Abstract
Cystic Fibrosis (CF), a common lethal inherited disorder defined by ion transport abnormalities, chronic infection and robust inflammation, is the result of mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a cAMP-activated chloride (Cl−) channel. Macrophages are reported to have impaired activity in CF. Previous studies suggest that Cl− transport is important for macrophage function therefore impaired Cl− secretion may underlie CF macrophage dysfunction. To determine if alterations in Cl− transport exist in CF macrophages, Cl− efflux was measured using N-[ethoxycarbonylmethyl]-6-methoxyquinolinium bromide (MQAE), a fluorescent indicator dye. The contribution of CFTR was assessed by calculating Cl− flux in the presence and absence of cftrinh-172. The contribution of calcium (Ca2+) modulated Cl− pathways was assessed by examining Cl− flux with varied extracellular Ca2+ concentrations, or following treatment with carbachol or thapsigargin, agents that increase intracellular Ca2+ levels. Our data demonstrate that CFTR contributed to Cl− efflux only in WT macrophages, while Ca2+-mediated pathways contributed to Cl− transport in CF and WT macrophages. Furthermore, CF macrophages demonstrated augmented Cl− efflux with increases in extracellular Ca2+. Taken together, this suggests that Ca2+-mediated Cl− pathways are enhanced in CF macrophages compared to WT macrophages.
INTRODUCTION
Cystic Fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes a Protein Kinase A (PKA) -activated chloride (Cl−) channel. In the absence of functional CFTR, defective Cl− secretion impairs mucociliary clearance and results in viscous secretions in which bacteria proliferate, leading to an influx of immune cells (1, 2). The subsequent robust inflammatory response contributes significantly to airway destruction, respiratory failure and shortened life expectancy. Recent reports suggest that airway inflammation occurs early in life and can be observed prior to bacterial colonization. Elevated numbers of neutrophils and increased IL-8, which may be partially macrophage-derived, have been noted without concomitant infection in the bronchoalveolar lavage (BAL) fluid of infants with CF (3). These findings suggest that if inflammation is present prior to infection then macrophages may be important in stimulating the influx of neutrophils into the airways of these patients. Hubeau et al provided additional evidence that macrophages may contribute to this process as they reported increased numbers of macrophages in CF-affected fetal lung tissue in the absence of acute infection or concurrent rise in other immune cells or inflammatory markers (4). Since macrophages are responsible for recruitment of immune cells to sites of inflammation, macrophage dysfunction in CF may result in altered responses to pathogenic stimuli.
If macrophage dysfunction contributes to the robust inflammatory response described in CF, it may be due to impaired Cl− transport, similar to the mechanism that underlies the pathology observed in CF-affected epithelia. Cl− flux has been described in macrophages at rest (5, 6), during phagocytosis (7), following stimulation when it is associated with increased intracellular calcium (Ca2+) levels (8) and during macrophage activation when it is accompanied by changes in membrane potential. Despite these reports, the exact Cl− pathways and their roles in macrophage function have not been fully defined.
The aim of this study was to evaluate the Cl− efflux pathways present in macrophages. More specifically, to define the contributions of CFTR and Ca2+ activated Cl− pathways to total Cl− flux. While, CFTR activity has been reported in WT macrophages (9), its functional significance remains a question that requires further investigation. Additionally, upregulation of Ca2+-activated Cl− channels (CaCCs) has been well described in airway epithelia in the absence of functional CFTR (10), but it is unknown if this relationship exists in non-epithelial cells. If this relationship is present in macrophages, then it may represent a potential pathway that can be targeted for novel therapeutic intervention. Cl− efflux was studied in murine bone marrow derived (BMD) WT and CF macrophages to compare the contribution of these Cl− efflux pathways to total Cl− transport.
METHODS
Animals
For all experiments outlined two murine models of CF were utilized- a ΔF508 model (Cftrtm1Kt)(11), and a cftr−/− model (Cftrtm1UNC)(12). Both models have been fully backcrossed on a Bl6 background. They were bred and maintained as previously described (13–15). All procedures were performed in accordance with protocols approved by the Yale University Institutional Animal Care and Use Committee.
BMD Macrophage isolation
BM was obtained from long bones (hip, femur, and tibia) of mice (2– 4 mos). Monocytic precursors were selected via Histopaque gradient. Following overnight culture, nonadherent macrophages were selectively grown in DMEM media (Invitrogen, Carlsbad, CA) with 10% FCS, L-glutamine, Penicillin/Streptomycin (100,000 units/ml), and 20ng/ml recombinant murine macrophage colony stimulating factor (PeproTech Inc., Rocky Hill, NJ). Macrophages were cultured at 37°C with 5% CO2 for 9–14d then harvested with Neutral Protease (Worthington Co., Lakewood, NJ); 5–30 × 106 cells were obtained/mouse. Cultured macrophages are F480+/MAC- 1+ as confirmed by flow cytometry (16). A suspension of 1 × 106 macrophages/ml concentration was used for experiments.
Fluorescent Dye Indicator Studies
Macrophages (~1× 105 cells), attached to glass coverslips precoated with Cell Tak (BD scientific laboratories, San Jose, CA), were incubated for 30 min at 37°C with N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide (MQAE-30mM) (17). MQAE (Invitrogen, Carlsbad, CA) is a Cl− sensitive fluorescent indicator dye that measures increases in Cl− concentration via a quenching mechanism. Reductions in cell Cl− give increases in fluorescent intensity indicative of decreased cytosolic Cl− concentration (18). Dye loading and subsequent experimentation were performed in a custom perfusion chamber mounted on an Olympus IX-71 inverted microscope (19). MQAE was excited at 354 ± 10 nm and emitted fluorescent light was measured at 460 ± 10 nm every 5s using a charge coupled device camera attached to a digital imaging system (20, 21). Typically, 10–20 macrophages were monitored simultaneously for each experiment. The rate of change in MQAE fluorescence (Δarbitrary fluorescent units (AFU)/Δtime(s)) was used to calculate Cl− efflux.
Initially macrophages were perfused at 3–4 ml/min with Cl−-containing solution (135 mM NaCl, 5mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, 2mM NaH2PO4, 2mM HEPES, 10mM glucose or previously described (20)) to allow for removal of extraneous dye. Following the initial perfusion with Cl−-containing buffer, the perfusate was changed to a Cl−-free solution (135 mM NaCyclamate, 3mM KGluconate, 0.5 mM CaCyclamate, 1.2 mM MgSO4, 2mM KH2PO4, 2mM HEPES, 10mM Glucose or previously described (20)) in which Cl− was substituted with cyclamate. In a subset of experiments the loading of MQAE was assessed by exposing cells to a final perfusion solution containing potassium thiocyanate (KSCN) (150 mM KSCN, 0.5 mM CaCyclamate, 1.2 mM MgSO4, 2mM KH2PO4, 2mM HEPES, 10mM Glucose) with Nigericin (10 µM) to measure the minimum specific fluorescence of the cells (20). The control Cl−-free solution, contained 0.5mM Ca2+, which is within the normal range for extracellular Ca2+ concentrations (22–25). The high Ca2+/Cl−-free solution had a Ca2+ concentration of 2mM, which can be found in tracheobronchial secretions (26, 27). Macrophages were assessed in a low Ca2+ (0.1mM)/Cl−-free solution for comparison. To ensure that the extracellular Ca2+ concentrations did not affect cell viability, assays were performed with Trypan blue in each experimental solution demonstrating ≥90% viability. Solutions were adjusted to a final pH of 7.4 at 37°C and an osmolarity of 300mOsmol.
To confirm the presence of Cl− movement, macrophages were assessed in the presence of 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB-100 µM), a broad inhibitor of Cl− channels (28), as cells transitioned from Cl−-containing to Cl−-free solutions. The contribution of CFTR to total Cl− flux was evaluated in the presence of the CFTR-specific inhibitor, cftrinh-172 (20µM). Macrophages were treated for 2 min with cftrinh-172 in the Cl−-containing solution, prior to assessing Cl− efflux in the control Cl−-free solution with cftrinh-172 still present. Rates of Cl− efflux following treatment with either inhibitor were compared with rates of Cl− efflux observed in the absence of the inhibitors. Vehicles alone (ethanol or DMSO) had no effect on efflux.
The effect of increasing intracellular Ca2+ concentrations on Cl– efflux were assessed indirectly following treatment with either carbachol or thapsigargin. Macrophages were treated with carbachol (100 µM) for 30 min, while loading with MQAE (29). Alternatively, macrophages were assessed following treatment with thapsigargin (1 µM) for 2 min in Cl−-containing solution prior to assessment in Cl−-free solution. Following treatment with either agent, Cl– efflux was assessed in either low Ca2+/Cl−-free solution with addition of EGTA (1mM) or in the control solution. Chemicals were purchased from Sigma Corporation unless specified.
Data Analysis
Maximal apparent Cl− efflux was calculated using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA) in conjunction with Microsoft Excel to compute the first derivative of the change in MQAE fluorescence over time (slope). The data are summarized as the mean Cl− efflux (ΔAFU/Δs) ± the SEM. An unpaired t-test with Welch’s correction (accounting for unequal variances) was performed to compare slopes between experimental conditions. A p value of <0.05 was considered statistically significant.
RESULTS
Chloride efflux is present in both CF-affected and wild type macrophages
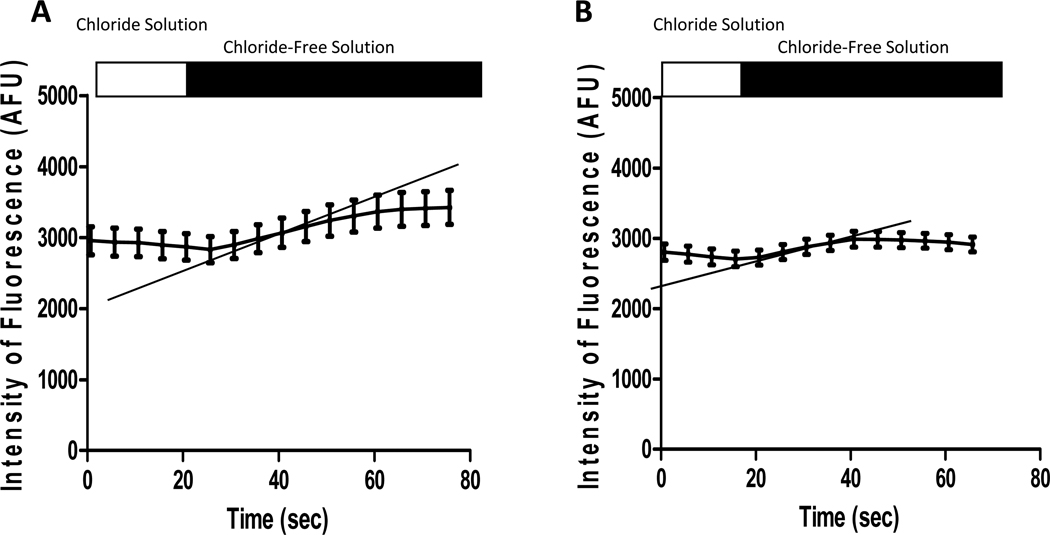
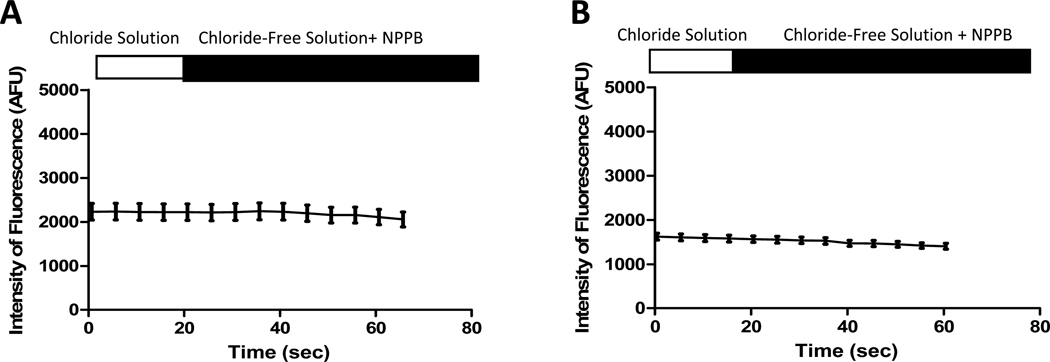
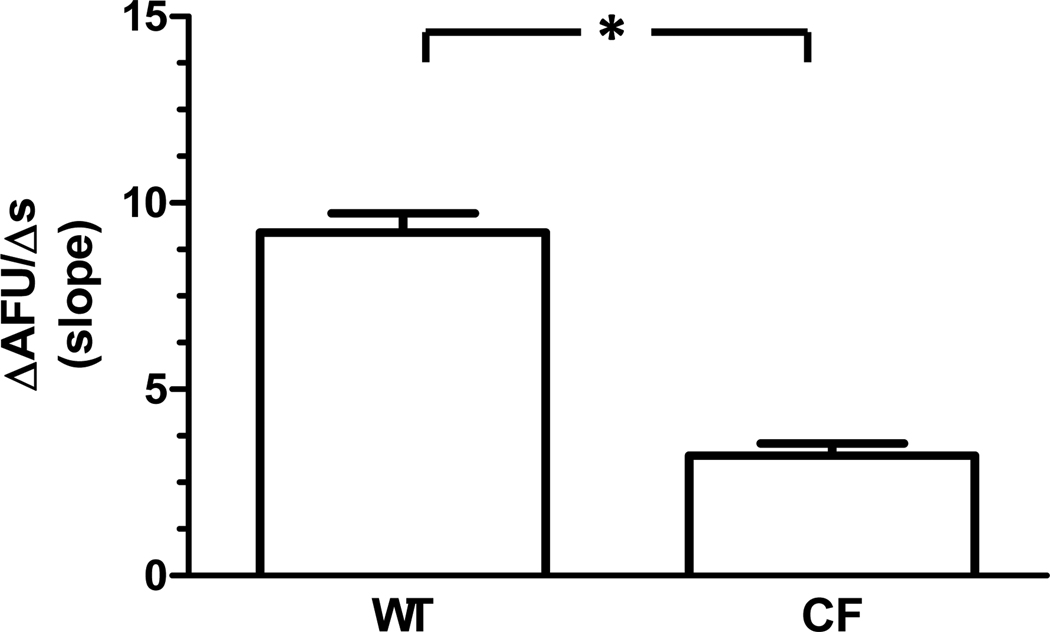
Changes in MQAE fluorescence in Cl−-containing and control Cl−-free solutions for both WT and CF macrophages are represented in Figure 1. Cl− efflux, calculated as the rate of change in MQAE fluorescence (ΔAFU/Δs), was present in both genotypes. Cl− efflux was confirmed via the absence of flux in both WT and CF macrophages in the presence of NPPB (Figure 2). The mean Cl− efflux (ΔAFU/Δs) was calculated for comparison between genotypes in control Cl−-free solution (Figure 3). The Cl− efflux observed in WT macrophages (9.21 ± 0.51 AFU/s) was significantly greater than CF macrophages (3.22 ± 0.32 AFU/s, p<0.0001). Furthermore, experiments performed with perfusion of KSCN solution demonstrated that MQAE fluorescence in macrophages was 4-fold greater than the background fluorescence.
Figure 1. Representative tracings of MQAE fluorescence in WT and cftr−/− macrophages following transition to Cl−-free solution.
All macrophages demonstrate an increase in MQAE fluorescence (AFU) in Cl−-free solution as Cl− exits the cell. The rate of change in fluorescence (i.e. slope: ΔAFU/Δs) represents Cl− efflux (shown with the gray lines). The data depicts the mean ± SEM values from 20 WT (A) and 31 cftr−/− macrophages (B).
Figure 2. Cl− efflux is inhibited in WT and CF macrophages by NPPB.
These representative tracings from WT (A, n=5) and ΔF508/ ΔF508 (B, n=23) macrophages, demonstrate that the addition of NPPB (100µM) abolishes the expected increase in fluorescence that occurs in Cl−-free solution.
Figure 3. Cl− efflux under control conditions in WT and CF macrophages.
WT macrophages (n= 157 from 8 mice) demonstrate greater Cl− efflux compared with CF macrophages (n= 222 total from 7 ΔF508/ ΔF508 mice and 7 cftr−/− mice) in control Cl−-free solution. *p<0.0001
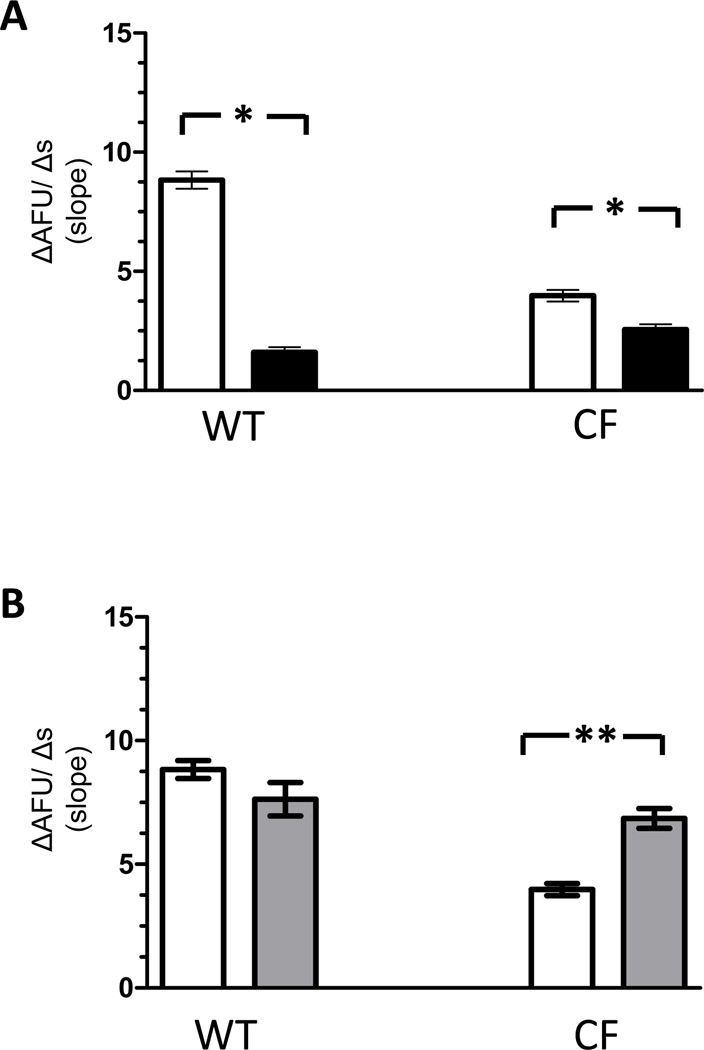
CFTR contributes to the chloride efflux in macrophages
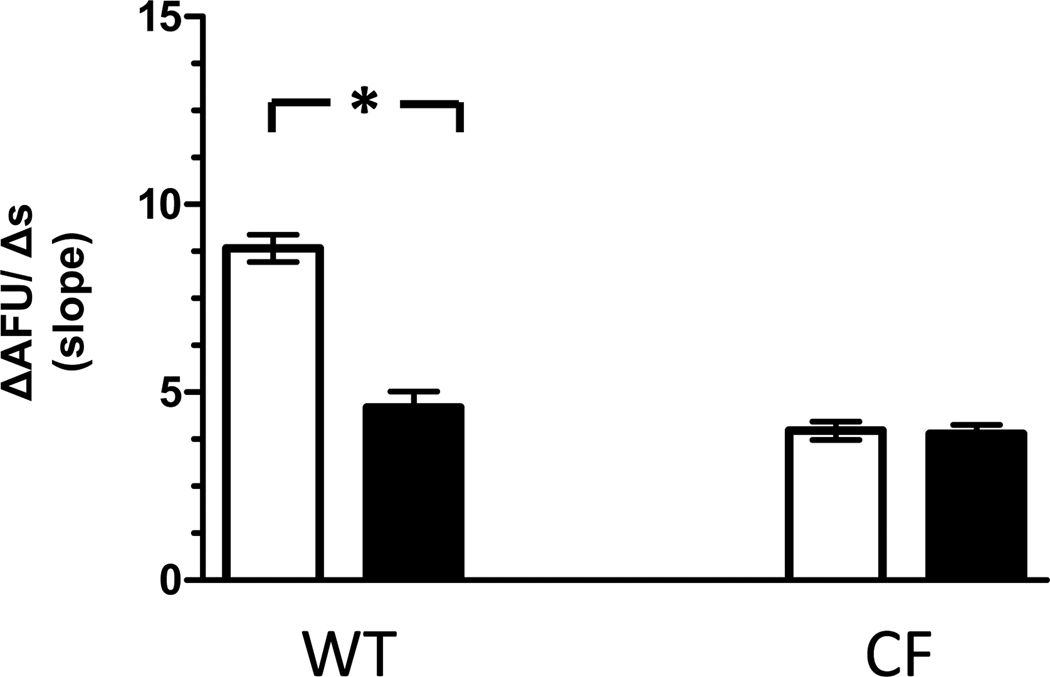
To assess the specific contribution of functional CFTR to Cl− efflux, macrophages were studied in the presence and absence of cftrinh-172 (20µM). Following exposure to cftrinh-172, Cl− efflux in WT macrophages was significantly reduced (4.6 ± 0.42 AFU/s, p<0.0001) compared to Cl− efflux observed under control conditions (Figure 4). In contrast, cftrinh-172 had no appreciable effects on Cl− efflux in CF macrophages (3.9 ±0.23 AFU/s, p=0.09). Of note in the presence of cftrinh-172, the rate of Cl− efflux observed in WT macrophages was equivalent to the rate observed in CF macrophages (p=0.15).
Figure 4. Effects of cftrinh-172 on Cl− efflux in WT and CF macrophages.
Cl− efflux is significantly reduced in WT macrophages (n= 92 from 6 mice) but is unchanged in CF macrophages (n= 177 from 7 ΔF508/ ΔF508 mice and 3 cftr−/− mice) in the presence of cftrinh-172 (■) compared with control conditions (□). * p<0.0001
The presence of Cl− efflux in CF and WT macrophages following treatment with cftrinh-172 suggests that non-CFTR dependent Cl− pathways contributed to total Cl− efflux in macrophages. Previous studies in CF-affected epithelia have described an upregulation of CaCCs (10), but this has not been studied in CF macrophages. Therefore, in subsequent experiments, the effects of Ca2+ on Cl− efflux were assessed in macrophages at various extracellular Ca2+ concentrations, or following treatment with either carbachol or thapsigargin.
Extracellular calcium concentrations increase chloride efflux in CF-affected macrophages
Extracellular Ca2+ concentrations affected Cl− efflux in both WT and CF macrophages. The rates of Cl− efflux were significantly diminished (1.6 ± 0.22 AFU/s and 2.56 ± 0.23 AFU/s respectively, p<0.0001) in low Ca2+ (0.1mM) solution compared with rates of Cl− efflux observed in control (0.5mM Ca2+) solution as shown in Figure 5A. In contrast, only CF-affected macrophages demonstrated a significant increase in Cl− efflux in high (2mM) Ca2+ solution (6.86 ± 0.4 AFU/s, p=0.0002) (Figure 5B). Similar changes were not observed in WT macrophages (7.64 ± 0.67 AFU/s, p=0.12).
Figure 5. Cl− efflux in WT and CF macrophages assessed in low and high extracellular Ca2+ solutions.
A) Both WT (n= 95 cells from 8 mice) and CF (n= 213 cells from 7 ΔF508/ΔF508 and 7 cftr−/− mice) macrophages demonstrate a significant reduction in Cl− efflux in low Ca2+ solution (■) compared with control solution (□). *p<0.0001 B) Cl− efflux is increased in CF macrophages (n= 192 from 5 ΔF508/ ΔF508 and 5 cftr−/− mice) in high Ca2+ solution ( ) compared with control solution (□). Similar changes are not present in WT macrophages (n= 96 from 6 mice). **p=0.0002
) compared with control solution (□). Similar changes are not present in WT macrophages (n= 96 from 6 mice). **p=0.0002
Because extracellular Ca2+ can ultimately affect intracellular Ca2+ levels, the effects of altering intracellular Ca2+ concentrations on Cl− efflux were subsequently assessed. Macrophages were exposed to carbachol, a combined muscarinic and nicotinic receptor agonist that stimulates Ca2+ release from intracellular stores (30). Additionally, macrophages were exposed to thapsigargin, a sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) pump inhibitor that prevents Ca2+ sequestration into the endoplasmic reticulum (31).
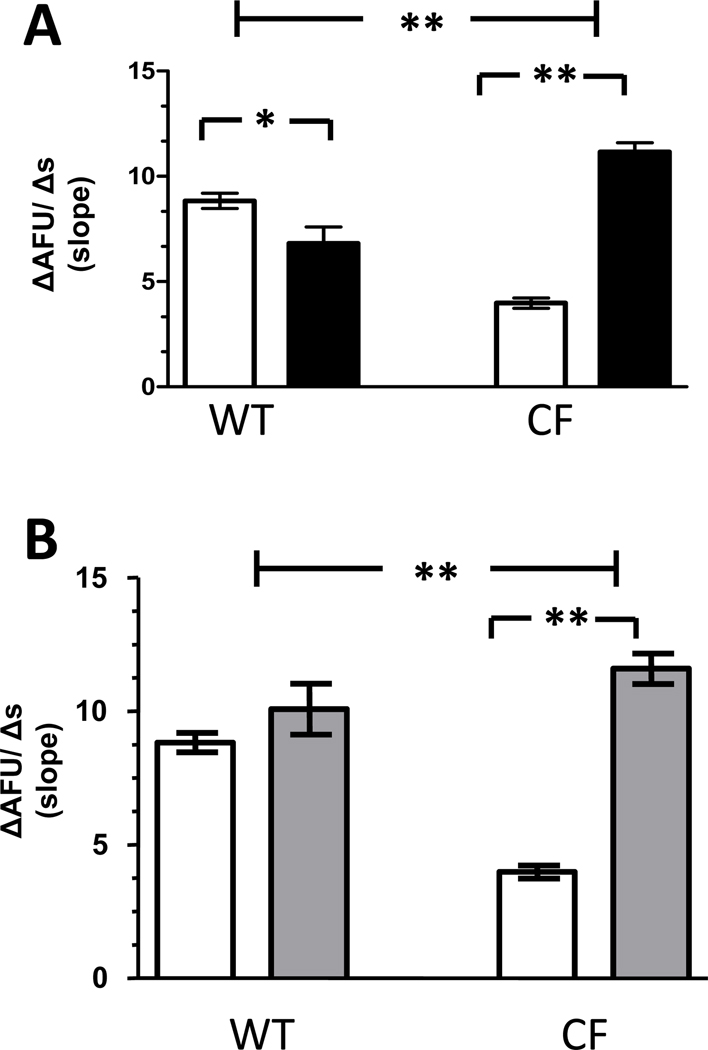
Intracellular calcium concentrations modulate chloride efflux in CF macrophages
Following treatment with carbachol, only CF macrophages demonstrated a significant augmentation of Cl− efflux (11.16 ±0.44 AFU/s, p<0.0001) (Figure 6A). The enhanced rate of Cl− efflux observed in CF macrophages was greater than the rate observed in WT macrophages under control conditions (8.84 ± 0.36 AFU/s, p<0.0001). In contrast, WT macrophages demonstrated a decrease in Cl− efflux following treatment with carbachol (6.82 ±0.77 AFU/s, p=0.019).
Figure 6. Cl− efflux following agents that increase intracellular Ca2+ concentrations.
A) Cl− efflux in macrophages assessed in control solution following carbachol treatment (■) compared with Cl− efflux under control conditions alone (□). (WT macrophages = 86 from 5 mice & CF macrophages = 203 from 5 ΔF508/ ΔF508 and 4 cftr−/− mice).*p=0.019, **p<0.0001 B) Cl− efflux in macrophages assessed in control solution following thapsigargin treatment ( ) compared with Cl− efflux under control conditions alone (□). (WT macrophages = 89 from 5 mice & CF macrophages = 77 from 4 ΔF508/ ΔF508 and 4 cftr−/− mice) **p<0.0001. Only CF macrophages demonstrate increases in Cl− efflux following treatment with either carbachol or thapsigargin.
) compared with Cl− efflux under control conditions alone (□). (WT macrophages = 89 from 5 mice & CF macrophages = 77 from 4 ΔF508/ ΔF508 and 4 cftr−/− mice) **p<0.0001. Only CF macrophages demonstrate increases in Cl− efflux following treatment with either carbachol or thapsigargin.
To confirm that the changes in Cl− flux observed following carbachol treatment were due to increased intracellular Ca2+ concentration rather than nonspecific effects, macrophages were assessed following treatment with thapsigargin. As shown in Figure 6B, Cl− efflux was significantly augmented in CF macrophages following treatment with thapsigargin (11.59 ±0.57 AFU/s, p<0.0001), providing support that Ca2+ mobilized in the setting of SERCA pump inhibition also enhanced Cl− efflux in these cells. In contrast, WT macrophages demonstrated no change in Cl− efflux following thapsigargin treatment (10.08 ±0.95 AFU/s, p=0.22).
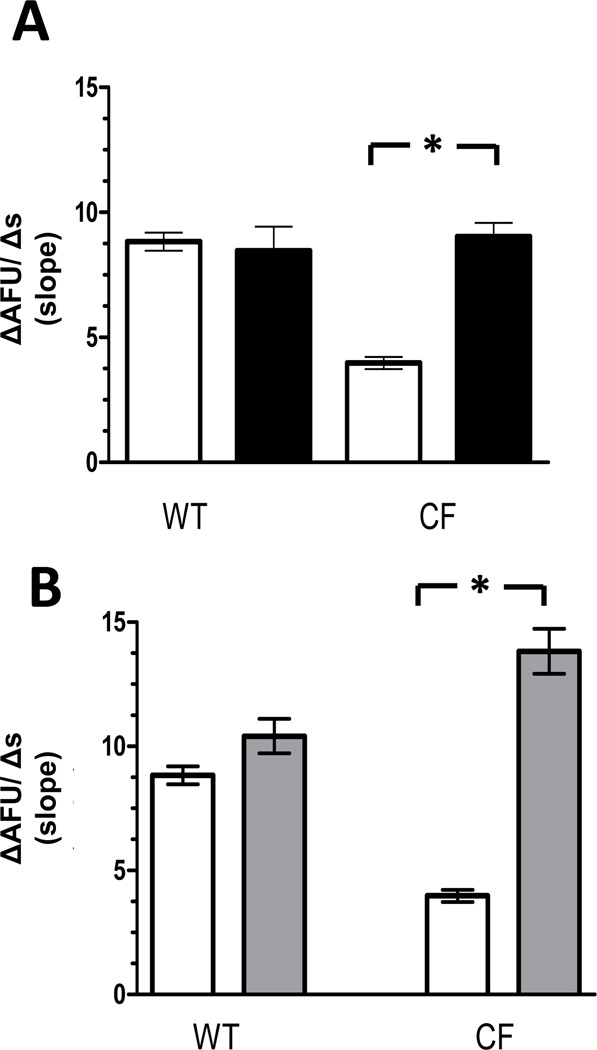
Lastly, to assess if Ca2+ entry contributed to the observed increase in Cl− efflux, macrophages were assessed following treatments with either carbachol (Figure 7A) or thapsigargin (Figure 7B), while being perfused with a low extracellular Ca2+ solution containing EGTA (1mM) to chelate external Ca2+ available. In the low Ca2+ solution, only CF macrophages demonstrated significant enhancement of Cl− efflux following carbachol (9.03 ± 0.55 AFU/s, p<0.0001) and thapsigargin (13.82 ± 0.91 AFU/s, p<0.0001). Interestingly, the rate of Cl− efflux observed in CF macrophages following thapsigargin treatment was greater than that observed in WT macrophages under all conditions studied. In contrast, rates of Cl− efflux were not similarly increased in WT macrophages following treatment with either carbachol (8.47 ± 0.96 AFU/s, p=0.723) or thapsigargin (10.41 ± 0.69 AFU/s, p=0.056). Taken together these data suggest that it is unlikely Ca2+ entry significantly contributed or modulated the rates of Cl− efflux observed following carbachol or thapsigargin treatments.
Figure 7. Cl− efflux assessed in low Ca2+ solution.
A): Cl− efflux in macrophages assessed in low Ca2+ solution following carbachol treatment (■) compared with Cl− efflux under control conditions alone (□). (WT= 79 macrophages from 4 mice & CF = 138 macrophages from 4 ΔF508/ ΔF508 and 3 cftr−/− mice). *p<0.0001 B): Cl− efflux in macrophages assessed in low Ca2+ solution following thapsigargin treatment ( ) compared with Cl− efflux under control conditions alone (□). (WT = 27 macrophages from 3 mice & CF = 130 macrophages from 4 ΔF508/ ΔF508 and 4 cftr−/− mice) *p <0.0001 Only CF macrophages demonstrate increases in Cl− efflux following treatment with either carbachol or thapsigargin.
) compared with Cl− efflux under control conditions alone (□). (WT = 27 macrophages from 3 mice & CF = 130 macrophages from 4 ΔF508/ ΔF508 and 4 cftr−/− mice) *p <0.0001 Only CF macrophages demonstrate increases in Cl− efflux following treatment with either carbachol or thapsigargin.
DISCUSSION
CF has been described as a disease of the epithelia (32). However, the possibility that CFTR dysfunction affects non-epithelial cells, including primary immune cells, has been raised on numerous occasions (1, 2). For instance the BAL specimens from asymptomatic CF infants demonstrate increased levels of IL-8 that could be macrophage-derived (3). Additionally, BAL specimens from older patients with CF demonstrate increased numbers of macrophages in combination with elevated levels of chemokines, known to attract peripheral monocytes (33). Also following stimulation with lipopolysaccharide, CF mice exhibit increased levels of BAL cytokines, that are largely macrophage-derived, compared with WT littermates (16). Moreover, comparable abnormalities of cytokine secretion are observed in their BMD macrophages (16, 34). These data suggest that there is a primary defect in CFTR-deficient monocytes that results in their increased activation. Despite these reports there is no consensus that CFTR dysfunction directly contributes to these findings and thus the role of CFTR in macrophages remains speculative.
Reports suggest that ion channel conductances likely influence immune cell function (35), therefore Cl− permeability may play a role in modulating macrophage activities. Previous studies demonstrated that swell-activated (36), voltage-gated (35), and Ca2+-dependent (8) Cl− pathways are present in macrophages. Additionally, the presence of CFTR has been reported in WT macrophages (9). To date the Cl− pathways that are present in CF macrophages, where CFTR is absent, are not well-characterized but may play a role in the CF inflammatory response. To our knowledge, this study is the first to compare the contributions of CFTR and Ca2+ modulated Cl− pathways to total Cl− transport in CF and WT macrophages.
Our results demonstrate that although Cl− efflux is present in both WT and CF macrophages, the contributions of CFTR and other Cl− pathways to the total Cl− efflux is different for each genotype. The contribution of CFTR to Cl− efflux in WT macrophages is demonstrated clearly by the decreased rate of Cl− efflux observed in WT macrophages treated with cftrinh-172. In addition, non-CFTR dependent Cl− efflux pathways are present in both CF and WT cells, as each genotype exhibits significant residual flux despite the absence of functional CFTR. Furthermore, these additional pathways are partially mediated by extracellular Ca2+ concentrations because a decrease in Cl− flux is observed in both genotypes when extracellular Ca2+ is reduced. Interestingly, only CF macrophages exhibit an increase in Cl− efflux when extracellular Ca2+ concentrations are raised.
The effects of extracellular Ca2+ concentrations on Cl− efflux were unexpected because the link between extracellular Ca2+ concentrations and Cl− flux is not overtly intuitive. It is possible that the presence of divalent cations may stabilize the open state of CFTR and allow for increased Cl− movement (37) as described previously. This would suggest that altering Ca2+ concentration may not only modulate Ca2+ mediated Cl− pathways but also potentially affect CFTR function in WT macrophages.
An alternative explanation for the effects of extracellular Ca2+ on Cl− efflux may be the presence of Ca2+ sensing receptors (CaSR) which have been described in BMD cells (38). Increases in extracellular Ca2+ concentrations would activate CaSRs leading to the release of intracellular Ca2+ stores (25), subsequently increasing Cl− flux via CaCCs. If this mechanism is present, then increased extracellular Ca2+ concentrations will result in an increase in Cl− efflux, while decreased extracellular Ca2+ concentrations should have the opposite effect. However, one must also postulate a difference in some portion of this pathway in CF or WT cells as only CF macrophages exhibited an augmentation of Cl− efflux when examined in high extracellular Ca2+ solution. Additionally, following modulation of intracellular Ca2+ concentrations indirectly with carbachol or thapsigargin, only CF macrophages demonstrated a significant rise in Cl− efflux. Together, these findings suggest that Ca2+ modulates Cl− secretory pathways in CF and WT macrophages differently.
Interestingly, similar findings have been described in cftr−/− epithelia (10) suggesting Ca2+ modulated Cl− pathways may represent an alternative route for augmenting Cl− efflux in the absence of functional CFTR protein in multiple cell types (39–41). One could speculate that under certain circumstances, such as an acute inflammatory response, extracellular Ca2+ levels, which range from 1–4mM in tracheobronchial secretions, could result in a more robust Cl− efflux in these CF macrophages to enhance their function.
However, the use of Ca2+ modulated Cl− pathways could also be detrimental in the overall CF inflammatory response. For instance, studies indicate that changes in Ca2+ mobilization and homeostasis within CF airway epithelia are linked with its predisposition to a hyperinflammatory phenotype (42–45). Also, Mueller et al recently described that altered intracellular Ca2+ mobilization in cftr−/− lymphocytes led to the induction of inflammatory signaling pathways and cytokine secretion (46). Thus the enhancement of Ca2+ modulated Cl− efflux pathways in our CF macrophages may be a potential mechanism by which macrophages directly contribute to the hyperinflammatory phenotype and airway pathophysiology observed in CF.
Acknowledgments
Statement of Financial Support: This study was funded by NIH 5T32HL07272 [to A.S.], CFF (SHENOY10DO) and (EGANG08G, 10G), and NIH (NHLBI) HL093004 [to MEE].
Abbreviations
- AFU
arbitrary fluorescent units
- BAL
Bronchoalveolar lavage
- BMD
Bone Marrow derived
- CaCC
Calcium Activated Chloride Channel
- CF
Cystic Fibrosis
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- KSCN
Potassium Thiocyanate
- MQAE
N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide
- NPPB
5-Nitro-2-(3-phenylpropylamino)benzoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brennan S. Innate immune activation and cystic fibrosis. Paediatr Respir Rev. 2008;9:271–279. doi: 10.1016/j.prrv.2008.05.008. , quiz 279-280. [DOI] [PubMed] [Google Scholar]
- 2.Döring G, Gulbins E. Cystic fibrosis and innate immunity: how chloride channel mutations provoke lung disease. Cell Microbiol. 2009;11:208–216. doi: 10.1111/j.1462-5822.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 3.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 4.Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol. 2001;108:524–529. doi: 10.1067/mai.2001.118516. [DOI] [PubMed] [Google Scholar]
- 5.Robin ED, Smith JD, Tanser AR, Adamson JS, Millen JE, Packer B. Ion and macromolecular transport in the alveolar macrophage. Biochim Biophys Acta. 1971;241:117–128. doi: 10.1016/0005-2736(71)90310-5. [DOI] [PubMed] [Google Scholar]
- 6.Castranova V, Bowman L, Miles PR. Transmembrane potential and ionic content of rat alveolar macrophages. J Cell Physiol. 1979;101:471–479. doi: 10.1002/jcp.1041010313. [DOI] [PubMed] [Google Scholar]
- 7.Ince C, Coremans JM, Ypey DL, Leijh PC, Verveen AA, van Furth R. Phagocytosis by human macrophages is accompanied by changes in ionic channel currents. J Cell Biol. 1988;106:1873–1878. doi: 10.1083/jcb.106.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holevinsky KO, Jow F, Nelson DJ. Elevation in intracellular calcium activates both chloride and proton currents in human macrophages. J Membr Biol. 1994;140:13–30. doi: 10.1007/BF00234482. [DOI] [PubMed] [Google Scholar]
- 9.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 10.Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca(2+)-mediated Cl- secretion in nasal epithelia of CF mice. Am J Physiol. 1994;266:C1478–C1483. doi: 10.1152/ajpcell.1994.266.5.C1478. [DOI] [PubMed] [Google Scholar]
- 11.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB, Jr, Capecchi MR, Welsh MJ, Thomas KR. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 13.Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006;59:191–195. doi: 10.1203/01.pdr.0000196720.25938.be. [DOI] [PubMed] [Google Scholar]
- 15.Weiner SA, Caputo C, Bruscia E, Ferreira EC, Price JE, Krause DS, Egan ME. Rectal potential difference and the functional expression of CFTR in the gastrointestinal epithelia in cystic fibrosis mouse models. Pediatr Res. 2008;63:73–78. doi: 10.1203/PDR.0b013e31815b4bc6. [DOI] [PubMed] [Google Scholar]
- 16.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barmeyer C, Ye JH, Sidani S, Geibel J, Binder HJ, Rajendran VM. Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflugers Arch. 2007;454:441–450. doi: 10.1007/s00424-007-0213-7. [DOI] [PubMed] [Google Scholar]
- 18.Verkman AS. Development and biological applications of chloride-sensitive fluorescent indicators. Am J Physiol. 1990;259:C375–C388. doi: 10.1152/ajpcell.1990.259.3.C375. [DOI] [PubMed] [Google Scholar]
- 19.Sidani SM, Kirchhoff P, Socrates T, Stelter L, Ferreira E, Caputo C, Roberts KE, Bell RL, Egan ME, Geibel JP. DeltaF508 mutation results in impaired gastric acid secretion. J Biol Chem. 2007;282:6068–6074. doi: 10.1074/jbc.M608427200. [DOI] [PubMed] [Google Scholar]
- 20.Egan ME, Glockner-Pagel J, Ambrose C, Cahill PA, Pappoe L, Balamuth N, Cho E, Canny S, Wagner CA, Geibel J, Caplan MJ. Calcium-pump inhibitors induce functional surface expression of Delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat Med. 2002;8:485–492. doi: 10.1038/nm0502-485. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman JF, Geibel JP. Fluorescent imaging of Cl- in Amphiuma red blood cells: how the nuclear exclusion of Cl- affects the plasma membrane potential. Proc Natl Acad Sci USA. 2005;102:921–926. doi: 10.1073/pnas.0408597102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970;170:404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- 23.Jin CH, Segawa A, Miyaura C, Tanaka H, Abe E, Suda T. Calcium is essential in the fusion of mouse alveolar macrophages induced by 1α,12-dihydroxyvitaminD3. J Cell Physiol. 1988;137:110–116. doi: 10.1002/jcp.1041370113. [DOI] [PubMed] [Google Scholar]
- 24.Holian A, Daniele RP. The role of calcium in the initiation of superoxide release from alveolar macrophages. J Cell Physiol. 1982;113:87–93. doi: 10.1002/jcp.1041130115. [DOI] [PubMed] [Google Scholar]
- 25.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 26.Joris L, Dab I, Quinton PM. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 27.Gilljam H, Ellin A, Strandvik B. Increased bronchial chloride concentration in cystic fibrosis. Scand J Clin Lab Invest. 1989;49:121–124. doi: 10.3109/00365518909105409. [DOI] [PubMed] [Google Scholar]
- 28.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittemore ER, Korotzer AR, Etebari A, Cotman CW. Carbachol increases intracellular free calcium in cultured rat microglia. Brain Res. 1993;621:59–64. doi: 10.1016/0006-8993(93)90297-z. [DOI] [PubMed] [Google Scholar]
- 30.Mathes C, Thompson SH. Calcium current activated by muscarinic receptors and thapsigargin in neuronal cells. J Gen Physiol. 1994;104:107–121. doi: 10.1085/jgp.104.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 33.Brennan S, Sly PD, Gangell CL, Sturges N, Winfield K, Wikstrom M, Gard S, Upham JW. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J. 2009;34:655–661. doi: 10.1183/09031936.00178508. [DOI] [PubMed] [Google Scholar]
- 34.Thomas GR, Costelloe EA, Lunn DP, Stacey KJ, Delaney SJ, Passey R, McGlinn EC, McMorran BJ, Ahadizadeh A, Geczy CL, Wainwright BJ, Hume DA. G551D cystic fibrosis mice exhibit abnormal regulation of inflammation in lungs and macrophages. J Immunol. 2000;164:3870–3877. doi: 10.4049/jimmunol.164.7.3870. [DOI] [PubMed] [Google Scholar]
- 35.Gallin EK. Ionic channels in leukocytes. J Leukoc Biol. 1986;39:241–254. doi: 10.1002/jlb.39.3.241. [DOI] [PubMed] [Google Scholar]
- 36.Eder C. Ion channels in microglia (brain macrophages) Am J Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- 37.Ikuma M, Welsh MJ. Regulation of CFTR Cl- channel gating by ATP binding and hydrolysis. Proc Natl Acad Sci USA. 2000;97:8675–8680. doi: 10.1073/pnas.140220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, Glowacki J, Brown EM. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res. 1997;12:1959–1970. doi: 10.1359/jbmr.1997.12.12.1959. [DOI] [PubMed] [Google Scholar]
- 39.Sellers ZM, De Arcangelis V, Xiang Y, Best PM. Cardiomyocytes with disrupted CFTR function require CaMKII and Ca(2+)-activated Cl(−) channel activity to maintain contraction rate. J Physiol. 2010;588:2417–2429. doi: 10.1113/jphysiol.2010.188334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L, Vankeerberghen A, Cuppens H, Eggermont J, Cassiman JJ, Droogmans G, Nilius B. Interaction between calcium-activated chloride channels and the cystic fibrosis transmembrane conductance regulator. Pflugers Arch. 1999;438:635–641. doi: 10.1007/s004249900108. [DOI] [PubMed] [Google Scholar]
- 41.Kunzelmann K, Mall M, Briel M, Hipper A, Nitschke R, Ricken S, Greger R. The cystic fibrosis transmembrane conductance regulator attenuates the endogenous Ca2+ activated Cl− conductance of Xenopus oocytes. Pflugers Arch. 1997;435:178–181. doi: 10.1007/s004240050498. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro CM. The role of intracellular calcium signals in inflammatory responses of polarised cystic fibrosis human airway epithelia. Drugs R D. 2006;7:17–31. doi: 10.2165/00126839-200607010-00002. [DOI] [PubMed] [Google Scholar]
- 43.Antigny F, Norez C, Cantereau A, Becq F, Vandebrouck C. Abnormal spatial diffusion of Ca2+ in F508del-CFTR airway epithelial cells. Respir Res. 2008;9:70. doi: 10.1186/1465-9921-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antigny F, Norez C, Becq F, Vandebrouck C. Calcium homeostasis is abnormal in cystic fibrosis airway epithelial cells but is normalized after rescue of F508del-CFTR. Cell Calcium. 2008;43:175–183. doi: 10.1016/j.ceca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 46.Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]