Abstract
Familial dysautonomia (FD) is caused by an intronic splice mutation in the IKBKAP gene that leads to partial skipping of exon 20 and tissue-specific reduction in I-κ-B kinase complex associated protein/ elongation protein 1 (IKAP/ELP-1) expression. Kinetin (6-furfurylaminopurine) has been shown to improve splicing and increase wild-type IKBKAP mRNA and IKAP protein expression in FD cell lines and carriers. To determine if oral kinetin treatment could alter mRNA splicing in FD subjects and was tolerable, we administered kinetin to eight FD individuals homozygous for the splice mutation. Subjects received 23.5 mg/Kg/day for 28 days. An increase in wild-type IKBKAP mRNA expression in leukocytes was noted after eight days in six of eight individuals; after 28 days the mean increase as compared to baseline was significant (p=0.002). We have demonstrated that kinetin is tolerable in this medically fragile population. Not only did kinetin produce the desired effect on splicing in FD patients, but also that effect appears to improve with time despite lack of dose change. This is the first report of a drug that produces in vivo mRNA splicing changes in individuals with FD and supports future long-term trials to determine if kinetin will prove therapeutic in FD patients.
INTRODUCTION
Familial dysautonomia (FD), also known as Riley Day syndrome or hereditary sensory and autonomic neuropathy type III, is a rare fatal autosomal recessive disease, which impairs the development of sensory and autonomic nerves (1). Affected patients have characteristic alacrima, depressed tendon reflexes and decreased pain and temperature perception, as well as gastrointestinal dysmotility (1, 2) and afferent baroreflex failure resulting in extreme blood pressure volatility (3). The leading causes of death include respiratory infections, renal failure and unexpected sleep death (4). Current treatments are limited to supportive measures and are not always effective.
In 2001, we discovered that the disease was caused by mutations in IKBKAP (I-κ-B kinase complex associated protein) gene, leading to a deficiency of I-κ-B kinase complex associated protein/ elongation protein 1 (IKAP) (5). IKAP, also known as elongation protein 1 (ELP-1), is an essential component of the human Elongator complex, which promotes the transcriptional elongation of a number of target genes (6). Ninety-nine percent of patients with FD are homozygous for the IVS20+6T>C mutation (5), which weakens the splice site, causing variable, tissue-specific skipping of exon 20 in the transcribed RNA message, resulting in reduced protein expression of IKAP. Interestingly, the splicing abnormality primarily affects neuronal tissue, which produces mostly the shortened or mutant IKBKAP mRNA and a minimal amount of functional protein product (5, 7). Other cells, like fibroblasts, produce almost equal levels of the normally spliced or wild-type IKBKAP mRNA and mutant IKBKAP mRNA.
As a result of a National Institute of Neurological Disorders and Stroke sponsored drug screen (8), kinetin (6-furfurylaminopurine), a dietary supplement, was shown to rescue the splicing abnormality and restore normal IKAP protein levels in FD fibroblasts and transformed lymphoblast lines after only one week in culture (9, 10). Recently, kinetin’s ability to correct the splicing defect was also demonstrated in induced pluripotent FD stem cell derived neural crest precursors from fibroblasts (11) and olfactory stem cells (12). Animal studies showed that kinetin was well absorbed orally, followed first-order elimination, was distributed into plasma and into the central nervous system, and was not a clastogenic agent (Sitek studies No. 0849-M208, No. 0849-1721.4, No. 0849-1731.C, SITEK Research Laboratories, Rockville, MD, 2005–2007).
Initial clinical studies of kinetin were performed in carriers of the IVS20+6T>C mutation, who despite being asymptomatic have some degree of mis-splicing and decreased levels of wild-type IKBKAP mRNA (13). Encouragingly, after receiving kinetin for 8 days, an increase in wild-type IKBKAP mRNA was found in circulating leukocytes. Here, we report the result of a pharmacokinetics study and one month trial of kinetin in patients with FD.
METHODS
Study Population
Eight individuals with FD (mean age 36 ± 9 yrs; 2 males) (Table 1), who were homozygous for the IVS20+6T>C mutation, were enrolled in the study. Subjects were recruited from the FD registry database at the Dysautonomia Center of the New York University School of Medicine. NYU School of Medicine Institutional Research Board approved the study and informed consent was obtained from all participants.
Table 1.
Demographics and pharmacokinetics
| Demographics | Administration | Pharmacokinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| # | Weight (kg) |
Gender | Age (y) |
Route | Total daily dose (mg) |
AUC (ng/mL*h) |
Tmax (h) |
Cmax (ng/mL) |
| 1 | 41 | F | 28 | Gastrostomy | 1000 | 21001 | 0.96 | 2715 |
| 2 | 46 | M | 30 | Oral | 1000 | 7195 | 1.74 | 1521 |
| 3 | 64 | F | 46 | Oral | 1500 | 10320 | 1.22 | 2267 |
| 4 | 51 | F | 33 | Gastrostomy | 1000 | 5542 | 1.81 | 724 |
| 5 | 50 | F | 24 | Oral | 1250 | 48889 | 3.85 | 4673 |
| 6 | 56 | F | 49 | Oral | 1250 | 33307 | 1.81 | 6521 |
| 7 | 45 | F | 37 | Oral | 1250 | 9925 | 1.42 | 2103 |
| 8 | 60 | M | 37 | Gastrostomy | 1500 | 26152 | 2.56 | 2715 |
Study preparation
Patients were admitted to the General Clinical Research Center (Bellevue Hospital, New York, NY). An indwelling venous catheter was inserted. Vital signs were measured and baseline bloods obtained to establish pre-treatment IKBKAP mRNA levels and safety parameters. The daily dose of kinetin for each patient was calculated based on weight as 23.5 mg/kg. This dose was identical to the dose used in the carrier study (13), which was extrapolated from cell culture data (9, 10). The initial kinetin dose was administered in the morning, 2 hours after eating. Doses ranged from 1000 mg to 1500 mg (Table 1). The kinetin was given in clear capsules containing 250 mg kinetin and 120 mg of maltodextrin (Nature’s Best Inc. Hauppauge, NY). Dosages were administered either via the gastrostomy tube or by mouth. Standard meals were served throughout the day.
Kinetin pharmacokinetics study
To determine the pharmacokinetics of kinetin, blood was drawn 2 hours before the single dose of kinetin was given. Blood samples were repeated after 0.5, 1, 1.5, 2, 3, 4, 6, 9, 12, and 24 hours. All blood samples were taken after 10 minutes in the seated position. At the 8-day follow-up visit, blood was obtained 2 hours after the morning kinetin dose to measure plasma kinetin levels.
One-month safety and tolerability study
After completing the pharmacokinetics study, patients were discharged from the General Clinical Research Center and instructed to continuing taking the same kinetin dose once daily for 28 consecutive days. Participants returned weekly for repeat safety bloods and clinical assessment.
Measurement of plasma kinetin levels
Whole blood was collected in heparinized tubes. After centrifugation (10 min @ 2000 × g), the plasma was extracted and stored in aliquots at −20° C. Kinetin levels were determined using a validated solid phase extraction method using caffeine (2.5 ng) as an internal standard and Varian Bond Elut 200 mg C18 extraction columns (Varian, Lake Forest, CA). One ml plasma was mixed with 70 µl of 1N acetic acid. The extraction columns were activated with 3 ml of acetonitrile and washed with 3 ml of distilled H2O. The kinetin containing plasma sample (1 ml), was applied to the extraction column followed by 2 ml of distilled H2O. The kinetin was eluted with the addition of 1 ml methanol. The samples were taken to dryness by vacuum centrifugation. Prior to analysis, the samples were reconstituted with 250 µl of the A component of the mobile phase buffer (5% acetonitrile/H2O pH = 3.0). 100 µl was injected into the analytical system via a WISP 717 autosampler (Waters, Milford, MA). The analysis employed a 15 minute linear gradient to 90% acetonitrile/H2O, (B component) followed by 5 minutes isocratic B, and then a 5 minute linear return to A mobile phase using Knauer type 42 pumps (Sonntek, Saddle River, NJ) controlled by Pyramid software (Axxiom Chromatography, Moorepark, CA). The reverse phase HPLC employed a Luna C18, 3µ analytical column (4.6 × 150 mm, Phenomenex, Torrance, CA) with UV detection at 280 nm. The assay for kinetin was linear over the range of 0.05 to 5.0 µg/ml. We utilized nonlinear models available in Winnonlin 5.1 (Pharsight, Sunnyvale, CA) to calculate values of maximum plasma concentration (Cmax) and times of maximal concentration (Tmax), the area under curve (AUC), elimination rate constant (Kel) and time of half-life (T1/2).
Measurement of IKBKAP mRNA levels
Blood samples for IKBKAP mRNA were obtained prior to administration of the first kinetin dose and then at 8 days and at 28 days. Five ml of blood was collected into two vacutainers using the PAXgene Blood RNA System containing the PreAnalytiX reagent (PreAnalytiX, Switzerland) to stabilize intracellular RNA.
As previously described (13, 14), RNA extraction and quantification were performed and the relative amount of wild-type IKBKAP was measured using semi quantitative RT-PCR. Briefly, the relative amount of spliced transcripts were determined using an Alpha 2000 Imager Analyzer (BioRad, Hercules, CA) and Image Quant QL (Amersham, Piscataway, NJ) software, using the integrated density value for each band. These values were then expressed as percent exon inclusion i.e., the percent of IKBKAP transcript produced that contains exon 20 (10).
Statistics
A repeated measure ANOVA was used to compare IKBKAP mRNA exon inclusion levels at baseline, day 8 and day 28. Statistical analyses were performed using SPSS (Version 14.0). Data are shown as mean ± SD, unless otherwise stated. P values of 0.05 or less were considered statistically significant.
RESULTS
Kinetin absorption
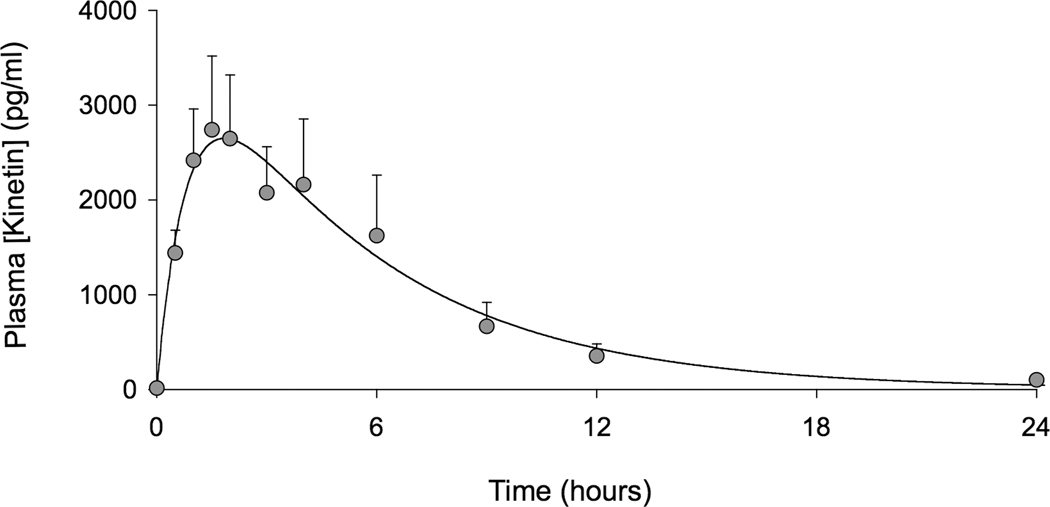
Seven of the eight subjects completed the trial; one subject (female, 37 years old) developed a community-acquired respiratory infection after 21-days of receiving kinetin and was withdrawn from the study. Based on the extrapolation of efficacy data obtained in cell culture studies (9), the target kinetin level was a plasma concentration (Cmax) of 2150 ng/mL. Only subjects #2 and #4 did not meet this target. The remaining participants either met or exceeded the target (Table 1). The mean Cmax was 2866±1889 ng/ml and Tmax=2.1 hr. The mean AUC was 20291±15203 ng/ml*hr. Kinetin was rapidly absorbed and then cleared according to first-order elimination kinetics (Figure 1). Eight days after receiving kinetin, repeated plasma kinetin levels two hours after dose administration were 2994±3168 ng/ml and did not show accumulation or slower metabolism.
Figure 1.
Venous plasma levels of kinetin after administration (n = 8, data are mean±SEM). Time zero represents the time of drug administration.
Effect on IKBKAP mRNA
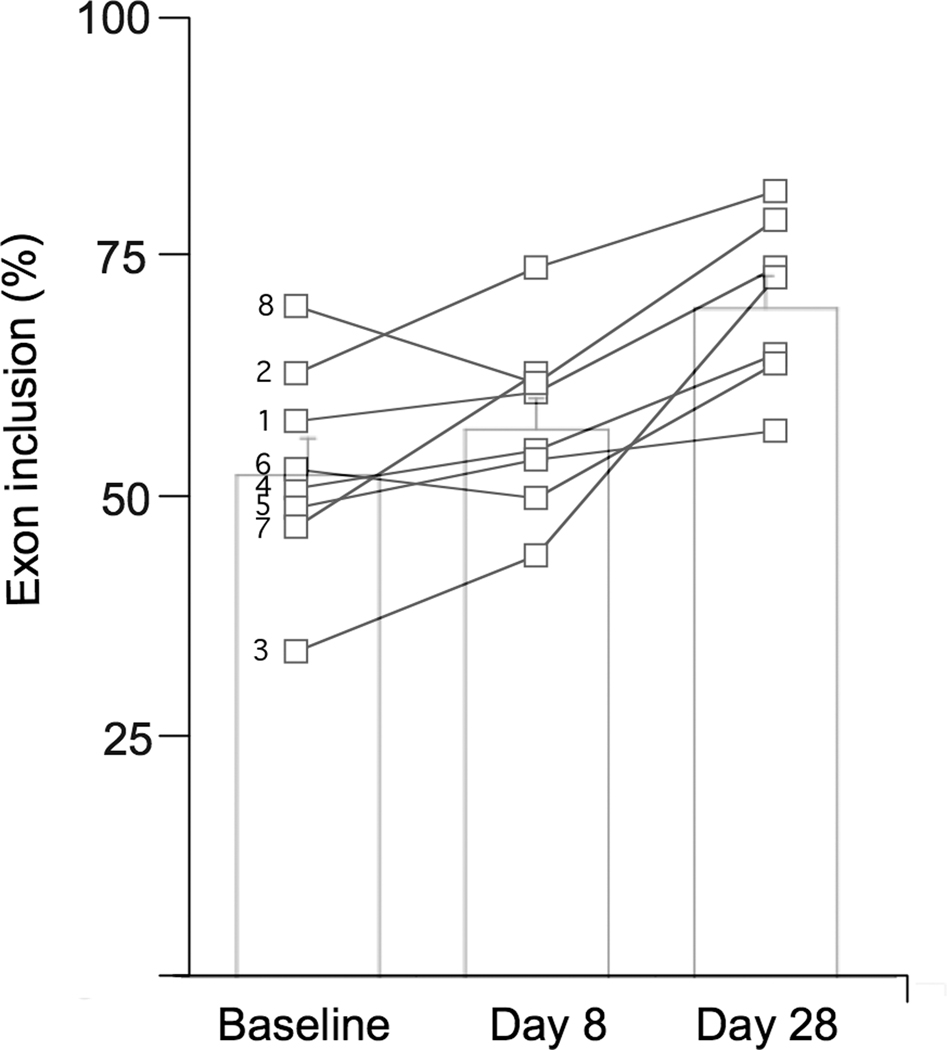
All patients with FD increased the amount of normal wild-type IKBKAP mRNA produced in white blood cells after taking kinetin. At baseline, the mean percentage exon inclusion (i.e., the percentage of correctly spliced IKBKAP mRNA) was 54 ±10%. This increased to 57±10% after receiving kinetin for 8 days and increased further to 71±9% after receiving kinetin for 28 days (p=0.002, Figure 2). There was no relationship between the kinetin dosages and peak concentration levels achieved or the effect on wild-type IKBKAP mRNA production.
Figure 2.
The percent of IKBKAP transcript produced that include exon 20 at baseline, after 8 days of receiving kinetin and after 28 days of receiving kinetin. Open squares show individual data. Subject number is to the left of the baseline values. Bar charts show mean±SEM; p =0.002.
Tolerability
Overall, kinetin was well tolerated with only minimal side effects reported over the 28-day period. Diarrhea occurred in two subjects. Mild to moderate nausea was reported in three participants. These side effects were anticipated as they were common complaints in all subjects before they began taking kinetin. Three subjects complained of headaches. The side effects were not severe enough to cause any of the subjects to drop out of the study over the 28 days.
Safety
Mild transitory changes in laboratory values were noted. Three patients with FD (#3, #6 and #8) had modest increases in liver enzymes. Subject #3 had the highest serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase values at 110 Units/L and 169 Units/L, respectively. Values in subjects #6 and #8 never exceeded 82 Units/L for either transaminase, and bilirubin and alkaline phosphatase values remained normal for all three subjects. One subject (#2) had a decrease in platelet count from his baseline 168,000/ml to 47,000/ml accompanied by a slight decrease in white blood count from baseline of 5300/ml to 3300/ml after 28 days after receiving kinetin, but had no overt signs of viral illness. The possibility of a laboratory error could not be excluded. All laboratory changes were reversible and normalized after discontinuation of kinetin. Blood pressure and heart rate in supine and upright positions were unaffected by kinetin administration. There were no changes in weight or differences noted in the neurological examination during the 28-day period.
DISCUSSION
Familial dysautonomia (FD) is caused by an intronic splice mutation in the IKBKAP gene that leads to partial skipping of exon 20 and tissue-specific reduction in IKAP/ELP-1 protein expression. Because kinetin has been shown to improve splicing and increase wild-type IKBKAP mRNA and IKAP protein expression in FD cell lines, we administered kinetin to eight FD individuals who are homozygous for the splice mutation and were able to demonstrate in vivo splicing changes.
To date, four substances have been identified that potentially increase total IKAP protein levels in FD cell lines, by either increasing total IKBKAP gene expression or modifying mRNA splicing and increasing wild-type IKBKAP mRNA production (9, 15–17). These compounds have been postulated as therapeutic agents for patients with FD. Although in vitro administration of tocotrienols was reported to increase total IKBKAP gene expression (16), its effectiveness could not be replicated by other recent studies (11, 17). Epigallocatechin gallate, like kinetin, reportedly modifies mRNA splicing (15), but its effectiveness in cell lines is modest when compared to kinetin (9, 11). Most recently, phosphatidylserine was shown to also increase total IKBKAP expression, however, its effects on IKAP levels in patients with FD are still unknown. To date, kinetin is the first of these compounds to be subjected to an objective assessment in humans regarding pharmacokinetics, impact on mRNA splicing and safety.
Our results address several fundamental issues relevant to kinetin’s possible role as a therapeutic agent for individuals affected with FD. First, we confirm that kinetin results in plasma levels equivalent to cell culture concentrations reported to affect IKBKAP splicing (9). Consistent with results from a previous study of oral administration of kinetin, kinetin elimination is almost complete by 12 hours following ingestion (13).
Second, our data shows that kinetin corrects the FD splicing defect in affected individuals and raises the level of normal IKBKAP mRNA in vivo. After 8 days of single daily dosing, normal splicing of IKBKAP had increased in six of eight subjects. Even more intriguing was the finding that the magnitude of splicing continued to improve without dosage change, as the levels of wild-type IKBKAP mRNA were greater in all subjects on day 28 than on day 8. It is possible that this is the result of a cumulative effect, similar to that seen in FD cell lines (10). Furthermore the kinetin was relatively well tolerated and safe over this 28-day period. Given the severity of FD and the possibility that kinetin may alter the natural history of the disease, some tolerance for minor side effects might be warranted.
Our data clearly demonstrates that kinetin can modify splicing in peripheral leukocytes. We anticipate that IKBKAP mRNA expression may be similarly affected in neural tissues where mis-splicing is most profound causing the deficits characteristic to this disorder. Gene expression studies in Hela cells following reduction of ELP-1 by RNAi has shown that a subset of Elongator target genes play a role in cell migration (6). This is consistent with previously described neuropathology in FD, such as arrested small fiber neuronal development resulting in variable degrees of disease severity (1). Because of its small molecular size (215.21), kinetin crosses the blood brain barrier quickly (Sitek study No. 0849-M208, SITEK Research Laboratories, Rockville, MD, 2005). Therefore we project that target levels of kinetin will be achieved in human neuronal tissue in order to improve splicing efficiency so that there is increased production of wild-type mRNA transcript and thus increased amounts of normal functioning IKAP. IKAP is an integral component of the human Elongator complex, a transcriptional regulator that impacts on a host of other target genes (6) some of which may be involved in maintenance of sensory and autonomic nervous systems. Thus by modifying activity of these target genes the natural history of FD may be altered and relentless progression attenuated. Long-term clinical trials of kinetin are warranted.
ACKNOWLEDGEMENTS
We would like to thank the staff of the General Clinical Research Center at Bellevue Hospital for their diligence in obtaining and preparing samples. We are grateful to the participants and their families for their continued support
Financial support:
These studies were supported by CTSI Grant # 1UL1RR029893 and grants from the NIH General Clinical Research Centers Program of the National Center for Research Resources M01 RR-00096: NIH NS058318, NS036326, U54NS065736: FDA FD-R-3731-01, and awards from the Dysautonomia Foundation Inc, New York, NY.
ABBREVIATIONS
- AUC
area under the curve
- Cmax
maximum plasma concentration
- ELP-1
elongation protein 1
- FD
familial dysautonomia
- IKBKAP
I-κ-B kinase complex associated protein gene
- IKAP
I-κ-B kinase complex associated protein
- Tmax
time of maximal concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Axelrod FB. Familial dysautonomia. Muscle Nerve. 2004;29:352–363. doi: 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod FB, Gold-von Simson G. Hereditary sensory and autonomic neuropathies: types II, III, and IV. Orphanet J Rare Dis. 2007;2:39. doi: 10.1186/1750-1172-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–1911. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod FB, Goldberg JD, Ye XY, Maayan C. Survival in familial dysautonomia: impact of early intervention. J Pediatr. 2002;141:518–523. doi: 10.1067/mpd.2002.127088. [DOI] [PubMed] [Google Scholar]
- 5.Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, Liebert CB, Chadwick B, Idelson M, Reznik L, Robbins C, Makalowska I, Brownstein M, Krappmann D, Scheidereit C, Maayan C, Axelrod FB, Gusella JF. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Close P, Hawkes N, Cornez I, Creppe C, Lambert CA, Rogister B, Siebenlist U, Merville MP, Slaugenhaupt SA, Bours V, Svejstrup JQ, Chariot A. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell. 2006;22:521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Cuajungco MP, Leyne M, Mull J, Gill SP, Lu W, Zagzag D, Axelrod FB, Maayan C, Gusella JF, Slaugenhaupt SA. Tissue-specific reduction in splicing efficiency of IKBKAP due to the major mutation associated with familial dysautonomia. Am J Hum Genet. 2003;72:749–758. doi: 10.1086/368263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heemskerk J, Tobin AJ, Bain LJ. Teaching old drugs new tricks. Meeting of the Neurodegeneration Drug Screening Consortium, 7–8 April 2002, Washington, DC, USA. Trends Neurosci. 2002;25:494–496. doi: 10.1016/s0166-2236(02)02236-1. [DOI] [PubMed] [Google Scholar]
- 9.Slaugenhaupt SA, Mull J, Leyne M, Cuajungco MP, Gill SP, Hims MM, Quintero F, Axelrod FB, Gusella JF. Rescue of a human mRNA splicing defect by the plant cytokinin kinetin. Hum Mol Genet. 2004;13:429–436. doi: 10.1093/hmg/ddh046. [DOI] [PubMed] [Google Scholar]
- 10.Hims MM, Ibrahim EC, Leyne M, Mull J, Liu L, Lazaro C, Shetty RS, Gill S, Gusella JF, Reed R, Slaugenhaupt SA. Therapeutic potential and mechanism of kinetin as a treatment for the human splicing disease familial dysautonomia. J Mol Med. 2007;85:149–161. doi: 10.1007/s00109-006-0137-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boone N, Loriod B, Bergon A, Sbai O, Formisano-Tréziny C, Gabert J, Khrestchatisky M, Nguyen C, Féron F, Axelrod FB, Ibrahim el C. Olfactory stem cells, a new cellular model for studying molecular mechanisms underlying familial dysautonomia. PLoS One. 2010;5:e15590. doi: 10.1371/journal.pone.0015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold-von Simson G, Goldberg JD, Rolnitzky LM, Mull J, Leyne M, Voustianiouk A, Slaugenhaupt SA, Axelrod FB. Kinetin in familial dysautonomia carriers: implications for a new therapeutic strategy targeting mRNA splicing. Pediatr Res. 2009;65:341–346. doi: 10.1203/PDR.0b013e318194fd52. [DOI] [PubMed] [Google Scholar]
- 14.Gold-von Simson G, Leyne M, Mull J, Rolnitzky LM, Goldberg JD, Berlin D, Axelrod FB, Slaugenhaupt SA. IKBKAP mRNA in peripheral blood leukocytes: a molecular marker of gene expression and splicing in familial dysautonomia. Pediatr Res. 2008;63:186–190. doi: 10.1203/PDR.0b013e31815ef74b. [DOI] [PubMed] [Google Scholar]
- 15.Anderson SL, Qiu J, Rubin BY. EGCG corrects aberrant splicing of IKAP mRNA in cells from patients with familial dysautonomia. Biochem Biophys Res Commun. 2003;310:627–633. doi: 10.1016/j.bbrc.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Anderson SL, Qiu J, Rubin BY. Tocotrienols induce IKBKAP expression: a possible therapy for familial dysautonomia. Biochem Biophys Res Commun. 2003;306:303–309. doi: 10.1016/s0006-291x(03)00971-9. [DOI] [PubMed] [Google Scholar]
- 17.Keren H, Donyo M, Zeevi D, Maayan C, Pupko T, Ast G. Phosphatidylserine increases IKBKAP levels in familial dysautonomia cells. PLoS ONE. 2010;5:e15884. doi: 10.1371/journal.pone.0015884. [DOI] [PMC free article] [PubMed] [Google Scholar]