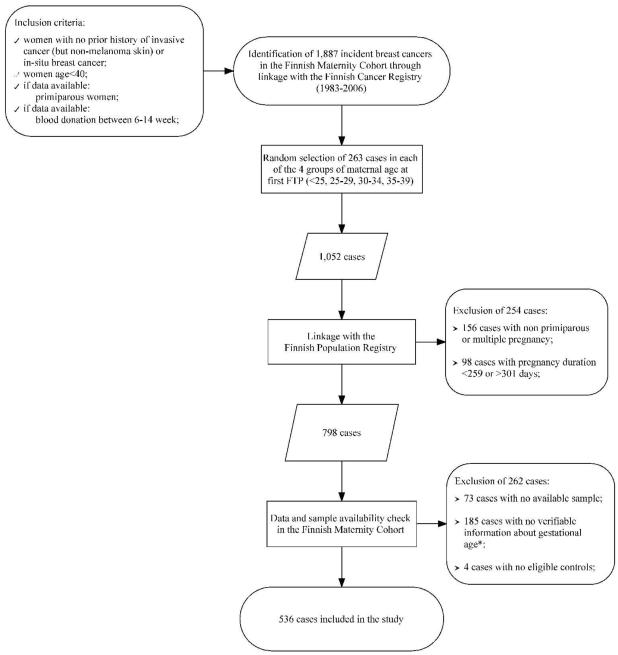
Figure 1.
Selection of study cases among women identified as potentially eligible after initial linkage of a restricted FMC file with data from the Finnish Cancer Registry. (Because of logistic difficulties, control eligibility was verified only for those matching a case included in the study.)
Footnote: Gestational age at blood donation was available only for women recruited after 1986 and all subjects recruited prior to that year (of which 171 were cases) were excluded from the study.
A linkage with the Finnish Population Registry led to the exclusion of 254 cases who did not meet the primiparous, singleton, or full-term pregnancy requirement. Cases with unverifiable information about gestational day at blood collection (necessary to account for hormone variations during pregnancy, n=185) or with no serum samples (n=73) were also excluded. Among the eligible, individually matched controls per each case, two were selected at random. For 23 cases only one eligible control was available and 4 cases with no eligible controls were further excluded. In total, 536 cases and 1,049 controls were retained for the study. Information on first FTP was obtained through a linkage with the Finnish Birth Registry and on malignant cancers diagnosed among first degree relatives of the study subjects from linkages with the Population and Cancer Registries.
Laboratory analyses
Hormonal analyses were performed at the University Hospital in Umeå, Sweden by technicians unaware of the case, control, or quality control status of the specimens. Samples of individually matched cases and controls were always included in the same laboratory run. A pool of serum from the cohort was created at the beginning of the study and 2 aliquots, undistinguishable from the test samples, were inserted in each laboratory run. Sex-steroids were quantified by High Performance Liquid Chromatography Tandem Mass Spectrometry on an Applied Biosystems API4000 triple stage quadrupole mass spectrometer. Laboratory quality controls most closely corresponding to the levels observed in the population: 2.5 ng/mL for estradiol and estrone and 50 ng/mL for progesterone, showed coefficients of variation (CV) of 10.3% or lower. Intra- and inter-run CV based on the blinded pool of quality controls were 9.1% or lower. Sex hormone binding globulin (SHBG) was quantified with a solid-phase competitive chemiluminescence assays on Immulite 2000 Siemens analyzer. The intra- and inter-run CV estimated from analyses of laboratory quality controls of 84 nmol/L were ≤5.5%, whereas those based on the quality controls from the blind pool were ≤7.6%. One progesterone measurement exceeding 3 times the interquartile range was set to missing.
Statistical analysis
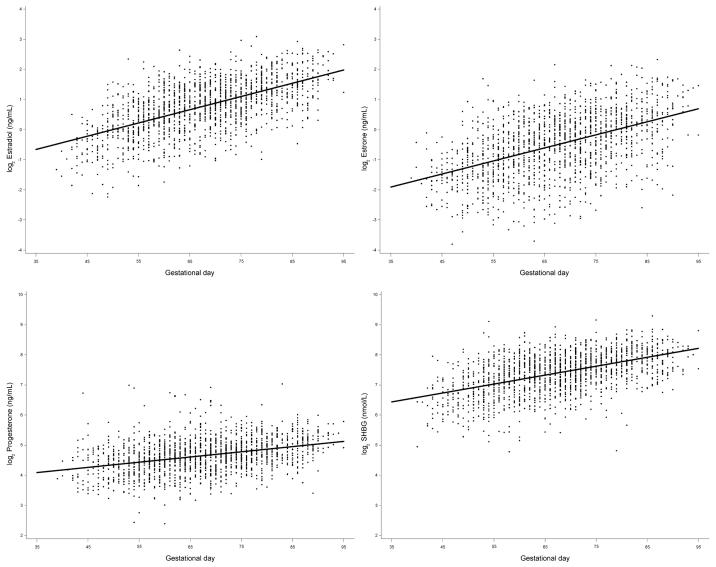
Prior to analysis, original hormone values were log2-transformed to normalize their distributions. As expected, hormone concentrations increased linearly with gestational age (Figure 2) and all statistical analyses were adjusted for gestational age.
Figure 2.
Distribution of log2 hormone concentrations (case and control subjects) by gestational day. The solid line shows the progression of hormone concentrations during pregnancy, estimated by linear regression.
The conditional logistic regression model (appropriate for the individually matched design) was used to calculate odds ratios (OR) and corresponding 95% confidence intervals (CI). Subjects were classified in quartiles using the frequency distribution of all controls. The associations of breast cancer with hormone concentrations were investigated in all women and in subgroups by the median of ages at first FTP (30 years) and diagnosis (40 years), and lag-time to diagnosis (10 years). The median cut-off points were chosen as they provide the greatest statistical power for the sub-group analyses. Coincidentally, they correspond to biologically relevant categories (epidemiological studies have shown that the protective effect of pregnancy is confined to women who give birth before age 307, in comparison with nulliparous women parous women are at decreased risk of breast cancer after age 40-458, and the transient increase in breast cancer risk after a pregnancy is evident for about 10 years6). Likelihood ratio tests were used to assess trends in ORs with assigned quantitative scores 1, 2, 3, and 4 for the categories.
Further analyses in finer sub-categories of age at first FTP (<25, 25-29, 30–34 and 35–39), age at diagnosis (<35, 35-39, 40-44 and ≥45) and lag-time (<5, 5-9, 10–14 and ≥15 years) and combinations of these were conducted. For analyses based on small number of cases, risk estimates were calculated on the continuous scale of the log2-hormone variables, a unit increase of which corresponds to a doubling of concentrations. Associations were also explored by histological subtypes of the tumors, parity to index date (cancer diagnosis) and according to categories of time between the first and subsequent pregnancies. Tests of homogeneity between the odds ratios in different subgroups were based on chi-square statistics.15 Adjustment for potential confounders (prior gravidity, parity by index date, characteristics of the first FTP and family history of breast cancer) mostly had only negligible effect on risk estimates (occasional estimates changed up to 4.8%) and were not retained in the final models. Estradiol models were additionally adjusted for progesterone and SHBG. Risk estimates for combined exposure to estradiol and progesterone (above or below the median) were also calculated. All analyses were repeated with hormone concentrations for each study subject computed as the difference (residual) between the assay value and the estimated mean value determined for the day of gestation when the sample was drawn using local linear regression.16 As results were very similar to those obtained from models including log2-transformed hormone values adjusted for gestational age, only the latter are presented. All tests of statistical significance were two-sided and considered significant if the p-values were <0.05.
The study was approved by the ethical committee of the National Institute for Health and Welfare, Finland.
RESULTS
Cases and controls were comparable in all characteristics listed in Table 1, except for family history of breast cancer, which was observed in twice as many cases than controls. For the cases, median age at diagnosis was 40.0 years. Only 26 cases were diagnosed after age 50. The proportions of women with lobular and localized disease were significantly higher in women diagnosed after age 40 than in women diagnosed before that age (19% versus 9% and 52% versus 41%, respectively).
Table 1.
Selected characteristics of study subjects and first FTP, median (range) or number (percent)
| Characteristic | Cases (536) | Controls (1,049) | P Value** |
|---|---|---|---|
| Age at first FTP, years | 29·9 (18·4-40·0) | 29·9 (18·0-40·0) | |
| Age at first FTP | |||
| < 25 | 123 (23) | 240 (23) | |
| 25 - 29 | 147 (27) | 291 (28) | |
| 30 - 34 | 137 (26) | 272 (26) | |
| 35 - 39 | 129 (24) | 246 (23) | |
| Gestational day at blood donation | 67 (39-95) | 68 (40-95) | 0·32 |
| Primigravida | 415 (79) | 818 (80) | 0·63 |
| Parity by index date (cancer diagnosis) | 0·41 | ||
| 1 | 197 (37) | 387 (37) | |
| 2 | 244 (46) | 451 (43) | |
| > 2 | 95 (18) | 211 (20) | |
| Time between first FTP and subsequent | 0.85 | ||
| pregnancy in multiparous women* | 2.36 (0.91-13.8) | 2.38 (0.87-12.8) | |
| Child gender | 0.85 | ||
| Boy | 274 (51) | 531 (51) | |
| Girl | 262 (49) | 518 (49) | |
| Child weight, g † | 3,490 (2,220-5,185) | 3,505 (1,630-5,530) | 0·86 |
| Child length, cm † | 50·0 (44·0-56·0) | 50·0 (41·0-58·0) | 0·58 |
| Use of assisted reproduction techniques† | 0·27 | ||
| No | 523 (99) | 1,017 (98) | |
| Yes | 7 (1) | 22 (2) | |
| Diabetes treated with insulin † | 0·73 | ||
| No | 529 (100) | 1,036 (100) | |
| Yes | 1 | 3 | |
| Hypertension during pregnancy † | 0·24 | ||
| No | 511 (96) | 1,012 (97) | |
| Yes | 19 (4) | 27 (3) | |
| Hospitalization during pregnancy † | 0·88 | ||
| No | 475 (90) | 933 (90) | |
| Yes | 55 (10) | 106 (10) | |
| Smoking † | 0·17 | ||
| No | 431 (83) | 873 (86) | |
| yes | 86 (17) | 143 (14) | |
| Socio-economic status | 0·36 | ||
| low | 191 (36) | 388 (37) | |
| high | 80 (15) | 126 (12) | |
| other | 25 (5) | 50 (5) | |
| missing | 240 (45) | 485 (46) | |
| ≥1 1st degree relative with breast cancer | 60 (11) | 48 (5) | <0·0001 |
| Strong family history of breast cancer ‡ | 22 (4) | 17 (2) | 0·003 |
| Age at cancer diagnosis, years | 40·0 (23·6-56·3) | - | |
| Lag-time to diagnosis, years | 10·1 (1·1-19·0) | - | |
| Tumor histology | |||
| Ductal | 414 (77) | - | |
| Lobular | 73 (14) | - | |
| Others / unknown | 49 (9) | - | |
| Tumor spread | |||
| Local | 227 (42) | - | |
| Regional or distant | 262 (49) | - | |
| Others / unknown | 47 (9) | - | |
| Estradiol §, ng/mL | 1·70 (1·64-1·76) | 1·70 (1·66-1·74) | 0·96 |
| Estrone §, ng/mL | 0·70 (0·70-0·73) | 0·71 (0·68-0·73) | 0·84 |
| Progesterone §, ng/mL | 25·0 (24·4-25·7) | 25·1 (24·6-25·6) | 0·87 |
| SHBG §, nmol/L | 168 (163-173) | 168 (164-171) | 0·92 |
by date of cancer diagnosis
Information from the Finnish Birth Registry is missing for 6 cases and 10 controls
First degree relatives with breast cancer diagnosed < age 50 or families with ≥ 2 breast cancer cases
Geometric mean (10th - 90th percentile)
p-values calculated by conditional logistic regression
Among study participants overall none of the hormones was related to risk of breast cancer. However, analyses by median ages at diagnosis and first FTP revealed a heterogeneity of the associations by age at diagnosis for estradiol, estrone and SHBG and by age at first FTP for estradiol (Table 2).
Table 2.
Odds ratios for breast cancer associated with quartiles of hormone concentrations within subgroups (conditional logistic regression)*
| Quartiles | Estradiol | Estrone | Progesterone | SHBG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases / Controls |
OR (CI) | Ptrend | Cases / Controls |
OR (CI) | Ptrend | Cases / Controls |
OR (CI) | Ptrend | Cases / Controls |
OR (CI) | Ptrend | |
| all women | ||||||||||||
| 1 | 145/260 | 1·00 [Reference] | 148/261 | 1·00 [Reference] | 137/258 | 1·00 [Reference] | 136/258 | 1·00 [Reference] | ||||
| 2 | 133/260 | 0·94 (0·70-1·28) | 124/262 | 0·86 (0·63-1·16) | 141/264 | 1·03 (0·76-1·39) | 124/260 | 0·94 (0·69-1·28) | ||||
| 3 | 117/261 | 0·84 (0·61-1·17) | 124/258 | 0·90 (0·66-1·22) | 119/260 | 0·90 (0·66-1·24) | 138/262 | 1·07 (0·78-1·47) | ||||
| 4 | 140/264 | 1·04 (0·73-1·17) | 0·98 | 139/265 | 1·00 (0·72-1·40) | 0·93 | 138/264 | 1·03 (0·73-1·44) | 0·91 | 137/263 | 1·10 (0·78-1·54) | 0·43 |
| age at first FTP < 30 | ||||||||||||
| 1 | 69/145 | 1·00 [Reference] | 74/147 | 1·00 [Reference] | 82/164 | 1·00 [Reference] | 69/137 | 1·00 [Reference] | ||||
| 2 | 59/119 | 1·13 (0·73-1·76) | 54/116 | 0·99 (0·64-1·53) | 74/144 | 1·08 (0·72-1·61) | 59/124 | 1·02 (0·65-1·60) | ||||
| 3 | 62/129 | 1·17 (0·74-1·85) | 64/124 | 1·15 (0·75-1·77) | 56/121 | 1·01 (0·65-1·55) | 62/122 | 1·12 (0·71-1·77) | ||||
| 4 | 79/130 | 1·63 (1·00-2·66) | 0·06 | 77/136 | 1·32 (0·84-2·07) | 0·20 | 57/95 | 1·32 (0·82-2·11) | 0·36 | 79/139 | 1·32 (0·83-2·09) | 0·21 |
| age at first FTP ≥ 30 | ||||||||||||
| 1 | 76/115 | 1·00 [Reference] | 74/114 | 1·00 [Reference] | 55/94 | 1·00 [Reference] | 67/121 | 1·00 [Reference] | ||||
| 2 | 74/141 | 0·75 (0·48-1·15) | 70/146 | 0·72 (0·47-1·11) | 67/120 | 0·96 (0·61-1·52) | 65/136 | 0·85 (0·55-1·32) | ||||
| 3 | 55/132 | 0·58 (0·36-0·93) | 60/134 | 0·69 (0·44-1·07) | 63/139 | 0·78 (0·49-1·25) | 76/140 | 1·02 (0·66-1·56) | ||||
| 4 | 61/134 | 0·62 (0·37-1·04) | 0·05 | 62/129 | 0·74 (0·45-1·20) | 0·21 | 81/169 | 0·80 (0·49-1·30) | 0·28 | 58/124 | 0·88 (0·53-1·44) | 0·85 |
| pint ** | 0·008 | 0·08 | 0·15 | 0·24 | ||||||||
| age at cancer diagnosis < 40 | ||||||||||||
| 1 | 62/137 | 1·00 [Reference] | 65/141 | 1·00 [Reference] | 75/147 | 1·00 [Reference] | 57/134 | 1·00 [Reference] | ||||
| 2 | 65/128 | 1·23 (0·79-1·91) | 56/120 | 1·08 (0·69-1·68) | 71/130 | 1·12 (0·73-1·72) | 56/124 | 1·19 (0·75-1·90) | ||||
| 3 | 63/130 | 1·28 (0·79-2·07) | 62/122 | 1·27 (0·81-2·00) | 54/136 | 0·84 (0·53-1·34) | 75/117 | 1·78 (1·11-2·86) | ||||
| 4 | 75/122 | 1·81 (1·08-3·06) | 0·03 | 82/134 | 1·63 (1·01-2·60) | 0·04 | 65/105 | 1·32 (0·80-2·19) | 0·53 | 76/138 | 1·62 (0·99-2·64) | 0·03 |
| age at cancer diagnosis ≥ 40 | ||||||||||||
| 1 | 83/123 | 1·00 [Reference] | 83/120 | 1·00 [Reference] | 62/111 | 1·00 [Reference] | 79/124 | 1·00 [Reference] | ||||
| 2 | 68/132 | 0·73 (0·47-1·12) | 68/142 | 0·69 (0·45-1·04) | 70/134 | 0·96 (0·63-1·48) | 68/136 | 0·78 (0·51-1·20) | ||||
| 3 | 54/131 | 0·59 (0·37-0·93) | 62/136 | 0·66 (0·43-1·01) | 65/124 | 0·98 (0·63-1·51) | 63/145 | 0·71 (0·46-1·08) | ||||
| 4 | 65/142 | 0·64 (0·40-1·04) | 0·05 | 57/131 | 0·63 (0·39-1·00) | 0·05 | 73/159 | 0·84 (0·53-1·32) | 0·48 | 61/125 | 0·79 (0·49-1·27) | 0·27 |
| pint ** | 0·004 | 0·005 | 0·19 | 0·04 | ||||||||
| lag-time to cancer diagnosis < 10 | ||||||||||||
| 1 | 75/136 | 1·00 [Reference] | 73/138 | 1·00 [Reference] | 61/119 | 1·00 [Reference] | 59/130 | 1·00 [Reference] | ||||
| 2 | 65/130 | 0·99 (0·65-1·51) | 58/126 | 0·91 (0·58-1·41) | 76/122 | 1·22 (0·79-1·87) | 65/121 | 1·30 (0·82-2·06) | ||||
| 3 | 58/140 | 0·84 (0·53-1·34) | 64/126 | 1·05 (0·68-1·61) | 50/141 | 0·73 (0·45-1·19) | 74/128 | 1·42 (0·90-2·23) | ||||
| 4 | 62/104 | 1·29 (0·77-2·18) | 0·52 | 65/121 | 1·18 (0·73-1·91) | 0·44 | 73/130 | 1·15 (0·69-1·89) | 0·92 | 62/130 | 1·25 (0·76-2·05) | 0·38 |
| lag-time to cancer diagnosis ≥ 10 | ||||||||||||
| 1 | 70/124 | 1·00 [Reference] | 75/123 | 1·00 [Reference] | 76/139 | 1·00 [Reference] | 77/128 | 1·00 [Reference] | ||||
| 2 | 68/130 | 0·91 (0·5-1·42) | 66/136 | 0·81 (0·54-1·23) | 65/142 | 0·84 (0·55-1·28) | 59/139 | 0·71 (0·46-1·10) | ||||
| 3 | 59/121 | 0·86 (0·54-1·37) | 60/132 | 0·76 (0·49-1·18) | 69/119 | 1·10 (0·72-1·68) | 64/134 | 0·84 (0·54-1·30) | ||||
| 4 | 75/160 | 0·88 (0·55-1·41) | 0·58 | 74/144 | 0·87 (0·55-1·37) | 0·53 | 65/134 | 0·89 (0·56-1·41) | 0·95 | 75/133 | 1·00 (0·63-1·58) | 0·82 |
| pint ** | 0·28 | 0·36 | 0·47 | 0·52 | ||||||||
Abbreviations: OR, odds ratio; CI, 95% confidence interval
Quartile cut-off points: estradiol (1: < 1.17; 2: 1.17-1.79; 3: 1.79-2.59; 4: ≥ 2.59 ng/mL), estrone (1: < 0.44; 2: 0.44-0.74; 3: 0.74-1.17; 4: ≥ 1.17 ng/mL), progesterone (1: < 19.5; 2: 19.5-25.3; 3: 25.3-31.8; 4: ≥ 31.8 ng/mL) and SHBG (1: < 127; 2: 127-177; 3: 177-234; 4: ≥ 234 nmol/L).
P for interaction
Estrogens were associated with significantly increased risk of breast cancer before age 40 and decreased risk (significant only for doubling of concentrations) for cancers diagnosed ≥40 years (Table 2). Risk estimates for estradiol were very similar in the bottom two (<35 and 35-39) and upper two (40-44 and ≥45) age at diagnosis categories (OR 1.70 (0.78-3.71), 1.72 (0.85-3.50), 0.64 (0.35-1.20) and 0.59 (0.27-1.29), respectively).
Estradiol concentrations tended to be inversely associated with breast cancer risk in women who had their first FTP at age 30 or older, while the opposite was observed for those with first FTP before age 30 (both associations of borderline significance). Analyses in quartiles of maternal age were less clear (e.g. for estradiol top quartile ORs were 1.29 (0.61-2.70), 1.69 (0.89-3.24), 0.51 (0.25-1.03) and 0.80 (0.38-1.69) for women age <25, 25-29, 30-34 and 35-39 at first FTP).
Combined analyses by medians of ages at first FTP and diagnosis are presented in Table 3. There was no indication for heterogeneity of the associations of breast cancer with any of the studied hormones by maternal age at first FTP in women diagnosed either before or after age 40. The heterogeneity by age at diagnosis, however, remained significant for doubling of estradiol in women above age 30 at first FTP and for doubling of estrone for women below age 30 (results for estradiol in this sub-group were in the same direction, but not significant (p=0.07)), suggesting that the observed differences by age at first pregnancy could have been influenced by the heterogeneity by age at diagnosis.
Table 3.
OR for breast cancer associated with doubling of hormone concentrations by ages at FTP and diagnosis * (conditional logistic regression)
| OR (CI) | P Value | OR (CI) | P Value | Pint** | |
|---|---|---|---|---|---|
| Age at diagnosis < 40 | Age at diagnosis ≥ 40 | ||||
| Age at first FTP < 30 | |||||
| Estradiol | 1·33 (1·04-1·71) | 0·03 | 0·85 (0·56-1·30) | 0·46 | 0·07 |
| Estrone | 1·26 (1·04-1·53) | 0·02 | 0·76 (0·55-1·04) | 0·09 | 0·007 |
| Progesterone | 1·22 (0·85-1·76) | 0·28 | 1·07 (0·59-1·92) | 0·83 | 0·70 |
| SHBG | 1·23 (0·93-1·63) | 0·15 | 0·87 (0·52-1·44) | 0·58 | 0·24 |
| Cases / Controls | 205/400 | 64/123 | |||
| Age at first FTP ≥ 30 | |||||
| Estradiol | 1·39 (0·82-2·34) | 0·22 | 0·71 (0·55-0·92) | 0·01 | 0·03 |
| Estrone | 1·05 (0·72-1·52) | 0·82 | 0·82 (0·68-1·00) | 0·05 | 0·27 |
| Progesterone | 0·69 (0·34-1·39) | 0·30 | 0·88 (0·64-1·21) | 0·45 | 0·53 |
| SHBG | 1·30 (0·69-2·44) | 0·41 | 0·81 (0·60-1·09) | 0·16 | 0·18 |
| Cases / Controls | 60/117 | 206/405 | |||
Abbreviations: OR, odds ratio; CI, 95% confidence interval
Subgroups defined by the medians of age at first FTP (< 30, ≥ 30 years) and age at cancer diagnosis (< 40, ≥ 40 years); None of the interaction tests of risk estimates in subgroups defined by median age at first FTP was rejected (smallest p value = 0·16, the remaining ≥ 0·38);
P for interaction by age at diagnosis
Analyses by the median lag-time to diagnosis did not indicate heterogeneity of the effect for any of the hormones. However, an increased risk with doubling of estradiol concentrations was observed in women diagnosed within 5 years of the first FTP both below and above age 30 and among women diagnosed before age 40 (Table 4). Combined analyses by age at first FTP, age at diagnosis and lag-time were not informative because of the interrelation between these variables resulting in limited number of subjects in some of the subgroups.
Table 4.
OR (95% CI) for breast cancer for doubling of estradiol concentrations in case women (and their controls) diagnosed within 5 years of first FTP and in those diagnosed ≥5-years in subgroups by median ages at first FTP and diagnosis
| Cases / Controls |
OR (CI) | P Value | Cases / Controls |
OR (CI) | P Value | Pint* | |
|---|---|---|---|---|---|---|---|
| Age at first FTP < 30 | Age at first FTP ≥ 30 | ||||||
| All women | 269 / 523 | 1·19 (0·96-1·47) | 0·11 | 266 / 522 | 0·82 (0·65-1·03) | 0·09 | |
| Lag < 5 | 28 / 56 | 1·63 (0·75-3·54) | 0·22 | 51 / 101 | 1·26 (0·76-2·08) | 0·37 | 0·59 |
| Lag ≥ 5 | 241 / 467 | 1·16 (0·93-1·44) | 0·20 | 215 / 421 | 0·73 (0·56-0·95) | 0·02 | 0·01 |
| Pint ** | 0·41 | 0·06 | |||||
| Age at diagnosis < 40 | Age at diagnosis ≥ 40 | ||||||
| All women | 265 / 517 | 1·34 (1·07-1·68) | 0·01 | 270 / 258 | 0·75 (0·60-0·94) | 0·01 | |
| Lag < 5 | 62 / 123 | 1·83 (1·10-3·04) | 0·02 | 17 / 34 | 0·41 (0·13-1·25) | 0·12 | 0·02 |
| Lag ≥ 5 | 203 / 394 | 1·23 (0·96-1·59) | 0·11 | 253 / 494 | 0·77 (0·61-0·97) | 0·03 | 0·01 |
| Pint ** | 0·17 | 0·28 | |||||
P for interaction by age at first FTP / age at diagnosis
P for interaction by lag time
Progesterone concentrations were not related to risk. In analyses in quartiles, SHBG concentrations were significantly associated with risk only in women with diagnosis before age 40, but after adjustment for estradiol the association was no longer significant. Adjustment of estradiol models for progesterone did not change the direction or the significance of the observed associations. Analyses by combined estradiol and progesterone exposure below or above the median yielded similar results to those overall. Adjustment for SHBG attenuated some of the associations and the results across quartiles of estradiol in women diagnosed below age 40 were no longer significant (1.60 (0.89-2.88), p=0.14), but the association remained different from that in women diagnosed at or after age 40 (pint<0.05).
Adjustment for potential confounders had only negligible effect on risk estimates. Results for ductal carcinoma were similar or stronger to those reported overall. Analyses excluding women with family history of breast cancer or women diagnosed after age 50 yielded almost identical results. In multiparous women at diagnosis, doubling of estradiol and estrone concentrations was associated with significantly increased risk in those diagnosed before age 40, with risk estimates very similar in women who had a subsequent pregnancy ≤ 2 years or > 2 years of first FTP. Results in uniparous and biparous women were largely similar to those overall, but did not reach statistical significance with the exception for estradiol in biparous women with lag-time < 10 years (OR 1.65 (1.01-2.68) and for estrone in women diagnosed above age 40 (OR 0.58 (0.38-0.88)) and lag-time ≥10 years (OR 0.72 (0.52-0.98)).
DISCUSSION
This study is the first to offer direct epidemiological evidence that estrogens during the early part of a first FTP are involved of the complex relationship between pregnancy and breast cancer. Our observations suggest that the effect of hormones differ by age at breast cancer diagnosis. The strongest associations were observed for estradiol, the most potent natural estrogen. In comparison with women with first trimester estradiol concentrations in the lowest quartile, those in the top quartile had 80% increase in risk of breast cancer occurring before age 40, while the same concentrations of the hormone decreased risk of cancer after that age by 36%. Associations with estrone, highly correlated with estradiol, followed similar patterns, whereas there was no association with progesterone.
Our results on first trimester hormones differ from those in the only other reported study on pregnancy sex steroids and risk of maternal breast cancer, where third trimester samples were analyzed.13 Estrogens may be most strongly related to risk during the first trimester, when the proliferation of breast epithelium is at its peak17-19, which can account for our findings and explain the lack of clear association in the study by Peck et al13. In contrast, as observed by Peck et al.13, progesterone concentrations could be of relevance in the latter parts of pregnancy, after the initial proliferation of breast ductal epithelium and sufficient expression of the PR-receptor induced by estrogens had set the stage necessary for progesterone to stimulate a complete lobulo-alveolar differentiation of the breast.17-19
Breast cancer is a heterogeneous disease. Epidemiological studies have shown that the effect of risk factors differs both by menopausal status and by tumor expression of estrogen and progesterone receptors (PR).10-12, 20, 21 Our findings concern mostly premenopausal breast cancer as 95% of the cases were diagnosed before age 50. Nevertheless, the proportion of hormone receptor-positive tumors is likely to differ by age at diagnosis, as it is about 11% higher in women diagnosed at age 40 to 49, as compared to those diagnosed at age 30-39, and the difference is larger across wider age-ranges.22 Additionally, the proportion of triple negative tumors decreases with age.23 Differences by tumor receptor expression are possibly of greatest relevance to our results, as pregnancy confers protection against hormone receptor-positive disease, is not associated with hormone receptor-negative breast cancer, and may increase risk of triple negative tumors.11, 12, 23
The decrease in maternal breast cancer after age 40 associated with elevated estrogens is in line with the well-established long-term protection conferred by pregnancy, and specifically against ER-positive disease, which increases in incidence with age.22, 24 Experimental data also support such interpretation, as treatment with estradiol and progesterone prior to carcinogen exposure has been shown to block the proliferation of ER-positive cells and to reduce the incidence of tumors which are largely (80%) hormone-responsive.2, 3, 25 The mechanisms underlying the protective effect have not yet been identified, but terminal differentiation of epithelial breast cells to a state less susceptible to transformation, reduced number of mammary stem cells, and changes in estrogen responsiveness of the parous glands have been proposed.1
We speculate that the increased risk of breast cancer before age 40 associated with estrogens in our data could be driven, at least in part, by a direct association of estrogens with ER-negative breast cancer (particularly triple negative tumors). In comparison with women diagnosed after 40, those diagnosed before that age have higher proportion of receptor-negative tumors and lower proportion of receptor-positive tumors 22. Animal models of parturition-induced receptor-negative breast cancer have demonstrated that despite the lack of estrogen receptors, these tumors require estrogen for their formation and progression.26 High background estrogen concentrations appear important in the development of receptor-negative tumors also in humans, as their incidence is higher before menopause and then plateaus and decreases with the transition to menopause and low estrogen environment.27 The association of circulating estrogens with ER-negative breast cancer remains to be characterized. Prospective studies in postmenopausal women (all including less than 42 ER-negative cases) have shown mixed results. 28-31 Similar risk estimates for the association of estradiol with both ER-positive and ER-negative disease were found in two studies 28,29 and in one a direct strong association with ER-positive, but not with ER negative disease was observed 30.
Another possible interpretation is that some women might be especially vulnerable to the massive push to cell proliferation that estrogens induce during pregnancy. Several large record-linkage studies have described a transient increase in maternal breast cancer that peaks about 5 years after delivery.5, 6 In line with these findings, the increase in breast cancer risk before age 40 associated with doubling of estradiol concentrations was stronger in women diagnosed within 5 years of their first pregnancy. One group of susceptible women could be those carriers of deleterious BRCA1 mutations, who have been shown to experience disturbed development and differentiation of the breast epithelium during pregnancy32, 33, to develop the disease at young age and possibly be at particularly elevated risk following pregnancy34. However, study results were unaffected when women with family history of breast cancer were excluded.
The major strengths of this large study in young women are its prospective design and that it was conducted within a well-defined cohort of primiparous women who delivered a child at term. The first completed pregnancy is associated with profound changes both in the maternal breasts and in hormone concentrations, which tend to be lower during subsequent pregnancies.35, 36 The matching on age of first FTP and the additional information on the first FTP, women’s overall parity and cancer occurrence in first degree relatives allowed us to control for several sources of potential confounding.
The major study limitation is the lack of information on receptor status of the tumors necessary to fully characterize and understand the observed associations with risk. Unfortunately this information is not collected centrally in Finland. Additionally, given the correlation between maternal age at first FTP pregnancy, age at cancer diagnosis and lag-time to diagnosis, a substantially larger study would be necessary to investigate in sufficient detail and with adequate statistical power the individual contribution of each of these factors as potential effect modifiers of the association of breast cancer with hormone concentrations during pregnancy. Study samples had been stored at relatively high temperature (−25°C), but cases and controls were tightly matched on date of sampling. Reassurance that our results were not influenced in a major way by possible analyte degradation is that hormone levels were uncorrelated with time in storage (as also reported previously37), and their concentrations varied with gestational age as expected. A large number of potentially eligible cases were excluded from the study. However, as the exclusions were made either at random (to reduce the number of cases because of financial constraints) or affected in non-differential way both the case and control subjects (e.g. exclusion of all women with blood donation prior to 1986 to be able to control for hormone variation with gestational day), it is unlikely that they have affected our results in a systematic way. Finally, we cannot exclude the possibility of chance findings and confirmation of our results in other and larger studies is necessary.
In conclusion, our results support the hypothesis that comparatively elevated estrogen concentrations during the first trimester of a first FTP could be involved in protection that a pregnancy affords the mother after age 40. Comparative elevations in the same hormones could be associated with an increase in risk of breast cancer before age 40. Confirmation of our findings is of interest, given the growing interest in the possibility of utilizing pregnancy hormones as chemopreventive agents in young women.38
Acknowledgements
This work was supported by research grants from the US National Cancer Institute [CA114329 and CA120061]. We gratefully acknowledge Yelena Afanasyeva, Pirjo Kontiokari, Annika Uimonen, and Sara Kuusiniemi for their excellent technical assistance in the conduct of the study.
Abbreviations
- FMC, FTP
full-term pregnancy
- SHBG
sex hormone binging globulin
- OR
odds ratio
- CI
confidence interval
- CV
coefficient of variation
- ER
estrogen receptor
- PR
progesterone receptor
Footnotes
No potential conflicts of interest were disclosed by any of the authors, but M. Lehtinen, who has received grants for his other epidemiological and vaccination studies from his employers the National Institute for Health and Welfare, the University of Tampere and from Merck and Co and GSK Biologicals.
REFERENCES
- 1.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14:907–33. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 2.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12:483–95. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 3.Sivaraman L, Medina D. Hormone-induced protection against breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:77–92. doi: 10.1023/a:1015774524076. [DOI] [PubMed] [Google Scholar]
- 4.Ewertz M, Duffy SW, Adami HO, Kvale G, Lund E, Meirik O, Mellemgaard A, Soini I, Tulinius H. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46:597–603. doi: 10.1002/ijc.2910460408. [DOI] [PubMed] [Google Scholar]
- 5.Albrektsen G, Heuch I, Hansen S, Kvale G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92:167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13:299–305. doi: 10.1023/a:1015287208222. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 8.Beral V, Reeves G. Childbearing, oral contraceptive use, and breast cancer. Lancet. 1993;341:1102. doi: 10.1016/0140-6736(93)92469-a. [DOI] [PubMed] [Google Scholar]
- 9.Pathak DR, Osuch JR, He J. Breast carcinoma etiology: current knowledge and new insights into the effects of reproductive and hormonal risk factors in black and white populations. Cancer. 2000;88(Suppl):1230–8. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1230::aid-cncr9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat. 2002;72:107–15. doi: 10.1023/a:1014891216621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, Fasching PA, Hein R, et al. Associations of Breast Cancer Risk Factors With Tumor Subtypes: A Pooled Analysis From the Breast Cancer Association Consortium Studies. J Natl Cancer Inst. 2010 Dec 29; doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:361–8. [PubMed] [Google Scholar]
- 14.Lehtinen M, Koskela P, Ogmundsdottir HM, Bloigu A, Dillner J, Gudnadottir M, Hakulinen T, Kjartansdottir A, Kvarnung M, Pukkala E, Tulinius H, Lehtinen T. Maternal herpesvirus infections and risk of acute lymphoblastic leukemia in the offspring. Am J Epidemiol. 2003;158:207–13. doi: 10.1093/aje/kwg137. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–77. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 16.Cleveland WS, Loader C. Smoothing by local regression: Principles and Methods. In: Schimek MG, editor. Statistical Theory and Computational Aspects of Smoothing. Springer; New York: 1996. pp. 113–20. [Google Scholar]
- 17.Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30:51–74. doi: 10.1210/er.2008-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shyamala G. Roles of estrogen and progesterone in normal mammary gland development insights from progesterone receptor null mutant mice and in situ localization of receptor. Trends Endocrinol Metab. 1997;8:34–9. doi: 10.1016/s1043-2760(96)00207-x. [DOI] [PubMed] [Google Scholar]
- 19.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 20.Anderson WF, Chen BE, Brinton LA, Devesa SS. Qualitative age interactions or effect modification suggest different cancer pathways for early-onset and late-onset breast cancers. Cancer Causes Control. 2007;18:1187–98. doi: 10.1007/s10552-007-9057-x. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer. 2009;124:698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 22.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg LU, Einarsdottir K, Friman EI, Wedren S, Dickman PW, Hall P, Magnusson C. Risk factors for hormone receptor-defined breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:2482–8. doi: 10.1158/1055-9965.EPI-06-0489. [DOI] [PubMed] [Google Scholar]
- 25.Sivaraman L, Hilsenbeck SG, Zhong L, Gay J, Conneely OM, Medina D, O’Malley BW. Early exposure of the rat mammary gland to estrogen and progesterone blocks co-localization of estrogen receptor expression and proliferation. J Endocrinol. 2001;171:75–83. doi: 10.1677/joe.0.1710075. [DOI] [PubMed] [Google Scholar]
- 26.Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–71. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 27.Anderson WF, Matsuno R. Breast cancer heterogeneity: a mixture of at least two main types? J Natl Cancer Inst. 2006;98:948–51. doi: 10.1093/jnci/djj295. [DOI] [PubMed] [Google Scholar]
- 28.yJacquotte A, Toniolo P, Levitz M, Shore RE, Koenig KL, Banerjee S, Strax P, Pasternack BS. Endogenous estrogens and risk of breast cancer by estrogen receptor status: a prospective study in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1995;4:857–60. [PubMed] [Google Scholar]
- 29.Baglietto L, Severi G, English DR, Krishnan K, Hopper JL, McLean C, Morris HA, Tilley WD, Giles GG. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:492–502. doi: 10.1158/1055-9965.EPI-09-0532. [DOI] [PubMed] [Google Scholar]
- 30.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–65. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 31.Sieri S, Krogh V, Bolelli G, Abagnato CA, Grioni S, Pala V, Evangelista A, Allemani C, Micheli A, Tagliabue G, Schunemann HJ, Menard S, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:169–76. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 32.Jernstrom H, Johannsson O, Borg A, Olsson H. Do BRCA1 mutations affect the ability to breast-feed? Significantly shorter length of breast-feeding amomg BRCA1 mutation carriers compared with their unaffected relatives. The Breast. 1998;7:320–4. [Google Scholar]
- 33.Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7:278–91. doi: 10.1046/j.1524-4741.2001.21033.x. [DOI] [PubMed] [Google Scholar]
- 34.Albrektsen G, Heuch I, Thoresen S, Kvale G. Family history of breast cancer and short-term effects of childbirths on breast cancer risk. Int J Cancer. 2006;119:1468–74. doi: 10.1002/ijc.22003. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein L, Depue RH, Ross RK, Judd HL, Pike MC, Henderson BE. Higher maternal levels of free estradiol in first compared to second pregnancy: early gestational differences. J Natl Cancer Inst. 1986;76:1035–9. [PubMed] [Google Scholar]
- 36.Arslan AA, Zeleniuch-Jacquotte A, Lukanova A, Afanasyeva Y, Katz J, Levitz M, Del PG, Toniolo P. Effects of parity on pregnancy hormonal profiles across ethnic groups with a diverse incidence of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2123–30. doi: 10.1158/1055-9965.EPI-06-0470. [DOI] [PubMed] [Google Scholar]
- 37.Holl K, Lundin E, Kaasila M, Grankvist K, Afanasyeva Y, Hallmans G, Lehtinen M, Pukkala E, Surcel HM, Toniolo P, Zeleniuch-Jacquotte A, Koskela P, et al. Effect of long-term storage on hormone measurements in samples from pregnant women: The experience of the Finnish Maternity Cohort. Acta Oncol. 2008;47:406–12. doi: 10.1080/02841860701592400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuma R. Mimicking pregnancy to reduce breast cancer risk. J Natl Cancer Inst. 2010;102:517–8. doi: 10.1093/jnci/djq146. [DOI] [PubMed] [Google Scholar]