Abstract
Regulatory CD4+CD25+Foxp3+ T cells (Treg) have received significant attention for their role in controlling immune responses. Although knowledge of Treg biology has burgeoned, wide gaps remain in our understanding of Treg function under both normal and pathological conditions. Pioneering studies demonstrated roles for Treg in cancer and autoimmune diseases, including experimental autoimmune encephalitis, and this knowledge is often applied to other pathologies including neurodegenerative conditions. However, differences between immunity in neurodegeneration and in malignancy or autoimmunity are often neglected. Thus, Treg manipulations in CNS neurodegenerative conditions often yield unexpected outcomes. Here, we explore how the immunology of neurodegeneration differs from that of cancer and autoimmunity and how these differences create confusion about the role of Treg in neurodegenerative conditions.
CNS injury and the immune system
Traumatic injuries in the central nervous system (CNS) are associated with an immune response. The regulation of this immune response by regulatory T cells has been intensively studied recently, but the results are equivocal, leading to great confusion in the field. We first provide here an introduction on CNS injury and describe a role for the immune system in CNS pathology and then discuss the regulation of CNS immunity and the associated confusion.
CNS injuries
Research on injuries to the CNS has yielded a vast amount of basic knowledge in recent years, but development of effective therapies in this area is still desperately needed. Acute mechanical or biochemical injury to the mammalian CNS often results in irreversible functional deficit [1] because of the poor ability of injured axons to regrow and a destructive cascade of events that causes the damage to spread to neurons that had escaped the primary injury [2]. This spread of damage, or secondary degeneration, is attributed to devastating changes in metabolic, oxidative, and trophic factors that inevitably follow the primary cellular damage [3]. Attempts to promote CNS recovery have focused on two goals: neuroprotection (the arrest of self-perpetuating degeneration) and stimulation of regrowth [2, 4]. In the absence of intervention, the extent of recovery is a function of the amount of tissue that escapes the initial injury minus the loss associated with the subsequent neurodegenerative process. This secondary loss of tissue makes the outcome significantly worse than could have been predicted by the severity of an initial injury and is shared across different CNS injury models (Box 1).
Box 1. Commonly used CNS injury models in rodents.
-
Primary damage is due to direct mechanical injury
Large area of secondary degeneration, killing previously uninjured neurons
Displays both apoptotic and necrotic cell death
Pure white matter injury; secondary degeneration of cell bodies (retinal ganglion cells) in the retina
Blood-brain barrier breakdown
Moderate T cell infiltrate
-
Primary damage is due to direct mechanical injury
Large area of secondary degeneration, killing previously uninjured neurons
Displays both apoptotic and necrotic cell death
Mixed grey and white matter degeneration in both primary and secondary insults
Blood-brain barrier breakdown
Moderate T cell infiltrate
-
Primary injury is due to loss of blood flow to CNS
Large area of secondary degeneration, killing previously uninjured neurons
Displays both apoptotic and necrotic cell death
Can affect grey or white matter, depending on the location of infarct: most common experimental stroke affects both grey and white matter
Blood-brain barrier breakdown
Moderate T cell infiltrate
-
Primary injury is due to pressure wave from explosion
Extent of secondary degeneration is largely unknown
Displays both apoptotic and necrotic cell death
Complete pathogenesis unknown, thought to be a diffuse axonal injury initially
Blood-brain barrier breakdown
Moderate T cell infiltrate
Lack of regeneration in the mammalian CNS
Unlike mammals, lower vertebrates are able to undergo extensive recovery after CNS injuries, even reconstituting the entire caudal end of a transected spinal cord [5]. The most obvious reason for this difference is that lower vertebrates are capable of neurogenesis, in which new neurons are produced from radial glia [6]. Other factors causing loss of neuroregenerative capacity in the mammalian CNS are mainly extrinsic to neurons, such as the production of inhibitory molecules by astrocytes and oligodendrocytes [7, 8] including Nogo, myelin associated glycoprotein (MAG), and oligodendrocytes myelin glycoprotein (OMgp) families, whose neutralization leads to an increase in axonal outgrowth [9].
Innate immune response to CNS injury
The brain has long been considered an “immune-privileged” site, protected by a blood-brain barrier (BBB) that is impenetrable to immune mediators, and hence seemingly unaffected by the immune system (other than resident microglia) [10]. It is now recognized, however, that activated immune cells can access the intact brain [11] and when the BBB breaks down after CNS trauma, a massive infiltration of immune cells to the CNS parenchyma is evident. Mechanisms contributing to the breakdown of the BBB include injury-induced immediate damage to the vasculature, as well as later damage due to inflammatory, apoptotic, and metabolic causes [12]. As soon as a few hours after injury, the pro-inflammatory cytokines interleukin (IL)-1β, tumor necrosis factor alpha (TNFα) and IL-6, which are secreted copiously into the injured tissue, bind to endothelial, neuronal, and glial cell receptors and upregulate the production of inflammatory mediators [13, 14], leading in turn to neutrophil influx [15]. As a consequence, the normally low expression of class I and II major histocompatibility complex (MHC) proteins in astrocytes and microglia is increased [16], enabling the injured tissue to initiate and sustain an immune response from the adaptive immune system. Adhesion molecules, also upregulated after injury, regulate the temporal segregation of infiltration by innate and adaptive immune cells, so that neutrophils enter the CNS shortly after injury but by 48 hours have greatly decreased in number [17]. In contrast, macrophages infiltrate 2 to 5 days post-injury and lymphocytic infiltration peaks on day 7 [18].
Mechanisms of peripheral and central tolerance
Adaptive immune responses are controlled at several levels throughout the body. First, during T-cell development a process of central tolerance occurs in the thymus, which results in deletion of many self-antigen specific T cells. In this process, thymocytes are positively selected for either CD4 or CD8 expression, then undergo negative selection in which cells with high avidity are deleted by apoptosis upon recognition of peptide–MHC complexes (including self-peptide–MHC complexes) expressed on dendritic cells or on thymic medullary epithelial cells [19].
Not all self-reactive T cells are deleted through central tolerance, however, as some receptors recognize low-avidity self-peptide–MHC complexes [20]. Control by naturally occurring regulatory CD4+CD25+Foxp3+ T cells (Treg), via prevention of peripheral T cell activation, is the most intensively studied mechanism for regulating the response of these self-reactive cells that have survived thymic selection. These cells have received a great deal of new interest due to their absence in the scurfy mouse mutant, which dies of devastating autoimmune disease by the age of 3 weeks. The mutation was traced to the X chromosome and mapped to the gene encoding the transcription factor forkhead box P3 (Foxp3) [21]. Expression of Foxp3 is sufficient for CD4+ lymphocytes to acquire a suppressive phenotype in mice [21], and conditional ablation of Foxp3 in lymphocytes confers a multisystem autoimmune disease with massive lymphoproliferation which, as in the scurfy mouse, kills the animal at about 3 weeks of age [21].
Regulatory and effector T cells
Regulatory T cells
Although Treg display the same T cell receptors as CD4+Foxp3− effector T cells (Teff), unlike Teff they do not proliferate in response to antigen presentation in vitro. Instead, they are stimulated to divide in response to IL-2, whose production is a hallmark of activated Teff, in vitro; in fact, some Tregs proliferate in response to IL-2 in vivo [22]. Although Treg constitutively express the high-affinity IL-2 receptor CD25, they are unable to produce IL-2 on their own [22]. Indeed, it was surprising to find that half of all Il2 knock-out mice died of autoimmune disease by 9 weeks of age [23] until it was realized that Treg require IL-2 to survive [22]. This constitutive expression of CD25 is thought to be one of the ways by which Treg suppress proliferation of Teff, that is, by acting as a sink for IL-2, which is also needed for Teff proliferation. Treg do require antigen stimulation to become suppressive, but once activated their suppressive ability seems not to be antigen-specific [24]. Whereas Treg can apparently utilize various mechanisms to suppress Teff, the most strongly suppressive mechanisms are contact-dependent [25]. Treg utilize the anti-inflammatory signaling molecule transforming growth factor (TGF)-β both by secreting it and expressing it on their surface [25]. One mechanism of TGFβ-mediated suppression is its ability to affect the differentiation of naïve T cells [26]. TGFβ, in the absence of other inflammatory signals, is able to induce Foxp3 expression and confer a suppressive phenotype to these cells. This action can be a double-edged sword because TGFβ in combination with the inflammatory cytokines IL-6 and IL-22 is able to induce IL-17 expression in T cells, a phenotype that has been associated with autoimmune disease and inflammation [27].
The ability of Treg to suppress immune responses is not constant, but can be modulated by signals from their extracellular environment. For example, after burn injuries the suppressive ability of Treg is decreased because of changes in both their secretion of anti-inflammatory cytokines and their surface expression of TGF-β. What kinds of signals can induce this change in suppression? There are a few examples of signals that increase Treg suppressive ability; the best studied is IL-2 (also known for its role in Treg development) [28]. By contrast, a multitude of factors are known to decrease the suppressive capacity of these cells. They include pro-inflammatory cytokines produced by lipopolysaccharide-activated APCs [29], TNF [30], B cells [31], glucocorticoid-induced TNF receptor ligands [32], IL-4 [33], Toll-like receptor (TLR)-2 signaling through Treg [34], and two factors suggested to be important in CNS trauma, namely, unmethylated CpG-island rich DNA segment signaling through TLR9 [35] and the neurotransmitter dopamine [36].
Autoimmune T cells are beneficial after CNS injury
Self-antigen-specific T cells are not exclusive to patients with autoimmune disease; T cells reactive against self-antigens also are commonly found in healthy controls [37]. Because of the toll taken by autoimmune diseases, it would appear to make sense biologically if such self-reactivity provided an evolutionary advantage. Whereas the role of self-reactive cells in fighting cancer has been known for some time [38], the role of the adaptive immune system in other sterile situations did not emerge until later. One of the first clues that self-reactivity by the adaptive immune system could be beneficial came from CNS injury models: passive immunization with T cells specific for myelin basic protein (MBP) increased neuronal survival after optic nerve crush while those without specificity to antigen that is presented in the CNS did not show benefit [39]. This seminal finding suggested that MBP-specific T cells, which cause experimental autoimmune encephalomyelitis (EAE) in susceptible strains, can actually be beneficial after CNS injury in both EAE-susceptible and resistant strains, provided that the immune response is well controlled. In fact, it seemed that this injury-attenuating response was not exclusive to MBP. Indeed, the proteolipid protein (PLP) and the myelin oligodendrocyte glycoprotein (MOG), both associated with myelin, could also exert beneficial effects in mice actively immunized with epitopes from these proteins prior to CNS injury [40]. The beneficial effects of CNS-directed adaptive immune responses to injury have by now been tested using several different experimental paradigms [41]. Further illustrating the importance of the adaptive immune system, neuronal survival after CNS injury in nude mice (which lack mature T cells) was significantly decreased relative to neuronal survival in similarly injured normal controls. The decrease in survival was reversed, however, upon adoptive transfer of T cells from naïve mice [42].
The identity of the cell type that mediates this beneficial effect after CNS injury is not yet known, and literature reports are conflicting. Some studies have indicated that TH1 cells have the greatest potential for such neuroprotection [43], whereas others suggest that the vital part of recovery from CNS injury derives from soluble molecules from TH2 cells [44]. In the absence of additional in vivo studies that corroborate either of these claims, this remains an area requiring further investigation.
The role of Treg and autoimmune T cells in autoimmune diseases and in cancer has been intensively studied. The best evidence regarding Treg and autoimmune Teff cell function in the CNS is available through extensive research with the EAE model, a commonly used model of multiple sclerosis, [45, 46], as well as from studies on experimental autoimmune uveitis (EAU) [47]. Although there is a neurodegenerative component in human multiple sclerosis and in some models of EAE, here we refer to EAE as an “autoimmune” and not as a “neurodegenerative” disorder. The autoimmune components in the animal models are crucial for disease initiation (we do not discuss here the EAE model induced by Theiler’s virus [48]) and therefore, it is plausible that induction of Treg would benefit the disease pathology, as has been previously described [49, 50]. In this manuscript, however, we propose that the differences between the nature of immunity in neurodegenerative conditions, both acute (physical injury) and chronic (e.g., Alzheimer’s, and Parkinson’s), and in malignancy or autoimmunity (including EAE) are often neglected. The basic principles of Treg biology are “borrowed” from other fields and applied to injury or chronic neurodegenerative conditions, which often results in unexpected outcomes and overall confusion. Below, we discuss the “confusion” in more details.
The simple, the complex, and the confused
Treg in CNS injury: the simple
Early experiments, though lacking the sophisticated methods available today, hinted that the presence of Treg limited the beneficial potential of autoimmune Teff [42]. The techniques used in those studies did not rely on the targeting of specific molecules, given that they were performed at a time when the molecular characteristics of Treg were just beginning to emerge. Although these techniques could not provide unequivocal answers, they were able to demonstrate a drop in Treg numbers and an increase in the proportion of Teff to be associated with increased neuronal survival after CNS injury. As an example, a three-day postnatal thymectomy resulted in a decrease in Treg relative to Teff in adult mice, permitting increased post-injury neuroprotection compared to non-thymectomized controls [42]. Similarly, low-dose irradiation, which triggers apoptosis preferentially in Treg and promotes proliferation of Teff, increased neuronal survival after injury to either the rodent optic nerve or spinal cord and following glutamate-mediated toxicity of retinal ganglion cells [51]. Moreover, several compounds that decrease the number or activity of Treg have also shown neuroprotective effects. Dopamine, which can decrease the suppressive ability of Treg through ERK1/2 signaling, increases neuroprotection when administered systemically [36]. In a more immune-relevant paradigm, the synthetic, bacterial-DNA mimetic CpG modulated the regulatory capacity of Treg, presumably by acting through Toll-like receptors, and increased neuronal survival by decreasing immune suppression [35]. The results of these experiments collectively support the simple hypothesis that Teff are required for neuroprotection after CNS injury and that this ongoing response is limited by Treg. In line with this hypothesis, the limiting of the beneficial Teff response by Treg can be viewed as “the evolutionary compromise between a need and a risk” [52].
Treg in CNS injury: the complex
The real situation, however, is not always that simple and cannot always be explained in terms of Treg-imposed restraint on a beneficial, but potentially risky, autoimmune response to injury. In Balb/c mice, transfer of splenocytes depleted of CD25+ cells into nude mice increased the beneficial effect on neuronal survival beyond the benefit that could be achieved by transferring whole splenocytes. In line with this, transfer of isolated CD4+CD25+ cells (i.e., Treg) from naive mice into injured, immune-competent Balb/c mice partially abolished the beneficial neuroprotective effect [53]. However, transfer of the same CD4+CD25+ population resulted in the opposite phenotype when the recipient mice were of the C57/B6 background; that is, neuroprotection in the C57/B6 background was increased following injection of exogenous Treg [53]. This finding was surprising because when the same two strains were subjected to immunization experiments with retina-specific antigens, neuronal survival in both strains was increased [53]. These results suggest that the nature of the endogenous response to injury in C57/B6 mice differs from that in Balb/c mice and that Treg manipulation might lead to neurodestruction or neuroprotection based on the genetic background of the recipient mouse. Interestingly, when Treg were transferred into injured, immune-compromised mice, the treatment was beneficial in mice of both genetic backgrounds. Moreover, upon injection into an immune-deficient host these Treg not only lose their suppressive activity [54] but also undergo proliferation and acquire effector function. It is, therefore, questionable whether these studies measure the effect of the injected CD4+CD25+ Tregs directly or the effect of the cells they differentiate into within immune-deficient hosts. Clearly, the nature of this “Treg” population requires further experimental clarification.
The findings described above raise concern with regard to the development of Treg-based therapies for clinical use. At this point it is obvious that we do not completely understand the complexity of Teff/Treg interactions in situations involving CNS injury or neurodegenerative disorders. Therefore, instead of a rush to examine the protective activity of Treg under different neurodegenerative conditions, what is needed is a thorough study and systematic analysis of the interactions between the cellular participants to understand the complexity.
Treg in CNS injury: the confused I (strains and immunity)
As noted above, confusion still reigns with regard to Treg function, and particularly to the protective properties of these cells under acute and chronic neurodegenerative conditions. Because most of the present knowledge in neuroimmunology comes from early studies on animal models of multiple sclerosis, both the commonly used cell lines and the acquired data are dominated by strains susceptible to autoimmune disease. Furthermore, because most existing transgenic and knockout mice are on a B6 background, this is the most intensively studied strain in neuroimmunogical investigations of CNS injury and chronic neurodegeneration. As described above, Treg have opposite effects when transferred into Balb/c and C57Bl/6J mice, and their effects after injury in other strains are as yet unknown. This finding alone should raise questions among experimenters as to the most relevant animal model of CNS neurodegeneration for use in studies of the immune response. The use of several mouse strains in any study carried out to support the claims for a neuroprotective therapy or agent is probably necessary. The failure to transfer most of the proposed therapies from the laboratory to the clinic might be due in no small measure to a failure to follow this practice. Moreover, mice that are lymphopenic or otherwise immune-compromised might not be reliable models for addressing the role of Treg in neurodegeneration because the basic properties of Treg change in immune-compromised hosts. Efforts at this stage should be aimed at understanding the immunobiology of Treg under neurodegenerative conditions (both in immune-competent and immune-compromised hosts), before translation into human therapeutics is considered. At some point in the future we might then be justified in shifting the focus to clinical trials of Treg-based neuroprotective therapies.
Treg in CNS injury: the confused II (anti-CD25 antibody)
The discovery that CD25 is highly expressed on Treg led researchers to realize that the use of antibodies against this molecule would result in Treg clearance via the FcγRIII-mediated mechanism [55]. Use of anti-CD25 antibody resulted in exacerbation of the infarct zone and functional deterioration in a stroke model of CNS injury [56], leading to the conclusion that depletion of Treg results in impaired recovery and that Treg are needed for neuroprotection after stroke. However, CNS injury results in presentation of self-antigens, leading to Teff activation and proliferation. Activated effector T cells express CD25, therefore, while CD25 serves as a marker for naturally occurring Treg in naive animals, it cannot distinguish between Treg from activated Teff in “challenged” mice, such as after CNS injury. Thus, mice that are treated with anti-CD25, which targets and depletes Treg in the initial stages of treatment will exhibit a faster Teff response (Teff are activated and proliferate more efficiently when Treg are removed). However, the anti-CD25 antibody is cleared slowly and will thus eventually target the emerging, activated, CD25-expressing Teff and result in their depletion as well. Therefore, exacerbation of CNS injury using anti-CD25 antibody does not necessarily imply that Treg are needed for a better recovery; it might also suggest that activated Teff, also eliminated by the antibody, are needed for neuroprotection. With the development of alternative tools to study the effect of Treg depletion, such as the expression of the diphtheria toxin receptor controlled by the Foxp3 promoter [57], many of the experiments whose results have been equivocal because of this dual targeting of the anti-CD25 antibody [56, 58, 59] need to be re-evaluated to determine the effect of each target individually.
Concluding remarks
A population of suppressor T cells were known to exist for many years, but the markers with which they could be recognized had not been identified [60]. This population was rediscovered as regulatory T cells approximately a decade ago. Treg express both CD25 (also expressed by activated Teff) and the transcription factor Foxp3 (uniquely expressed by Treg) [61, 62]. Early studies suggest opposite activities for Treg function in autoimmune disease and cancer: an enhanced functioning of Treg would be needed in autoimmune diseases to suppress the ongoing inflammatory response, whereas in cancer Treg function would need to be attenuated, allowing effector T cells to be easily activated to fight off the malignancy (Figure 1). Since that early period, a role for Treg has been proposed in nearly every clinical condition. In the field of neurodegeneration, there is some consensus that Treg regulate the response to CNS injury [63, 64]. The original studies on Treg in CNS injuries [42] suggested that Treg activity serves as a restraining self-checking mechanism to prevent excessive reaction on the part of the beneficial immunity activated spontaneously in response to CNS injury. Subsequent studies assigned a more direct neuroprotective role to Treg, bypassing the immune response. It was proposed that Treg, based on their suppressive function in the periphery, also suppress the characteristic glial response to injury in the CNS, assumed to be destructive to neuronal survival [53]. Although the data obtained from animal models in these later studies is intriguing, it is important to remember that after an injury to the CNS, the spontaneous immune response itself plays a role in the overall effect that injected, activated Treg will exert on neuronal survival. The answer, as is typical in biology, depends on the timing and strength of the response and how the response-related signals integrate with the signals of their downstream target cells, the Teff, to achieve an immune response that is robust and properly controlled (Figure 1). Because these parameters have yet to be verified, they preclude, at least at this point, any application of potential Treg therapies in clinical situations. The good news is that after a decade of largely inconclusive findings on the precise role of Treg in CNS injury, we are reaching a point where newly available molecular tools should facilitate more elegant studies of these cells and help to clear up the remaining confusion and controversy (Box 2).
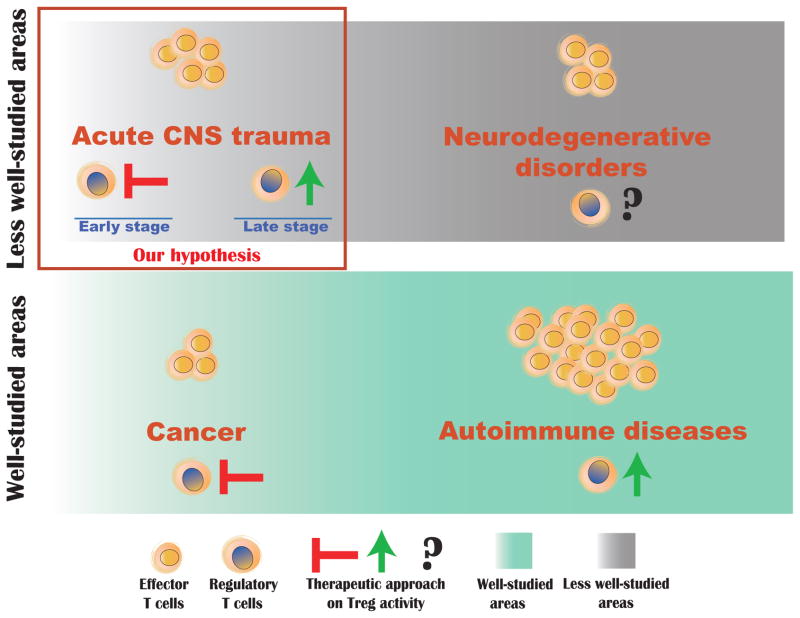
Figure 1. Model of proposed Treg-based therapeutic interventions in different pathological conditions.
While the role of Treg is well studied in cancer and autoimmune diseases (green shaded areas), its role in CNS acute and chronic neurodegenerative conditions (grey shaded) is understudied. In autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE), increasing suppression of Tregs would ameliorate symptoms of the disease, whereas inhibiting the Treg suppression would be the therapeutic aim in cancer. We propose that in acute CNS injury Treg suppression needs to be inhibited immediately after injury to allow a beneficial autoimmune response, but then in the late stage of injury this suppression needs to be reinstated to prevent destructive autoimmune attack from developing.
Box 2. Outstanding questions in the field.
Is the adaptive immune system playing different roles in grey matter injury and white matter injuries?
Is the adaptive immune system playing different roles in traumatic and ischemic injuries?
What are the roles of adaptive vs. innate immune responses in CNS injuries?
Do Tregs have any direct effect on neuroprotection, or do they only work via suppressing Teff and what is the mechanism of T cell mediated neuroprotection?
Is the timing of Treg depletion vital for neuroprotection?
Are certain CNS antigens more neuroprotective than others?
Are T cells initially activated in the tissue or in the draining lymph nodes?
Glossary.
White matter – areas in the brain containing myelinated axons in the CNS. The most prominent in the brain is the corpus callosum, which connects the two hemispheres
Grey Matter – areas of the brain containing cell bodies and unmyelinated axons
Ischemia – a decrease in blood flow, as seen in stroke
Infarct zone – area damaged by an ischemic event
FCγRIII – a receptor on innate immune cells that recognizes antibody and which mediates phagocytosis of material that is bound to an antibody
Lymphopenic – having a decreased number of circulating lymphocytes
Retinal ganglion cells – the retinal neurons whose axons make up the optic nerve
Experimental autoimmune encephalomyelitis – experimental model of multiple sclerosis induced by passive transfer of CNS-antigen specific T cells or immunization with CNS antigens
Experimental autoimmune uveitis – autoimmune model of the eye induced by immunization with retinal antigens or passive transfer of T cells specific to retinal antigens
Thymectomy – removal of the thymus
Lymphoproliferation – an expansion of lymphocytes
Thymic medullary epithelial cells – cells in the thymus that are important in maintaining self-tolerance. These cells express AIRE, and therefore can display an expanded repertoire of self-antigens
Parenchyma – The area of the brain inside the BBB, consisting of astrocytes, oligodendrocytes, microglia, and neurons
Foxp3 – a transcription factor that regulates a set of genes, including CTLA4 and TGFβ, conferring suppressive ability to CD4+ lymphocytes
CD25 – alpha chain of IL-2 receptor. Expressed on all Treg and on activated Teff cells (as well as some other leukocytes)
Acknowledgments
We thank Shirley Smith for editing the manuscript. This work was supported in part by an award to J. Kipnis from the National Institute of Neurological Disorders and Stroke (R01NS061973).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERNCES
- 1.Mizell M. Limb regeneration: induction in the newborn opossum. Science. 1968;161:283–286. doi: 10.1126/science.161.3838.283. [DOI] [PubMed] [Google Scholar]
- 2.Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 3.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 4.Thuret S, et al. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MJ, Waxman SG. Caudal spinal cord of the teleost Sternarchus albifrons resembles regenerating cord. Anat Rec. 1983;205:85–92. doi: 10.1002/ar.1092050111. [DOI] [PubMed] [Google Scholar]
- 6.Walder S, et al. Up-regulation of neural stem cell markers suggests the occurrence of dedifferentiation in regenerating spinal cord. Dev Genes Evol. 2003;213:625–630. doi: 10.1007/s00427-003-0364-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen MS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 8.Rolls A, et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 2008;5:e171. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nat Rev Neurosci. 2009;10:713–723. doi: 10.1038/nrn2707. [DOI] [PubMed] [Google Scholar]
- 10.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 11.Hickey WF, et al. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 12.Abbott NJ, et al. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 13.Lee SC, et al. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 14.Sawada M, et al. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 15.Wong D, Dorovini-Zis K. Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J Neuroimmunol. 1992;39:11–21. doi: 10.1016/0165-5728(92)90170-p. [DOI] [PubMed] [Google Scholar]
- 16.Lassmann H, et al. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28:236–243. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- 17.Guth L, et al. The unique histopathological responses of the injured spinal cord. Implications for neuroprotective therapy. Ann N Y Acad Sci. 1999;890:366–384. doi: 10.1111/j.1749-6632.1999.tb08017.x. [DOI] [PubMed] [Google Scholar]
- 18.Popovich PG, et al. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Klein L, et al. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 20.Benichou G, et al. Immunogenicity and tolerogenicity of self-major histocompatibility complex peptides. J Exp Med. 1990;172:1341–1346. doi: 10.1084/jem.172.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontenot JD, et al. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 22.Setoguchi R, et al. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignali DAA, et al. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Stockinger B, et al. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Kohm AP, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 29.Kubo T, et al. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 30.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piao J, et al. Enhancement of T-cell-mediated anti-tumour immunity via the ectopically expressed glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) on tumours. Immunology. 2009;127:489–499. doi: 10.1111/j.1365-2567.2008.03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S, et al. B cell-deficient NOD. H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pace L, et al. Cutting edge: IL-4-induced protection of CD4+CD25-Th cells from CD4+CD25+ regulatory T cell-mediated suppression. J Immunol. 2006;176:3900–3904. doi: 10.4049/jimmunol.176.7.3900. [DOI] [PubMed] [Google Scholar]
- 34.Sutmuller RP, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson TV, et al. Bacterial DNA confers neuroprotection after optic nerve injury by suppressing CD4+CD25+ regulatory T-cell activity. Invest Ophthalmol Vis Sci. 2007;48:3441–3449. doi: 10.1167/iovs.06-1351. [DOI] [PubMed] [Google Scholar]
- 36.Kipnis J, et al. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. J Neurosci. 2004;24:6133–6143. doi: 10.1523/JNEUROSCI.0600-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzanti B, et al. T-cell response to myelin basic protein and lipid-bound myelin basic protein in patients with multiple sclerosis and healthy donors. J Neuroimmunol. 1998;82:96–100. doi: 10.1016/S0165-5728(97)00194-X. [DOI] [PubMed] [Google Scholar]
- 38.Finn OJ. Molecular origins of cancer - Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 39.Moalem G, et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 40.Fisher J, et al. Vaccination for neuroprotection in the mouse optic nerve: Implications for optic neuropathies. J Neurosci. 2001;21:136–142. doi: 10.1523/JNEUROSCI.21-01-00136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beers DR, et al. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kipnis J, et al. Neuroprotective autoimmunity: Naturally occurring CD4(+)CD25(+) regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kipnis J, et al. Myelin specific Th1 cells are necessary for post-traumatic protective autoimmunity. J Neuroimmunol. 2002;130:78–85. doi: 10.1016/s0165-5728(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 44.Wolf SA, et al. Neuroprotection by T-cells depends on their subtype and activation state. J Neuroimmunol. 2002;133:72–80. doi: 10.1016/s0165-5728(02)00367-3. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor RA, Anderton SM. Foxp(3+) regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, et al. TGF-beta-Induced Myelin Peptide-Specific Regulatory T Cells Mediate Antigen-Specific Suppression of Induction of Experimental Autoimmune Encephalomyelitis. J Immunol. 2010;184:6629–6636. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon EJ, et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 49.Cabbage SE, et al. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J Immunol. 2007;178:887–896. doi: 10.4049/jimmunol.178.2.887. [DOI] [PubMed] [Google Scholar]
- 50.Reddy J, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kipnis J, et al. Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur J Neurosci. 2004;19:1191–1198. doi: 10.1111/j.1460-9568.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz M, Kipnis J. Autoimmunity on alert: naturally occurring regulatory CD4(+)CD25(+) T cells as part of the evolutionary compromise between a ‘need’ and a ‘risk’. Trends Immunol. 2002;23:530–534. doi: 10.1016/s1471-4906(02)02322-0. [DOI] [PubMed] [Google Scholar]
- 53.Kipnis J, et al. Dual effect of CD4(+)CD25(+) regulatory T cells in neurodegeneration: A dialogue with microglia. Proc Natl Acad Sci U S A. 2004;101:14663–14669. doi: 10.1073/pnas.0404842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavin MA, et al. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 55.Setiady YY, et al. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 56.Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 57.Lahl K, et al. Selective depletion of Foxp3(+) regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren X, et al. CD4(+)FoxP3(+) regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2010 doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tenorio EP, et al. Depletion with PC61 mAb before Toxoplasma gondii infection eliminates mainly Tregs in BALB/c mice but activated cells in C57BL/6J mice. FEMS Immunol Med Microbiol. 2011 doi: 10.1111/j.1574-695X.2011.00805.x. [DOI] [PubMed] [Google Scholar]
- 60.Rich RR, Pierce CW. Biological expressions of lymphocyte activation. II Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973;137:649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 62.Sakaguchi S, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 63.Huang XY, et al. CD 4+T cells in the pathobiology of neurodegenerative disorders. J Neuroimmunol. 2009;211:3–15. doi: 10.1016/j.jneuroim.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu YW, et al. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz M. Optic nerve crush: protection and regeneration. Brain Res Bull. 2004;62:467–471. doi: 10.1016/S0361-9230(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 66.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 67.Springer JE, et al. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 68.Lo EH, et al. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 69.Tu W, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abrahamson EE, et al. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp Neurol. 2006;197:437–450. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 71.DeKosky ST, et al. Traumatic brain injury--football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]