Abstract
Post-operative cognitive dysfunction (POCD) is a clinical phenomenon characterized with cognitive decline in patients after anesthesia and surgery. It has been shown that interleukin-1β (IL-1β) contributes to the cognitive impairment of mice after surgery and isoflurane anesthesia. This study is designed to determine whether isoflurane alone increases inflammatory cytokines and causes cell injury and cognitive impairment. Four-month-old male Fisher 344 rats were exposed to or were not exposed to 1.2% isoflurane for 2 h. Two weeks later, rats were subjected to Barnes maze and fear conditioning tests. Although animals exposed to or non-exposed to isoflurane developed spatial learning, animals exposed to isoflurane had significant impairments in long-term spatial memory assessed by Barnes maze. They also had impaired hippocampus-dependent learning and memory in fear conditioning test. IL-1β in the hippocampus was increased at 6 h after isoflurane exposure. Isoflurane also increased activated caspase 3 in the hippocampus and decreased the neuronal density in the CA1 region. However, isoflurane did not change the amount of β-amyloid peptide in the cerebral cortex at 29 days after isoflurane exposure when cognitive impairment was present. These results suggest that isoflurane increases inflammatory cytokine expression and causes cell injury in the hippocampus, which may contribute to isoflurane-induced cognitive impairment in rats.
Keywords: Cell injury, cognitive functions, cytokines, isoflurane, rats
1. Introduction
Post-operative cognitive dysfunction (POCD) is a recognized clinical entity (Baranov, et al., 2009). Although POCD may last for a short period (days or weeks) in most patients after cardiac and non-cardiac surgeries, POCD in some patients can last for months or longer (Moller, et al., 1998, Monk, et al., 2008, Newman, et al., 2001). In addition, it has been shown that POCD is associated with an increased mortality (Monk, et al., 2008, Steinmetz, et al., 2009). It is known that patients with an advancing age (≥ 60 years) have an increased incidence of POCD (Moller, et al., 1998, Monk, et al., 2008). However, younger adults can also suffer from POCD (Monk, et al., 2008).
In today’s practice, most patients are having surgery under general anesthesia (Clergue, et al., 1999). Thus, it is conceivable that general anesthesia/anesthetics may contribute to POCD. However, strong clinical evidence to support this contribution has not been reported and may be difficult to obtain because studies to address this issue will require anesthetizing a large number of people without surgery. In contrast, in vitro and animal studies have suggested that volatile anesthetics, the most commonly used anesthetics, may play a role in POCD. For example, rats exposed to volatile anesthetics develop cognitive impairment (Culley, et al., 2003, Culley, et al., 2004a), although volatile anesthetic neuroprotective effects and improvement of cognitive functions after anesthetic exposure have also been reported (Culley, et al., 2003, Komatsu, et al., 1993, Li, et al., 2009, Rammes, et al., 2009, Statler, et al., 2006b, Statler, et al., 2006a). Activated caspase 3 expression and β-amyloid peptide (Aβ) production are increased in mouse brains after volatile anesthetic exposure (Xie, et al., 2008). Aβ oligomerization in vitro can be induced by volatile anesthetics (Eckenhoff, et al., 2004). It has been proposed that Aβ overproduction, oligomerization and accumulation in the brain contribute to the development of Alzheimer’s disease (AD) (Selkoe, 2004), the most common form of dementia in the elderly patients.
The pathogenesis of volatile anesthetic-induced cognitive impairment is not fully understood. It has been known that neuroinflammation induces cognitive impairment (Sanderson, et al., 2009). A recent study showed that neuroinflammation played an important role in cognitive dysfunction of young adult mice after an open tibia fixation under isoflurane anesthesia (Cibelli, et al., 2010). Activated caspase 3, a key enzyme to induce cell apoptosis, can be increased in mouse brains (Xie, et al., 2008). Pathological changes including neuroinflammation, Aβ accumulation and neurodegeneration are key features of AD brains (Mrak, et al., 2001, Selkoe, 2004). Thus, this study was designed to determine whether these pathological features and cognitive impairment occurred after isoflurane exposure. We exposed 4-month old Fisher 344 rats to isoflurane. We then evaluated their cognitive functions, Aβ levels and cell death in the brain, as well as neuroinflammation as reflected by the levels of the proinflammatory cytokines interleukin 1β (IL-1β) and tumor necrosis factor-α (TNF-α).
2. Materials and Methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996.
2.1. Animal groups
Four-month-old male Fisher 344 rats weighing 290 – 330 g from Charles River Laboratories International, Inc. (Wilmington, MA) were divided into two groups: control and isoflurane. Animals in the isoflurane group were exposed to 1.2% isoflurane for 2 h. Since isoflurane was carried by 100% O2, rats in the control group were kept in a chamber gassed with 100% O2 for 2 h and were not exposed to isoflurane at any time. Two sets of experiments were performed. In the first set of experiments, rats were exposed to or were not exposed to isoflurane and their hippocampi were harvested at 6 or 16 h after the isoflurane exposure for measuring cytokines and activated caspase 3. In the second set of experiments, control animals and animals exposed to isoflurane were subjected to Barnes maze and fear conditioning tests. Their brains were harvested after these tests for determining neuronal density, the levels of cytokines and activated caspase 3 in the hippocampus, and Aβ in the cerebral cortex.
2.2. Isoflurane exposure
As we described before (Lee, et al., 2008), anesthesia was induced by placing rats in a chamber gassed with 3% isoflurane in oxygen. Rats were intubated with a 14-gauge catheter. They were mechanically ventilated with isoflurane carried by 100% O2 to maintain end tidal isoflurane concentration at 1.2%. The inhaled and exhaled gas concentrations were monitored continuously with a DatexTM infrared analyzer (Capnomac, Helsinki, Finland). The ventilator settings usually were 2 ml as the tidal volume at a rate of 60 breaths/min and were adjusted to maintain the end tidal CO2 at ~32 mmHg. Rectal temperature was maintained at 37°C ± 0.5°C. Heart rate and pulse oximeter oxygen saturation (SpO2) were measured continuously during anesthesia with a MouseOxTM Pulse Oximeter (Harvard Apparatus, Holliston, MA). This isoflurane exposure was for 2 h. Rats were extubated when they were responsive. All animals were recovered for 20 min in a chamber gassed with 100% O2 and at 37°C. They then were placed in their home cage.
2.3. Barnes maze
Two weeks after isoflurane exposure, animals were subjected to Barnes maze. Barnes maze is designed to test spatial learning and memory. Animals were placed in the middle of a circular platform with 20 equally spaced holes (SD Instruments, San Diego, CA). One of the holes was connected to a dark chamber that was called target box. Animals were encouraged to find this box by aversive noise (85 dB) and bright light (200 W) shed on the platform. Animals went through a spatial acquisition phase that took 4 days with 3 min per trial, 2 trials per day and 15 min between each trial. Animals then went through the reference memory phase to test the short-term retention on day 5 and long-term retention on day 12. One trial on each of these two days was performed. No test was performed during the period from day 5 to day 12. The latency and number of errors to find the target box during each trial were recorded with the assistance of ANY-Maze video tracking system (SD Instruments). The numbers of pokes in each hole during the reference memory phase also were recorded.
2.4. Fear conditioning
One day after Barnes maze test, rats were subjected to fear conditioning test. Fear conditioning is a very sensitive and non-effort-dependent test of learning and memory (Kim, et al., 1992). Each animal was placed in a test chamber wiped with 70% alcohol and subjected to 3 tone-foot shock pairings (tone: 2000 Hz, 85 db, 30 s; foot shock: 1 mA, 2 s) with an intertrial interval 1 min in a relatively dark room. Animal was removed from this test chamber 30 s after the conditioning training. The animal was placed back to the chamber 24 h later for 8 min in the absence of tone and shock. The amount of time with freezing behavior was recorded in an 8 s interval. The animal was placed 2 h later in a test chamber that had different context and smell from the first test chamber (this second chamber was wiped with 1% acetic acid) in a relatively light room. Freezing was recorded for 3 min without the auditory conditioning stimulus. The auditory stimulus then was turned on for 3 cycles, each cycle for 30 s followed by 1-min inter-cycle interval (4.5 min in total). The freezing behavior in the 4.5 min was recorded. The order of the context and tone fear conditioning tests was arranged in a way so that half of the animals in each group were tested first by contextual test and the other half by tone test. Freezing behavior recorded in the video was scored by an observer who was blind to group assignment. These tests test hippocampus-dependent (context-related) and hippocampus-independent (tone-related) learning and memory functions (Kim, et al., 1992).
2.5. Brain tissue harvest
Thirty minutes after fear conditioning test, rats were deeply anesthetized with isoflurane and perfused transcardially with saline. Brains were removed. The right hippocampi were dissected out immediately for the Western blotting of various proteins and enzyme-linked immunosorbent assay (ELISA) of IL-1β and TNF-α. The left cerebral hemisphere at Bregma −3.5 to −4.5 was used for Nissl staining. Cerebral cortex from Bregma −1 to −3 was harvested for ELISA of Aβ.
2.6. Nissl staining
The cerebral block containing hippocampus was fixed in 10% neutral buffered formalin overnight and then paraffin embedded. Coronal 8 μm sections were prepared and subjected to Nissl staining (Zhao, et al., 2004). These sections were examined by an observer blinded to the group assignment of the sections. The number of positively stained cells (neurons) in a high magnification field (× 400, ~0.2 mm2) in the CA1 and CA3 regions was counted. Three determinations, each on different locations in these two brain regions, were performed for each section. Three sections were used for each rat. These 9 determinations were averaged to yield a single number (density of the neurons) for each brain region of individual rat.
2.7. Western blotting
Hippocampal tissues were homogenated in RIPA buffer (catalogue number: 89900; Thermo Scientific, Worcester, MA) containing protease inhibitor cocktail (catalogue number: P2714; Sigma, St Louis, MO) and protease inhibitor mixture (catalogue number: 1697498; Roche Applied Science, Indianapolis, IN). Homogenates were centrifuged at 13,000 g at 4°C for 30 min. The supernatant was saved and its protein concentration was determined by Bradford assay. Fifty microgram proteins per lane were electrophoresed on a polyacrylamide gel and then blotted onto a polyvinylidene difluoride membrane. After being blocked with Protein-Free T20 Blocking Buffer (catalogue number: 37573; Thermo Scientific, Lot LB141635), membranes were incubated with the following primary antibodies: rabbit monoclonal anti-cleaved caspase-3 antibody (1:1000; catalogue number: 9664; Cell Signaling Technology, Inc., Danvers, MA) and mouse monoclonal anti-β-actin polyclonal antibody (1:2000; catalogue number: A2228; Sigma). Appropriate secondary antibodies were used. Proteins were visualized using a Genomic and Proteomic Gel Documentation (Gel Doc) Systems from Syngene (Frederick, MD). The protein band intensities of caspase 3 were normalized by the corresponding band intensities of β-actin from the same samples. The results from animals under various experimental conditions were then normalized by those of the corresponding control animals.
2.8. Quantification of IL-1β, TNFα and Aβ
Brain tissues were homogenized on ice in 20 mM Tris–HCl buffer (pH = 7.3) containing protease inhibitors (10 μg/ml aproteinin, 5 μg/ml peptastin, 5 μg/ml leupeptin, and 1 mM phenylmethanesulfonylfluoride). Homogenates were centrifuged at 10,000 g for 10 min at 4°C. The supernatant was then ultracentrifuged at 150,000 g for 2 h 4°C. Bradford protein assay of the supernatant was performed for each sample. ELISA kits for measuring rat IL-1β and TNFα (catalogue number: DY501 and RTA00, respectively; R&D Systems, Minneapolis, MN) were used to quantify the contents of these cytokines in the samples according to the manufacturer’s instructions. The quantity of IL-1β and TNFα in each brain sample was standardized to the protein contents. The results from animals under various experimental conditions were then normalized by the mean values of the corresponding control animals in each ELISA assay.
Aβ was extracted from brain tissues as described before (Gravina, et al., 1995). Briefly, parietal cerebral cortex was homogenized in 8 × volume of 70% glass distilled formic acid. The homogenates were kept at 4°C for 15 min and then centrifuged at 100,000 g for 1 h. The formic acid extract layer between a thin overlying lipid layer and a small pellet was removed and used for Aβ1–42 quantification by a Aβ1–42 ELISA kit (catalog number KMB3441; Invitrogen, Carlsbad, CA). The level of the Aβ1–42 in each brain sample was standardized to the brain tissue weight. The results from animals under various experimental conditions were then normalized by the mean values of the corresponding control animals in each ELISA assay.
2.9. Statistical analysis
All data in this study, including physiological results and results from behavioral tests, Western blotting and ELISA, were parametric. They are presented as means ± S.D. (n ≥ 4) and analyzed by Student’s t test, Rank Sum test or by one way repeated measures analysis of variance followed by the Tukey test (for the data generated during the training sessions of Barnes maze). A P < 0.05 was accepted as significant. All statistical analyses were performed with the SigmaStat (Systat Software, Inc., Point Richmond, CA, USA).
3. Results
No animals had an episode of hypoxia (defined as SpO2 < 90%) during the isoflurane exposure. The SpO2 and heart rates were 98 ± 1% and 344 ± 29 beats/min, respectively, during the exposure.
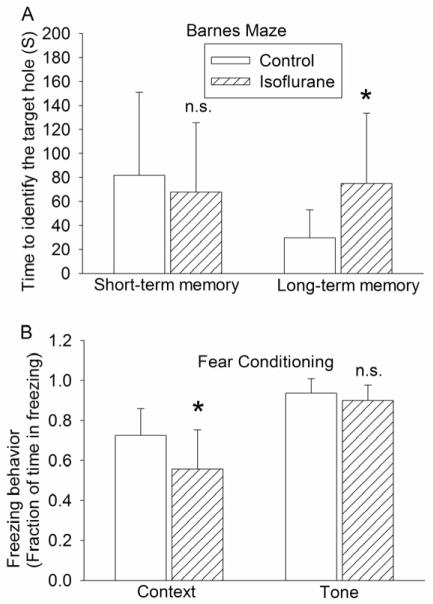
Control rats and rats exposed to isoflurane took less time to find the target hole with increased training sessions [F(7, 10) = 3.022 (P < 0.01) for the control group and F(7, 10) = 3.573 (P < 0.01) for the isoflurane exposure group]. As a result, they took significantly less time to find the target hole in the last training session on the fourth day than that in the first training session on the first day (Fig. 1). These results suggest that animals in control and isoflurane exposure groups developed spatial learning. However, animals exposed to isoflurane needed much longer time to find the target hole than control rats during the reference memory tests occurred at 8 days after the last training session [t(20) = −2.388] (Fig. 2). Isoflurane-exposed rats also had decreased freezing behavior compared with control rats in the contextual fear conditioning test [t(20) = 2.340]. However, the freezing behavior during the tone fear conditioning test was not different between the control rats and isoflurane-exposed rats (Fig. 2).
Fig. 1. Performance in the training sessions of Barnes maze.
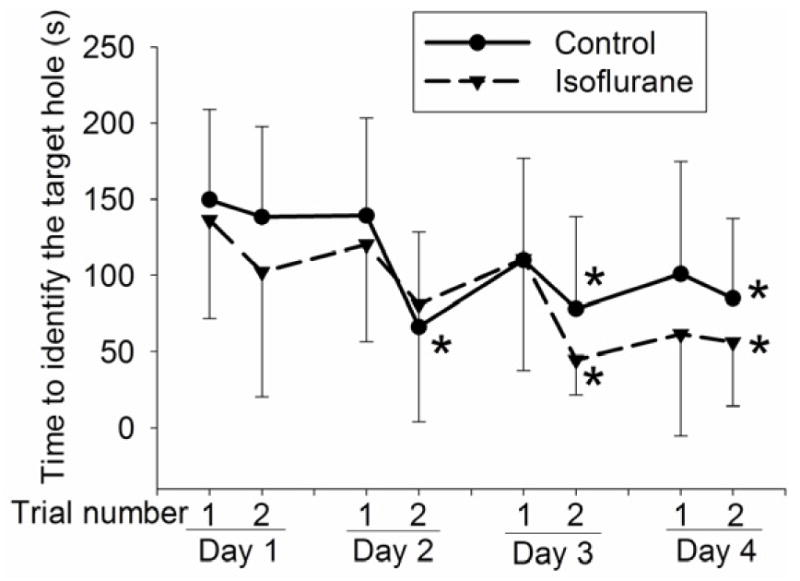
Four-month-old Fisher 344 rats were exposed to or were not exposed to 1.2% isoflurane for 2 h. They were subjected to Barnes maze 2 weeks later. Results are mean ± S.D. (n = 11). Statistical results are F(7, 10) = 3.022 and P < 0.01 for the control group and F(7, 10) = 3.573 and P < 0.01 for the isoflurane exposure group. * P < 0.05 compared with the corresponding data in the first trial on day 1. The differences between the data at other time-points and those in the first trial on day 1 within the same group were not significant.
Fig. 2. Performance in the reference memory sessions of Barnes maze and fear conditioning test.
Four-month-old Fisher 344 rats were exposed to or were not exposed to 1.2% isoflurane for 2 h. They were subjected to Barnes maze 2 weeks later or fear conditioning 27 days later. Results are mean ± S.D. (n = 11). * P < 0.05 compared with the control group [t(20) = −2.388 for the comparison in long-term memory test of the Barnes maze and t(20) = 2.340 for the comparison in the contextual fear conditioning test]; n.s. means non-significant for the comparisons between the data of isoflurane exposure group and the control group.
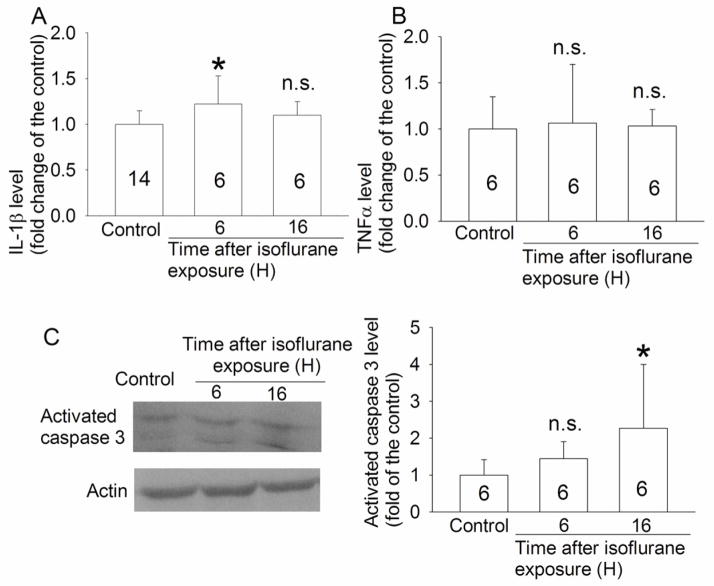
To determine the possible mechanisms for isoflurane-induced cognitive impairments, we measured the expression of proinflammatory cytokines and a cell injury marker in the hippocampus. The hippocampal IL-1β level in rats exposed to isoflurane 6 h ago was significantly higher than that in control animals [t(18) = −2.203]. However, the TNFα level in the hippocampus was not different between control and isoflurane exposed animas. In contrast, activated caspase 3 was increased at 16 h after isoflurane exposure [T = 24 with n(small) = 6 and n(big) = 6] (Fig. 3). Two caspase 3 fragments at 20 KDa and 17 KDa were detected. These fragments are subunits of activated caspase 3 (Liu, et al., 1996). The final results presented in the bar graphs of figure 3 were the sum of these two fragments.
Fig. 3. Expression of interleukin (IL) 1β, tumor necrosis factor (TNF)-α and activated caspase 3 in rat hippocampi.
Four-month-old Fisher 344 rats were exposed to or were not exposed to 1.2% isoflurane for 2 h. Hippocampi were harvested at 6 or 16 h after the isoflurane exposure. Results are mean ± S.D. (n = 6 – 14, the number of animals for each experimental condition is indicated inside each bar in the figure). * P < 0.05 compared with the control group [t(18) = −2.203 for the comparison of IL-1β content between control group and the group at 6 h after isoflurane exposure and T = 24 with n(small) = 6 and n(big) = 6 for the comparison of activated caspase 3 content between control group and group at 16 h after isoflurane exposure]; n.s. means non-significant for the comparisons between the data of isoflurane exposure group and the control group.
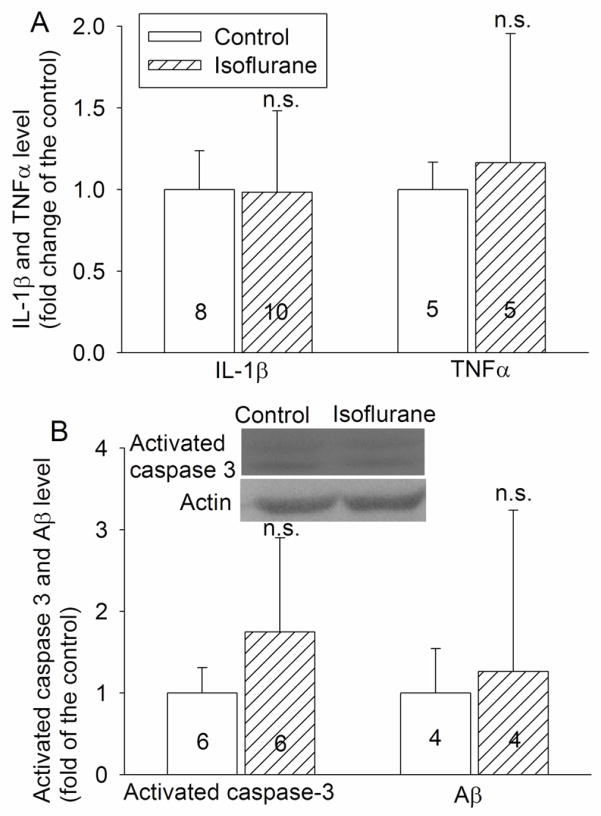
Different from the findings in the acute phase after isoflurane exposure, the IL-1β and TNFα concentrations as well as the activated caspase 3 expression in the hippocampi of animals exposed to isoflurane 29 days ago were not different from those of control rats (Fig. 4). Similarly, the Aβ1–42 levels in the parietal cerebral cortex were not different between the two groups of animals at this time-point (Fig. 4).
Fig. 4. Expression of interleukin (IL) 1β, tumor necrosis factor (TNF)-α, activated caspase 3 and β amyloid peptide (Aβ) in rat brains.
Four-month-old Fisher 344 rats were exposed to or were not exposed to 1.2% isoflurane for 2 h. IL-1β, TNFα and activated caspase 3 in the hippocampus and Aβ in the parietal cerebral cortex were measured 29 days after the isoflurane exposure. Results are mean ± S.D. (n = 4 – 10, the number of animals for each experimental condition is indicated inside each bar in the figure). n.s. means non-significant for the comparisons between the data of isoflurane exposure group and the control group.
Consistent with the possibility of cell injury as indicated by increase of activated caspase 3 expression in the hippocampus soon after isoflurane exposure, the neuronal density in the CA1 region at 29 days after isoflurane exposure was significantly lower than control animals [t(9) = 3.062]. Similar direction of change was observed in CA3 region but the difference was not statistically significant (Fig. 5).
Fig. 5. Neuronal density in the hippocampus.
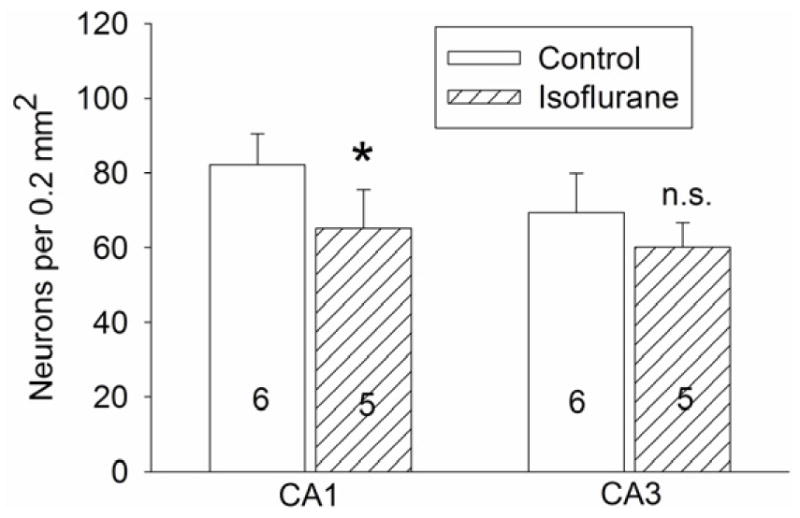
Four-month-old Fisher 344 rats were exposed to or were not exposed to 1.2% isoflurane for 2 h. The hippocampus was harvested at 29 days after the isoflurane exposure for Nissl staining. Results are mean ± S.D. (n = 5 – 6, the number of animals for each experimental condition is indicated inside each bar in the figure). * P < 0.05 compared with the control group [t(9) = 3.062 for the comparison in the CA1 region]; n.s. means non-significant for the comparisons between the data of isoflurane exposure group and the control group.
4. Discussion
We performed our study under clinically relevant conditions. General anesthesia maintained with anesthetics including volatile anesthetics at 0.5 to 1.3 minimum alveolar concentrations (MAC, one MAC is the concentration at which 50% of animals do not have a motor response to painful stimuli) for 2 h or longer is commonly performed in the clinical practice. One MAC of isoflurane in rats is ~1.5% (Stratmann, et al., 2009). Cognitive function tests on the animals were initiated at 2 weeks after isoflurane exposure. Most patients have already been discharged from hospital by this time. Existence of POCD will significantly affect their daily activity and quality of life at home or at work.
Our results showed that exposure of rats to 1.2% isoflurane for 2 h did not affect the performance of rats in the training sessions of Barnes maze, suggesting that isoflurane does not affect the acquisition/learning of spatial reference tasks. However, rats in the isoflurane exposure group had a poorer performance in the long-term spatial reference memory test than control rats. Isoflurane exposed rats also had poorer performance in the contextual fear conditioning. These results suggest that isoflurane impairs hippocampus-dependent learning and memory as well as the recall/memory but not the acquisition of spatial reference tasks. Impairment of spatial learning and memory in young adult rats after being exposed to the combination of isoflurane and nitrous oxide has been reported (Culley, et al., 2004b). However, it is not known from this previous study which phase of learning and memory process is affected by anesthesia. A recent study showed that exposure of 2 – 3-month old mice to 1% isoflurane, but not 1.5 and 2% isoflurane, impaired the acquisition of spatial reference tasks but did not affect spatial reference recall/memory when they were tested within 5 days after isoflurane exposure (Valentim, et al., 2010). However, another recent study demonstrated that 2-month old rats did not have an impairment in fear conditioning test and spatial reference learning but had improved long-term memory at 4 months after one-MAC isoflurane anesthesia for 4 h (Stratmann, et al., 2009). The reasons for these different findings among our study and the three previous studies are not known. Differences in animal species and strains, methods of anesthetic exposure, and time to perform the leaning and memory tests may have contributed to these discrepancies.
Mechanisms for volatile anesthetics-induced cognitive impairment are poorly understood. Since Aβ has been hypothesized to contribute to the development of AD (Selkoe, 2004), it is possible that increased Aβ in the brain and its subsequent neurotoxicity play a role in the anesthetic-induced cognitive impairment in the animals. Consistent with this possibility, Aβ oligomerization in vitro is increased when exposed to volatile anesthetics at clinically relevant concentrations (Eckenhoff, et al., 2004). Those anesthetics also increase Aβ production from cell cultures (Xie, et al., 2007). Mice exposed to 1.4% isoflurane for 2 h had an increased expression of Aβ in their cerebral cortices quantified at 24 h after isoflurane exposure (Xie, et al., 2008). However, no study has quantified cognitive impairments and Aβ accumulation in the brains of the same wild-type animals to determine the possible relationship between them after anesthetic exposure. We showed here that isoflurane impaired the cognitive functions of rats. However, isoflurane did not increase the Aβ concentrations in the brains of the same animals at a time when they had significant cognitive impairments. Thus, our results suggest that Aβ may not play a role in isoflurane-induced cognitive impairments in young adult rats.
Neuroinflammation is an important component of neuropathology for AD (Cameron, et al.). Neuroinflammation can also impair learning and memory (Sanderson, et al., 2009). A recent study showed that tibial surgery under anesthesia with isoflurane and buprenorphine induced a rapid increase of IL-1β in the brain and hippocampal inflammation within 3 days after the surgery in young adult mice. These animals also had significant memory impairments assessed at 3 days after the surgery and anesthesia (Cibelli, et al., 2010). However, the anesthesia alone did not increase IL-1β level and cause inflammation in the mouse hippocampus in the same study (Cibelli, et al., 2010). In contrast, our results showed that hippocampal IL-1β level was increased at 6 h after isoflurane exposure.
Our results also showed a significant increase of activated caspase 3 expression at 16 h after isoflurane exposure. Since this increase is after the IL-1β elevation and IL-1β has been shown to activate caspase 3 in the brain (Martin, et al., 2002, Zarifkar, et al., 2010), it is possible that the increase of activated caspase 3 is caused by IL-1β. Since our results also showed a decreased neuronal density, it is conceivable that the increased IL-1β and activated caspase 3 may have ultimately led to neurodegeneration in the hippocampus. Thus, our results suggest a pathway of IL-1β-caspase 3-neuronal injury/death. Isoflurane-induced caspase 3 activation in mouse brain tissues has been reported (Xie, et al., 2008). However, no study has reported whether this activation results in cell death and brain structure changes. Our results indicate this possibility because the neuronal density in the hippocampus of isoflurane exposed rats was decreased. Of note, our study showed that the expression of IL-1β, TNFα and activated caspase 3 was not different between control and isoflurane exposed animals at 29 days after isoflurane exposure, suggesting that there is no ongoing neuroinflammation and cell injury at this time-point.
Since neuroinflammation impairs cognitive functions (Sanderson, et al., 2009) and IL-1β plays a critical role in the memory impairment of mice after anesthesia and surgery (Cibelli, et al., 2010), it is natural to think that activation of IL-1β-caspase 3-neuronal injury/death pathway may contribute to cognitive impairment after isoflurane exposure. However, this connection can not be firmly established with the data presented here. Future studies with using specific inhibitors for each component of this pathway are needed to provide evidence for this connection.
In addition to the failure to firmly establish the relationship of the neurochemical and neuropathological changes with cognitive impairment after isoflurane exposure, our study has other limitations. First, we intubated and mechanically ventilated the rats during isoflurane exposure. This experimental design was aimed to simulate clinical situations because most patients under general anesthesia are intubated and ventilated. This practice also reduces the occurrence of hypoxia and hypercarbia. However, it is difficult to know whether intubation and mechanical ventilation contribute to the activation of the IL-1β-caspase 3-neuronal injury/death pathway and cognitive impairments because it is not possible to study a group of rats that are intubated and ventilated without anesthesia. However, isoflurane has been shown to activate caspase 3 in cell cultures (Xie, et al., 2007). Thus, it is very likely that the neurochemical, neuropathological and cognitive changes in the animals of isoflurane exposure group are caused by isoflurane. Second, our study is a pure animal investigation. There is no clinical evidence in the literature to suggest the applicability of our findings to humans.
In conclusion, our study showed that isoflurane impaired the long-term spatial reference memory and hippocampus-dependent learning and memory in young adult rats. Isoflurane may activate the IL-1β-caspase 3-neuronal injury/death pathway in the hippocampus. This activation may contribute to the cognitive impairment of these rats after isoflurane exposure.
Highlights.
Isoflurane impairs cognitive function of young adult rats.
Isoflurane induces a transient increase of interleukin-1β in rat hippocampus
Isoflurane may reduce neuronal density in the hippocampus
Acknowledgments
This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland and the Robert M. Epstein Professorship endowment, University of Virginia.
Abbreviations
- Aβ
β-amyloid peptide
- AD
Alzheimer’s disease
- IL-1β
interleukin-1β
- MAC
minimum alveolar concentrations
- POCD
post-operative cognitive dysfunction
- SpO2
pulse oximeter oxygen saturation
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baranov D, Bickler PE, Crosby GJ, Culley DJ, Eckenhoff MF, Eckenhoff RG, Hogan KJ, Jevtovic-Todorovic V, Palotas A, Perouansky M, Planel E, Silverstein JH, Wei H, Whittington RA, Xie Z, Zuo Z. Consensus statement: First International Workshop on Anesthetics and Alzheimer's disease. Anesth Analg. 2009;108:1627–30. doi: 10.1213/ane.0b013e318199dc72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiol Dis. 37:503–9. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clergue F, Auroy Y, Pequignot F, Jougla E, Lienhart A, Laxenaire MC. French survey of anesthesia in 1996. Anesthesiology. 1999;91:1509–20. doi: 10.1097/00000542-199911000-00045. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004a;99:1393–7. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004b;100:309–14. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–9. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Gravina S, Ho L, Eckman C, Long K, Otvos L, Jr, Younkin L, Suzuki N, Younkin S. Amyloid beta protein in Alzheimer's disease brain. J Biol Chem. 1995;270(7013):7–16. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Nogaya J, Anabuki D, Yokono S, Kinoshita H, Shirakawa Y, Ogli K. Memory facilitation by posttraining exposure to halothane, enflurane, and isoflurane in ddN mice. Anesth Analg. 1993;76:609–12. doi: 10.1213/00000539-199303000-00028. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–62. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, Horrobin DF, Campbell VA, Lynch MA. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem. 2002;277:34239–46. doi: 10.1074/jbc.M205289200. [DOI] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and Alzheimer's disease. Neurobiol Aging. 2001;22:903–8. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgansberger W, Ohl F, Kochs EF, Blobner M. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–36. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Cunningham C, Deacon RM, Bannerman DM, Perry VH, Rawlins JN. A double dissociation between the effects of sub-pyrogenic systemic inflammation and hippocampal lesions on learning. Behav Brain Res. 2009;201:103–11. doi: 10.1016/j.bbr.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–38. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Dixon CE, Clark RS, Jenkins L, Kochanek PM. Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J Neurotrauma. 2006b;23:97–108. doi: 10.1089/neu.2006.23.97. [DOI] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Holubkov R, Dixon CE, Clark RS, Jenkins L, Kochanek PM. Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 2006a;1076:216–24. doi: 10.1016/j.brainres.2005.12.106. [DOI] [PubMed] [Google Scholar]
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113:1099–108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–54. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, Rastegar K, Moosavi M. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol. 2010;634:84–8. doi: 10.1016/j.ejphar.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in the neonatal rats. Anesthesiology. 2004;101:695–702. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]