Abstract
Background
The benefit of implantable defibrillators (ICDs) for primary prevention remains debated. We analysed the implications of prophylactic ICD implantation according to the guidelines in 2 tertiary hospitals, and made a healthcare utilisation inventory.
Methods
The cohort consisted of all consecutive patients with coronary artery disease (CAD) or dilated cardiomyopathy (DCM) receiving a primary prophylactic ICD in a contemporary setting (2004–2008). Follow-up was obtained from hospital databases, and mortality checked at the civil registry. Additional data came from questionnaires sent to general practitioners.
Results
There were no demographic differences between the 2 centres; one had proportionally more CAD patients and more resynchronisation therapy (CRT-D). The 587 patients were followed over a median of 28 months, and 50 (8.5%) patients died. Appropriate ICD intervention occurred in 123 patients (21%). There was a small difference in intervention-free survival between the 2 centres. The questionnaires revealed 338 hospital admissions in 52% of the responders. Device-related admissions happened on 68 occasions, in 49/276 responders. The most frequently reported ICD-related admission was due to shocks (20/49 patients); for other cardiac problems it was mainly heart failure (52/99). Additional outpatient visits occurred in 19%.
Conclusion
Over a median follow-up of 2 years, one fifth of prophylactic ICD patients receive appropriate interventions. A substantial group undergoes readmission and additional visits. The high number of admissions points to a very ill population. Overall mortality was 8.5%. The 2 centres employed a similar procedure with respect to patient selection. One centre used more CRT-D, and observed more appropriate ICD interventions.
Keywords: Complications, Coronary artery disease, Dilated cardiomyopathy, Health care resources, Implantable cardioverter defibrillator, Primary prevention, Mortality, Sudden cardiac death, Ventricular arrhythmias
Introduction
Implantable defibrillator (ICD) therapy is without doubt the most effective therapy available to prevent sudden cardiac death (SCD) in selected patient groups [1–5]. Convincing evidence of the effectiveness of primary prophylactic ICD therapy exists for coronary artery disease (CAD) and dilated cardiomyopathy (DCM) [6–10]. Like most state-of-the-art therapeutic modalities, ICD therapy is costly [11–17]. With the current improvement in heart failure therapy, the recent increased number of device recalls, and the trend towards more lead failure, the debate about prophylactic ICD therapy has shifted from ‘effectiveness’ toward ‘cost-effectiveness’ [18–22]. Nevertheless, some authors remain convinced that a serious underutilisation continues to exist in Europe [23, 24].
The costs of a therapy cannot be assessed without assessing its clinical benefit. In fact, only few modern therapies—mostly those that prevent costly events—are cost-effective. In contrary, ICD therapy prevents SCD, an event that costs little to nothing to treat, when an ICD is in place [25]. Cost-effectiveness analyses showed that a large part of the lifetime costs of a primary prophylactic ICD patient occur at the time of ICD implantation. However, it takes years to accrue the benefit of a primary prophylactic ICD since this effect is time-dependent. In this perspective, the health care costs that accumulate during these years (i.e. hospitalisation costs, costs for unscheduled outpatient consultations) should also be taken into account when analysing the cost-effectiveness, especially when considering that primary prevention ICD candidates are heart failure patients with a potential high need for specialised care. Unfortunately, no data on the burden of medical care are available in a real world setting for this kind of patients in the Netherlands.
The aim of this study was to analyse the frequency of hospitalisation and unscheduled outpatient consultations in primary prophylactic ICD patients, as documented in the hospital records of 2 major university hospitals, supplemented with data obtained with a survey among general practitioners, who could provide data on otherwise unknown admissions in other institutions.
Methods
Study population
The study population consisted of all consecutive patients with CAD or DCM, who received an ICD according to the ESC/AHA/ACC 2006 guidelines for primary prophylaxis of sudden cardiac death in the Erasmus MC between January 2004 and July 2008, and of all similar consecutive patients in the Amsterdam University Medical Centre (AMC) in the year 2006. The presence of CAD had to be documented clinically by a history of a myocardial infarction according to the definitions by ACC/AHA/ESC, if coronary artery bypass grafting (CABG) or a percutaneous coronary intervention (PCI) had been performed, or if significant coronary artery stenosis was documented with coronary angiography. DCM was defined as a primary myocardial disease with dilatation without valvular or congenital aetiology. Patients with channelopathies and specific diseases as arrythmogenic right ventricular cardiomyopathy and the Brugada syndrome were excluded. All ICDs were implanted by a transvenous technique in the cardiac catheterisation laboratory, or if necessary by a cardiac surgeon (e.g. for placement of epicardial leads).
Data collection
Baseline characteristics included age, gender, underlying disease, presence of prior myocardial infarction, and left ventricular function at the time of ICD implantation. Follow-up started at the time of ICD implantation, with conventional ICD control. All patients were followed at 3-month intervals and were advised to contact the outpatient clinic after a symptomatic event. At each follow-up visit, arrhythmic events with stored electrograms (EGMs) were retrieved from the device’s memory. Two independent reviewers analysed the stored electrograms to classify the arrhythmia and assess the appropriateness of device classification and therapy. In case of disagreement, a third reviewer was consulted to provide the final diagnosis.
The electronic hospital files and the electronic ICD database were reviewed to record and understand the impact of complications. Procedure-related complications were defined as occurring within 30 days after implantation.
In order to make a better health care utilisation inventory, we sent questionnaires to the general practitioners of 464 of the included patients (all AMC and the first 355 Erasmus MC patients). This questionnaire examined the frequency and duration of hospital admissions for ICD-related problems, hospital admissions for non-ICD related cardiac problems, and hospital admissions for other conditions. We also asked for the number of additional outpatient visits. Regular outpatient control visits and admissions for elective ICD generator replacements because of battery depletion were not taken into account. Follow-up for vital status was obtained by consulting the civil registry. The duration of the hospitalisation was only obtained from the AMC sample. Follow-up was completed until 31 December 2008.
Statistical analysis
Continuous variables are expressed as the mean ± SD, or as the median with the interquartile range (IQR) when not normally distributed. Categorical variables are expressed as frequency (percentage). Group differences were analysed with Chi-square tests and independent sample T-tests where appropriate. The assumption of normality was checked for all variables tested. If not normally distributed, we used non-parametric tests. Alpha was set at 0.05 for all statistical tests. Cumulative survival and event-free rates of appropriate ICD therapy were calculated according to the Kaplan-Meier method and were compared with the log-rank test. Patients who underwent heart transplantation were censored alive from the moment of transplantation.
Results
Study population
The study population consisted of a total group of 587 patients: 478 patients originated from the Erasmus MC Rotterdam, 109 patients from the AMC Amsterdam. The baseline characteristics of the study population are presented in Table 1. The median follow-up till death or the closure date was 28 months (IQR 14–37 months). The follow-up of the Erasmus MC patients was shorter (Table 2). There were slightly more patients with CAD in the group from the Erasmus MC. The mean ejection fraction was the same. There were 5% of the patients in NYHA class I, 51% in NYHA class II, 44% in class III, and only 1% in class IV. Heart transplantation was performed in 17 patients of a total of 41 patients who were on the transplantation list of the Erasmus MC. There were significant differences in the device type used in the two centres, with the Erasmus MC using more cardiac resynchronisation therapy systems with defibrillation (CRT-D).
Table 1.
Baseline characteristics
| Characteristic | Erasmus MC n = 478 | AMC n = 109 | p value |
|---|---|---|---|
| Mean follow-up (days) | 762 ± 446 | 969 ± 185 | <0,0001 |
| Age (years) | 59 ± 12 | 61 ± 12 | NS |
| Male gender | 374 (78%) | 84 (77%) | NS |
| Coronary artery disease | 319 (67%) | 61 (56%) | 0.023 |
| Dilated cardiomyopathy | 159 (33%) | 45 (41%) | |
| LVEF (%) | 25 ± 8 | 22 ± 6 | NS |
| ICD type | |||
| Single chamber | 200 (42%) | 65 (61%) | <0.001 |
| Dual chamber | 74 (15%) | 38 (35%) | |
| CRT-D | 204 (43%) | 4 (4%) | |
CRT-D cardiac resynchronisation therapy defibrillator; LVEF left ventricular ejection fraction; NA not available
Table 2.
Follow-up data from hospital records
| Characteristic | Erasmus MC n = 478 | AMC n = 109 | p value |
|---|---|---|---|
| Mean follow-up (days) | 762 ± 446 | 969 ± 185 | <0.0001 |
| Appropriate shocks/ATP | 105 (22%) | 18 (16.5%) | NS |
| Mean time to first appropriate event (days) | 313 ± 315 | 414 ± 271 | NS |
| Late complications (patients) | 38 (7.9%) | 9 (8.3%) | NS |
| Mortality | 41 (8.7%) | 9 (8.2%) | NS |
ATP anti-tachycardia pacing
Mortality
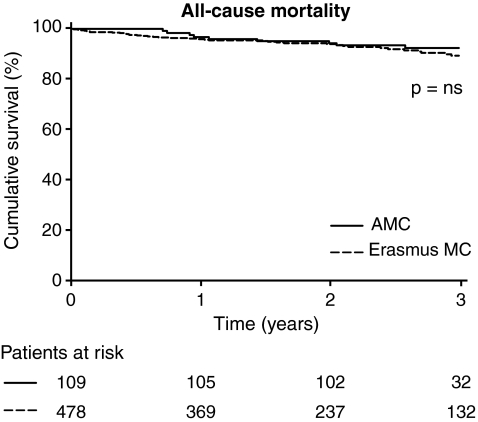
During the reported follow-up, 50 patients (8.5%) died (Table 2), at a median time since implantation of 359 days (IQR 159–759 days). The survival curves for Erasmus MC patients and AMC patients are depicted in Fig. 1. No significant differences in mortality was observed between Erasmus MC patients and AMC patients (log rank = 0.573; NS).
Fig. 1.
Kaplan-Meier analysis, comparing mortality in both centres
ICD interventions
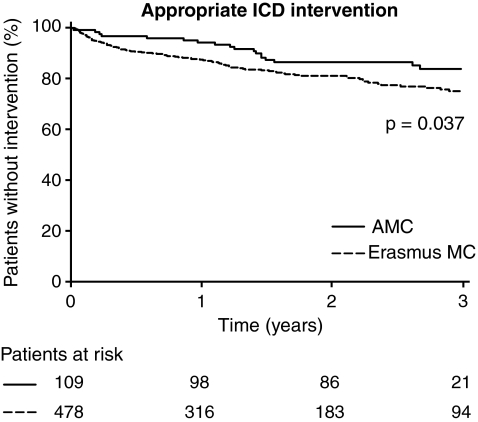
During follow-up, 123 patients (21%) experienced at least one episode of ventricular tachyarrhythmia triggering appropriate ICD intervention, including 16/50 of the deceased patients (32%), and 9/41 patients (22%) who were on the heart transplantation list. The median interval to the first appropriate ICD intervention was 251 days (IQR 65–507 days). Differences in this parameter between the 2 institutions were not significant. Figure 2 shows the Kaplan-Meier curves for appropriate ICD interventions for patients originating from both centres. Nevertheless, the cumulative incidence of appropriate ICD interventions was significantly higher in Erasmus MC patients compared with AMC Amsterdam patients (log rank = 4.340; p-value = 0.037).
Fig. 2.
Kaplan-Meier analysis, comparing the rate of first appropriate ICD interventions (shocks and antitachycardia pacing) in both centres
Readmissions for lead and ICD problems
As obtained from the patient records, there were 54 late complications in 47 patients (8%), similar for both hospitals. This included 8 lead repositions, 12 lead replacements of which 3 were because of a recall (Sprint Fidelis). A total number of 27 ICD replacements occurred, including 15 replacements within 36 months and 3 pocket infections. There were no recalls for pulse generators in this time frame in both institutions.
Hospitalisation and unscheduled outpatient consultations
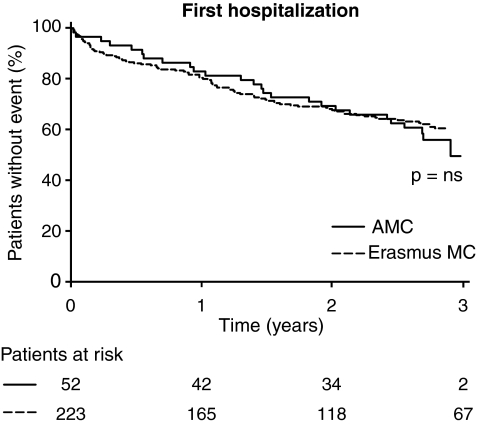
A questionnaire was sent to 464 patients. A completed questionnaire was received from 276 patients, making the response rate 59%. A total number of 338 hospital admissions were reported, occurring in 144 patients (52% of the responders, and 31% of the group to whom a questionnaire was sent). In total, 79/144 were admitted twice; of these, 44 a third time, and 27 at least 4 times. The median interval between ICD implantation and first hospitalisation was 407 days (IQR 132–800 days). The cumulative incidence of first hospitalisation after ICD implantation is depicted in Fig. 3. No significant difference is observed between the centres.
Fig. 3.
Kaplan-Meier analysis, comparing the rate of the first hospital readmission after ICD implantation in both centres
Analysis of the questionnaires is presented in Table 3. The median duration of hospitalisation was 2.5 days (IQR 1–12 days).
Table 3.
Hospitalisation (≥1) and unscheduled outpatient consultations per patient as obtained from the questionnaires
| Characteristic | All (n = 276) | Rotterdam (n = 224/355) | Amsterdam (n = 52/109) | p |
|---|---|---|---|---|
| ICD-related hospital admission | 49/276 (18%) | 40/224 (18%) | 9/52 (17%) | NS |
| Cardiac hospital admission | 99/276 (36%) | 78/224 (35%) | 21/52 (40%) | NS |
| Other hospital admission | 58/276 (21%) | 48/224 (21%) | 10/52 (19%) | NS |
| Additional outpatient cardiac visit | 51/276 (19%) | 38/224 (17%) | 13/52 (25%) | NS |
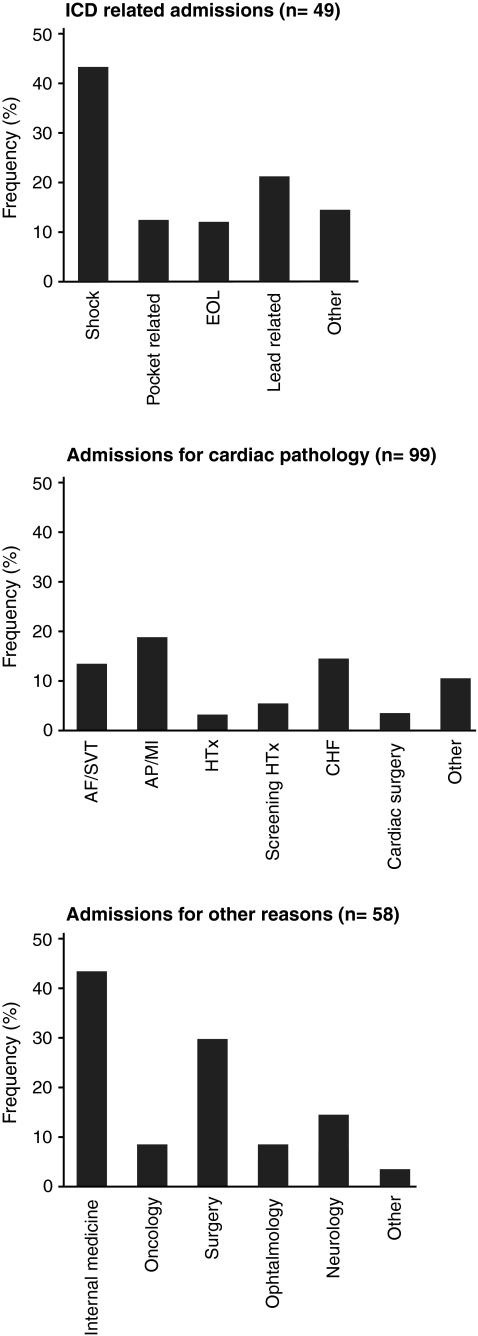
From the 338 reported hospital admissions, 68 (20%) were ICD related; 180 (53%) were for other cardiac reasons, and 90 (27%) were for non-cardiac pathology. Figure 4 shows the distribution of the reasons per admission category. Of 49 patients with an ICD-related admission, 20 were admitted because of a shock (41%); of 99 patients with other cardiac admissions, heart failure or associated conditions were the reason in 52 occasions (53%). The largest non-cardiological reason for hospital admission was related to internal medicine (25/58 patients).
Fig. 4.
Histograms, with the reasons for ICD-related readmission, cardiac-related hospital readmission, and the reasons for non-cardiac hospital admissions. AF: atrial fibrillation; AMI: acute myocardial infarction; AP: angina pectoris; CHF: congestive heart failure; EOL: end-of-life (battery depletion), HTx: heart transplantation; SVT: supraventricular tachycardia
Additional unscheduled cardiac outpatient consultations were observed in 51 (19%) of the patients (Table 3).
Discussion
This study of patients in two different Dutch university hospitals shows that in spite of an apparent different attitude, but with formal adherence to the actual guidelines, the outcome of the patients was completely comparable with respect to a hard endpoint as mortality, and to a softer endpoint as hospitalisation [26]. Nevertheless, we have seen differences in the underlying cardiac disease of the patients (the prevalence of coronary artery disease was higher in Rotterdam), and in the type of implanted defibrillation systems (higher number of CRT-D; also in Rotterdam). The only different outcome between the 2 centers was the occurrence of appropriate ICD therapy, which can not be explained at first glance.
Mortality
The mortality is less than 10% in both centres, even with a median follow-up of more than 2 years. These data correspond with recent real world data [27, 28]. The landmark trials (MADIT, MADIT II, SCDHeFT) all had a mortality rate in the active arm of 14, 22 and 18%, respectively, at 3 years [1–3]. This implicates that today, the practice in the Netherlands can still be compared with the trials as they were published. The outcome of the heart transplantation patients, a typically censored group, was not different in this respect from the others.
Appropriate ICD interventions (shocks/antitachycardia pacing)
In both centres, approximately one fifth of the patients received appropriate ICD interventions, over a median follow-up of more than 2 years. This intervention rate is high, and supports the idea that the right patients were selected. This patient selection was in line with the actual guidelines and was more or less conservative, meaning that early post infarct patients were avoided, in line with recent studies [29, 30]. It is striking that one centre had a higher rate of device therapy than the other, which might be attributed to the presence of patients with advanced heart failure (as might be suggested by the higher proportion of CRT-D) or simply to different programming strategies. The latter is a very likely explanation. Although, there was no obvious difference in the standard programming of rate cut-offs for arrhythmia detection. On the other hand, differences in time to intervention, and the use of ATP may certainly influence the rate of interventions [31]. It is reassuring to observe that this higher intervention rate is not associated with a different mortality or real morbidity. Further, in both centres, the rate of inappropriate interventions was low as is reported elsewhere [32].
Morbidity
The readmission rate was very high, and the questionnaire did not really yield a different outcome in respect to what was known from the hospital files e.g. for lead reintervention. This supports the idea that the questionnaire was reliable as well as the observation that these patients were very ill, with a high readmission rate, also for non-cardiac causes. This might be an indication that more attention should be given to comorbidity in general, before implantation [33–35]. It is known that diabetes, renal failure and high age contribute to the morbidity after implantation. Further, not all cardiac admissions occurred because of device therapy. Only a minority of admissions were reported to occur because of shock therapy. The most important reason for admission remains heart failure, which confirms previous findings [38, 39].
Limitations
It might be that our method (the survey) resulted in over-reporting, as general practitioners might have responded more frequently if they had encountered problematic patients. However, it was the only way to understand what was happening with the patients at home and in the referral centre. Further, the rate of specific complications as reported coincided with the rate as known from the hospital records.
Conclusions
The 2 centres behaved in a similar way with respect to patient selection. Mortality and readmission were the same during follow-up. However, one centre more often used CRT, and observed a higher ICD therapy rate for ventricular arrhythmias.
Further, the high readmission rate, mainly for heart failure, is a reason to organise a good follow-up for these patients. It is clear that the referring cardiologist still has a role after implantation. This role could even increase when it is accepted that ICD control is performed in those centres. On the other hand, the high early complication rate demands a tight control to improve the quality at the time of implantation. One important measure could be to maintain a limited number of experienced implanting centres [36, 37]. It is clear that patient selection, according to the actual guidelines, results in a reproducible outcome, even in different centres.
Acknowledgments
Conflicts of interest
T. Smith: nothing to declare. P. van Dessel: nothing to declare. D.A.M.J. Theuns received research grants from Biotronik, Boston Scientific, St Jude Medical, and he is a consultant to Cameron Health. A. Muskens and R.T. van Domburg: nothing to declare. A.A.W. Wilde: nothing to declare. L. Jordaens received research grants and speaker fees from Biotronik, Boston Scientific, Cameron Health, Medtronic, Sorin, and St Jude Medical.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities: Executive Summary. Heart Rhythm. 2008;5:934–955. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia–AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–1712. doi: 10.1016/S0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 7.Bänsch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–1458. doi: 10.1161/01.CIR.0000012350.99718.AD. [DOI] [PubMed] [Google Scholar]
- 8.Kadish A, Dyer A, Daubert JP, et al. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. . N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 9.Desai AS, Fang JC, Maisel WH, et al. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–2879. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 10.Theuns DAMJ, Smith T, Hunink MGM, et al. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronisation in patients with ischaemic or nonischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12:1564–1570. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 12.Zwanziger J, Hall J, Dick AW, et al. The cost effectiveness of implantable cardioverter-defibrillators. Results from the multicenter automatic defibrillator implantation trial (MADIT)-II. JACC.. 2006;47:2310–2318. doi: 10.1016/j.jacc.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or Amiodarone in chronic stable heart failure. Circulation. 2006;114:135–142. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 14.Caro JJ, Ward A, Deniz HB, et al. Cost-benefit analysis of preventing sudden cardiac deaths with an implantable cardioverter defibrillator versus Amiodarone. Value Health. 2007;10:13–22. doi: 10.1111/j.1524-4733.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 15.Cowie MR, Marshall D, Drummond M, et al. Lifetime cost-effectiveness of prophylactic implantation of a cardioverter defibrillator in patients with reduced left ventricular systolic function: results of Markov modelling in a European population. Europace. 2009;11:716–726. doi: 10.1093/europace/eup068. [DOI] [PubMed] [Google Scholar]
- 16.Neyt M, Thiry N, Ramaekers D, et al. Cost effectiveness of implantable cardioverter-defibrillators for primary prevention in a Belgian context. Appl Health Econ Health Policy. 2008;6:67–80. doi: 10.2165/00148365-200806010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Van Brabandt H, Neyt M. Pitfalls in health-economic evaluations: the case of cost-effectiveness of prophylactic implantable cardioverter-defibrillator therapy in Belgium. Europace. 2009;11:1571–1573. doi: 10.1093/europace/eup290. [DOI] [PubMed] [Google Scholar]
- 18.Hauser RG. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005;45:2022–2025. doi: 10.1016/j.jacc.2005.02.077. [DOI] [PubMed] [Google Scholar]
- 19.Maisel WH, Moynahan M, Zuckerman BD, et al. Pacemaker and ICD generator malfunctions: analysis of Food and Drug Administration annual reports. JAMA. 2006;295:1901–1906. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- 20.Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. doi: 10.1161/CIRCULATIONAHA.106.663807. [DOI] [PubMed] [Google Scholar]
- 21.Maisel WH, Kramer DB. Implantable cardioverter-defibrillator lead performance. Circulation. 2008;117:2721–2723. doi: 10.1161/CIRCULATIONAHA.108.776807. [DOI] [PubMed] [Google Scholar]
- 22.Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6:605–610. doi: 10.1016/j.hrthm.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Borleffs C, Wilde A, Cramer J, et al. Clinical implementation of guidelines for cardioverter defibrillation implantation: lost in translation? Neth Heart J. 2007;15:129–132. doi: 10.1007/BF03085968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camm AJ, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the ‘enigma’? Europace. 2010;12:1063–1069. doi: 10.1093/europace/euq282. [DOI] [PubMed] [Google Scholar]
- 25.Camm J, Klein H, Nisam S. The cost of implantable defibrillators: perceptions and reality. Eur Heart J. 2007;28:392–397. doi: 10.1093/eurheartj/ehl166. [DOI] [PubMed] [Google Scholar]
- 26.Wilde A, Simmers T. Primary prevention, are we on the right track ? Neth Heart J. 2009;17:92–94. doi: 10.1007/BF03086225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santini M, Russo M, Botto G, et al. Clinical and arrhythmic outcomes after implantation of a defibrillator for primary prevention of sudden death in patients with post-myocardial infarction cardiomyopathy: The Survey to Evaluate Arrhythmia Rate in High-risk MI patients (SEARCH-MI) Europace. 2009;11:476–482. doi: 10.1093/europace/eun349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gradaus R, Block M, Brachmann J, et al. Mortality, morbidity, and complications in 3344 patients with implantable cardioverter defibrillators: results fron the German ICD Registry EURID. Pacing Clin Electrophysiol. 2003;26:1511–1518. doi: 10.1046/j.1460-9592.2003.t01-1-00219.x. [DOI] [PubMed] [Google Scholar]
- 29.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 30.Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 31.Wathen MS, DeGroot PJ, Sweeney MO, et al. PainFREE Rx II Investigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 32.Theuns DA, Rivero-Ayerza M, Goedhart DM, et al. Morphology discrimination in implantable cardioverter-defibrillators: consistency of template match percentage during atrial tachyarrhythmias at different heart rates. Europace. 2008;10:1060–1066. doi: 10.1093/europace/eun194. [DOI] [PubMed] [Google Scholar]
- 33.Pellegrini CN, Lee K, Olgin JE, et al. Impact of advanced age on survival in patients with implantable cardioverter defibrillators. Europace. 2008;10:1296–1301. doi: 10.1093/europace/eun253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cygankiewicz I, Gillespie J, Zareba W, et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter-defibrillators. Heart Rhythm. 2009;6:468–473. doi: 10.1016/j.hrthm.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Theuns DAMJ, Schaer B, Soliman OII, et al. The prognosis of implantable defibrillator patients treated with cardiac resynchronization therapy: comorbidity burden as predictor of mortality. Europace. 2011;13:62–69. doi: 10.1093/europace/euq328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Khatib SM, Lucas FL, Jollis JG, et al. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol. 2005;46:1536–1540. doi: 10.1016/j.jacc.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 37.Curtis JP, Luebbert JJ, Wang Y, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator. JAMA. 2009;301:1661–1670. doi: 10.1001/jama.2009.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–2817. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]