Abstract
Aim
Aim of this study is to determine the prevalence of Barrett's Esophagus (BE) in first degree relatives of patients with esophageal adenocarcinoma (EAC) and Barrett's' high grade dysplasia (HGD).
Methods
After Institutional Review board approval first degree relatives of patients with EAC/HGD underwent unsedated ultrathin trans-nasal endoscopy (UUTNE) with biopsy. BE was suspected if any salmon colored epithelial tongues were seen above the gastro-esophageal junction. A diagnosis of BE was made only if biopsy from these areas confirmed columnar lined epithelium with intestinal metaplasia.
Results
From 23 families 47 first degree relative underwent UUTNE and one patient underwent routine upper endoscopy with sedation as part of this study. The mean age of cases was 44.4 yrs. All patients tolerated the procedure well and there were no procedure related complications. BE was suspected in 16 (34%) patients and confirmed in 13/16 (27.7%) patients. There was 4 long segment (> 3cm) and 9 short segment (<3 cm) of BE.
Conclusion
There is a significantly higher than expected prevalence of BE in first degree relatives of EAC/HGD patients. This should be taken in to consideration to develop further screening guidelines. Further work is need to confirm these findings. Un-sedated trans-nasal endoscopy is a safe and well-tolerated method for BE screening.
Keywords: Barrett's esophagus, prevalence, trans-nasal endoscopy, esophageal adenocarcinoma
Introduction
During the past two decades, the incidence of esophageal adenocarcinoma (EAC) has increased nearly 700% in the United States [1-4]. Despite advances in chemo-radiotherapy and surgical techniques, the prognosis for patients who have EAC remains dismal. This is primarily due to the advanced stage of disease at diagnosis for most patients. It has been well established that EAC develops through a metaplasia-dysplasia-carcinoma sequence secondary to chronic gastroesophageal reflux. Barrett's esophagus (BE) is the metaplasia stage and increases patients' risk of developing cancer by 100-fold [5-6]. Current guidelines recommend placing patients with BE in scheduled surveillance programs to detect the progression of the dysplasia-carcinoma sequence [7]. Patients in surveillance programs have earlier detection of EAC and a better prognosis when EAC is found than do those patients who present de novo [8].
Diagnosis of BE requires endoscopic visualization of the columnar-lined epithelium above the gastroesophageal junction that on biopsy shows intestinal metaplasia [9]. Because of improved understanding of the disease and improved endoscopic technology, over the years the required minimum length of visualized columnar epithelium has progressively decreased from 3cm to any visible mucosal breaks above the squamo-columnar junction. Chandrasoma and DeMeester have argued that even a normal-looking Z-line that, upon biopsy, shows columnar epithelium with intestinalization should be regarded as BE (i.e., cardiac intestinal metaplasia) [9].
The current guidelines for BE screening place an emphasis on the duration of reflux symptoms (>5-10 years), along with race and gender. However, BE is often asymptomatic and may in fact be symptom protective, leading to improvement or even disappearance of typical GERD symptoms upon development of BE [10]. The prevalence of asymptomatic BE is high, especially among the elderly [11,12]. BE was found during upper endoscopy screening in up to 8% of GERD-asymptomatic patients who were undergoing colonoscopy for positive fecal occult blood. Based on representative studies, the prevalence of BE is estimated to be between 1-3% of the adult Swedish population [13]. It is estimated that only one out of 20 patients who have BE are currently diagnosed and under surveillance. This implies that only a small percentage of patients who have EAC have an antecedent diagnosis of BE, and that the majority present in the late stages of the disease. Current screening practices lack the sensitivity to identify patients who are at high risk for BE and can be placed in surveillance programs. There clearly is a need to develop improved screening criteria for BE to identify a population at higher risk.
An ever-increasing number of malignancies are being included in the realm of “familial” or “hereditary” neoplasms. Several cases of familial aggregation of BE and EAC have been reported. The aim of our study was to determine the prevalence of columnar-lined epithelium with intestinalization (BE) in first-degree relatives of patients with known EAC and BE with high-grade dysplasia.
Materials and methods
The present study is part of the Familial Barrett's Consortium. The Consortium encompasses Case Western Reserve University, University Hospitals of Cleveland, Fred Hutchinson Cancer Research Center, John Hopkins University, Mayo Clinic, University of North Carolina, Cleveland Clinic, and Creighton University Medical Center (CUMC) and is headed by Dr Amitabh Chak. This consortium is supported by an NIH RO1DK070863 grant (NIH IK24DK02800). The patients in this study are from CUMC only.
Index patients and relatives
After IRB approval, patients with EAC and high-grade dysplasia (HGD) undergoing treatment at the esophageal center at CUMC were contacted and asked to fill out a detailed personal and family questionnaire.
Permission was taken from the index patients to contact the relatives whom he or she had listed on the questionnaire (Appendix 1). A pedigree chart was prepared based on the information provided by the index patients. The eligible first-degree relatives (ages 19 years or older) of the index patients were mailed questionnaires asking them details about their health (Appendix 2) and inviting them to undergo a screening endoscopy. The screening endoscopy was done at no cost to the patients and was supported by funding through the Health Future Foundation at Creighton University.
Screening endoscopy
After filling out a screening questionnaire, patients underwent an unsedated, ultrathin trans-nasal endoscopy. Two percent lidocaine hydrogen chloride jelly (5-10ml) (Copley Pharmaceutical Inc., Pennsauken, MA, USA) was used for topical anesthesia. The ultrathin endoscope (GIF-N30 Olympus®) was introduced trans-nasally and the distal end of the esophagus and proximal stomach examined. Retroflex view was also routinely obtained to assess the GEJ. Using NBI® to improve visualization and characterization, any columnar-lined esophagus above the squamo-columnar junction was considered suspicious. During the entire procedure, the candidates were able to talk and communicate with the endoscopist. Photographs were taken of the gastroesophageal junction. Biopsies were taken using pediatric forceps (Radial Jaw, Boston Scientific® Natick, MA) from suspicious areas and placed in formalin. Patients with 3 or more cm of columnar metaplasia in the distal esophagus were deemed to have long segment BE.
Biopsy and histology
Biopsies were obtained from all areas suspected for BE. Circumferential biopsies were taken at 2 cm intervals for long segments. Biopsies were send in formalin separately at different levels (if more than one level obtained). Biopsies were interpreted by one of two experienced pathologists (ZG and CD) who were blinded to family history and endoscopic findings. After paraffin embedding, samples were stained with haematoxyllin-eosin and with Alcian blue for confirmation of intestinal metaplasia.
Statistical testing was done with Person's Chi-square test. A p value less than 0.05 was considered significant.
Results
The BE and EAC databases at Creighton University contained 174 patients overall. The 542 eligible first-degree relatives of these probands were mailed questionnaires asking them details about their health. We received 157 completed questionnaires from 74 families; of these, 95 belonged to 40 families in which the proband had EAC/HGD. In the remaining, 62 relatives belonged to 34 families in which the proband had non-dysplastic Barrett's. Those indentified were advised to undergo upper endoscopy screening (priority was given to family members of EAC/HGD probands), and were given the option of undergoing unsedated ultrathin trans-nasal endoscopy (UUTNE) by the senior authors (SKM, THL) at no cost. The study includes 23 probands (EAC/HGD) and their first degree relatives. There were a total of 141 first degree relatives of which 42 had died prior to the study leaving 99 potential screening candidates. The cohort of this study is formed by 47 (48%) first degree relatives belonging to these families who have undergone screening endoscopy. Of these, 46 have undergone UUTNE, and one patient underwent routine upper endoscopy with sedation.
Probands
The mean age of the probands was 69.2 yrs (52-88 yrs), with 21 males and 2 females. There were 22 probands who had EAC and one who had HGD (male, 61 yrs). In each patients the malignancy / dysplasia was located in the distal esophagus. BE was noted on final pathology in 14/23 cases.
First-degree relatives
The mean age was 44.4 yrs (26-81 yrs); 27 were male and 20 female (in 44 cases children, in 2 cases parent and in 1 case sibling of the proband). Forty reported a history of reflux symptoms, while 31 were taking acid-suppressive medications. One patient underwent routine sedated upper endoscopy near his home, and endoscopic findings and pathology slides were obtained for review at our institution. The remaining 46 patients underwent UUTNE by SKM or THL at our institution. Mean time for the procedure was 4.2 minutes. There were no complications and the procedure was well-tolerated. In one case, the procedure was performed peroral because of a previous nasal fracture. Hiatal hernias were found in 17 cases (36.2%) (mostly less than 2cm reducible), endoscopic esophagitis was noted in 15 cases (grades A and B) and BE was suspected in 16 (34%) patients. Mean length of BE in patients with LSBE was 4 cm. Seven patients had 0.5 to 1 cm columnar lined epithelium. Median length of the BE was 1 cm. The endoscopic findings are summarized in Table 1.
Table 1. Endoscopic findings in the first degree relatives.
| pathological alteration | n=47 (%) |
|---|---|
| esophagitis Grade A * | 11 (23.4%) |
| esophagitis Grade B | 4 (8.5%) |
| esophagitis Grade C | 0 |
| esophagitis Grade D | 0 |
| possible Barrett's esophagus | 16 (34%) |
| Hiatal hernia | 17 (36.2%) |
Los Angeles Classification of reflux esophagitis (10th World Congress of Gastroenterology, LA, USA, 1994)
Histological findings
Columnar epithelium with intestinal metaplasia was noted in 13 cases, with a mean age of 46.5 yrs: 48 years (40-58 years) in males and 41 years (26-59 years) in females. It was more than twice as frequent in men (men/women = 10/27 vs. 3/20). Of the 13 patients who had BE, four had long-segment disease and nine had short-segment (SSBE, less than 3cm intestinal metaplasia above the gastro-esophageal junction). The age distributions of screened relatives and those with BE are shown in Table 2. In two families, there was more than one case of BE per family; in four families, there was more than one case of GERD per family. In patients who had BE, eight (61.5%) had suffered from heartburn for more than 10 years, while 12 (92.3%) reported subjective “reflux” pain. Of those who had BE, eight (61.5%) were smokers, three (23%) were alcohol abusers, and six (46.2%) were coffee drinkers. These factors and the reflux symptoms of patients with BE or without BE are shown in Table 3.
Table 2. Age distribution of screened relatives and those with BE.
| Relatives | relatives with BE | |||
|---|---|---|---|---|
| n = 47 (%) | n = 13 (%) | |||
| 20-29 yrs | 8 | 17 | 1 | 7.7 |
| 30-39 yrs | 10 | 21.3 | 1 | 7.7 |
| 40-49 yrs | 10 | 21.3 | 4 | 30.8 |
| 50-59 yrs | 14 | 29.8 | 7 | 53.8 |
| 60-69 yrs | 2 | 4.3 | 0 | 0 |
| 70+ yrs | 3 | 6.3 | 0 | 0 |
Table 3. Reflux symptoms and habits of relatives with/without BE.
| relatives without BE | relatives with BE | |||
|---|---|---|---|---|
| n=34 (%) | n=13 (%) | |||
| reflux + | 23 | 67.6 | 12 | 92.3 |
| reflux - | 11 | 32.4 | 1 | 7.7 |
| smoker | 14 | 41.2 | 8 | 61.5 |
| non-smoker | 20 | 58.8 | 5 | 38.5 |
| alcohol + | 13 | 38.2 | 3 | 23 |
| non-alcoholic * | 21 | 61.8 | 10 | 77 |
non-alcoholic: less than one drink a week ( one drink = 12oz beer, 4oz wine or 1½ oz of liquor)
There was one additional case of oxynto-cardiac mucosa and 2 cases with >15 eosinophils per hpf. In these patients with high eosinophils/hpf a repeat EGD was recommended after 6-8 weeks of PPI therapy (as severe reflux can also have a high eosinophilic infiltrate).
Patients with reflux proven histologically were started on proton pump inhibitor (PPI) therapy and have been advised to undergo surveillance per standard guidelines. Patients who were suspected of having BE per endoscopy that was not confirmed histologically have been advised to undergo a routine endoscopy within the next year to rule out sampling error due to the use of pediatric biopsy forceps (used during UUTNE). Patients with reflux esophagitis have been recommended to undergo repeat endoscopy in 5 years. Patients were treated with acid suppressive therapy and were counseled in regards to surgical options especially if they had severe symptoms.
Discussion
The meteoritic rise in incidence of EAC during the past 20 years has shown no sign of leveling off. The nature of this disease has undergone a dramatic change in our lifetime. Chronic gastroesophageal reflux is the only identifiable risk factor for the development and progression of the BE-EAC sequence. In the western world, dietary and lifestyle choices have increased the prevalence of GERD in adults, placing a large proportion of the adult population at risk of developing BE and EAC [17]. Current guidelines from the American Gastroenterological Association recommend that patients who experience reflux symptoms for more than five years undergo screening endoscopy for BE so that, if necessary, they can be placed in surveillance programs. Up to 40% of patients who have BE may be asymptomatic and, thus, may not receive recommended screening.
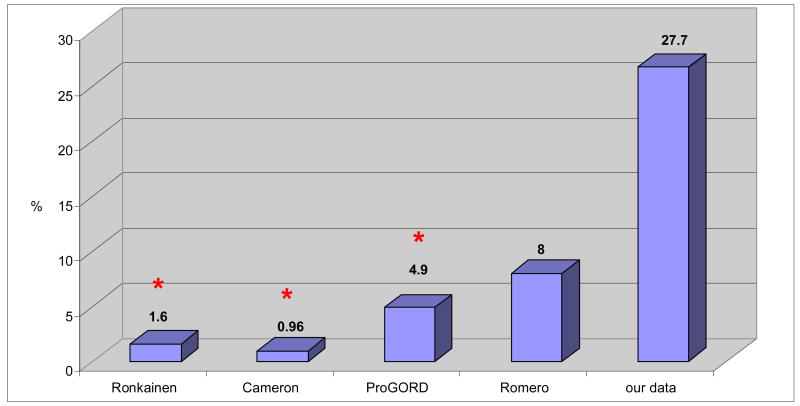
Obtaining endoscopic and pathological confirmation in this very large cohort of people is impractical. In the carcinoma sequence, BE is the metaplasia stage and is associated with a 100-fold increase in cancer risk. A randomized study to calculate the prevalence of BE in the general population of Sweden was conducted by Ronkainen. One thousand endoscopies were performed from a randomized pool. Reflux esophagitis was noted in 40% of patients, and BE was found in 1.6% [13]. Another objective study of BE in the general population was reported by Cameron (MN, USA). Altogether, seven cases of long-segment-BE were found in 733 autopsies (0.96%) [14]. If only symptomatic patients are considered, the ProGORD study reports the prevalence of BE in patients with reflux symptoms at 4.9% [15]. The GOSPE study described BE in 0.32% of patients without subjective symptoms [10]. In another study, 25% of elderly patients undergoing colonoscopy for fecal occult blood and who underwent upper endoscopy screening were found to have BE. Of these, 8% were asymptomatic in regard to reflux symptoms [12].
Several authors have argued that screening and surveillance of BE does not reduce the disease's incidence. They cite that less than 5% of patients who have EAC have an antecedent history of BE [18]. However, the results of screening and surveillance may be improved by identifying high-risk groups and ensuring they receive earlier detection and treatment. There is a great need to develop risk factors to identify a high-risk population that can be targeted for BE screening and surveillance. In addition, with the growing number of ablative technologies becoming available, we can alter the natural progression of disease and probably alter the metaplasia-dysplasia sequence.
A growing number of malignancies are being considered “familial” or “hereditary” neoplasms [19]. With the advancements in the understanding of the multi-step genetic changes involved in the progression from normal to malignant tissue, several studies describe familial aggregation of reflux and BE that predict that those who have a genetic predisposition will then develop BE [20-23]. Romero found that the prevalence of BE in the family members of patients who had BE was 8% [24]. The aim of our study was to measure the prevalence of BE in the families of patients who had EAC. In 47 relatives of EAC patients, 13 (27.7%) had BE. (Table 4) This is significantly higher than reported in the studies previously described (i.e., Ronkainen [13], Cameron [14], ProGORD [15]). Of these 13 patients, one was completely asymptomatic. This suggests that it may not be sufficient to screen only symptomatic relatives of EAC patients.
Table 4. The prevalence of BE.
|
Ronkainen: prevalence of BE in the whole population (endoscopic study)
Cameron: prevalence of BE in the whole population (autopsy study)
ProGORD: prevalence of BE in patients with GERD
Romero: prevalence of BE in relatives of patients with BE
Our data: prevalence of BE in relatives of patients with EAC
statistically significant alteration (Person's Chi-square test, p<0.05)
Another controversial issue is the relevance of SSBE as a risk factor for progression to EAC [25]. With improved understanding of the disease, the diagnostic criteria for BE has changed several times during the past 40 years. Traditionally, not long ago, a minimum of 3 cm of columnar epithelium was a requirement for a diagnosis of BE [26]. It was subsequently discovered that intestinal metaplasia is the marker of an epithelium at risk of dysplasia/neoplasia [27]. More recently it was realized that even shorter segments of columnar-lined epithelium (less than 3 cm) with intestinal metaplasia have a risk of malignant progression and are considered as BE [28].
Chandrasoma and DeMeester have argued that the presence of columnar epithelium with intestinal metaplasia at the gastroesophageal junction represents abnormal mucosa at risk of progression to adenocarcinoma. We agree with their view that an arbitrary requirement of a minimum length (1 cm, 3 cm, or any other) is erroneous. We consider any columnar epithelium with intestinal metaplasia abnormal.
When screening a wider segment of the populations, cost is an important consideration. In most centers in the United States, the protocol for standard trans-oral endoscopy is to perform it while the patient is under sedation. Jobe et al have shown that UTTNE is equivalent to conventional esophago-gastro-duodenoscopy for BE surveillance [29]. Much of the cost and the risk of the procedure is related to conscious sedation. In our protocol, unsedated trans-nasal endoscopy and biopsy was performed. After examination, patients were asked how uncomfortable the procedure was. Only one patient experienced discomfort in the form of temporary gagging, and all were satisfied with this method. We can conclude that unsedated trans-nasal esophago-gastroscopy using an ultra-thin endoscope is a safe, economic method with good patient acceptance.
Limitations
Our study has several limitations. Foremost being a relatively small cohort from a large number of eligible relatives. Forty-seven (48%) eligible first degree relatives of probands with EAC/HGD underwent screening endoscopy. However, this is an ongoing effort and further information is being gathered. Second shortcoming can be that majority of the patients complain of reflux symptoms which is a confounding factor in true “familial” risk of BE. However, previous studies (proGORD [15]) have shown only a 4.9% prevalence of BE in patients with reflux symptoms. In our cohort we have found a significantly higher prevalence of BE which cannot be explained only by existence of reflux symptoms. Lastly it can be criticized that there is no control group in this study. We agree that the ideal situation would be to have a control group of first degree relatives of patients with non –dysplastic Barrett's and/or non-Barrett's reflux patients. With limited resources we decided to first embark on the highest risk group probands, i.e those with HGD/EAC. However, if we use the general population BE prevalence available from other studies [13] as comparison there is clearly a significantly and alarmingly higher prevalence of BE in first degree relatives of this group of probands with EAC/HGD.
Conclusion
The presence of EAC/ HGD is a risk factor for BE in first-degree relatives. Reflux symptoms are not the sine qua-non of BE; therefore, asymptomatic relatives of patients who have EAC/HGD should also be offered screening. Unsedated trans-nasal endoscopy is a safe, economic, and well-tolerated method that holds promise to substantially decrease cost while increasing the applicability of BE screening.
Supplementary Material
Acknowledgments
grant support: NIH RO1DK070863
Footnotes
Conflict of Interest: The authors disclose no conflicts.
Arpad Juhasz (acquistion of data, analysis and interpretation of data, drafting of the manuscript)
Sumeet K Mittal (study suprevisor)
Tommy H Lee (statistical analysis)
Caishu Deng (pathological examinations)
Amitabh Chak (critical revision of manuscript for important intellectual content)
Henry T Lynch (critical revision of manuscript for important intellectual content)
References
- 1.Cameron AJ. Epidemiology of columnar lined esophagus and adenocarcinoma. Gastroenterol Clin N Am. 1997;26:487–494. doi: 10.1016/s0889-8553(05)70308-3. [DOI] [PubMed] [Google Scholar]
- 2.Haggitt RC. Adenocarcinoma in Barrett's esophagus: a new epidemic? Hum Pathol. 1992;23:475–476. doi: 10.1016/0046-8177(92)90121-i. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasoma PT, DeMeester TR. GERD – Reflux to Esophageal Adenocarcinoma. Elsevier; 2006. pp. 6–7. [Google Scholar]
- 4.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 5.Weinstein W, Ippoliti AF. The diagnosis of Barrett's esophagus: goblets, goblets, goblets. Gastrintest Endosc. 1996;44:91–95. doi: 10.1016/s0016-5107(96)70239-0. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 7.Rees JR, Lao-Sirieix P, Wong A, et al. Treatment for Barrett's oesophagus. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD004060. doi: 10.1002/14651858.CD004060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Bright T, Schloithe A, Bull JA, et al. Outcome of endoscopy surveillance for Barrett's oesophagus. ANZ J Surg. 2009 Nov;79(11):812–6. doi: 10.1111/j.1445-2197.2009.05107.x. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasoma PT, DeMeester TR. GERD – Reflux to Esophageal Adenocarcinoma. Elsevier; 2006. p. 34. [Google Scholar]
- 10.Gruppo Operative per lo Studio delle Precancerosi dell Esophago (GOSPE) Barrett's esophagus: epidemiological and clinical results of a multicentric survey. Int J Cancer. 1991;48:364. [PubMed] [Google Scholar]
- 11.Ward EM, Wolfsen HC, Achem SR, et al. Barrett's esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol. 2006;101:12–17. doi: 10.1111/j.1572-0241.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461–467. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 13.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Cameron AJ, Zinsmeister AR, Ballard DJ, et al. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1991 Sep;101(3):875–876. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 15.Malfertheiner P, Lind T, Willich S, et al. Prognostic influence of Barrett's oesophagus and Helicobacter pylori infection on healing of erosive gastro-oesophageal reflux disease (GORD) and symptom resolution in non-erosive GORD: report from the ProGORD study. Gut. 2005;54:746–751. doi: 10.1136/gut.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero Y, Cameron AJ, Schaid DJ, et al. Barrett's esophagus: prevalence in symptomatic relatives. Am J Gastroenterol. 2002;97:1127–1132. doi: 10.1111/j.1572-0241.2002.05665.x. [DOI] [PubMed] [Google Scholar]
- 17.Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Dig Dis Sci. 1976;21:953–956. doi: 10.1007/BF01071906. [DOI] [PubMed] [Google Scholar]
- 18.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 19.Jochem VJ, Fuerst PA, Fromkes JJ. Familial Barrett's esophagus associated with adenocarcinoma. Gastroenterology. 1992;102:1400–1402. [PubMed] [Google Scholar]
- 20.Trudgill NJ, Kapur KC, Riley SA. Familial clustering of reflux symptoms. Am J Gastroenterol. 1999;94:1172–1178. doi: 10.1111/j.1572-0241.1999.01060.x. [DOI] [PubMed] [Google Scholar]
- 21.Fahmy N, King JF. Barrett's esophagus: an acquired condition with genetic predisposition. Am J Gastroenterol. 1993;88:1262–1265. [PubMed] [Google Scholar]
- 22.Chak A, Lee T, Kinnard MF, Brock W, Faulx A, Willis J, Cooper GS, Sivak MV, Goddard KAB. Familial aggregation of Barrett's esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323–328. doi: 10.1136/gut.51.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Elston R, Barnholtz-Sloan J, Falk G, Grady WM, Kinnard M, Mittal SK, Willis JE, Markowitz S, Brock W, Chak A. A segregation analysis of Barrett's esophagus and associated adenocarcinomas. Cancer Epidemiol Biomarkers Prev. 2010 Mar;19(3):666–674. doi: 10.1158/1055-9965.EPI-09-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero Y, Cameron AJ, Locke GR, et al. Familial aggregation of gastroesophageal reflux in patients with Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 1997;113:1449–1456. doi: 10.1053/gast.1997.v113.pm9352846. [DOI] [PubMed] [Google Scholar]
- 25.Chak A, Faulx A, Kinnard M, et al. Identification of Barrett's esophagus in relatives by endoscopic screening. Am J Gastroenterol. 2004;99:2107–2114. doi: 10.1111/j.1572-0241.2004.40464.x. [DOI] [PubMed] [Google Scholar]
- 26.Barrett NR. The lower esophagus lined by columnar epithelium. Surgery. 1957;41:881–94. [PubMed] [Google Scholar]
- 27.Shafer RB. Adenocarcinoma in Barrett's columnar-lined esophagus. Arch Surg. 1971 Sep;103(3):411–3. doi: 10.1001/archsurg.1971.01350090093021. [DOI] [PubMed] [Google Scholar]
- 28.Grassi A, Giannarelli D, Lacopini F, et al. Prevalence of intestinal metaplasia in the distal esophagus in patients endoscopically suspected for short Barrett's esophagus. J Exp Clin Cancer Res. 2006;25:297–302. [PubMed] [Google Scholar]
- 29.Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett's esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006 Dec;101(12):2693–703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


