Abstract
Background
Social determinants of prostate cancer survival and their relation to racial/ethnic disparities thereof are poorly understood. We analyzed whether census tract-level socioeconomic status (SES) at diagnosis is a prognostic factor in men with prostate cancer and helps explain racial/ethnic disparities in survival.
Methods
We used a retrospective cohort of 833 African-American and white, non-Hispanic men diagnosed with prostate cancer at four Chicago-area medical centers between 1986 and 1990. Tract-level concentrated disadvantage (CD), a multi-dimensional area-based measure of SES, was calculated for each case using 1990 U.S. census data. Its association with prostate cancer-specific survival was measured using Cox proportional hazard models adjusted for case and tumor characteristics, treatment, and healthcare system (private sector vs. Veterans Administration [VA]).
Results
Tract-level CD associated with an increased risk of death from prostate cancer (highest vs. lowest quartile, hazard ratio [HR] = 2.37, p < .0001). However, the association was observed in the private sector and not in the VA (per 1 standard deviation [SD] increase, HR = 1.33, p < .0001 and HR = 0.93, p = .46, respectively). The multivariate HR for African Americans before and after accounting for tract-level CD was 1.30 (p = .0036) and 0.96 (p = .82), respectively.
Conclusion
Census tract-level SES is a social determinant of prostate-specific mortality and helps account for racial/ethnic disparities in survival. An equal-access healthcare system may moderate this association.
Impact
This study identifies a potential pathway for minimizing disparities in prostate cancer control. The findings need confirmation in a population-based study.
Keywords: socioeconomic status, prostatic neoplasms, survival
INTRODUCTION
The epidemiology of prostate cancer in the United States is characterized by disparities in survival by race and place. African Americans have worse prostate cancer-specific survival rates than those of Whites, Hispanics and other racial/ethnic groups (1). African Americans also tend to present with more advanced and higher histologic grade tumors than other racial/ethnic groups, and taking these differences into account explains some but not all of the disparity in survival (2). Consequently, researchers have investigated biological predisposition, lifestyle, and treatment differences as other component causes of U.S. racial/ethnic disparities in survival (3, 4).
Geographic variation in fatal prostate cancer and tumor-level predictors of survival has also been documented. In their analysis of U.S. prostate cancer mortality rates from 1970 through 1989, Jemal et al. identified clusters of contiguous counties with rates that were statistically significantly higher than those in the rest of the country. Prostate cancer mortality clusters among whites were predominantly in the northwest, whereas those among African Americans were mainly in the southeast (5). Klassen et al. conducted a smaller area analysis of prognostic tumor characteristics among 20,928 men diagnosed with prostate cancer in the state of Maryland between 1992 and 1997 (6). They examined variation in tumor stage and histologic grade at the census block-group level and were able to detect clusters of advanced-stage and high-histologic grade tumors. Adjustment for case and block-group social and demographical characteristics altered the pattern of clusters for both stage and grade. The results of these studies suggest that factors associated with ‘place’ influence the prognosis of prostate cancer. These factors could include, among other things, social determinants of health and disease.
Social determinants of prostate cancer-specific survival and their relation to racial/ethnic disparities are poorly understood. Area-level socioeconomic status (SES) is a plausible prognostic factor in prostate cancer, because gradients thereof associate with inequalities in geographical access to healthcare, individual-level insurance status, and other circumstances that can lead to clinically important variation in cancer detection, staging, treatment, and long-term cancer care (7–9). However, in contrast to individual-level SES, the relation between area-level SES and racial/ethnic disparities in prostate cancer-specific survival has been more difficult to establish (10). Race and ethnicity are often highly correlated with aggregate measures of socioeconomic status such as mean per capita household income (11). Furthermore, area-level SES is multidimensional, composed of poverty, housing, employment, education, racial composition, and occupational domains (12). However, studies of area-level SES and prostate cancer survival that incorporate multidimensional global measures of SES are scarce (13, 14). Also, given the likely complexity of the area-level SES and cancer survival relationship, it would be desirable to link the study design to a conceptual framework or mechanistic model that guides the selection and analysis of the relevant variables (15).
Therefore, the primary objective of this study was to analyze whether area-level socioeconomic status is a prognostic factor in men diagnosed with prostate cancer using a multidimensional global measure of area-level SES. The analysis was not focused on the geospatial dimension of an association between area-level SES and prostate cancer-specific survival. Rather, it was concerned with area-level of SES as a non-spatial characteristic of the environment. The secondary objective was to determine whether an association helped explain observed racial/ethnic disparities in prostate cancer-specific survival. We hypothesized that the socioeconomic status of the census tract of residence at the time of a diagnosis of prostate cancer would be an independent predictor of cancer-specific survival after accounting for patient demographic and clinical characteristics, prognostic tumor parameters, first-course treatment and healthcare setting, and that this association would help explain disparities in prostate cancer-specific survival between African American and non-Hispanic whites. These hypotheses were tested in a retrospective cohort of men diagnosed with prostate cancer in the Chicago area using an analysis guided by the MacArthur Model for the Pathway from Socioeconomic Status to Health (16). In this model, the association of race/ethnicity with health is mediated by individual-level SES, environmental resources and constraints (e.g., neighborhood factors) and psychosocial factors. These, in turn, influence downstream factors that are more proximal determinants of health. These factors include access to health care, the physical environment, health behaviors, and biologic responses that may determine the development and course of disease.
MATERIALS AND METHODS
Cohort Selection and Baseline Variables
Cohort selection and the baseline variables collected have been described in detail previously (17). Briefly, our cohort consisted of all African-American and non-Hispanic white men diagnosed with adenocarcinoma of the prostate (International Classification of Diseases, 9th revision, Clinical Modification, code 187.0) at four Chicago-area medical centers between January 1, 1986 and December 31, 1990 (n=1163) (18). Two of the medical centers were private university-affiliated, and two were in the Veterans Health Administration (VA) system. Cases were identified through the tumor registry at each facility. Data were extracted from medical records by trained medical records abstractors blinded to the hypotheses under study. These data included case demographics, tumor characteristics, processes of diagnosis and treatment, comorbidities at the time of diagnosis, and follow-up information. Stage assignments were based on the American Joint Committee on Cancer TMN (tumor, node, metastasis) staging system (19). Stage was classified as localized (tumor confined to prostate gland or extracapsular extension without lymph node involvement [T1b-3 any, N0, M0]; regional (tumor confined to prostate gland or extracapsular extension with regional lymph node involvement [T1b-3 any, N1, M0] or tumor invading adjacent structures other than seminal vesicles without distant metastases [T4, N any, M0]); distant (tumor metastases at distant sites [T any, N any, M1]); or unstaged. Tumor differentiation was classified as well, moderately, or poorly differentiated per the pathologist’s report. First-course treatment was defined as any treatment directed at the primary tumor received within 4 months of initiating therapy (20, 21). Comorbidity at the time of diagnosis was measured using the Charlson index (22). We also created an indicator variable for healthcare system, VA vs. the private sector. This variable reflects access independent of the ability to pay (‘equal access’) vs. access dependent on the ability to pay.
Multidimensional Global Measure of SES at the Census Tract Level
The measure of census tract-level SES used in this analysis was based on one derived in the Chicago area population by Browning and Cagney (23). They examined the social context of Chicago neighborhoods in detail using information from the 1990 U.S. decennial census. A factor analysis of 10 variables tapping into various aspects of structural disadvantage revealed a dimension they referred to as “concentrated disadvantage.” The measure was dominated by high factor loadings for percentage in census tract population living below the poverty line, unemployed, residing in a female-headed household, under age 18 years (factor loading of 0.85 for each), and African American (factor loading of 0.60). The resulting formula weighted each variable by its factor loading: 0.85 × (% in poverty + %unemployed + % in female-headed household + % age under 18 years) + 0.60 × (% African-American). The result has been shown to predict self-rated physical health and asthma severity in the general Chicago population (23, 24).
In our study, we did not use percentage of African-American ethnicity to calculate tract-level concentrated disadvantage. Firstly, the major focus of our analysis was to identify determinants of prostate cancer survival disparities between African-American and non-Hispanic white males in the Chicago area. Secondly, we wanted to analyze our area-based measure of SES as an independent prognostic variable rather than a confounder, and the concentrated disadvantage measure using the original Browning and Cagney formula was likely highly correlated with the racial composition of the census tracts. Also, additional analyses in our study cohort, which consisted primarily of older men with prostate cancer, supported substituting the percentage under age 18 years with (100 - % college graduate). Consequently, the formula we used to calculate census tract-level concentrated disadvantage scores based on 1990 U.S. census data was a simple sum of the following: % in poverty + % unemployed + % female-headed households + (100 - % college graduate) (25). As with the original formula, this modified version is a multidimensional area-based measure of SES, and it does not have an analogous value at the individual level. Therefore, the results of analyses of its impact on the prognosis of individual patients are less susceptible to ecologic confounding compared to those based on a single-variable aggregate measure of SES (e.g., percent poverty), especially in the context of the research questions being addressed in this study (26, 27).
Vital Status and Underlying Cause of Death
Follow-up of the cohort ended on December 31, 2006, with death from prostate cancer as the underlying cause as the primary outcome of interest. The tumor registries of the participating medical centers served as our primary source of vital status. Each tracked vital status through active surveillance and obtained certified copies of death certificate for all known decedents. For known decedents for whom death certificates were available through the registries, the underlying cause of death was determined by review of the death certificate by an independent physician reviewer who was blinded to the hypotheses under study. For known decedents for whom death certificate were not available through the registries and for cases whose vitals status as of December 31, 2006 could not be verified, we determined vital status and underlying cause of death through the National Death Index. All causes of death were coded according to the International Classification of Diseases using the ninth revisions for deaths occurring before 1999 and the tenth revision for deaths occurring thereafter (28, 29) The comparability ratio for malignant neoplasms of the prostate between the tenth and ninth is 1.0134 (30).
Exclusions
Of the 1007 cases with medical records available for review, 174 were excluded for the following reasons: cancers were T1a-stage lesions, which are considered clinically insignificant (n = 90); incomplete data (n = 53); and the case’s residential address at diagnosis could not be matched to its’ corresponding census tract geocode (n = 31). This left 833 cases (320 African Americans and 513 non-Hispanic whites) for statistical analysis.
Nesting of Cases by Medical Center and by Census Tract
Our sampling frame was at the medical center level. The number of cases at each of the four medical centers was 169, 208, 222, and 234, respectively. The 833 cases represented 562 unique census tracts. Of these census tracts, 47% contained 1 case, 26% contained 2 cases, and the rest contained 3 to 8 cases.
Statistical Analysis
We used a 2-sample t test, analysis of variance, and chi-square analysis to compare baseline demographic and clinical characteristics of between African American and non-Hispanic whites and across quartiles of census tract-level concentrated disadvantage. Post-diagnosis log-log survival distributions by quartile of concentrated disadvantage at diagnosis were computed after accounting for case race/ethnicity (African-American vs. non-Hispanic White), tumor differentiation (moderate and poor vs. well), tumor stage (local/region vs. distant/unstaged), age (continuous), Charlson comorbidity score (continuous), first-course treatment, and healthcare system (private sector vs. VA). The Wald chi-square statistic was used to compare the resulting survival distributions across quartiles. Localized and regional-stage cases were combined, because they would be candidates for aggressive primary therapy. Unstaged cases were combined with distant cases because their respective survival distributions were not significantly different (p = .61).
Cox proportional hazard models measured the association of African-American ethnicity with prostate cancer-specific survival after sequential adjustment for tumor characteristics, age and comorbidities, first-course treatment (surgery, radiation, diethylstilbestrol, castration, experimental, or observation-only), concentrated disadvantage (quartiles, with the lowest quartile as the reference category) and healthcare system. The sequence of the adjustments was guided by the MacArthur Model. Based on this conceptual model, we evaluated the following four statistical models:
Model 1: λ(t;Z)=λ0(t) exp(β1race/ethnicity)
Model 2: λ(t;Z)=λ0(t) exp(β1race/ethnicity + β2stage + β3differentiation + β4age + β5Charlson score + β6first-course treatment + Interactions)
Model 3: λ(t;Z)=λ0(t) exp(β1race/ethnicity + β2stage + β3differentiation + β4age + β5Charlson score + β6first-course treatment + β7concentrated disadvantage quartiles + Interactions).
Model 4: λ(t;Z)=λ0(t) exp(β1race/ethnicity + β2stage + β3differentiation + β4age + β5Charlson score + β6first-course treatment + β7concentrated disadvantage quartiles + β8healthcare system + Interactions)
where Z denotes the variables incorporated into each model.
Model 1 estimated the crude hazard of prostate cancer-specific mortality for African Americans vs. non-Hispanic Whites. Model 2 estimated the multivariate hazard of prostate cancer-specific mortality for African Americans vs. non-Hispanic Whites as function of prognostic tumor parameters, first-course treatment, and competing hazards of death from other causes. Model 3 evaluated the multivariate hazard of prostate cancer-specific mortality for African Americans vs. non-Hispanic Whites after accounting for census tract-level concentrated disadvantage. Model 4 then takes healthcare system into account. Using product terms, we tested for interaction between independent variables and incorporated statistically significant ones where applicable. Models 2 thru 4 were also adjusted for diagnosis year, and deaths from causes other than prostate cancer were analyzed as censored observations. We used Schoenfield residuals and time-dependent coefficients to test the proportional hazards assumption in each model evaluated (31). No violations of the proportional hazards assumptions were observed (p = .12 to .83). Regression diagnostics were also performed on all multivariate models to detect any evidence of collinearity among the independent variables. Collinearity problems occur when any one independent variable reflects a near linear combination of one or more other independent variables in the model. Indicators of potential problems in this regard include a variance inflation factor greater than 10 and a condition index greater than 30, especially when the proportion of variance in the parameter estimate was greater than 0.5 (32). The statistical analyses were performed with the statistical package SAS version 9.2 (SAS Institute, Inc. Cary, NC) (33). Cox proportional hazards were analyzed using the ‘PHREG’ procedure with the ‘COVSANDWICH’ option to account for the nesting of cases by medical center. In this option, the standard errors for the model parameters are computed using the robust ‘sandwich’ variance estimator of Lin and Wei (34). We repeated the analysis to account for the nesting of cases by census tract, but results were essentially the same as those without the sandwich variance estimate.
RESULTS
Baseline characteristics and mortality outcomes of the cohort are summarized in Table 1. Cases were followed on average for 8.6 years, with an overall mortality rate of 75.5 percent. Approximately forty percent of the deaths were due to prostate cancer (n=256). African Americans were significantly more likely than their non-Hispanic white counterparts to be diagnosed with advanced stage disease (p < .0001) and to die of prostate cancer (p = .0064). At the time of diagnosis, African Americans resided in census tracts with higher concentrated disadvantage scores compared with those of non-Hispanic whites (mean = 124.4 vs. 80.8, respectively, p < .0001). This was reflected in the increasing numbers of African-Americans with increasing quartile of concentrated disadvantage (p < .0001). The likelihood of being diagnosed at an advanced tumor stage also increased with increasing quartile of concentration disadvantage (p = .0058). The probability of death due to prostate cancer increased as well, but the trend did not reach statistical significance (overall, p = .24 and highest vs. lowest quartile, p = .052).
Table 1.
Characteristics of 833 Men with Prostate Cancer Overall and Stratified by Race/Ethnicity and Quartile of Census Tract-Level Concentrated Disadvantage at Diagnosis: 4 Chicago-Area Medical Centers, 1986–1990
| Race/Ethnicity | Census Tract-Level Concentrated Disadvantage Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Overall (n=833) |
White, Non- Hispanic (n=513) |
African- American (n=320) |
pa,b | Lowest 1st Quartile |
2nd Quartile |
3rd Quartile |
Highest 4th Quartile |
pb,c |
| Follow-up time, y, Mean (SD) | 8.6 (6.9) | 9.4 (6.9) | 7.4 (6.5) | <.0001 | 10.7 (7.1) | 8.6 (7.0) | 7.4 (6.3) | 7.6 (6.6) | <.0001 |
| Deaths, N (%) | |||||||||
| Any cause | 622 (75.5) | 349 (70.0) | 268 (83.8) | <.0001 | 126 (60.3) | 152 (73.1) | 174 (83.7) | 175 (84.1) | <.0001 |
| Prostate cancer | 256 (30.7) | 140 (27.3) | 116 (36.3) | .0064 | 55 (26.3) | 61 (29.3) | 67 (32.2) | 75 (35.1)d | .24 |
| African-American, N (%) | 320 (38.4) | n/a | n/a | 8 (3.4) | 20 (9.6) | 111 (53.4) | 181 (87.0) | <.0001 | |
| Age at diagnosis, y, Mean (SD) | 69.0 (7.8) | 68.8 (7.8) | 69.3 (7.8) | .29 | 68.8 (8.1) | 68.5 (7.6) | 69.5 (7.8) | 69.1 (7.8) | .56 |
| Charlson Comorbidity Score, Mean (SD) | 1.8 (2.1) | 1.6 (2.0) | 2.2 (1.7) | .0017 | 1.2 (1.7) | 1.6 (1.9) | 2.3 (2.3) | 2.0 (2.2) | <.0001 |
| Stage, N (%) | |||||||||
| Distant/Unknown | 419 (50.3) | 226 (44) | 193 (60.3) | <.0001 | 98 (46.9) | 91 (43.8) | 105 (50.5) | 125 (60.1) | .0058 |
| Differentiation, N (%) | |||||||||
| Well | 187 (22.4) | 126 (24.6) | 61 (19.1) | 45 (21.5) | 52 (25.0) | 45 (21.6) | 45 (21.6) | ||
| Moderate | 314 (37.7) | 194 (37.8) | 120 (37.5) | 77 (36.8) | 80 (38.5) | 75 (36.1) | 82 (39.4) | ||
| Poor | 332 (39.9) | 193 (37.6) | 139 (43.4) | .114 | 87 (41.6) | 76 (36.5) | 88 (42.3) | 81 (38.9) | .89 |
| 1st-Course Treatment(s), N (%) | |||||||||
| Surgery | 260 (31.2) | 180 (35.1) | 80 (25.0) | .0022 | 79 (37.8) | 70 (33.7) | 52 (25.0) | 59 (28.3) | .0253 |
| Radiation | 279 (33.5) | 201 (39.2) | 78 (24.4) | <.0001 | 86 (41.2) | 81 (38.9) | 67 (32.2) | 45 (21.6) | <.0001 |
| Castration | 203 (24.4) | 112 (21.8) | 91 (28.4) | .031 | 46 (22.0) | 48 (23.1) | 49 (23.6) | 60 (28.9) | .37 |
| Diethylstilbesterol | 37 (4.4) | 19 (3.7) | 18 (5.6) | .19 | 6 (2.9) | 11 (5.3) | 12 (5.8) | 8 (3.9) | .45 |
| Observation only | 55 (6.6) | 25 (4.9) | 30 (9.4) | .011 | 9 (4.3) | 11 (5.3) | 15 (7.2) | 20 (9.6) | .14 |
| Experimental | 57 (6.8) | 21 (4.1) | 36 (11.3) | <.0001 | 10 (4.8) | 6 (2.9) | 15 (7.2) | 26 (12.5) | .0007 |
| Healthcare Setting, N (%) | |||||||||
| Private sector | 456 (54.7) | 342 (66.7) | 144 (35.6) | 171 (81.8) | 127 (61.1) | 98 (47.1) | 60 (28.9) | ||
| VHA | 377 (45.3) | 171 (33.3) | 206 (64.4) | <.0001 | 38 (18.2) | 81 (39.9) | 110 (52.9) | 148 (71.1) | <.0001 |
| Census Tract-Level | 98 (32.9) | 80.8 (21.3) | 124.4 (30.3) | <.0001 | |||||
| Concentrated Disadvantage Score, Mean (SD) [range] | [37.2 to 235.7] | [37.2 to 205.0] | [52.8 to 235.7] | ||||||
Note: SD = standard deviation; VHA = Veterans Health Administration
t-test for continuous variables
Chi-square test for categorical variables
Analysis of Variance for continuous variables
Versus 1st quartile, p = .052
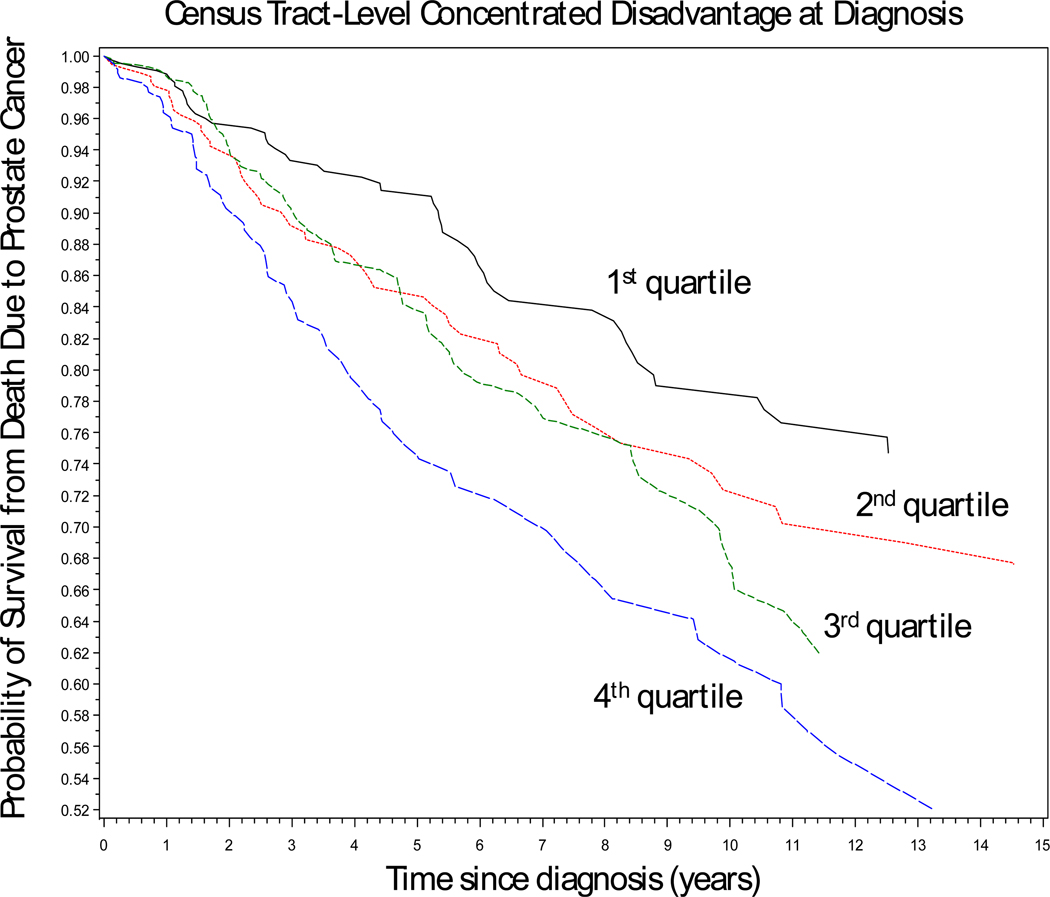
Figure 1 shows prostate cancer-specific survival by quartile of census tract-level concentrated disadvantage at diagnosis after adjustment for race/ethnicity, age, tumor characteristics, first-course treatment, Charlson comorbidity score, and healthcare system. A statistically significant trend in survival across quartiles was observed (p-trend = .006). The survival distributions began to diverge by the second year of follow-up, with the greatest contrast being between the first and fourth quartiles. The survival distributions in the second and third quartiles of concentrated disadvantaged appeared to be similar, but these too diverged after the eighth year of follow-up.
Figure 1.
Log-log plot of prostate cancer-specific survival for 833 men diagnosed with prostate cancer between 1986 and 1990 stratified by quartile of census tract-level concentrated disadvantage at diagnosis: Chicago Metropolitan Area (p-trend = 0.006). The individual survival curves are adjusted for race/ethnicity, age, tumor characteristics, first-course treatment, Charlson comorbidity score, and healthcare system (private sector vs. Veterans Health Administration), and treatment date.
Table 2 presents the results of Cox proportional hazards models that measure the association between race/ethnicity and prostate cancer-specific survival before and after sequential adjustment for baseline clinical characteristics, census tract-level concentrated disadvantage, and healthcare system. Initially, African Americans were significantly more likely to die of prostate cancer relative to non-Hispanic whites (model 1 - hazard ratio [HR] = 1.58, p = .0051). Moreover, the excess risk among African Americans persisted after accounting for prognostic tumor parameters, age, comorbidity, and first-course treatment (model 2 - multivariate HR = 1.30, p = .0036). After accounting for census tract-level concentrated disadvantage at diagnosis (model 3), the association between African-American ethnicity and prostate cancer-specific survival disappeared (multivariate HR = 0.98, p = .82); at the same time, an inverse association between concentrated disadvantage and survival was observed (highest vs. lowest quartile, multivariate HR = 1.77, p = .0043, p trend = .0029). Regression diagnostics did not detect any potential collinearity of concern between race/ethnicity and concentrated disadvantage or any other combination of independent variables in the model (proportion of variance 2.1 × 10−7 to 0.58, condition index 1 to 3.6, and variance inflation factor 1.1 to 2.8). The final model incorporates healthcare system (VA vs. private sector). The inverse association between census tract-level concentrated disadvantage and prostate cancer-specific survival persisted (highest vs. lowest quartile, multivariate HR = 2.37, p < .0001, p trend < .0001). Furthermore, a statistically significant interaction with healthcare setting was observed (p = .0043). Other statistically significant interactions were not detected.
TABLE 2.
Prostate Cancer-Specific Mortality Hazard Ratios for African Americans vs. Non-Hispanic Whites Before and After Sequential Adjustment for Baseline Clinical Characteristics, Census Tract-Level Concentrated Disadvantage, and Healthcare Setting: 4 Chicago-Area Medical Centers, 1986–1990, with Follow-up Through 2006
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | (95% CI) | p | HR | (95% CI) | p | HR | (95% CI) | p | HR | (95% CI) | p |
| Race/Ethnicity | ||||||||||||
| White, non-Hispanic (n=513) | 1.00 | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |||||
| African-American (n=320) | 1.58 | (1.15, 2.19) | .0051 | 1.30 | (1.09, 1.56) | .0036 | 0.98 | (0.83, 1.16) | .82 | 0.96 | (0.83, 1.11) | .61 |
| Tumor Characteristics | ||||||||||||
| Stage | ||||||||||||
| Local/Regional | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | ||||||
| Distant/Unknown | 2.43 | (1.69, 3.48) | <.0001 | 2.42 | (1.70, 3.44) | <.0001 | 2.40 | (1.66, 3.46) | <.0001 | |||
| Differentiation | ||||||||||||
| Well | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | ||||||
| Moderate | 1.46 | (0.97, 2.18) | .067 | 1.49 | (1.02, 2.16) | .037 | 1.52 | (1.07, 2.16) | .020 | |||
| Poor | 2.49 | (1.61, 3.84) | <.0001 | 2.59 | (1.74, 3.87) | <.0001 | 2.60 | (1.75, 3.86) | <.0001 | |||
| Age and Coexistent Illness | ||||||||||||
| Age, y | 1.00 | (0.99, 1.01) | .13 | 1.01 | (1.00, 1.01) | .0056 | 1.01 | (1.00, 1.01) | .021 | |||
| Charlson comorbidity score | 0.99 | (0.91, 1.07) | .72 | 0.98 | (0.91, 1.05) | .55 | 0.98 | (0.91, 1.05) | .55 | |||
| 1st-Course Treatment | ||||||||||||
| Surgery | 0.38 | (0.27, 0.54) | <.0001 | 0.38 | (0.27, 0.53) | <.0001 | 0.37 | (0.26, 0.53) | <.0001 | |||
| Radiation | 1.28 | (0.80, 2.03) | .30 | 1.30 | (0.84, 2.02) | .24 | 1.26 | (0.83, 1.94) | .28 | |||
| Castration | 2.05 | (1.40, 2.99) | .0002 | 2.05 | (1.40, 3.01) | .0002 | 2.03 | (1.39, 2.96) | .0003 | |||
| Diethylstilbesterol | 2.25 | (1.19, 4.25) | .013 | 2.20 | (1.06, 4.57) | .035 | 2.18 | (1.06, 4.47) | .033 | |||
| Observation only | 0.45 | (0.26, 0.81) | .0072 | 0.44 | (0.23, 0.82) | .0094 | 0.44 | (0.23, 0.84) | .013 | |||
| Experimental | 1.39 | (1.22, 1.59) | <.0001 | 1.39 | (1.18, 1.63) | <.0001 | 1.42 | (1.19, 2.71) | .0002 | |||
| Concentrated Disadvantage | ||||||||||||
| 1st Quartile (lowest) | 1.00 | (reference) | 1.00 | (reference) | ||||||||
| 2nd Quartile | 1.30 | (1.01, 1.66) | .036 | 1.44 | (1.19, 1.73) | .0002 | ||||||
| 3rd Quartile | 1.50 | (1.23, 1.83) | <.0001 | 1.55 | (1.14, 2.09) | .0049 | ||||||
| 4th Quartile (highest) | 1.77 | (1.20, 2.63) | .0043 | 2.37 | (1.76, 3.18) | <.0001 | ||||||
| p-trend | .0029 | <.0001 | ||||||||||
| Healthcare Setting | ||||||||||||
| Private Sector | 1.00 | (reference) | ||||||||||
| VHA | 1.93 | (1.23, 3.04) | .0046 | |||||||||
| Interaction Terms | ||||||||||||
| VHA*CD Quartile 1 | 1.00 | (reference) | ||||||||||
| VHA*CD Quartile 2 | 0.57 | (0.38, 0.88) | .011 | |||||||||
| VHA*CD Quartile 3 | 0.65 | (0.37, 1.14) | .13 | |||||||||
| VHA*CD Quartile 4 | 0.42 | (0.23, 0.76) | .0043 | |||||||||
Note: HR = hazard ratio; CI = confidence interval; VHA = Veterans Health Administration. Models 2 thru 4 are also adjusted for diagnosis year.
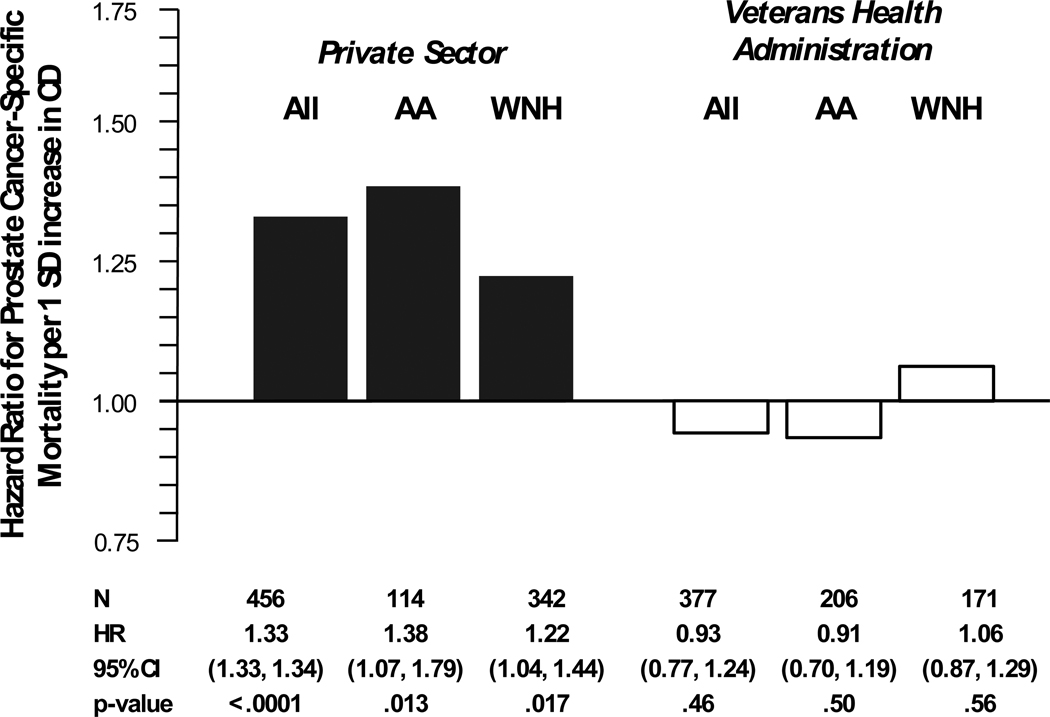
Figure 2 illustrates the difference in the association between census tract-level concentrated disadvantage and prostate cancer-specific survival in cases diagnosed and treated in the private sector versus those in the VA healthcare system. Census tract-level concentrated disadvantage was risk factor for death due to prostate cancer in the private sector (per 1 standard deviation [SD] increase, multivariate HR = 1.33, p < .0001) but not in the VA system (per 1 SD increase, multivariate HR = 0.93, p = .46). Furthermore, this modification of the association between tract-level concentrated disadvantage and prostate cancer-specific survival by healthcare system was evident for both African Americans and non-Hispanic whites.
Figure 2.
Multivariate hazard ratios for prostate cancer-specific mortality per 1 standard deviation increase in census tract-level concentrated disadvantage for 833 men diagnosed with prostate cancer between 1986 and 1990, private sector vs. Veterans Health Administration healthcare system: Chicago area. Hazard ratios are adjusted for age, tumor characteristics, first-course treatment, Charlson comorbidity score, and in the case for all patients in either healthcare setting, race/ethnicity. Note: SD = standard deviation, CD = concentrated disadvantage, AA = African Americans, WNH = White, Non-Hispanic, and CI = confidence interval.
DISCUSSION
In this study, census tract-level socioeconomic status was an independent predictor of in prostate cancer-specific survival based on a multi-dimensional measure of tract-level SES and helped account for racial disparities in survival. Two other studies have analyzed the impact of area-level SES on prostate cancer-specific survival in the United States using multidimensional measures of area-level SES. Robbins et al. evaluated whether differences in prostate cancer survival between 122,374 black men and non-Hispanic white men diagnosed with prostate cancer in the state of California between 1995 and 2004 were reduced or eliminated after accounting for differences in age, stage, treatment, grade, diagnosis year, and block-group SES (13). The cases were identified through the state’s population-based cancer registry data, and block group level SES was characterized using an index that incorporated seven 1990 U.S. census indicators of income, housing, education, and occupation to create a composite SES score (35) After simultaneous adjustment for the other prognostic factors under study, higher block group-level SES scores associated with a significantly lower risk of death from prostate cancer in both black and non-Hispanic white men (highest vs. lowest quintile, multivariate HR = 0.77 [95% CI = 0.60 to 0.99] and 0.66 [95% CI = 0.60 to 0.72], respectively). White al. conducted as similar population-based analysis in a multi-ethnic cohort of 87,449 men diagnosed with prostate cancer in the state of Texas between 1995 and 2002 (14). The authors measured block group-level SES using a principal component analysis-derived composite variable that incorporated median household income, percentage of men living below poverty, percentage with a college education, percentage with a management/professional occupation, and median home value based on the 2000 US census (36). After simultaneous adjustment for age, tumor stage, grade, diagnosis year, and rural residence, block group-level SES was significantly associated with prostate cancer-specific survival (lowest vs. highest quintile, multivariate HR = 1.36 [95% CI = 1.36 to 1.49]). Our results are consistent with the findings or these two studies.
White et al. also explored the impact of area-level SES and rural residence on racial/ethnic differences in prostate cancer-specific survival by comparing the hazard ratios for race/ethnicity across three models (table 5 in their paper). Model 1 consisted only of race/ethnicity only (non-Hispanic black, Hispanic, and Asian/Pacific Islander vs. non-Hispanic white); model 2 added age, stage, grade, and diagnosis year; and model 3 added block group SES and rural residence to the variables in model 2. The hazard ratio (95% CI) for non-Hispanic blacks vs. non-Hispanic whites in these models were 2.01 (1.89–2.17), 1.84 (1.71–1.97), and 1.70 (1.58–1.83), respectively. The authors did not evaluate the influence of SES and rural residence separately. However, the sequence of adjustments and trend in the black-white disparity in prostate cancer survival are similar to that in our study.
The mechanisms underlying the association of area-level SES with prostate cancer-specific survival in these studies are not clear. However, in our study, the absence of the association in the VA suggests that healthcare system-related factors after diagnosis may be responsible. In fact, our findings suggest that a diagnosis of prostate cancer in an equal-access system protects against the adverse effects of area-based socioeconomic gradients on prostate cancer survival. These gradients may also associate with inappropriate variation in care after treatment, including the intensity of surveillance, timeliness and nature of responses to changes in cancer status, and the detection and treatment of new health problems. Unfortunately, data on these relationships are scarce and, thus, are a critical gap in our understanding of the prognostic impact of area-level SES on cancer-specific survival not only in prostate cancer but in cancer in general. Finally, area-level SES is inversely associated with allostatic load, which has been conceptualized by McEwen and Seeman as the cumulative physiologic burden imposed by stress (37–39). Measures of allostatic load typically reflect the activity of multiple health regulatory pathways such as immunity/inflammation, energy balance and others implicated in carcinogenesis and proposed as targets for cancer control (40–43). Consequently, area-level SES may have a biological influence on the course of prostate cancer either at the tumor level or through the host’s response to the disease and its treatment.
Our study has several important limitations. The cohort was derived from a case series at four Chicago area medical centers rather than a population-based sampling frame. As a result, the choice of medical centers could have exaggerated differences in census tract-level SES and survival between our African-American and non-Hispanic white case and between medical centers. Of note, our age-adjusted prostate cancer mortality hazard ratio for African Americans vs. non-Hispanic whites was 1.56 (95% CI = 1.14 to 2.14), which is comparable to that reported by others (1, 13, 14). Also, we did not account for changes in tract-level SES between diagnosis and end of follow-up or patterns of care after first-course therapy and other post-treatment factors. Consequently, we were not able use our data to further clarify potential mechanisms involved the association that we observed between census tract-level SES and prostate cancer-specific survival. Finally, the men in our study were largely diagnosed just prior to the introduction of the prostate specific antigen (PSA) test for screening and early detection. Consequently, our cohort had a higher proportion of men who were diagnosed at an advanced-stage than would be expected in the PSA era.
In conclusion, much work remains on identifying the social determinants of disparities in prostate cancer prognosis. In our study, the socioeconomic status of the census tract of residence at diagnosis predicted prostate cancer-specific survival independent of age, race/ethnicity, prognostic tumor parameters, comorbidities, and initial treatment. Tract-level SES also helped account for differences in cancer-specific survival observed between African Americans and non-Hispanic whites. Furthermore, being diagnosed in an equal access healthcare system mitigated the influence of census tract-level SES on survival. Healthcare system factors, patterns of cancer care, and tumor/host biology after initial treatment are plausible mediators of the association. Unfortunately, data on these and other potential mediating relationships are scarce. Future research should focus on identifying the underlying mechanisms for the association of census tract-level SES with prostate cancer-specific survival. The results of this study are consistent with the view that minimizing differences in healthcare access is a pathway to minimizing disparities in cancer control (44). However, our results need to be corroborated using a population-based study design that is guided by an appropriate conceptual framework for identifying social disparities in prostate cancer.
Acknowledgements
The authors are grateful support from the University of Illinois at Chicago Cancer Center.
Grant Support
National Cancer Institute (P50 CA106743-S1 to RBW),
American Cancer Society (RSGT-09-286-01-CPHPS to VLF)
Robert Wood Johnson Foundation, Harold Amos Medical Faculty Development Program, (AMFDP 033363 to VLF)
Department of Veterans Affairs, Health Services Research and Development (97–317 to VLF)
Financial Support
Freeman, VL (NIH/NCI R01CA129140; NIH/NCMHD P60MD003424; ACS RSGT-09-286-01-CPHPS)
Ricardo, AC (None to report)
Campbell, RT (NIH/NCI P50CA106743, R01 CA116750; NIH/NIA AG023424; NIH/NCMHD P60MD003424; CDC R18DP001140; ACS RSGT-09-286-01-CPHPS)
Barrett, RE (NIH/NCI P50CA106743; NIH/NCMHD P60MD003424; ACS RSGT-09-286-01-CPHPS)
Warnecke, RB (NIH/NCI P50CA106743; NIH/NCMHD P20 MD001816, P60MD003424)
Footnotes
Potential Conflicts of Interests: None
REFERENCES
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. Bethesda, MD: National Cancer Institute; 2010. [Last accessed 2/6/2011]. SEER Cancer Statistics Review, 1975–2007. http://seer.cancer.gov/csr/1975_2007/ based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Brawley OW, Jani AB, Master V. Prostate cancer and race. Curr Probl Cancer. 2007;31:211–225. doi: 10.1016/j.currproblcancer.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Evans S, Metcalfe C, Ibrahim F, Persad R, Ben-Shlomo Y. Investigating Black-White differences in prostate cancer prognosis: A systematic review and meta-analysis. Int J Cancer. 2008;123:430–435. doi: 10.1002/ijc.23500. [DOI] [PubMed] [Google Scholar]
- 4.Tewari A, Horninger W, Pelzer AE, Demers R, Crawford ED, Gamito EJ, et al. Factors contributing to the racial differences in prostate cancer mortality. BJU. 2005;96:1247. doi: 10.1111/j.1464-410X.2005.05824.x. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Kulldorff M, Devesa SS, Hayes RB, Fraumeni JF., Jr A geographic analysis of prostate cancer mortality in the United States, 1970–89. Int J Cancer. 2002;101:168–174. doi: 10.1002/ijc.10594. [DOI] [PubMed] [Google Scholar]
- 6.Klassen AC, Kulldorff M, Curriero F. Geographical clustering of prostate cancer grade and stage at diagnosis, before and after adjustment for risk factors. Int J Health Geogr. 2005;4:1. doi: 10.1186/1476-072X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Luo W. Assessing spatial and nonspatial factors in healthcare access in Illinois: Towards an integrated approach to defining health professional shortage areas. Health and Place. 2005;11:131–146. doi: 10.1016/j.healthplace.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. Ca Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 9.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. Ca Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 10.Gilligan T. Social disparities and prostate cancer: mapping the gaps in our knowledge. Cancer Causes Control. 2005 Feb;16:45–53. doi: 10.1007/s10552-004-1291-x. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian SV, Jones K, Duncan C. Multilevel methods in public health research. In: Kawachi I, Berkman L, editors. Neighborhoods and health. New York, NY: Oxford University Press; 2003. pp. 65–111. [Google Scholar]
- 12.Messer LC, Kaufman JS. “Using census data to approximate neighborhood effects.”. In: Oaks JM, Kaufman JS, editors. Methods in Social Epidemiology. San Francisco, CA: Jossey-Bass; 2006. [Google Scholar]
- 13.Robbins AS, Yin D, Parikh-Patel A. Differences in prognostic factors and survival among White men and Black men with prostate cancer, California, 1995–2004. Am J Epidemiol. 2007;166:71–78. doi: 10.1093/aje/kwm052. [DOI] [PubMed] [Google Scholar]
- 14.White A, Coker AL, Du XL, Eggleston KS, Williams M. Racial/ethnic disparities in survival among men diagnosed with prostate cancer in Texas. Cancer. 2011;117(5):1080–1088. doi: 10.1002/cncr.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macintyre S, Ellaway A. Neighborhoods and health: an overview. In: Kawachi I, Berkman L, editors. Neighborhoods and health. New York, NY: Oxford University Press; 2003. pp. 20–44. [Google Scholar]
- 16.Adler N. Institute of Medicine. Examining the health disparities research plan of the National Institutes of Health: Unfinished business. Washington DC: National Academies Press; 2006. Overview of Health Disparities; pp. 121–174. Figure D-9. [PubMed] [Google Scholar]
- 17.Freeman VL, Durazo-Arvizu R, Keys LCM, Johnson MP, Schafernak K, Patel VK. Racial differences in survival among men with prostate cancer and comorbidity at time of diagnosis. Am J Public Health. 2004 May;94(5):803–808. doi: 10.2105/ajph.94.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Classification of Diseases, Ninth Revision, Clinical Modification. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 19.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 5th ed. Philadelphia, Pa: Lippincott-Raven; 1997. [Google Scholar]
- 20.Schmidt JD, Mettlin CJ, Natarajan N, Peace BB, Beart RS, Jr, Winchester DP. Trends in patterns of care for prostatic cancer 1974–1983: results of surveys by the American College of Surgeons. J Urol. 1987;136:416–421. doi: 10.1016/s0022-5347(17)44889-0. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan N, Murphy GP, Mettlin C. Prostate cancer in blacks: an update from the American College of Surgeon’s pattern of care studies. J Surg Oncol. 1989;40:232–236. doi: 10.1002/jso.2930400406. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Alex KL, MacKenzi CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Browning CR, Cagney KA. Neighborhood structural disadvantage, collective efficacy, and self-rated physical health in an urban setting. J Health Soc Behav. 2002;43:383–399. [PubMed] [Google Scholar]
- 24.Cagney KA, Browning CR. Exploring neighborhood-level variation in asthma and other respiratory diseases: the contribution of social context. J Gen Intern Med. 2004;19:229–236. doi: 10.1111/j.1525-1497.2004.30359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Census Bureau. Washington, DC: US Dept of Commerce; 1993. US Census Bureau. 1990 Census of Population - Social and Economic Characteristics. Publication CP-2. [Google Scholar]
- 26.Szklo M, Nieto JF, editors. Epidemiology: Beyond the Basics. 2nd ed. Sudbury, MA: Jones and Bartlett; 2004. p. 17. [Google Scholar]
- 27.Diez-Roux A. The study of group-level factors in epidemiology: Rethinking variables, study designs, and analytical approaches. Epidemiol Rev. 2004;26:104–111. doi: 10.1093/epirev/mxh006. [DOI] [PubMed] [Google Scholar]
- 28.Geneva, Switzerland: World Health Organization; 1980. International Classification of Diseases, Ninth Revision. [Google Scholar]
- 29.Geneva, Switzerland: World Health Organization; 1992. International Classification of Diseases, Tenth Revision. [Google Scholar]
- 30.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. National vital statistics reports. no. 2. vol 49. Hyattsville, Maryland: National Center for Health Statistics; 2001. [Last accessed 2/17/2009]. Comparability of cause of death between ICD–9 and ICD–10: Preliminary estimates; p. 22. http://www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_02.pdf Table 1. [PubMed] [Google Scholar]
- 31.Therneau TM, Grambsch PM. Extending the Cox Model. New York, NY: Springer; 2000. Modeling Survival Data. [Google Scholar]
- 32.Belsey DA, Kuh E, Welsch RE. Regression Diagnostics. New York: John Wiley and Sons; 1980. [Google Scholar]
- 33.Statistical Analysis System (SAS), Institute, Inc., version 9.2. North Carolina: Cary; [Google Scholar]
- 34.Wei LJ, Lin DY, Weissfield L. Regression analysis of incomplete failure time data by using the marginal distributions. J Amer Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 35.Yost K, Perkins C, Cohen R, Morris C, Wight W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 36.Coker A, Eggleston K, Du XL, Ramondetta L. Ethnic disparities in cervical cancer survival among Medicare eligible women in a multiethnic population. Int J Gynecol Cancer. 2009;19:13–20. doi: 10.1111/IGC.0b013e318197f343. [DOI] [PubMed] [Google Scholar]
- 37.Merkin SS, Basurto-Dávila R, Karlamangla A, Bird CE, Lurie N, Escarce J, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol. 2009;19:194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 39.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 41.Geronimus AT, Hicken M, Keene D, Bound J. ‘Weathering’ and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005 Jul;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 43.Hursting SD, Lasinger LM, Colbert LH, Rogers CJ, Wheatley KW, Nunez NP, et al. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr Cancer Drug Target. 2007;7:484–491. doi: 10.2174/156800907781386623. [DOI] [PubMed] [Google Scholar]
- 44.Haynes MA, Smedley BD, editors. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medically underserved. Institute of Medicine; 1999. [PubMed] [Google Scholar]