Abstract
Background
Inflammation and pulmonary diseases, including interstitial lung diseases, are associated with increased lung cancer risk. Circulating levels of surfactant protein-D (SP-D) and Krebs von Lungren-6 (KL-6) are elevated in interstitial lung disease patients, and may be useful markers of processes contributing to lung cancer.
Methods
We conducted a nested case-control study, including 532 lung cancer cases, 582 matched controls and 150 additional controls with chest x-ray (CXR) evidence of pulmonary scarring, in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Serum SP-D and KL-6 levels were measured using enzyme immunoassay. Logistic regression was used to estimate the associations of SP-D and KL-6 with lung cancer and CXR scarring.
Results
Cases had higher levels than controls for SP-D (median 118.7 vs. 105.4 ng/ml, p-value=0.008) and KL-6 (372.0 vs. 325.8 μg/ml, p-value=0.001). Lung cancer risk increased with SP-D (p-trend=0.0003) and KL-6 levels (p-trend=0.005). Compared to the lowest quartile, lung cancer risk was elevated among those with the highest quartiles of SP-D (odds ratio [OR]=1.87; 95% confidence interval [CI] 1.32–2.64), or KL-6 (OR=1.58; 95% CI 1.11–2.25). Among controls, participants with CXR scarring were more likely than those without scarring to have elevated levels of SP-D (quartile 4 vs. quartile 1: OR=1.67; 95% CI: 1.04–2.70; p-trend=0.05) but not of KL-6 (OR=1.04; 95% CI: 0.64–1.68; p-trend=0.99).
Conclusion
Circulating levels of SP-D and KL-6 are associated with subsequent lung cancer risk.
Impact
Our findings support a potential role for interstitial lung disease in lung cancer etiology or early detection, but additional research is needed.
Keywords: lung cancer, inflammation, epidemiology, infections in the etiology of cancer
Introduction
Lung cancer is the most common malignancy and causes the greatest number of cancer-related deaths worldwide, with 1.61 million new cases and 1.38 million deaths in 2008 (1). Tobacco has been well established as the major cause of lung cancer for over 50 years (2), increasing lung cancer risk 15 to 30-fold (3). More recently, studies have examined the role of inflammation in the etiology of lung cancer (4). Both infectious and non-infectious lung diseases that cause extensive and prolonged pulmonary inflammation are associated with an increased risk of lung cancer. Pulmonary tuberculosis, Chlamydia pneumoniae infection, chronic bronchitis and emphysema have each been associated with increased lung cancer risk (5–10). Further, increased lung cancer risk is observed in people with asymptomatic pulmonary scarring (11) and with interstitial lung diseases, such as idiopathic pulmonary fibrosis (12–14).
Idiopathic pulmonary fibrosis and other interstitial lung diseases are associated with measurably elevated serum levels of two glycoproteins: surfactant protein D (SP-D) and Krebs von Lungren-6 (KL-6) (15, 16). SP-D is a component of pulmonary surfactant, plays a role in innate immunity, and modulates inflammation in the lung (16). SP-D levels are also elevated in patients with chronic obstructive pulmonary disease (COPD), particularly during COPD exacerbations (17, 18). KL-6 is a glycoprotein classified as a MUC1 mucin (15). It has been used as a diagnostic marker for interstitial lung diseases (19), and is expressed by a number of adenocarcinomas, including lung adenocarcinoma (15). SP-D and KL-6 are both produced by pulmonary alveolar type II cells, which proliferate during lung injury (20). In addition, SP-D is expressed by Clara cells, and KL-6 is expressed by respiratory bronchiolar epithelial cells and serous cells of the bronchial glands (15, 16). While increased serum levels of SP-D and KL-6 have been evident for specific lung diseases (15–18, 21–23), little is known about their utility in detecting more limited lung damage among relatively healthy people.
Although lung cancer risk is elevated in people with clinically evident lung diseases, it is unknown whether subclinical levels of lung damage and disease are associated with development of lung cancer. In the current study, we measured serum levels of SP-D and KL-6 in lung cancer cases and controls from the screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Our goal was to evaluate a largely healthy population, in order to assess whether low levels of lung damage or disease increase subsequent lung cancer risk. We carefully adjusted for cigarette smoking to assess the independent associations of SP-D and KL-6 with lung cancer risk.
Methods
Study Population and Laboratory Methods
Study participants were enrolled in the PLCO trial, which recruited approximately 155,000 people from the general population at ten U.S. screening centers from 1992 to 2001 (24). Participants aged 50–74 years were randomized to receive either screening for prostate, lung, colorectal and ovarian cancers, or routine health care. Participants were excluded if they were undergoing treatment for cancer, or had a history of prostate, lung, colorectal or ovarian cancer. Lung cancer screening included a chest x-ray (CXR) at baseline and yearly follow-up intervals. Additionally, baseline blood samples were obtained and demographic, behavioral and dietary information was collected via questionnaire. Lung cancer cases were ascertained through periodic self-report and death certificate review, and confirmed by medical chart abstraction.
Our case-control study was nested within the screening arm of PLCO (Supplemental Figure 1). Of the 898 lung cancer cases that occurred during PLCO follow-up through December 31, 2004, we included 532 in our analysis. Cases were excluded if they had a missing baseline questionnaire (n=17), a history of cancer at baseline (n=57), multiple cancers during follow-up (n=88), incomplete tobacco use information (n=11), if they did not give consent for their samples to be used (n=31) or did not have an available serum specimen (n=162). Controls (i.e., “matched controls”; n=582) were matched to cases based on the following criteria: age at randomization (55–59, 60–64, 65–69, 70–74 years), sex, year of randomization (1993–1995, 1996–1997, 1998–1999, 2000–2001), length of follow-up during the study (1-year intervals), smoking status (current, former, never), cumulative smoking at baseline for current and former smokers (0–29, 30–39, 40–49, 50+ pack-years), and time since quitting for former smokers (<15 and 15+ years). We selected one control for each case who was a current or former smoker, and three controls for each case who was a never smoker.
As part of the PLCO trial, study radiologists blinded to participant characteristics determined whether radiological findings interpreted from CXRs were suggestive of lung cancer (11). Further, radiologists recorded other CXR abnormalities on trial evaluation forms, including the presence and location of “scarring/pulmonary fibrosis/honeycombing.” While the matched controls described above included some subjects with evidence of pulmonary scarring on baseline CXRs (n=53), we selected 150 additional such controls (i.e., “scarred controls”, including 50 controls with right lung scarring, 50 controls with left lung scarring, and 50 controls with bilateral scarring). These scarred controls were matched to the original “matched controls” by age, sex and smoking status.
Enzyme immunoassays were used to measure levels of SP-D (BioVendor, LLC; Candler, NC), KL-6 (SankoJanyaku Co., Ltd; Tokyo, Japan) and a third marker, e-selectin (Bender MedSystems, Inc.; Burlingame, CA), in serum samples collected from participants at PLCO baseline. We also included 10 serum samples collected from patients with biopsy-confirmed idiopathic pulmonary fibrosis as positive controls. Average SP-D levels in positive controls were 318.5 ng/ml compared to 46.3 ng/ml in healthy blood donors, and average KL-6 levels in positive controls were 1,465 μg/ml compared to 276 μg/ml in healthy blood donors. Because e-selectin levels were not found to be elevated in patients with idiopathic pulmonary fibrosis (not shown), we did not assess this biomarker further. Mean coefficients of variation within each plate calculated from 50 quality control samples tested in pairs on the same plate were 17.3% for SP-D and 18.3% for KL-6. KL-6 was tested in single runs and SP-D was tested in duplicate. Duplicate SP-D measurements for each participant were averaged for analysis. In a previous study (25), high-sensitivity C-reactive protein (CRP) was measured in baseline serum samples using a chemiluminescent immunoassay (Diagnostic Products Corporation, Los Angeles, CA).
The study was approved by institutional review boards at the National Cancer Institute and the National Human Genome Research Institute, and all participants provided written informed consent.
Statistical Analysis
Participant characteristics were compared between cases and matched controls, using the chi-squared test and tests of medians (26). Geometric mean levels of SP-D and KL-6 were also compared across participant characteristics among matched controls. With data from the cases and matched controls, unconditional logistic regression was used to measure the associations of SP-D and KL-6 with lung cancer risk, adjusting for the matching factors. SP-D and KL-6 levels were categorized into quartiles based on the distribution of these markers in controls. In an additional logistic regression model, we also included baseline CXR scarring and levels of both SP-D and KL-6. We evaluated models separately according to lung cancer histology (squamous cell carcinoma, adenocarcinoma, small cell carcinoma and large cell carcinoma). Further analyses were carried out stratified by time from baseline blood collection to lung cancer/control selection or by smoking status. As controls were matched to cases on follow-up time, time from baseline was defined among controls as time from baseline blood collection to the date of cancer diagnosis in the matched case. We also utilized linear regression to assess the association between time from baseline and levels of SP-D and KL-6 among cases.
In a control-only analysis (both matched and scarred controls), we assessed the associations of SP-D and KL-6 levels with CXR scarring using unconditional logistic regression, adjusted for age, sex, smoking status, time since quitting, cumulative smoking, and randomization year. Associations with any scarring, unilateral scarring, and bilateral scarring were examined in separate models.
Results
Table 1 presents the characteristics of 532 lung cancers and 582 matched controls in our study. Lung cancers were predominantly adenocarcinomas (n=189) and squamous cell carcinomas (n=114), but also included 68 small cell carcinomas and 30 large cell carcinomas. No significant differences were observed for the matching characteristics, with the exception of smoking status, where by design we included an excess of controls who were never smokers. As reported previously (11), cases were more likely to have CXR evidence of scarring than controls (71 cases vs. 53 controls, p-value=0.02). Cases were also more likely than controls to have a history of emphysema or chronic bronchitis at baseline (p-value=<0.001 and p-value=0.02, respectively).
Table 1.
Characteristics of study subjects from the PLCO trial.
| Lung cancer cases, n (%) | Matched controls, n (%) | P-value | |
|---|---|---|---|
| Total | 532 (100) | 582 (100) | |
| Age at randomization, years | |||
| 55–59 | 96 (18) | 104 (18) | 0.68 |
| 60–64 | 145 (27) | 163 (28) | |
| 65–69 | 182 (34) | 182 (31) | |
| 70–74 | 109 (20) | 133 (23) | |
| Sex | |||
| Female | 169 (32) | 205 (35) | 0.22 |
| Male | 363 (68) | 377 (65) | |
| Randomization year | |||
| 1993–95 | 220 (41) | 246 (42) | 0.99 |
| 1996–97 | 164 (31) | 175 (30) | |
| 1998–99 | 109 (20) | 120 (21) | |
| 2000–01 | 39 (7) | 41 (7) | |
| Race | |||
| White | 470 (88) | 525 (90) | 0.06 |
| Black | 42 (8) | 28 (5) | |
| Asian | 9 (2) | 6 (1) | |
| Pacific Islander | 9 (2) | 21 (4) | |
| American Indian | 2 (0.4) | 2 (0.3) | |
| Smoking status | |||
| Never | 33 (6) | 109 (19) | <0.001 |
| Current | 208 (39) | 206 (35) | |
| Former | 291 (55) | 267 (46) | |
| Cumulative smoking, pack-years* | |||
| 1–30 | 126 (25) | 119 (25) | 0.95 |
| 30–40 | 136 (27) | 126 (27) | |
| 40–50 | 42 (8) | 45 (10) | |
| 50–230 | 195 (39) | 183 (39) | |
| Years since quitting** | |||
| 0.5–15 | 185 (64) | 167 (63) | 0.80 |
| 15–53 | 106 (36) | 100 (37) | |
| Body mass index | |||
| 14.0–18.5 | 6 (1) | 3 (0.5) | 0.54 |
| 18.6–25.0 | 184 (35) | 189 (33) | |
| 25.1–30.0 | 231 (44) | 269 (47) | |
| 30.1–52.5 | 104 (20) | 112 (20) | |
| Regular aspirin use | |||
| No | 235 (44) | 270 (46) | 0.51 |
| Yes | 294 (56) | 312 (54) | |
| Regular ibuprofen use | |||
| No | 393 (74) | 401 (69) | 0.06 |
| Yes | 138 (26) | 181 (31) | |
| History of chronic bronchitis | |||
| No | 472 (89) | 539 (93) | 0.02 |
| Yes | 59 (11) | 42 (7) | |
| History of emphysema | |||
| No | 470 (89) | 550 (95) | <0.001 |
| Yes | 61 (11) | 31 (5) | |
| Scarring on baseline chest x-ray | |||
| No | 461 (87) | 529 (91) | 0.02 |
| Yes | 71 (13) | 53 (9) | |
Analysis was limited to former and current smokers only.
Analysis was limited to former smokers only.
Table 2 describes levels of SP-D and KL-6 among matched controls. Age was not significantly associated with levels of SP-D or KL-6, but men had significantly higher levels of both SP-D and KL-6 compared to women. SP-D levels were also higher in whites than other racial groups. Significant differences in SP-D were observed by smoking status, with the highest levels observed in current smokers, but no significant trends in SP-D were observed with cigarettes smoked per day, pack-years smoked, or years since smoking cessation among former smokers. SP-D levels decreased with increasing body mass index and with regular ibuprofen use. KL-6 was positively associated with increasing CRP levels, and there was a borderline positive association for SP-D. KL-6 levels did not vary significantly by measures of smoking, and neither marker differed according to history of chronic bronchitis or emphysema. KL-6 and SP-D were correlated with each other among controls (r=0.30; p<0.0001).
Table 2.
Levels of surfactant protein-D and KL-6 among matched controls in the PLCO trial.
| SP-D (ng/ml) | KL-6 (μg/ml) | |||||
|---|---|---|---|---|---|---|
| Geometric mean | 95% CI | p-value† | Geometric mean | 95% CI | p-value† | |
| Age at randomization | ||||||
| ≤59 | 94.6 | 85.2–105.0 | 0.08 | 344.6 | 314.9–377.2 | 0.26 |
| 60–64 | 105.3 | 96.9–114.5 | 337.1 | 313.7–362.4 | ||
| 65–69 | 101.0 | 93.3–109.3 | 342.1 | 319.6–366.3 | ||
| 70+ | 110.6 | 100.8–121.3 | 366.4 | 338.2–396.8 | ||
| Gender | ||||||
| Male | 108.1 | 102.3–114.2 | 0.004 | 361.4 | 344.8–378.9 | 0.003 |
| Female | 94.5 | 87.7–101.8 | 320.8 | 300.9–341.9 | ||
| Race | ||||||
| White | 108.6 | 103.8–113.5 | <0.0001 | 347.7 | 334.0–362.0 | 0.38 |
| Black | 74.2 | 61.1–90.0 | 372.4 | 312.9–443.2 | ||
| Asian | 57.2 | 37.7–86.9 | 297.8 | 204.5–433.8 | ||
| Pacific Islander | 56.6 | 45.3–70.7 | 316.1 | 258.6–386.5 | ||
| American Indian | 41.6 | 20.2–85.7 | 214.3 | 111.8–411.0 | ||
| Smoking status | ||||||
| Never | 92.9 | 83.9–102.9 | 0.03 | 334.2 | 306.0–365.0 | 0.41 |
| Current | 110.3 | 102.4–118.8 | 341.0 | 319.8–363.6 | ||
| Former | 102.1 | 95.7–109.0 | 356.1 | 336.6–376.8 | ||
| Cigarettes smoked per day* | ||||||
| 10–20 | 101.3 | 95.4–107.5 | 0.91 | 348.1 | 330.7–366.4 | 0.57 |
| 21–30 | 106.1 | 96.6–116.6 | 345.3 | 318.3–374.5 | ||
| 31–40 | 104.3 | 91.9–118.4 | 342.2 | 306.9–381.5 | ||
| 41–100 | 105.4 | 91.2–121.8 | 346.2 | 305.7–392.1 | ||
| Cumulative smoking, pack-years* | ||||||
| 1–30 | 93.3 | 84.6–102.9 | 0.06 | 360.6 | 331.2–392.7 | 0.66 |
| 30–40 | 113.0 | 102.8–124.3 | 335.4 | 308.8–364.4 | ||
| 40–50 | 104.0 | 88.7–121.9 | 387.9 | 337.7–445.5 | ||
| 50–230 | 109.7 | 101.4–118.7 | 343.3 | 320.5–367.6 | ||
| Years since quitting** | ||||||
| 0.5–15 | 104.0 | 95.8–112.9 | 0.47 | 357.4 | 332.0–384.8 | 0.87 |
| 15–53 | 99.0 | 89.1–110.1 | 354.0 | 321.8–389.4 | ||
| Body mass index | ||||||
| 14.0–25.0 | 111.5 | 103.2–120.4 | 0.03 | 353.5 | 330.7–378.0 | 0.38 |
| 25.1–30.0 | 99.4 | 93.2–106.1 | 345.9 | 326.9–366.0 | ||
| 30.1–52.5 | 98.0 | 88.6–108.4 | 336.7 | 308.5–367.4 | ||
| History of chronic bronchitis | ||||||
| No | 102.6 | 97.9–107.4 | 0.41 | 346.1 | 332.6–360.1 | 0.73 |
| Yes | 110.2 | 93.4–130.0 | 355.2 | 308.1–409.5 | ||
| History of emphysema | ||||||
| No | 102.5 | 97.9–107.3 | 0.33 | 346.9 | 333.6–360.8 | 0.73 |
| Yes | 113.1 | 93.3–137.1 | 336.9 | 285.4–397.5 | ||
| Regular aspirin use | ||||||
| No | 101.3 | 94.9–108.2 | 0.48 | 348.7 | 329.7–368.8 | 0.77 |
| Yes | 104.7 | 98.5–111.2 | 344.7 | 327.2–363.2 | ||
| Regular ibuprofen use | ||||||
| No | 106.2 | 100.7–112.0 | 0.05 | 350.6 | 334.8–367.1 | 0.38 |
| Yes | 96.5 | 89.2–104.5 | 337.8 | 315.4–361.7 | ||
| C-reactive protein | ||||||
| 0.30–0.99 mg/L | 97.5 | 88.6–107.2 | 0.11 | 321.2 | 296.0–348.4 | 0.04 |
| 1.0–2.4 mg/L | 102.7 | 94.4–111.8 | 351.0 | 326.3–377.5 | ||
| 2.5–5.3 mg/L | 102.8 | 94.1–112.2 | 345.3 | 320.2–372.3 | ||
| 5.4–147.0 mg/L | 109.0 | 99.8–119.1 | 366.4 | 339.7–395.3 | ||
Analysis was limited to former and current smokers only.
Analysis was limited to former smokers only.
p-value is for overall difference or trend.
Cases had higher serum levels than controls for both SP-D (median 118.7 vs. 105.4 ng/ml, p-value=0.008) and KL-6 (372.0 vs. 325.8 μg/ml, p-value=0.001). As shown in Table 3, lung cancer risk increased with SP-D level (p-trend=0.0003), and participants with levels in the fourth quartile of SP-D had nearly twice the risk of lung cancer, compared to the first quartile (odds ratio [OR]=1.87; 95% confidence interval [CI] 1.32–2.64). Likewise, lung cancer risk increased with KL-6 levels (p-trend=0.005, Table 3), and participants with KL-6 levels in the highest quartile had 60% higher risk than participants in the lowest quartile (OR=1.58; 95% CI 1.11–2.25). Further adjustment for history of emphysema or chronic bronchitis did not alter the associations between the markers and lung cancer (results not presented). In models that additionally included quartiles of CRP, we observed independent associations with lung cancer for SP-D (p-trend=0.0006) and CRP (p-trend=0.003), and for KL-6 (p-trend=0.01) and CRP (p-trend=0.003). In a model that included SP-D, KL-6, and CXR scarring, SP-D and KL-6 each were independently associated with lung cancer risk (p-trend=0.001 and p-trend=0.03, respectively; Table 3). Additionally, we dichotomized SP-D and KL-6 at the median and examined joint categories. Compared to those below the median for both SP-D and KL-6, those with higher levels of both markers had double the risk of lung cancer (OR=2.01; 95% CI 1.43–2.83). No significant interactions were observed between SP-D and KL-6 (p-interaction=0.24), SP-D and CXR scarring (p-interaction=0.91), or KL-6 and CXR scarring (p-interaction=0.87).
Table 3.
Associations of serum levels of SP-D and KL-6 with subsequent risk of lung cancer.
| Lung cancer cases, n (%) | Matched controls, n (%) | Adjusted odds ratio1 | 95% CI | Mutually adjusted odds ratio2 | 95% CI | |
|---|---|---|---|---|---|---|
| SP-D, ng/ml | ||||||
| 16.5–71.7 | 97 (18) | 146 (25) | 1.00 | 1.00 | ||
| 71.8–105.3 | 120 (23) | 145 (25) | 1.25 | 0.87–1.79 | 1.19 | 0.83–1.72 |
| 105.4–143.8 | 122 (23) | 146 (25) | 1.17 | 0.81–1.68 | 1.13 | 0.79–1.63 |
| 143.9–615.5 | 193 (36) | 145 (25) | 1.87 | 1.32–2.64 | 1.77 | 1.24–2.51 |
| P-trend=0.0003 | P-trend=0.001 | |||||
| KL-6, μg/ml | ||||||
| 178.8–231.0 | 98 (18) | 145 (25) | 1.00 | 1.00 | ||
| 231.1–325.7 | 113 (21) | 146 (25) | 1.11 | 0.77–1.59 | 1.08 | 0.75–1.56 |
| 325.8–465.7 | 160 (30) | 145 (25) | 1.66 | 1.17–2.35 | 1.60 | 1.13–2.28 |
| 465.8–4225.9 | 161 (30) | 146 (25) | 1.58 | 1.11–2.25 | 1.43 | 1.00–2.05 |
| P-trend=0.005 | P-trend=0.03 | |||||
Abbreviations: CI confidence interval
Separate models were used for each marker. Estimates were adjusted for age, sex, smoking status, time since quitting, cumulative smoking, and randomization year.
A single model included quartiles of both markers. Estimates were adjusted for age, sex, smoking status, time since quitting, cumulative smoking, randomization year, and scarring on baseline chest x-ray.
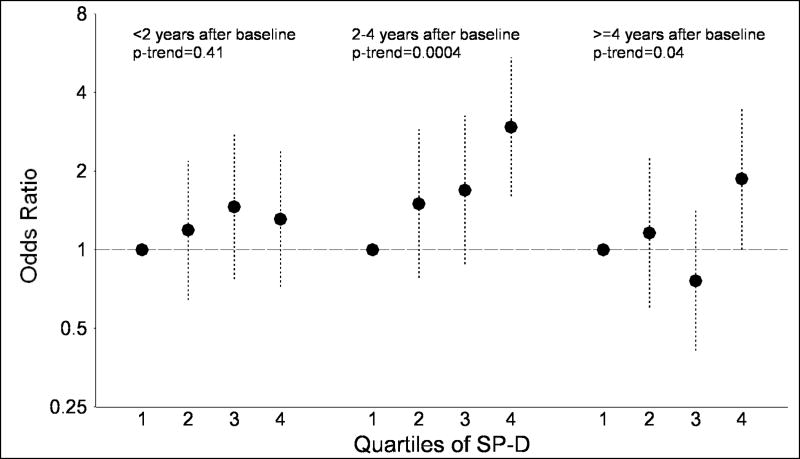
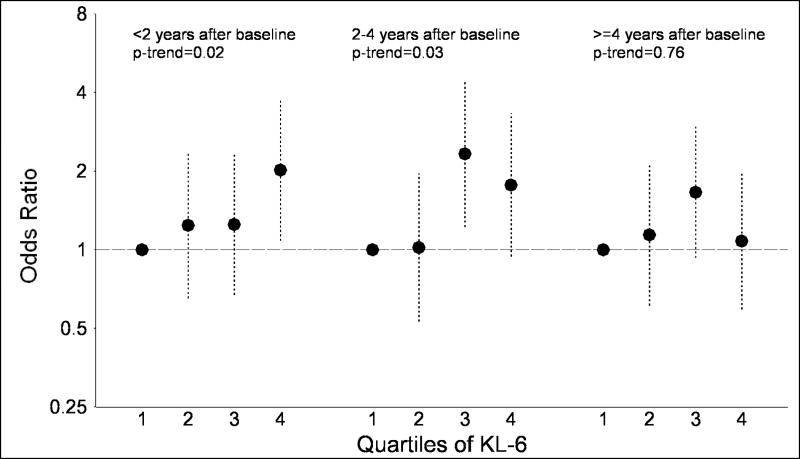
Additionally, we examined the association of SP-D and KL-6 with lung cancer stratified by the time following the baseline blood collection (Figures 1A and 1B). For SP-D, no association with lung cancer risk was observed within the two years after blood collection (quartile 4 vs. quartile 1: OR=1.31; p-trend=0.41). However, significant associations were observed after blood collection in the 2.0–4.0 year period (quartile 4 vs. quartile 1: OR=2.95; p-trend=0.0004) and 4.1–10.6 year period (OR=1.87; p-trend=0.04). In contrast, associations between KL-6 and lung cancer risk were observed in the time periods 0.03–1.9 years (quartile 4 vs. quartile 1: OR=2.02; p-trend=0.02) and 2.0–4.0 years (OR=1.77; p-trend=0.03) following blood collection, but no association was observed ≥4.1 years after blood collection (OR=1.08; p-trend=0.76). Tests for interaction with time from baseline were not significant for SP-D (p-interaction=0.16) or KL-6 (p-interaction=0.15). Similarly, when the analysis was limited to lung cancer cases, KL-6 levels were significantly higher (p=0.0003) in cases arising 0.03–1.9 years after blood collection compared to≥4.1 years after blood collection. In contrast, SP-D levels tended to be lower (p=0.10) in cases 0.03–1.9 vs. ≥4.1 years after blood collection.
Figure 1.
The figure presents odds ratios and 95% confidence intervals for lung cancer across quartiles of surfactant protein D (SP-D; figure 1A) and Krebs von Lungren-6 (KL-6; figure 1B), stratified by time since baseline blood collection (<2.0 years, 2.0–4.0 years and 4.1+ years). P-values for trend across quartiles are also presented.
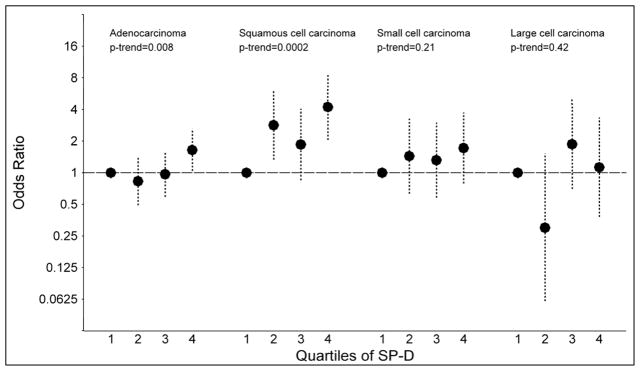
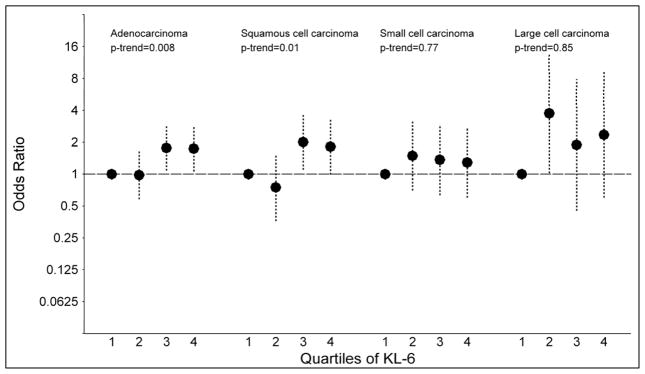
Associations between the serum markers and specific histological types of lung cancer were statistically significant for adenocarcinoma (quartile 4 vs. quartile 1: SP-D: OR=1.65; p-trend=0.008 and KL-6: OR=1.74; p-trend=0.008) and squamous cell carcinoma (quartile 4 vs. quartile 1: SP-D: OR=4.22; p-trend=0.0002 and KL-6: OR=1.82; p-trend=0.01; Figures 2A and 2B). No significant trends were observed for small cell (quartile 4 vs. quartile 1: SP-D: OR=1.72; p-trend=0.21 and KL-6: OR=1.29; p-trend=0.77) or large cell carcinomas (quartile 4 vs. quartile 1: SP-D: OR=1.13; p-trend=0.42 and KL-6: OR=2.36; p-trend=0.85).
Figure 2.
The figure presents odds ratios and 95% confidence intervals for lung cancer across quartiles of surfactant protein D (SP-D; figure 2A) and Krebs von Lungren-6 (KL-6; figure 2B), stratified by tumor histology (adenocarcinoma, squamous cell carcinoma, small cell carcinoma and large cell carcinoma). P-values for trend across quartiles are also presented.
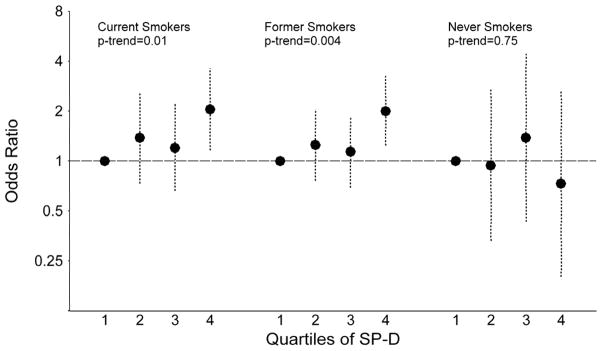
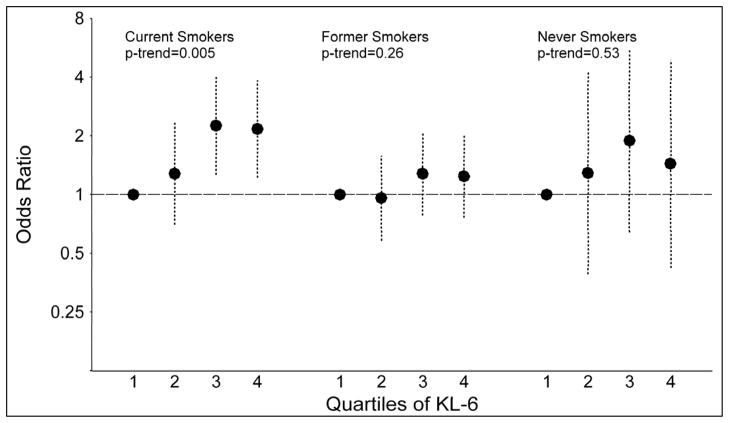
When stratified by smoking status, associations were observed between SP-D levels and lung cancer among current smokers (quartile 4 vs. quartile 1: OR=2.05; p-trend=0.01) and former smokers (OR=2.00; p-trend=0.004), but not among never smokers (OR=0.73; p-trend=0.75; Figures 3A and 3B). The association between KL-6 and lung cancer was limited to current smokers (quartile 4 vs. quartile 1: OR=2.17; p-trend=0.005), with no associations observed among former (OR=1.24; p-trend=0.26) or never smokers (OR=1.44; p-trend=0.53). Nonetheless, tests of interaction with smoking status were not significant for SP-D (p-interaction=0.81) or KL-6 (p-interaction=0.79).
Figure 3.
The figure presents odds ratios and 95% confidence intervals for lung cancer across quartiles of surfactant protein D (SP-D; figure 3A) and Krebs von Lungren-6 (KL-6; figure 3B), stratified by smoking status (current, former and never smoker). P-values for trend across quartiles are also presented.
Among controls, those with CXR scarring had marginally higher SP-D levels than those without scarring (median 105.0 vs. 113.9 ng/ml, p=0.12), and as shown in Table 4, participants with the highest levels were more likely to have CXR scarring (quartile 4 vs. quartile 1: OR=1.67; 95% CI: 1.04–2.70; p-trend=0.05). Also, higher SP-D levels were strongly associated with an increased frequency of bilateral CXR scarring (p-trend=0.005), but not unilateral lung scarring (p-trend=0.68). In contrast, KL-6 levels were similar in participants with and without scarring (median 321.6 vs. 325.5 μg/ml, p=0.56), and no association was observed with bilateral or unilateral scarring (Table 4).
Table 4.
Associations of serum levels of SP-D and KL-6 with chest x-ray scarring among controls.
| No scarring | Any scarring | Unilateral scarring | Bilateral scarring | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n | OR* | 95% CI | n | OR* | 95% CI | n | OR* | 95% CI | |
| SP-D, ng/ml | ||||||||||
| 17.1–71.7 | 135 | 39 | 1.00 | 26 | 1.00 | 13 | 1.00 | |||
| 71.8–105.3 | 131 | 52 | 1.36 | 0.84–2.22 | 35 | 1.36 | 0.77–2.40 | 17 | 1.30 | 0.60–2.80 |
| 105.4–143.8 | 136 | 48 | 1.31 | 0.80–2.15 | 33 | 1.34 | 0.75–2.39 | 15 | 1.23 | 0.56–2.73 |
| 143.9–506.6 | 127 | 64 | 1.67 | 1.04–2.70 | 32 | 1.22 | 0.68–2.19 | 32 | 2.51 | 1.24–5.09 |
| p-trend=0.05 | p-trend=0.68 | p-trend=0.005 | ||||||||
| KL-6, μg/ml | ||||||||||
| 178.8–231.0 | 135 | 47 | 1.00 | 29 | 1.00 | 18 | 1.00 | |||
| 231.1–325.7 | 135 | 56 | 1.14 | 0.72–1.82 | 35 | 1.16 | 0.67–2.03 | 21 | 1.13 | 0.57–2.24 |
| 325.8–465.7 | 132 | 53 | 1.16 | 0.72–1.84 | 34 | 1.20 | 0.69–2.08 | 19 | 1.09 | 0.54–2.18 |
| 465.8–4225.9 | 127 | 47 | 1.04 | 0.64–1.68 | 28 | 1.00 | 0.56–1.78 | 19 | 1.07 | 0.53–2.17 |
| p-trend=0.99 | p-trend=0.86 | p-trend=0.93 | ||||||||
Abbreviations: OR: odds ratio; CI: confidence interval
Models were adjusted for age, sex, smoking status, time since quitting, cumulative smoking, and randomization year
Discussion
We observed significant associations between two circulating markers of interstitial lung disease and subsequent lung cancer risk in a large cohort study, the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. After adjustment for cigarette smoking, lung cancer risk was increased 1.9-fold during follow-up for those in the top quartile of SP-D, and 1.6-fold for those in the top quartile of KL-6, compared to people with the lowest levels of these markers.
These associations with lung cancer risk are biologically plausible, because elevated circulating SP-D and KL-6 levels have been detected in patients with a number of pulmonary diseases, including idiopathic pulmonary fibrosis (15, 21), idiopathic pulmonary alveolar proteinosis (22), and acute respiratory distress syndrome (16, 23). Individuals with COPD also have elevated SP-D levels (17, 18). Both glycoproteins are produced by type II alveolar cells, which proliferate and replace type I alveolar cells during lung injury (27). Subsequently, the reparative type II cells transform into type I cells or undergo epithelial-to-mesenchymal transition (28). However, a disproportionately large number of type II alveolar cells persist in the presence of interstitial fibrosis and secrete high SP-D and KL-6 levels detectable in serum or plasma (27). Idiopathic pulmonary fibrosis has been associated with increased lung cancer risk and mortality in a number of studies (12–14), and may cause lung cancer through diffuse pulmonary inflammation (29). Chronic pulmonary inflammation could be an initiator or a promoter in the development of lung cancer, causing direct genotoxic injury due to a highly oxidative microenvironment, alteration of gene methylation, or increased cellular proliferation during tissue repair (4, 30).
In our study, current smoking status was associated with increased SP-D levels. However, we did not observe significant trends with cigarettes smoked per day, pack-years smoked or years since quitting. A prior study also observed increased SP-D levels in smokers (17), while other studies have not (21, 31). We found no difference in KL-6 levels by smoking status, consistent with one prior study (31). The lack of a consistent association between these markers and cigarette smoking suggests SP-D and KL-6 reflect processes that have effects on lung cancer that are independent of smoking, and are not merely markers of smoking-related lung damage.
Participants in PLCO were drawn from the general population, so they were largely healthy and free of severe pulmonary conditions. Thus, it is not surprising that SP-D and KL-6 levels observed in our study were much lower than those observed in patients with interstitial lung disease. For example, in our study the median level of SP-D among matched controls was 105 ng/mL, much lower than the median of approximately 400 ng/mL in a prior study of patients with pulmonary fibrosis (21), and of 318.5 ng/mL observed in our positive control subjects with pulmonary fibrosis. These considerations highlight that, among our subjects, elevations in SP-D and KL-6 mostly reflect mild pulmonary disease or damage in the absence of clinical illness. CRP was positively correlated with SP-D and KL-6, indicating that levels of SP-D and KL-6 may increase in the setting of lung inflammation. Nonetheless, our results showing independent associations with lung cancer risk suggest that SP-D, KL-6 and CRP each reflect somewhat independent processes that impact lung carcinogenesis.
Along these lines, we found a relationship between SP-D levels and scarring on the baseline CXR, which was particularly strong for bilateral scarring, but KL-6 levels were not associated with CXR scarring. One possible explanation could be that KL-6 is a sensitive marker for the detection and monitoring of certain interstitial lung diseases, but it may not be as sensitive for the detection of much less severe or early pulmonary scarring. It is also important to recognize that a degree of measurement error exists in the detection of scarring by CXRs, as manifest by only fair agreement in the assessment of scarring across consecutive CXRs in PLCO (32). Thus, it is possible that some scarring diagnoses in our study were misclassified and that some of the CXR findings may correspond to other conditions (e.g., congestive heart failure or atelectasis), which would have biased the associations of SP-D and KL-6 with CXR scarring toward the null.
Our results regarding the time intervals over which the associations between these markers and lung cancer risk were apparent are also informative (Figures 1A and 1B). For SP-D, an association with lung cancer risk was observed after more than two years following measurement of serum levels, and no association was observed over a shorter interval. If lung damage promotes cancer over an extended period of time, then perhaps the associations observed at longer latencies represent prolonged exposure to lung damage, while associations observed proximal to cancer diagnosis do not. In contrast, KL-6 was predominantly associated with lung cancer over shorter intervals. The association with lung cancer during the two years immediately following measurement suggests that an alternative explanation for our findings for KL-6 may be reverse causality due to a disease effect, i.e., that the presence of a cancer leads to production of KL-6. Indeed, KL-6 is secreted by some lung cancer tumors and has previously been considered as a possible diagnostic marker of lung adenocarcinoma (33, 34). Whether reverse causality could explain the associations between KL-6 and lung cancer observed over the longer interval of 2–4 years, or for squamous cell carcinoma, remains unclear.
Significant associations between each fibrosis marker and lung cancer were limited to adenocarcinomas and squamous cell carcinomas. Though we did not observe associations for small and large cell carcinomas, our analyses were limited by a small number of cases. Further, significant trends in lung cancer risk with increasing levels of fibrosis markers were limited to current and former smokers for SP-D, and current smokers only for KL-6. However, the number of cases who were never smokers was small, and because the interactions of SP-D and KL-6 with smoking status were not significant, we cannot conclude that the associations vary by smoking status.
The main strength of our study was the measurement of two circulating markers that are produced in the lung, and are associated with both lung damage and severe pulmonary diseases. We utilized pre-diagnostic sera, so that levels of these markers could be evaluated as predictors of subsequent lung cancer risk. Further, we were able to carefully control for potential confounding factors, including smoking, both in the study design and data analysis. A limitation was the potential measurement error of assessing pulmonary scarring with CXRs (11, 32). Further, our statistical power was limited for certain sub-analyses, due to a smaller number of cases.
In conclusion, we observed positive associations between circulating levels of SP-D and KL-6 and subsequent risk of lung cancer. Our results add support to a model whereby pulmonary disease, inflammation and scarring contribute to the etiology of lung cancer (4, 5, 7, 9–14, 35–38). Future research should further examine the role of interstitial lung disease and fibrosis in the etiology of lung cancer, and the measurement of serum levels of SP-D and KL-6, particularly in smokers. As KL-6 is secreted by some lung tumors, its use as an early detection marker should be further explored. The use of SP-D and KL-6 has been advocated for the clinical monitoring of interstitial lung diseases. These markers, along with other known predictors, may also offer promise to identify individuals at high risk of developing cancer.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Human Genome Research Institute, and of the Division of Cancer Epidemiology and Genetics and by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS.
The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute; the Screening Center investigators and staff of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial; Mr. Tom Riley and staff, Information Management Services, Inc.; Ms. Barbara O’Brien and staff, Westat, Inc.; Mr. Tim Sheehy and staff, DNA Extraction and Staging Laboratory, SAIC-Frederick, Inc; and Ms. Jackie King and staff, BioReliance, Inc. Most importantly, we acknowledge the study participants for their contributions to making this study possible.
Reference List
- 1.Ferlay J, Shin HR, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 (Internet) Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 2.Spitz MR, Wu X, Wilkinson A, Wei Q. Cancer of the Lung. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3. New York, NY: Oxford University Press, Inc; 2006. pp. 638–58. [Google Scholar]
- 3.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung cancer (Amsterdam, Netherlands) 2004;45 (Suppl 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert review of anticancer therapy. 2008;8(4):605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 5.Engels EA, Shen M, Chapman RS, et al. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. Int J Cancer. 2009;124(5):1183–7. doi: 10.1002/ijc.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Gaydos CA, Agreda P, et al. Chlamydia pneumoniae infection and risk for lung cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1498–505. doi: 10.1158/1055-9965.EPI-09-1261. [DOI] [PubMed] [Google Scholar]
- 7.Koshiol J, Rotunno M, Consonni D, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One. 2009;4(10):e7380. doi: 10.1371/journal.pone.0007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiels MS, Albanes D, Virtamo J, Engels EA. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, Beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):672–8. doi: 10.1158/1055-9965.EPI-10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz AG, Cote ML, Wenzlaff AS, et al. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J Thorac Oncol. 2009;4(3):291–9. doi: 10.1097/JTO.0b013e3181951cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner AV, Wang Z, Kleinerman RA, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol. 2001;30(1):118–24. doi: 10.1093/ije/30.1.118. [DOI] [PubMed] [Google Scholar]
- 11.Yu YY, Pinsky PF, Caporaso NE, et al. Lung cancer risk following detection of pulmonary scarring by chest radiography in the prostate, lung, colorectal, and ovarian cancer screening trial. Arch Intern Med. 2008;168(21):2326–32. doi: 10.1001/archinte.168.21.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161(1):5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 13.Le Jeune I, Gribbin J, West J, et al. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101(12):2534–40. doi: 10.1016/j.rmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Harris JM, Johnston ID, Rudd R, Taylor AJ, Cullinan P. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax. 2010;65(1):70–6. doi: 10.1136/thx.2009.121962. [DOI] [PubMed] [Google Scholar]
- 15.Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest. 1999;46(3–4):151–8. [PubMed] [Google Scholar]
- 16.Takahashi H, Sano H, Chiba H, Kuroki Y. Pulmonary surfactant proteins A and D: innate immune functions and biomarkers for lung diseases. Curr Pharm Des. 2006;12(5):589–98. doi: 10.2174/138161206775474387. [DOI] [PubMed] [Google Scholar]
- 17.Lomas DA, Silverman EK, Edwards LD, et al. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J. 2009;34(1):95–102. doi: 10.1183/09031936.00156508. [DOI] [PubMed] [Google Scholar]
- 18.Shakoori TA, Sin DD, Ghafoor F, Bashir S, Bokhari SN. Serum surfactant protein D during acute exacerbations of chronic obstructive pulmonary disease. Dis Markers. 2009;27(6):287–94. doi: 10.3233/DMA-2009-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakashima T, Yokoyama A, Inata J, et al. Mucins carrying selectin ligands as predictive biomarkers of disseminated intravascular coagulation complication in ARDS. Chest. 2011;139(2):296–304. doi: 10.1378/chest.09-3082. [DOI] [PubMed] [Google Scholar]
- 20.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2(1):33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene KE, King TE, Jr, Kuroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19(3):439–46. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 22.Lin FC, Chen YC, Chang SC. Clinical importance of bronchoalveolar lavage fluid and blood cytokines, surfactant protein D, and Kerbs von Lungren 6 antigen in idiopathic pulmonary alveolar proteinosis. Mayo Clin Proc. 2008;83(12):1344–9. doi: 10.1016/S0025-6196(11)60782-9. [DOI] [PubMed] [Google Scholar]
- 23.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160(6):1843–50. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 24.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi AK, Caporaso NE, Katki HA, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28(16):2719–26. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown GW, Mood AM. On median tests for linear hypotheses. Proc Second Berkeley Symp on Math Statist and Prob. 1951:159–66. [Google Scholar]
- 27.Bowden DH. Alveolar response to injury. Thorax. 1981;36(11):801–4. doi: 10.1136/thx.36.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samet JM. Does idiopathic pulmonary fibrosis increase lung cancer risk? Am J Respir Crit Care Med. 2000;161(1):1–2. doi: 10.1164/ajrccm.161.1.ed14-99. [DOI] [PubMed] [Google Scholar]
- 30.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5(1):46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Kanoh S, Motoyoshi K. Serum surfactant protein-A, but not surfactant protein-D or KL-6, can predict preclinical lung damage induced by smoking. Biomarkers. 2008;13(4):385–92. doi: 10.1080/13547500801903651. [DOI] [PubMed] [Google Scholar]
- 32.Pinsky PF, Freedman M, Kvale P, et al. Abnormalities on chest radiograph reported in subjects in a cancer screening trial. Chest. 2006;130(3):688–93. doi: 10.1378/chest.130.3.688. [DOI] [PubMed] [Google Scholar]
- 33.Inata J, Hattori N, Yokoyama A, et al. Circulating KL-6/MUC1 mucin carrying sialyl Lewisa oligosaccharide is an independent prognostic factor in patients with lung adenocarcinoma. Int J Cancer. 2007;120(12):2643–9. doi: 10.1002/ijc.22613. [DOI] [PubMed] [Google Scholar]
- 34.Kohno N, Akiyama M, Kyoizumi S, et al. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Jpn J Clin Oncol. 1988;18(3):203–16. [PubMed] [Google Scholar]
- 35.Park SK, Cho LY, Yang JJ, et al. Lung cancer risk and cigarette smoking, lung tuberculosis according to histologic type and gender in a population based case-control study. Lung cancer (Amsterdam, Netherlands) 2010;68(1):20–6. doi: 10.1016/j.lungcan.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Wang XR, Yu IT, Chiu YL, et al. Previous pulmonary disease and family cancer history increase the risk of lung cancer among Hong Kong women. Cancer Causes Control. 2009;20(5):757–63. doi: 10.1007/s10552-008-9289-4. [DOI] [PubMed] [Google Scholar]
- 37.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst. 2002;94(11):826–35. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- 38.Liang HY, Li XL, Yu XS, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009;125(12):2936–44. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.