Abstract
The high-throughput - next generation sequencing (HT-NGS) technologies are currently the hottest topic in the field of human and animals genomics researches, which can produce over 100 times more data compared to the most sophisticated capillary sequencers based on the Sanger method. With the ongoing developments of high throughput sequencing machines and advancement of modern bioinformatics tools at unprecedented pace, the target goal of sequencing individual genomes of living organism at a cost of $1,000 each is seemed to be realistically feasible in the near future. In the relatively short time frame since 2005, the HT-NGS technologies are revolutionizing the human and animal genome researches by analysis of chromatin immunoprecipitation coupled to DNA microarray (ChIP-chip) or sequencing (ChIP-seq), RNA sequencing (RNA-seq), whole genome genotyping, genome wide structural variation, de novo assembling and re-assembling of genome, mutation detection and carrier screening, detection of inherited disorders and complex human diseases, DNA library preparation, paired ends and genomic captures, sequencing of mitochondrial genome and personal genomics. In this review, we addressed the important features of HT-NGS like, first generation DNA sequencers, birth of HT-NGS, second generation HT-NGS platforms, third generation HT-NGS platforms: including single molecule Heliscope™, SMRT™ and RNAP sequencers, Nanopore, Archon Genomics X PRIZE foundation, comparison of second and third HT-NGS platforms, applications, advances and future perspectives of sequencing technologies on human and animal genome research.
Keywords: CHIP-chip, Chip-seq, De novo assembling, High-throughput next generation sequencing, Personal genomics, Re-sequencing, RNA-seq
Introduction
The completion of the first human genome drafts (Yamey 2000) was just a start of the modern DNA sequencing era which resulted in further invention, improved development toward new advanced strategies of high-throughput DNA sequencing, so called the “high-throughput next generation sequencing” (HT-NGS). These developed HT-NGS strategies addressed our anticipated future needs of throughput sequncing and cost, in a way which enabled its potential multitude of current and future applications in mammalian genomic research. Additionally in these advanced laboratory methodologies, a scope of new generation of bioinformatics tools has further emerged as an essential prerequisite to accommodate further strategic development and improvement of output results. The HT-NGS is one of the great challenges of today’s genomic research. For the future direction, we need the in-depth genome sequence information and analysis for most of the mammals, including human to fully understand genome variation of economic traits, genetic susceptibility to diseases, and pharmacogenomics of drug response. The leading genome research centers and scientists have publicly recognized that these are the core enabling goals for the next decade genomics research. The National Human Genome Research Institute (NHGRI) has echoed this need through its vision for genomics research (Collins et al. 2003). The NHGRI has categorized new sequencing approaches into those that offer near-term and revolutionary benefits with a 100-fold cost reduction per base pair (bp) within the next five years. To extend the near-term, i.e., of within the next 5–10 years, the revolutionary benefits should advance the field with a 10,000-fold cost reduction per base pair which in turn to attain the “US$ 1000 genome”.
Year 2011 is celebrated as the 10th anniversary since the human genome was first sequenced (www.nature.com/natureconferences/hg10years/index.html). During this period, tremendous success has been achieved in the fields of decoding of human genome, technological advancement of new era of human genome applications, toward personalized genomes and discovery of rare variants, leveraging genome sequencing to impact on cancer researches and mammalian evolution and population structure. The past decade has witnessed a revolution in the field of human genomics research. Today, a more global approach is being embraced which has not only given a rise to the field of systems biology, but has also touched all areas of biological and medical research, as well as bringing them closer together and blurring the lines that previously defined them as individual disciplines of research. The horizons and expectations have broadened due to the technological advances in the field of genomics, especially the HT-NGS and its wide range of applications such as: chromatin immunoprecipitation coupled to DNA microarray (ChIP-chip) or sequencing (ChIP-seq), RNA sequencing (RNA-seq), whole genome genotyping, de novo assembling and re-assembling of genome, genome wide structural variation, mutation detection and carrier screening, detection of inherited disorders and complex human diseases, DNA library preparation, paired ends and genomic captures, sequencing of mitochondrial genome and personal genomics (for the detailed description, see: Table 2). Besides the advancement of sequencing techniques, the past decade will be remembered as the decade of the genome research. Since the publications of first composite genomes of human (Lander et al. 2001; Venter et al. 2001) - many draft genomes from other organisms have been published (www.ensembl.org/info/about/species.html). The speed with which new genomes can now be sequenced has been facilitated by the development of potential HT-NGS technologies and assembly methods. It is now possible to assemble de novo a large genome, a good example can be derived from the recent genome assembly of the giant panda (Li et al. 2010b) which utilized the only short reads provided by next-generation DNA sequencing.
Table 2.
An overviews of impact HT-NGS technology on human genome researches (Only year 2010 and first issues of 2011 literatures were included)
| Applications | Description | References |
|---|---|---|
| 1. Whole genome genotyping | Typing of Human leukocyte antigen (HLA) by HT-NGS: A three step procedure of HLA typing was introduced. In first step, HLA-A, -B, -C, -DRB1, and -DQB1 were amplified with long-range PCR. In second step, amplicons were sequenced using the 454 GS-FLX platform. In third step, sequencing data were analyzed with Assign-NG software. | Lind et al. 2010 |
| HT-NGS in prenatal diagnosis tests: A comprehensive review on impact of HT NGS on prenatal diagnosis tests. | Raymond et al. 2010 | |
| In utero disease screening: A new study demonstrates the feasibility of genome-wide fetal genotyping using non-invasive next-generation sequencing of the mother's blood | Burgess 2011 | |
| 2. De Novo assembling and re-assembling of the human genome. | Re-sequencing of genome by DNA pools: study proposed a novel statistical approach, CRISP (Comprehensive Read analysis for Identification of SNPs from Pooled sequencing] that is able to identify both rare and common variants. The CRISP approach can detect 80-85% of SNPs identified using individual sequencing while achieving a low false discovery rate (3-5%). | Bansal 2010 |
| Re-sequencing of genome and HT-NGS platform: study evaluated the comparative performance of the Illumina Genome Analyzer and Roche 454 GS FLX for the re-sequencing of 16 genes associated with hypertrophic cardiomyopathy (HCM). Study concluded the feasibility of combining LR-PCR with NGS platforms for targeted re-sequencing of HCM-associated genes. | Dames et al. 2010 | |
| De novo assembling of the human genome: study proposed a novel method for de novo assembly of human genomes from short read sequences. Method successfully assembled N50 contig size of 7.4 and 5.9 kilobases (kb) and scaffold of 446.3 and 61.9 kb of Asian and African human genome. | Li et al. 2010a | |
| Assembling of the human genome: A comprehensive review on recent development of software packages in analyzing new generation sequencing data. | Nagarajan and Pop 2010 | |
| HT-NGS in ancient genome research: study sequenced the complete genome of a 4,000-year-old human with 20-fold coverage which providing a fresh look at human population history. | Shapiro and Hofreiter 2010 | |
| Epigenetics | DNA methylation and HT-NGS: study compared two different bisulfite conversion whole methylome sequencing methods using NGS SOLiD platform. | Bormann et al. 2010 |
| HT-NGS in epigenomics: study presented the methylation detection reagents and their application to microarray and sequencing platforms. Study also proposed an international coordination to standardize methylome platforms and to create a full repository of methylome maps from tissues and unique cell types. | Fouse et al. 2010 | |
| Profiling genome methylation patterns at single-base resolution: Study provides new insights into the conservation and divergence of DNA methylation in eukaryotes and their regulation of gene expression. | Bhaijee et al. 2011 | |
| Database for whole genome methylation maps at single-cytosine resolution: NGS methylation database (NGSmethDB: http://bioinfo2.ugr.es/NGSmethDB/gbrowse/) for human, mouse and Arabidopsis genome, comprised of wide range of tissues including the differential tissue methylation or the changes occurring along pathological conditions. | Hackenberg et al. 2011 | |
| ChIP-seq | Study of gene expression regulation through HT-NGS: Study indentified both coding and regulatory regions of PPARG gene-novel nucleotide variations and haplotypes associated to human diseases by DNA-seq, defining a PPARγ binding map by ChIP-Seq, and unraveling the wide and intricate gene pathways regulated by PPARG by RNA-Seq. | Costa et al. 2010a |
| Advance statistical methods for Chip-seq mapping: Improved method to predict the de novo motif discovery in the peak environments by investigating the human growth-associated binding protein (GABPalpha) based on ChIP-seq observations. | Jiao et al. 2010 | |
| Genome wide structural variation detection in human population | HT-NGS in 1000 genome project: Pilot study by whole-genome sequencing of 179 individuals from four populations, to develop and compare different strategies for genome-wide sequence variation using HT NGS platforms. | Durbin et al. 2010 |
| Study of fine scale human population structural variation: to implicate in population structure for the distribution and discovery of disease-causing genetic variants in diverse human genomes, using HT-NGS sequencing data. | Henn et al. 2010 | |
| Detection of disease-causing mutations in patients with monogenic inherited diseases: The Retinitis pigmentosa (RP): Study demonstrates that next-generation sequencing is an effective approach for detecting novel, rare mutations causing heterogeneous monogenic disorders such as RP. With the addition of this technology, disease-causing mutations can now be identified in 65% of autosomal dominant RP cases | Bowne et al. 2011 | |
| Detecting structural variations in the human genome using next generation sequencing: A comprehensive review on application of HT-NGS technology in identification of sequencing-based algorithms for detection of structural variations of human genome. | Xi et al. 2010 | |
| Mutation detection and carrier screening | "Functional genomic fingerprinting" (FGF) in mutation detection: Study proposed a selective enrichment of functional genomic regions (the exome, promoterome, or exon splice enhancers) approach (FGF) to HT-NGS, in response to discovery of causal mutations for disease and drug response. | Senapathy et al. 2010 |
| Target HT-NGS in disease mutation detection: Study identified a mutation in a gene and have shown its association with autosomal-recessive cerebellar ataxia, by combining SNP array-based linkage analysis and targeted resequencing of relevant sequences in the linkage interval with the use of next-generation sequencing technology. | Vermeer et al. 2010 | |
| Microarray-based target enrichment in HT-NGS: Study allowed the parallel, large-scale analysis of complete genomic regions for multiple genes of a disease pathway and for multiple samples simultaneously, thus provides an efficient tool for comprehensive diagnostic screening of mutations. | Amstutz et al. 2011 | |
| Pre-conceptional carrier screening of 448 severe recessive childhood diseases: An economic way of carrier screening by HT-NGS is possible and available to the general population with severe recessive childhood disorders | Bell et al. 2011 | |
| Detection of inherited disorders | Detection of monogenic inherited disorders: Study revealed the identification of human monogenic disorders by sequencing of all exons in the human genome (exome sequencing). | Kuhlenbäumer et al. 2011 |
| Role of HT-NGS neurogenetics and psychiatric disorders: Comprehensive review of impact of HT-NGS on last two decades on brain research including large number of neurological and psychiatric disorders. | Zoghbi and Warren 2010 | |
| Impact of HT-NGS to understand the genetic causes of disorders of sex development (DSD): a combined approach of comparative genomic hybridization, sequencing by hybridization with HT-NGS was presented to understand the genetic basis of human sexual determination and differentiation. | Bashamboo et al. 2010 | |
| Complex human diseases | HT-NGS in exploiting the complex disease traits: comprehensive review on the experimental design considerations, data handling issues and required analytical developments tools in mapping genetic traits using NGS. | Day-Williams and Zeggini 2010 |
| Genome-wide association studies (GWAS) using HT-NGS: Systematically identifying the genetic risks that lead or predispose to complex diseases by HT-NGS. | Singleton et al. 2010 | |
| HT-NGS in clinical diagnosis: principles of sequencing library preparation, sequencing chemistries, and NGS data analysis for targeted re-sequencing of genes implicated in hypertrophic cardiomyopathy. | Voelkerding et al. 2010 | |
| HT-NGS in identifying the causal variants of human disease: A comprehensive review on identification of causal variant typically involves in the vicinity of disease-associated SNPs including protein coding, regulatory, and structural sequences. | Kingsley 2011 | |
| Cancer research | Analysis of HT-NGS data in cancer genomics: Introduction to set up of an integrate database for multiple cancers and tumor genomes to understand a coherent picture of the genetic basis of cancer. | Ding et al. 2010 |
| Impact of HT-NGS on surgical oncology: Fast growing HT-NGS technology enables to identify the causal mutations responsible for driving cancer initiation and metastasis and raises significant expectations for improving oncologic outcomes. | Katsios et al. 2010 | |
| Understanding the cancer genomes through HT-NGS: icluding somatic genome alterations, cancer biology, diagnosis and therapy through whole-genome, whole-exome and whole-transcriptome HT-NGS approaches. | Meyerson et al. 2010 | |
| HT-NGS in cancer researches: A Comprehensive review on HT-NGS applications to cancer genome, particularly, the glioblastoma multiforme that identified the gene encoding isocitrate dehydrogenase 1 (IDH1), as target for cancer-driving mutations. | Pfeifer and Hainaut 2011 | |
| Understanding of the potential actions of SOX2 in carcinogenesis: identification of 4883 SOX2 binding regions in the GBM cancer genome using the HT-NGS Chip-seq technology | Fang et al. 2011 | |
| RNA sequencing | MicroRNA expressing profiling by HT-NGS: study proposed an alternative improved method to generate high quality miRNA sequencing libraries for the Illumina genome analyzer. | Buermans et al. 2010 |
| RNA-seq in HT-NGS: A comprehensive review on RNA-Seq for transcriptome studies supported by HT-NGS platforms. Study also addressed how to determine accurately the expression levels of specific genes, differential splicing, allele-specific expression of transcripts and many biological-related issues utilized in RNA-Seq experiments. | Costa et al. 2010b | |
| Classification of Small non-coding RNAs (ncRNAs) using HT-NGS: Study demonstrated a scoring system called alignment of pattern matrices score (ALPS) that only uses the relative positions and lengths of reads of NGS data, to classify ncRNAs (http://www.bio.ifi.lmu.de/ALPS). | Erhard and Zimmer 2010 | |
| Construction of complex miRNA repertoire database: A comprehensive survey of miRNA sequence variations from human and mouse samples using next generation sequencing platforms. Study device a method to construct a database to determine the most abundant sequence and the degree of heterogeneity for each individual miRNA species that catalogs the entire repertoire of miRNA sequences (http://galas.systemsbiology.net/cgi-bin/isomir/find.pl) | Lee et al. 2010 | |
| Analysis of miRNA profiling in HT-NGS: introduction of an efficient procedure to prepare the small RNA libraries for Illumina sequencing and analyses of the resultant sequence data for measuring microRNA abundance. | Morin et al. 2010 | |
| RNA-seq and HT-NGS: study introduced an efficient procedure for performing RNA-Seq using the Illumina sequencing platform | Nagalakshmi et al. 2010 | |
| RNA-seq and HT-NGS: a comprehensive review on RNA-Seq including the technical issues accompanying RNA-seq data generation and analysis. | Marguerat and Bähler 2010 | |
| HT-NGS in miRNA: First complete characterization of the "miRNAome" in a primary human cancer: study identified genetic variants of miRNA genes, and screen for alterations in miRNA binding sites in a patient with acute myeloid leukemia. | Ramsingh et al. 2010 | |
| HT-NGS in functional genomics: A comprehensive review on contribution NGS-based technologies in functional genomics research with a special focus on gene regulation by transcription factor binding sites. | Werner 2010 | |
| Annotation and mining of HT-NGS data: study proposed a novel database (The deepBase) to facilitate the comprehensive annotation and discovery of small RNAs from transcriptomic data. | Yang et al. 2011 | |
| Library preparation, paired ends and genomic captures for NGS platforms | Library preparation in HT-NGS: study presented a robust and cost-effective preprocessing method for DNA sample library construction using a unique 6 bp DNA barcode, which allowed multiplex sample processing and sequencing of 32 libraries in a single run using Applied Biosystems SOLiD sequencer. | Farias-Hesson et al. 2010 |
| Paired-end sequencing in HT-NGS: study proposed a NovelSeq pipeline (http://compbio.cs.sfu.ca/strvar.htm) to detect and characterize multiple types of genetic variation (SNPs, structural variation, etc.). | Hajirasouliha et al. 2010 | |
| Library preparations for tissue specific expression profiling in HT-NGS: study compared NGS with two alternative technologies, cap analysis of gene expression (CAGE) and serial analysis of gene expression (SAGE) and identified 196 novel regulatory regions with preferential use in proliferating or differentiated cells. These CAGE and SAGE libraries provides consistent expression levels and can enrich current genome annotations with tissue-specific promoters and alternative 3'-UTR usage. | Hestand et al. 2010 | |
| Genomic capture in HT-NGS: study developed an accurate, thorough, and cost-effective identification of inherited mutations for breast and ovarian cancer, through a genomic assay to capture, sequence, and detect all mutations in 21 genes, including BRCA1 and BRCA2, with inherited mutations that predispose to breast or ovarian cancer. | Walsh et al. 2010 | |
| Sequencing of mitochondrial genome | Annotation of mitochondrial genome HT-NGS: study proposed a high-throughput sequencing and bioinformatics pipeline for mt genomics, which have implications for the annotation and analysis of other organelles (e.g. plastid or apicoplast genomes) and virus genomes as well as long, contiguous regions in nuclear genomes. | Jex et al. 2010 |
| HT NGS in mitochondrial genome: Study developed and proposed a pipeline for sequencing and de novo assembly of multiple mitochondrial genomes without the costs of indexing. | McComish et al. 2010 | |
| Sequencing of complete four F-type mitochondrial genomes (15 761 bp) from the European freshwater bivalve Unio pictorum (Unionidae): Comparison of mitochondrial genomes revealed very low nucleotide diversity within the species which may have the potential importance for environmental management policies. | Soroka and Burzynski 2010 | |
| Personal genomics | Exploring the personal human genome by total integrated archive of short-read and array (TIARA): Set up of improved database for accurate detection of personal genomic variations, such as SNPs, short indels and structural variants (SVs). | Hong et al. 2011 |
First generation DNA sequencers
Historically in 1975, the Sanger introduced the concept of DNA sequencing method in his pioneered Croonian lecture (Sanger 1975) and later on, published a rapid method for determining sequences in DNA by primed synthesis with DNA polymerase (Sanger and Coulson 1975). In the year of 1977, two landmark articles for DNA sequencing were published, i.e., the Frederick Sanger’s enzymatic dideoxy DNA sequencing technique based on the chain-terminating dideoxynucleotide analogues (Sanger et al. 1977) and the Allan Maxam and Walter Gilbert’s chemical degradation DNA sequencing technique in which terminally labeled DNA fragments were chemically cleaved at specific bases and separated by gel electrophoresis (Maxam and Gilbert 1977). These two prominent elite laboratories were responsible for the introduction of the first automated DNA sequencers led by Caltech (Smith et al. 1986), which was subsequently commercialized by Applied Biosystems (ABI), the European Molecular Biology Laboratory (EMBL) (Ansorge et al. 1986, 1987) and Pharmacia-Amersham, later General Electric (GE) healthcare. This refinement and commercialization of the sequencing method led to its broad dissemination throughout the global research community.
In the first automated fluorescent DNA sequencing equipment, a complete gene locus for the hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene was sequenced, using for the first time the paired-end sequencing approach (Edwards et al. 1990). In 1996, ABI introduced the first commercial DNA sequencer that utilized a slab gel electrophoresis by the ABI Prism 310. Two years later, the considerable labor of pouring slab gels was replaced with automated reloading of the capillaries with polymer matrix by ABI Prism 3700 with 96 capillaries. This automated DNA sequencer was successfully utilized in the sequencing of the first human genome in 2003 taking into account 13-years of efforts of the human genome project consortium, and with an estimated cost of $2.7 billion. In the following years, another landmark was achived by the DNA sequencing of the first small phage genome (5386 bases in length) and sequencing of the human genome of upto ∼ 3 billion bases (Lander et al. 2001; Venter et al. 2001). It is remarkable that such progress has been made using methods that are refinements of the basic ‘dideoxy’ method introduced by Sanger in 1977.
Birth of HT-NGS
In 2000, Jonathan Rothberg founded 454 Life Sciences, which further developed the first commercially available NGS platform, the GS 20. The GS instrument was introduced in 2005, developed by 454 Life Sciences (www.454.com), as the first NGS system on the market. The developed technique was successfully validated by combining single-molecule emulsion PCR with pyrosequencing (shotgun sequencing procedure) of the entire 580 069 bp of the Mycoplasma genitalia genome at 96% coverage and 99.96% accuracy in a single GS 20 run (Margulies et al. 2005). In the following years, Roche applied science acquired 454 Life sciences and extended further the new version of the 454 instrument, i.e., the GS FLX titanium. Sharing the same technological principle in both GS 20 and GS FLX titanium, the flow cell is referred to as a "picotiter well" plate, which is made from a fused fiber-optic bundle. On a separate front, single-molecule PCR in microcompartments consisting of water-in-oil emulsions was also developed by Roche HT-NGS platform (Tawfik and Griffiths 1998). In general, the principle of pyrosequencing technique is based on the “sequencing by synthesis”. It differs from Sanger sequencing because, it depends on the detection of pyrophosphate release on nucleotide incorporation, rather than chain termination with dideoxynucleotides. The technique was developed by joint efforts of Swedish group (the teams of M. Ronaghi, M. Uhlen, and P. Nyren) in Stockholm (Ronaghi et al. 1996). They first described a sequencing approach based on chemiluminescent detection of pyrophosphate released during polymerase-mediated deoxynucleoside triphosphate (dNTP) incorporation (Nyren et al. 1993, Nyren 2007) and the real-time DNA sequencing, utilizing this release of pyrophosphate detection (Ronaghi et al. 1998). In pyrosequencing the DNA synthesis is performed within a complex reaction that includes ATP sulfurylase and luciferase enzymes and adenosine 5′ phosphosulfate and luciferin substrates in such a way that, the pyrophosphate group releases upon addition of a nucleotide, resulting in the production of detectable light.
The HT-NGS techniques, which are new opportunities and a great impact on mammalian genomics research were selected as the methods of the year in 2007 (Schuster et al. 2008). However, the road to gain the acceptance of these novel technologies was not an easy one. The first step of the HT-NGS technique consisted in detecting the next added fluorescently labeled base (reversible terminator) in the growing DNA chain by means of a sensitive CCD camera. This was performed on a large number of DNA samples in parallel, attached either to a planar support or to beads, on DNA chips, minimizing reaction volumes in a miniaturized microsystem. In the next step the terminator was converted into a standard nucleotide and the dye was removed. This cycle and the process were repeated to determine the next base in the sequence. The principle described in this application is in part very quasi to that used today in the so-called next-generation devices, commercialized by Roche, Illumina-Solexa, ABI, Helicos and other companies.
Principle of HT-NGS involves the DNA molecules, which are sequenced in a massively parallel fashion in a flow cell (Mardis 2008a, b; Metzker 2010). The sequencing is conducted in either a stepwise iterative process or in a continuous real-time manner. By virtue of this highly parallel process, each clonal template or single molecule is “individually” sequenced and can be counted among the total sequences generated. The high-throughput combination of qualitative and quantitative sequence information generated has allowed advanced genome analyses that were previously, either not technically possible or cost prohibitive.
Second generation HT-NGS platforms
The second generation HT-NGS platforms can generate about five hundred million bases of raw sequence (Roche) to billions of bases in a single run (Illumina, SOLiD). These novel methods rely on parallel, cyclic interrogation of sequences from spatially separated clonal amplicons (26 μm oil-aqueous emulsion bead [Roche: pyrosequencing chemistry], 1 μm clonal bead [SOLiD: sequencing by sequential ligation of oligonucleotide probes], clonal bridge [Illumina: sequencing by reversible dye terminators]). Currently, these (above mentioned) three leading second generation HT-NGS platforms (Fig. 1) are commercially available and the race for more additional platforms are continuously on the horizon (for comprehensive reviews on complete laboratory methods, technical aspacts of sample preparation and resulting sequencing data analysis of Roche, Illumina, SOLiD platforms, see: Mardis 2008a, b, 2009, 2010; Metzker 2010). In 2008, the US National Human Genome Research Institute (NHGRI) has initiated funding for a series of projects as part of its revolutionary genome sequencing technologies program and aimed toward its target goal of sequencing a human genome for $1000 or less (http://www.genome.gov/27527585). Recently in December 2010, the NHGRI consortium has published the most comprehensive map of human genetic variation using next-generation DNA sequencing technologies to systematically characterize the genetic differences among 179 individuals from four populations and 697 individuals from seven populations in three pilot studies (Durbin et al. 2010). These pilot studies of the “1000 genomes project” laid a critical foundation for studying human genetic variation, and aimed to create a comprehensive, publicly available map of genetic variation, that will ultimately collect sequence from 2,500 people from multiple populations worldwide and underpin future genetics research (http://www.genome.gov/27541917).
Fig. 1.
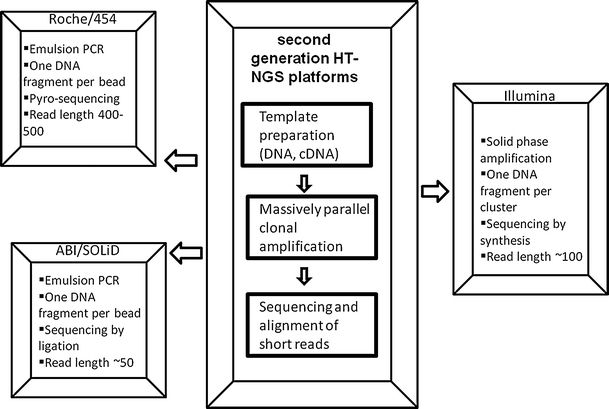
Advanced technological features of three leading second generation HT-NGS platforms
Third generation HT-NGS platforms
In the previously discussed second generation HT-NGS platforms, the principle was based on the emulsion PCR amplification of DNA fragments, to make the light signal strong enough for reliable base detection by the CCD cameras. Although the PCR amplification has revolutionized DNA analysis, but in some instances it may introduce base sequence errors or favor of certain sequences over others, thus changing the relative frequency and abundance of various DNA fragments that existed before amplification. To overcome this, the ultimate miniaturization into the nanoscale and the minimal use of biochemicals, would be achievable if the sequence could be determined directly from a single DNA molecule, without the need for PCR amplification and its potential for distortion of abundance levels. This sequencing from a single DNA molecule is now called as the “third generation of HT-NGS technology” (Schadt et al. 2010). The concept of sequencing-by-synthesis without a prior amplification step, i.e., single-molecule sequencing is currently pursued by a number of companies and described below in Sects. 5.1 to 5.7.
Heliscope™ single molecule sequencer
One of the first techniques for sequencing from a single DNA molecule was introduced by Braslavsky et al. 2003 and licensed by Helicos biosciences as the first commercial single-molecule DNA sequencing system in 2007. The principle of Heliscope sequencer relies on “true single molecule sequencing” (tSMS) technology. The tSMS technology begins with DNA library preparation through DNA shearing and addition of poli-(A) tail to generated DNA fragments (Ozsolak et al. 2010), followed by hybridization of DNA fragments to the poli-(T) oligonucleotides which are attached to the flow cell and simultaneously sequenced in parallel reactions. The sequencing cycle consists of DNA extension with one, out of four fluorescently labeled nucleotides, followed by nucleotide detection with the Heliscope sequencer. The subsequent chemical cleavage of fluorophores allows the next cycle of DNA elongation to begin with another fluorescently labeled nucleotide, which enables the determination of the DNA sequence (Harris et al. 2008). The Heliscope sequencer is capable of sequencing up to 28 Gb in a single sequencing run and takes about 8 days. It can generate short reads with a maximal length of 55 bases. In a recent development, Helicos announced that it has developed a new generation of “one-base-at-a-time” nucleotides which allow more accurate homopolymer and direct RNA sequencing (Ozsolak and Milos 2011a, b).
Single molecule real time (SMRT™) sequencer
The principle of SMRT sequencer relies on single molecule real time sequencing by synthesis method provided on the sequencing chip containing thousands of zero-mode waveguides (ZMWs). The sequencing reaction of a DNA fragment is performed by a single DNA polymerase molecule, which is attached to the bottom of each ZMW so that each DNA polymerase resides at the detection zone of ZMW (Fig. 2).
Fig. 2.
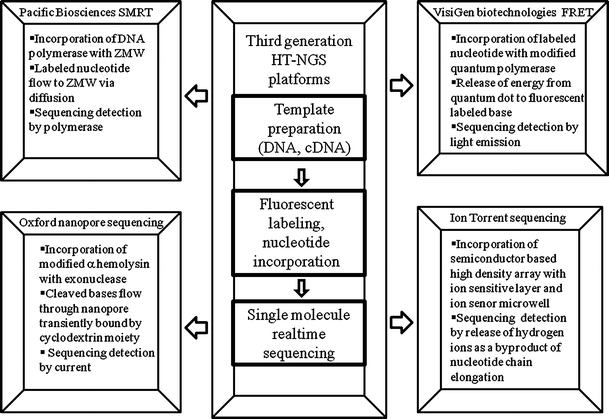
Advanced technological features of four leading third generation HT-NGS platforms
During the sequencing reaction, the DNA fragment is elongated by DNA polymerase with dNTP’s that are fluorescently labeled (each nucleotide is labeled with a fluorophore of different color) at the terminal phosphate moiety. The DNA sequence is determined with CCD array on the basis of fluorescence nucleotide detection, which is performed before nucleotide incorporation, while the labeled dNTP forms a cognate association with the DNA template. The fluorescence pulse is stopped after phosphodiester bond formation, which causes the release of a fluorophore that diffuses out of ZMW. Subsequently, the labeled nucleotide incorporation and detection allow us to determine the DNA sequence (Levene et al. 2003; Eid et al. 2009). The SMRT sequencer was designed and is still being developed by the Pacific Biosciences (www.pacificbiosciences.com). Although the SMRT instrument has recently been available on the market, the company claims that the SMRT analyzer can be capable of obtaining 100 Gb per hour with reads longer than 1000 in a single run.
Single molecule real time (RNAP) sequencer
A different single-molecule DNA sequencing approach, i.e., RNA polymerase (RNAP), has been proposed by (Greenleaf and Block 2006) in which the RNAP is attached to one polystyrene bead, whilst the distal end of a DNA fragment is attached to another bead. Each bead is placed in an optical trap and the pair of optical traps levitated the beads. The RNAP interacts with the DNA fragment and the transcriptional motion of RNAP along the template, changes the length of the DNA between the two beads. This leads to displacement of the two beads that can be registered with precision in the Angstrom range, resulting in single-base resolution on a single DNA molecule. By aligning four displacement records, each with a lower concentration of one of the four nucleotides, in a role analogous to the primers used in Sanger sequencing and for calibration using the known sequences flanking to the unknown sequenced fragment, it is possible to deduce the sequence information. The technique demonstrates that the movement of a nucleic acid enzyme and the very sensitive optical trap method, which may allow extraction of sequence information directly from a single DNA molecule.
Nanopore DNA sequencer
In contrary to all DNA sequencers mentioned above, sequencing a DNA molecule with the Nanopore DNA sequencer is free of nucleotide labeling and detection. This technique was developed from studies on translocation of DNA through various artificial nanopores. The DNA sequencing with Nanopore instrument relies on the converting of electrical signal of nucleotides by passing through a nanopore which is an α-hemolysin pore covalently attached with cyclodextrin molecule – the binding site for nucleotides. The principle of this technique is based on the modulation of the ionic current through the pore as a DNA molecule traverses it, revealing characteristics and parameters (diameter, length and conformation) of the molecule (Fig. 2). During the sequencing process the ionic current that passes through the nanopore is blocked by the nucleotide, i.e., the previously cleaved by exonuclease from a DNA strand that interacts with cyclodextrin. The time period of current block is characteristic for each base and enables the DNA sequence to be determined (Astier et al. 2006; Rusk 2009). However, further improvements and modifications in the technique, for example, increasing the number of parameters measured during the translocation of the DNA enabling single-base resolution, could lead to a rapid nanopore-based DNA sequencing technique.
Real time single molecule DNA sequencer platforms developed by VisiGen Biotechnologies
The VisiGen biotechnologies (www.visigenbio.com) introduced a specially engineered DNA polymerase, which acting as a ‘real-time sensor’ for modified nucleotides with a donor fluorescent dye and incorporated close to the active site involved in selection of the nucleotides during synthesis (Fig. 2). All four nucleotides to be integrated were modified, each with a different acceptor dye. During the synthesis, when the correct nucleotide is found, it selected and entered into the active site of the enzyme, and the donor dye label in the polymerase came into close proximity with the acceptor dye on the nucleotides and energy was transferred from donor to acceptor dye giving rise to a fluorescent resonant energy transfer (FRET) light signal (Selvin 2000). The frequency of this signal varied depending on the label incorporated in the nucleotides, so that by recording frequencies of emitted FRET signals was possible to determine base sequences, at the speed at which the polymerase can integrate the nucleotides during the synthesis process (usually a few hundred per second). The acceptor fluorophore is removed during nucleotide incorporation, which ensures that there are no DNA modifications that might slow down the polymerase during synthesis. The company is currently working on its first version of the instrument, which can generate around 4 Gb of data per day. The single-molecule approach requires no cloning and no amplification, which eliminates a large part of the cost, relative to current technologies. In addition, read lengths for the instrument are expected to be around 1 kb, longer than any current platform.
Multiplex polony technology
Run by the privately-funded personal genome project (PGP) and lead by Prof. G Church’s research group (www.personalgenomes.org), has developed and introduced the multiplex polony technology (Mitra et al. 2003; Shendure et al. 2005). In this technique, several hundred sequencing templates are deposited onto thin agarose layers and sequences are determined in parallel. This metod presents increase of several orders of magnitudes in the number of samples which can be analyzed simultaneously. It has the advantage, in terms of large reduction of the reaction volumes, requiring smaller amounts of reagents and the resulting at a lower cost. The designed instrument, i.e., Danaher Motion Polonator model G.007, is capable of 10 to 35 Gbp per module per 2.5 day run. Instrument can couple with 200 of these modules to collect 100 diploid genomes at 30X coverage in 5 days, with the remaining 5 days used for repeating any weak runs to assure 98% coverage at 1E-5 accuracy. With the significant reduced volume of reagents, the cost per unit volume is lower about 10-fold and the company hopes to meet the goal of $1000 per genome soon.
The Ion Torrent sequencing technology
In a recent advancement, the first PostLightTM sequencing technology (Ion Torrent) has been introduced (http://www.iontorrent.com/). This technology creates a direct connection between the chemical and the digital information, enabling fast, simple, massively scalable sequencing. It utilizes the simple nucleic acid Watson’s chemistry to incredibly powerful, proprietary of semiconductor technology -The Moore’s law (Moore 1965). The principle of Ion Torrent semiconductor technology is based on a well-characterized biochemical process, in which a nucleotide is incorporated into a strand of DNA by a polymerase, resulting in a release of hydrogen ion as a byproduct (Fig. 2). The technological device uses a high-density array of micro-machined wells to perform this biochemical process in a massively parallel way with each well holding a different DNA template. Beneath the wells is an ion-sensitive layer and beneath that a proprietary Ion sensor. The massive parallel sequencing at Ion Personal Genome Machine (PGM™) sequencer works on the basis of “base” principle. For example, if nucleotide A is added to a DNA template and it is incorporated into a strand of DNA and then a hydrogen ion will be released. The charge from that ion will change the pH of the solution and can be detected directly by the ion sensor without scanning, cameras and light. In this way, the PGM™ sequencer sequentially floods the chip with one nucleotide after another. The designed PGM™ system enables to perform wide range of sequencing application such as, multiplexing amplicons, transcriptome, small RNA, and ChIP-Seq. paired-end sequencing and methylation.
In terms of issues related to genomics data quality and analysis, a substantial $10 million funding has been offered by the Archon Genomics X PRIZE (AGXP), in order to generate rapid, accurate and complete human DNA sequences to global research community (editorial discussion: Toward a medical grade human genome sequence. Nat Genet. 2011 Mar, 43 [3]: 173). Because so many genome researchers have a stake, AGXP offers to help with a process of community consultation to help evolve fair and efficient methods to validate contestant genome data at high degrees of accuracy and completeness (Kedes et al. 2011). Since the launch of AGXP in 2006, there have been important advances in validation protocols of DNA sequencing technologies both in terms of speed and reduction in costs (Sutton et al. 2011). However, no current human genome sequence is fully complete, fully accurate or certain to contain all rearrangements or information of chromosome phasing (haplotype). Highly repetitive and other genome-wide regions remain difficult to sequence but are likely to be critical in defining heritable features. Hence the ideals of the X Prize remain as critical for the future of human genetics and genetic medicine as ever.
Comparison of second and third HT-NGS platforms
Unlike the second HT-NGS technologies, that rely on PCR to grow clusters of a given DNA template, attaching the clusters of DNA templates to a solid surface that is subsequently imaged as the clusters are sequenced by synthesis in a phased approach, the third HT-NGS technologies interrogate single molecules of DNA in a such a way that no synchronization (a limitation of second HT-NGS) is required (Whiteford et al. 2009), thereby overcoming issues related to the biases introduced by PCR amplification and dephasing. Furthermore, third HT-NGS technologies have the potential to exploit more fully, the high catalytic rates and high processivity of DNA polymerase, or avoid any biology or chemistry altogether to radically increase read length (from tens of bases, to tens of thousands of bases per read) and time to result (from days, to hours, or minutes). Besides this, the third HT-NGS technologies may offer the following advantages over second HT-NGS technologies: i) higher throughput, ii) faster turnaround time (e.g., sequencing metazoan genomes at high fold coverage in minutes), iii) longer read lengths to enhance de novo assembly and enable direct detection of haplotypes and even whole chromosome phasing, iv) higher consensus accuracy to enable rare variant detection, v) small amounts of starting material (theoretically only a single molecule may be required for sequencing), and vi) low cost, where sequencing the human genome at high fold coverage for less than $1000 is now a reasonable goal for the community.
In the past six years, an influx of plenty of original as well as comprehensive review papers related to both second and third generation HT-NGS platforms have been published. Thereby, the comparison of second HT-NGS platforms (Roche/454, SOLiD, and Illumina) and third HT-NGS platforms (Helicos and Pacific Biosciences etc.) are summarized in Table 1, illustrating the similarities and differences in these technologies, according to several metrics. For examples, in terms of technological features, both platforms work sequencing by synthesis, however, Second HT-NGS platform deals with washing and scan of many copies of the DNA molecules in comparison to direct physical inspection of the DNA molecule and its resolution in real time (i.e., no protracted cycles of hybridization or successive enzymatic steps) in third HT-NGS platforms. Among other dissimarities are the RNA sequencing, where, second HT-NGS platform only performed the cDNA sequencing whereas direct RNA sequencing in case of third HT-NGS platforms. Regarding data analysis, both platforms have complexity because of large data volume. In second HT-NGS platforms the mojor challenges are the short reads which can be complicated in genome assembly and alignment algorithms, whereas new signal processing challenges are still prominent in the third HT-NGS platforms.
Table 1.
Comparison of second and third HT NGS platforms
| Companies | Roche GS FLX | Illumina-Sollexa | Life Technologies | Helicos Biosciences | Pacific Biosciences |
|---|---|---|---|---|---|
| Company homepage | http://www.454.com/index.asp | http://www.solexa.com/ | http://www3.appliedbiosystems.com/AB_Home/ | http://www.helicosbio.com/ | http://www.pacificbiosciences.com |
| Platforms | GS FLX Titanium, GS Junior | HiSeq 2000, Genome Analyzer IIX, Genome Analyzer IIE, iScanSQ | ABI SOLiD, SOLiD 4 | HeliScope | SMRT |
| Template preparation | Clonal-ePCR on bead surface | Clonal bridge enzymatic amplification on glass surface | Clonal-ePCR on bead surface | Single molecule detection | Single molecule detection |
| Sample requirements | 1 μg for shotgun library, 5 μg for paired end | <1 g for single or paired-end libraries | <2 μg for shotgun library, 5–20 μg for paired end | <2 μg, single end only | Not available (NA) |
| Detection method | Light emitted from secondary reactions initiated by release of pyrophosphate | Fluorescent emission from incorporated dye-labelled nucleotides | Fluorescent emission from ligated dye-labelled oligonucleotides | Real time detection of fluorescent dye in polymerase active site during incorporation | Real time detection of fluorescent dye in polymerase active site during incorporation |
| Length of library prep/feature generation (days) | 3–4 | 2 | 2–4.5 | 1 | NA |
| Method of feature generation | Bead-based/emulsion PCR | Isothermal ‘bridge amplification’ on flow cell surface | Bead-based/emulsion PCR | Single molecule sequencing | Single molecule real time sequencing by synthesis |
| Paired ends/separation | 3 kb(2 × 110 p) | 200 bp (2 × 36 bp) | 3 kb(2 × 25 bp) | 25–55 bp | NA |
| Chemistry | Pyrosequencing | Reversible Dye Terminators | Oligonucleotide Probe Ligation | Reversible Dye Terminators | Phospho-linked Fluorescent Nucleotides |
| Bases/template | ∼400 | ∼75 (35–100) | 35–50 | 35 | 800–1000 |
|
Templates run |
1,000,000 | 40,000,000 | 85,000,000 | NA | NA |
| Data production/day | 400 MB/run/7.5 hr | 3,000 MB/run/6.5 days | 4,000 MB/run/6 days | 8 days | 0.02 days |
| Maximum samples | 16 regions/plate | 8 channels/flow cell | 16chambers/2 slides | NA | NA |
| Raw accuracy | 99.5% | >98.5% | 99.94% | >99% | NA |
| Sequencing method | Pyrosequencing | Reversible dye terminators | Sequencing by ligation | One base-at-a-time | Sequencing by synthesis |
| Read lengths | 400 bases | 36 bases | 35 bases | Longer than 1000 | Longer than 1000 |
| Sequencing run time | 10 h | 2-5 days | 6 days | 12 | <1 |
| Total Throughput bases/run (Gb) | 0.40–0.60 Gb, 0.035 Gb | 3–6 Gb | 10–20 Gb | 28 GB | 100 Gb per hour |
| Throughput/day (Gb) | ~1 | 1.5 | 1.7–2 | 2.5 | ~1 |
| Estimated system cost | $500,000 | ∼$400,000 | $525,000 | Lower than second NGS | Lower than second NGS |
| Consumable cost per single-end run (paired-end run) | $5000 | $3000 | $4000 | Lower than second NGS | Lower than second NGS |
| Cost per run (total direct) | $8439 | $8950 | $17,447 | Lower than second NGS | Lower than second NGS |
| Cost per Mb | $84.39 | $5.97 | $5.81 | Lower than second NGS | Lower than second NGS |
With the progressive advent of HT-NGS technologies, DNA sequencing costs have been drastically reduced (Table 1). Now, it is feasible to sequence hundreds or even thousands of genes for a single individual with a suspected genetic disease or complex disease predisposition. Along with the benefits offered by these technologies, there are a number of challenges that must be addressed before wide-scale sequencing becomes accepted in genome research practices. Molecular diagnosticians will need to become comfortable with, and gain confidence with, these new platforms, which are based on radically different technologies compared to the standard DNA sequencers in routine diagnostic today. Since 2001, when the technology that sequenced the human genome on the basis of capillary electrophoresis of individual fluorescently labeled Sanger sequencing method, the advent of next-generation sequencing platforms have dramatically increased the speed at which DNA sequence can be acquired, while reducing the costs by several orders of magnitude compared to their predecessors (Fig. 3). This is because of the basic mechanisms for data generation had changed radically, producing far more sequence reads per instrument run and at a significantly lower expense. Figure 3 illustrates how the resulting HT-NGS information has both enhanced our knowledge and expanded the impact of the genome on biomedical research (Mardis 2011).
Fig. 3.
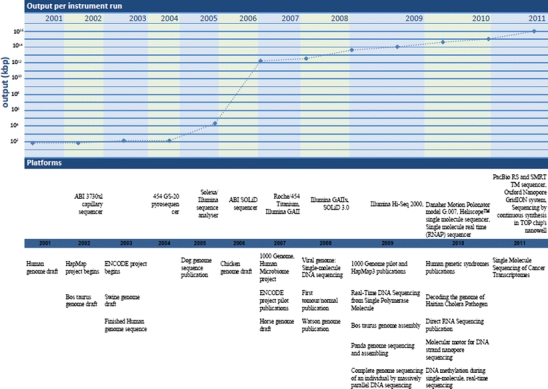
Changes in instrument capacity over the past decade, and the timing of major sequencing projects (Figure updated from Madris, 2011). On top: increasing scale of data output per run plotted on a logarithmic scale. At middle: timeline representing major milestones in massively parallel sequencing platform introduction and instrument revisions. On bottom: the time lines of several projects, milestones and publications citing different sequencing technologies
These next-generation platforms generate shorter reads with lower quality, when compared to the Sanger platform. The reduction in read length and quality necessitated the development of bioinformatics tools to assist in either the mapping of these shorter reads to reference sequences or de novo assembly. The development of these new techniques aims toward meeting the demand for sequence information in various fields of research, such as study of genomics and evolution, forensics, epidemiology and diagnostics and applied therapeutics.
Applications and advances of sequencing technologies on human genome research
The landmark of sequencing of human genome was accomplished by two groups, i.e., the publicly funded Human Genome Group (HGP) and Celera Groups. Both groups utilized different strategies. The HGP group produced a working draft of the human genome by a map-based strategy, while Celera, to sequence the human genome by the whole-genome shotgun (WGS) approach (Fig. 4). The availability of sequence material obtained through different approaches greatly facilitated the ability of the entire scientific community to interpret the data. The strategy of HGP originally established by the publicly funded effort and was based on the localizing bacterial artificial chromosomes (BACs) containing large fragments of human DNA within the framework of a landmark-based physical map. Ideally, sequencing would have been done on a clone-by-clone basis, with clones selected from the minimum BAC tiling path. The key to the HGP's strategy was the subsequent 'mapping' step in which the BACs were each positioned on the genome's chromosomes by looking for distinctive marker sequences, called sequence tagged sites (STSs), whose location had already been pinpointed. In this way, the BACs provided a high-resolution map of the entire genome (Fig. 4). The working draft, although containing some gaps and ambiguities in order, is extremely useful in such efforts as identifying disease-associated genes. Simultaneously, the idealized strategy of Celera was to avoid the up-front mapping phase by subcloning random fragments of the human genome directly. Sequencing of both ends of fragments in libraries of different sizes facilitated ordering. While saving time and effort at the beginning, the Celera approach made the assembly process much more dependent on algorithms and computer time. In their efforts to reach their goals, the idealized strategies evolved into hybrids in which the HGP selected more clones arbitrarily and Celera made use of BAC maps and sequence generated by the HGP (Fig. 4).
Fig. 4.
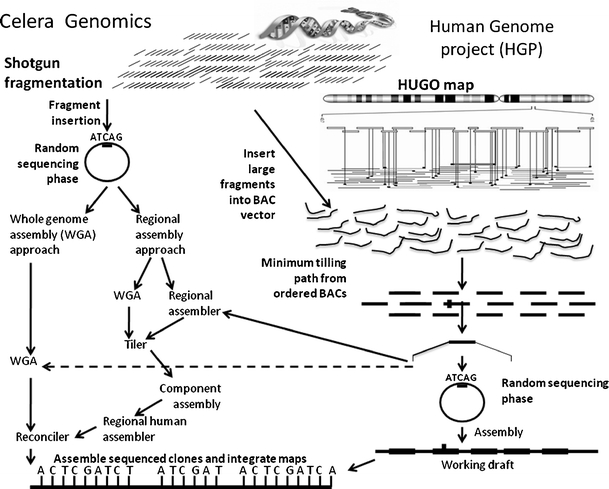
Two strategies for generating the first complete human genome sequence: On right, the Human Genome Project (HGP) map-based strategy (Lander et al. 2001) showing the localizing bacterial artificial chromosomes (BACs) containing large fragments of human DNA within the framework of a landmark-based physical map. On left, the Celera’s strategy (Venter et al. 2001) showing the whole genome shotgun sequencing approach
Since the introduction of HT-NGS platform in 2005, the production of large numbers of low-cost reads made the NGS platforms useful for many applications on human genomes research particularly, the de novo genome sequencing, whole-genome resequencing or more targeted sequencing, cataloguing the transcriptomes of cells tissues and organisms (RNA–seq), genomic variation and mutation detection, genome-wide profiling of epigenetic marks and chromatin structure using methyl– seq, DNase–seq and ChIP–seq (chromatin immunoprecipitation coupled to DNA microarray) and personal genomics (Table 2).
De Novo, resequencing and targeted sequencing
In general, the HT-NGS platforms made de novo assembly of most organisms including human, a lengthy and costly endeavor. In humans, such an endeavor had already commenced with the publication of several complete genomes, for example: using the Roche 454 technology to 7.5x human genome coverage (Wheeler et al. 2008), human genome sequences of Chinese (Wang et al. 2008), an African (Pushkarev et al. 2009), and two Korean individuals (Ahn et al. 2009; Kim et al. 2009), all were done using the Illumina Genome Analyzer and sequenced around 20x haploid genome coverage with the exception of the African male’s genome which was also resequenced on ABI SOLiD system (McKernan et al. 2009). More recently, James Lupski’s genome was sequenced to 30x base coverage using ABI’s SOLiD System (Lupski et al. 2010). Resequencing of human genome was not limited to the second generation platforms. Steven Quake’s genome, for example, was sequenced to 90% genome coverage on Helicos’ single-molecule sequencing platform (Pushkarev et al. 2009). The whole genome genotyping approach on HT-NGS effectively enables unlimited multiplexing and unconstrained single nucleotide polymorphism (SNP) selection, for example typing of HLA genotypes in human (Lind et al. 2010) and genome-wide fetal genotyping using non-invasive HT-NGS of the mother's blood (Burgess 2011).
RNA sequencing
HT-NGS is also finding application in the study of small RNAs. For example, a comprehensive study of miRNA in acute myeloid leukaemia performed by HT-NGS identified differentially expressed miRNAs binding sites for acute myeloid leukaemia (Ramsingh et al. 2010). In recent studies, several efficient procedures have been introduced to perform RNA-Seq using the Illumina sequencing platform (Buermans et al. 2010; Nagalakshmi et al. 2010) including the technical issues (Marguerat and Bähler 2010), Construction of complex miRNA repertoire database (Lee et al. 2010), preparation the small RNA libraries and analyses of the resultant sequence data for measuring microRNA abundance (Morin et al. 2010), as well as annotation and discovery of small RNAs from transcriptomic data (Yang et al. 2011). RNA seq using Illumina and 454 technologies has also been found to be a powerful tool for detecting novel gene fusions in cancer cell lines and tissues (Maher et al. 2009). Understanding the transcriptome is essential for interpreting the functional elements of the genome and revealing the molecular constituents of cells and tissues, and also for understanding development and disease. The specific aims of transcriptomics are: (1) to catalog all transcripts in a context of cell types for a species, including mRNAs, non-coding RNAs and small RNAs, (2) to determine the transcriptional structure of genes, in terms of their start sites, 5’- and 3’-ends, splicing patterns and other post-transcriptional modifications and (3) to quantify the expression levels of each transcript during development or under different physiologic and pathological conditions. With the availability of faster and cheaper HT-NGS platforms, more transcriptomic analyses are performed using a recently-developed deep sequencing approach (Wang et al. 2009). The short reads produced by HT-NGS technologies, particularly Illumina and SOLiD, are arguably suitable for gene expression profiling. RNA-Seq has been used to accurately monitor gene expression of specific genes to determine the differential splicing, allele-specific expression of transcripts and many biological-related issues utilized in RNA-Seq experiments (Costa et al. 2010b).
Epigenetics
The HT-NGS technologies offer the potential to substantially accelerate epigenomic research (the study of heritable gene regulation that does not involve the DNA sequence itself but its modifications and higher-order structures), including posttranslational modifications of histones, the interaction between transcription factors and their direct targets, nucleosome positioning on a genome-wide scale and the characterization of DNA methylation patterns (Bormann et al. 2010; Fouse et al. 2010; Bhaijee et al. 2011). Histone modification and methylation of DNA are two important epigenetic mechanisms that regulate the transcriptional status of genes. Using ChIP-Seq (chromatin immunoprecipitation and direct sequencing) technology, post-translational modifications of histones and the location of transcription factors can be studied at the whole-genome level (Neff and Armstrong 2009), whereas methylated DNA immunoprecipitation (meDIP) and bisulphite protocols can be used to study the methylation of DNA itself (Popp et al. 2010). For example, using ChIP-seq on HT-NGS platform, the binding sites for a transcription factor (TF) and the human growth-associated binding protein (GABP alpha) were directly sequenced instead of being hybridized on a chip-array and unraveling the wide and intricate gene pathways regulated by PPARG gene (Costa et al. 2010a) and predicted the de novo motif discovery (Jiao et al. 2010). This ChIP-Seq on HT-NGS platform allows now researchers to improve both quantity and quality of produced data. Among other prevalent high-throughput approaches, protein-DNA interactions have been studied by the combination of chromatin immunoprecipitation with DNA microarray (ChIP-chip). Contrarily, ChIP-seq technique inherits two advantages from the HT-NGs platforms, firstly, it is not limited by the microarray content and secondly, it does not depend on the efficiency of probe hybridization. The ChIP-seq approach was recently used to identify binding sites of two transcription factors, STAT1 and NRSF in human cells (Robertson et al. 2007; Euskirchen et al. 2007). Both studies compared their findings with those generated by ChIP-chip, demonstrating that ChIP-seq had better resolution and required fewer replicates.
Genomic variation and mutation detection
NGS promises to facilitate the genome-wide human population structural variation studies (Xi et al. 2010; Henn et al. 2010) by uncovering all of the common and rare genetic variation in human populations (Bowne et al. 2011). Indeed, the “1000 Genomes Project” has made great progress to date toward this goal (Durbin et al. 2010). With a comprehensive genetic map of all human variation produced by NGS, researchers will be able to perform more detailed experiments to detect genetic variation underlying the response to medicines. HT-NGS patforms have also found application in high throughput mutation detection and carrier screening using a method called functional genomic fingerprinting (FGF). The method implies a selective enrichment of functional genomic regions (the exome, promoterome, or exon splice enhancers) approach in response to discovery of causal mutations for disease and drug response (Senapathy et al. 2010). The target enrichment based on microarray also allowed the parallel, large-scale analysis of complete genomic regions for multiple genes of a disease pathway, and for multiple samples simultaneously, thus providing an efficient tool for comprehensive diagnostic screening of mutations (Amstutz et al. 2011). The carrier screening by HT-NGS is also feasible to the general population with severe recessive childhood disorders (Bell et al. 2011) and in mutation detection associated with autosomal-recessive cerebellar ataxia, by combining SNP array-based linkage analysis and targeted resequencing of relevant sequences in the linkage interval (Vermeer et al. 2010).
Cancer research and biomarkers
HT- NGS technological advances are also driving the development of novel diagnostic and therapeutic approaches to the treatment of cancer (Meyerson et al. 2010), as researchers re-sequence the tumor and normal genomes comparision from specific types of cancer (Pfeifer and Hainaut 2011). In cancer genome, it enables rapid identification of patient-specific rearrangements in solid tumours (Ding et al. 2010, McBride et al. 2010). A further interesting application of the technology is ‘personalized’ biomarkers, which has recently been developed to detect the presence of tumour-specific genomic rearrangements in plasma samples from patients to generate a tumour-specific biomarker. Genomic rearrangements were identified specific to the tumour, which were not present in the patient's normal somatic tissue. Digital PCR assays were then designed across rearrangement breakpoints to provide a sensitive tumour-specific biomarker which was successfully used to monitor residual disease following treatment (Leary et al. 2010). Furthermore, HT-NGS technology also enables to identify the causal mutations responsible for driving cancer initiation and metastasis and raises significant expectations for improving oncologic outcomes (Katsios et al. 2010) and identification of 4883 SOX2 binding regions in the Glioblastoma (GBM) cancer genome (Fang et al. 2011). Small RNAs could provide a further application of NGS in biomarker discovery (Lee et al. 2010). MicroRNAs (miRNAs) are implicated in the control of protein translation and are present in blood plasma. NGS could be used to assay tissues or blood plasma to generate whole-genome miRNA profiles, which could then be mined for biomarker signatures.
Applications and advances of sequencing technologies on animal genome research
Discussion pertaining to the economically important farm animals in context to impact of HT-NGS technology is also a scope of this publication. The updated assembled animal genome server is available for the following species: cat (Felis catus), chicken (Gallus gallus), cow (Bos taurus), dog (Canis lupus familiaris), horse (Equus caballus) and pig (Sus scrofa) (http://www.ensembl.org/info/about/species.html). Additionally, the preview of genome assemble is also available for the sheep (Ovis aries) (http://pre.ensembl.org/Ovis_aries/Info/Index) and turkey (Meleagris gallopavo) genome (http://pre.ensembl.org/Meleagris_gallopavo/Info/Index). The important characteristics features of these assembled animal genomes are summarized in Table 3.
Table 3.
Characteristics of assembled domestic animal genomes
| Assembly and Gene-build features | Cat (Felis catus), | Chicken (Gallus gallus) | Cow (Bos taurus) | Dog (Canis lupus familiaris) | Horse (Equus caballus) | Pig (Sus scrofa) | Sheep (Ovis aries) | Turkey (Meleagris gallopavo) |
|---|---|---|---|---|---|---|---|---|
| Sequencing strategy (fold coverage) | Whole-genome shotgun (1.87×) | Whole-genome shotgun/BAC and other clones (6.6×) | Whole-genome shotgun/BAC and other clones (7.1×) | Whole-genome shotgun/BAC and other clones (7.5×) | Whole-genome shotgun/BAC and other clones (6.8×) | Whole-genome shotgun (0.66×) and Minimal tile-path BAC by BAC (6×) | Whole-genome shotgun (3×) | BAC/other large clone shotgun (−) |
| Genome length (Assembly) | 1.64 Gb (CAT) | 1.05 Gb (WASHUC2) | 2.91 Gb (Btau4.0) | 2.38 Gb (CanFam2.0 | 2.47 Gb (EquCab 2) | ~2.1 and 2.26 Gbs (Sscrofa9) | 2.78 Gb (OAR1.0) | 1.08 Gb (UMD2) |
| Web resources | http://www.ensembl.org/Felis_catus/Info/Index/ | http://genome.wustl.edu/genomes/view/gallus_gallus, and http://www.ensembl.org/Gallus_gallus/Info/Index/ | http://genomes.arc.georgetown.edu/drupal/bovine, http://www.hgsc.bcm.tmc.edu/projectspecies-m-Bovine.hgsc?pageLocation=Bovine, and http://www.ensembl.org/Bos_taurus/Info/Index/ | http://www.broadinstitute.org/mammals/dog, and http://www.ensembl.org/Canis_familiaris/Info/Index/ | http://www.broadinstitute.org/mammals/horse, and http://www.ensembl.org/Equus_caballus/Info/Index | http://www.piggenome.dk/ http://www.piggenome.org/, http://www.sanger.ac.uk/Projects/S_scrofa/, and http://www.ensembl.org/Sus_scrofa/Info/Index/ | http://www.sheephapmap.org/, http://www.livestockgenomics.csiro.au/sheep/, and https://isgcdata.agresearch.co.nz/ | http://www.ensembl.org/Meleagris_gallopavo/Info/Index/ |
| Refrences | Pontius et al. 2007 | Hillier et al. 2004 | Elsik et al. 2009 | Lindblad-Toh et al. 2005 | Wade et al. 2009 | Wernersson et al. 2005 | http://www.sheephapmap.org | https://www.vbi.vt.edu/ |
| Sequencing organization | Agencourt Bioscience/ Broad Institute | Washington University Genome Sequencing Center | Baylor HGSC: The Bovine Genome Sequencing and Analysis Consortium. 2009 | Broad Institute/MIT Center for Genome Research | Broad Institute/MIT Center for Genome Research | The Sino-Danish pig genome sequencing project and Wellcome Trust Sanger Institute | AgResearch/Baylor HGSC/CSIRO/University of Otago | Virginia Bioinformatics Institute/ USDA Beltsville/ University of Maryland |
| Release year | 2006 | 2004 | 2009 | 2005 | 2009 | 2005 and 2009 | 2008 | 2009 |
| Database version | 60.1i | 60.2p | 60.4i | 60.2p | 60.2 g | 60.9 d | 57 | 57 |
| Base Pairs | 1,642,698,377 | 1,050,947,331 | 3,247,516,410 | 2,384,996,543 | 2,428,773,513 | 2,389,078,169 | 1,201,946,309 | 941,191,869 |
| Golden Path Length | 4,055,847,588 | 1,100,480,441 | 2,918,205,644 | 2,531,673,953 | 2,474,912,402 | 2,262,596,414 | 2,860,496,367 | 1,087,496,503 |
| Known protein-coding genes | 231 | 14,923 | 19,241 | 2,321 | 15,355 | 621 | Not available (NA) | 11,145 |
| Projected protein-coding genes | 13,061 | 1,544 | 1,416 | 13,512 | 2,275 | 11,899 | NA | NA |
| Novel protein-coding genes | 1,756 | 269 | 391 | 3,472 | 2,806 | 4,973 | NA | NA |
| Pseudogenes | 1,284 | 96 | 686 | 1,742 | 4,400 | 520 | NA | NA |
| RNA genes | 2,930 | 1102 | 3,936 | 3,613 | 2,118 | 2,447 | NA | NA |
| Gene exons | 195,263 | 182,492 | 225,837 | 216,305 | 211,815 | 159,909 | NA | NA |
| Gene transcripts | 19,262 | 23,392 | 31,599 | 30,914 | 29,159 | 22,050 | NA | NA |
The first initiative toward animal genome sequencing started, just after the human genome sequencing conducted in 2001 (Lander et al. 2001; Venter et al. 2001) to advocate public funding for animal genomics research from several universities, private industries, producer groups and Animal Genome Research (AGR) scientific group. At the beginning, AGR under the National Academy of Sciences (NAS) organized a public workshop on “exploring horizons for domestic animal genomics” (Pool and Waddell 2002). The workshop objective was to identify research goals, public and private fundings to generate high-coverage, draft genome sequences of the major domestic animal species, i.e., cattle, pig, horse, sheep, chicken, dog and cat. In the initial phase, two “white papers” were released in support for sequencing and assembling the cattle genome (Gibbs et al. 2002) and pig genome (Rohrer et al. 2002). Since then, considerable progress has been achieved toward the whole genome sequence of domestic animals. In 2006, latest progress and updates on bovine (Womack 2006), porcine (Mote and Rothschild 2006), sheep (Cockett 2006), horse (Chowdhary and Raudsepp 2006), chicken (Burt 2006), canine (Galibert and André 2006) and feline (Murphy 2006) genome sequencing were comprehensively reviewed and published. For the recent progress and development in domestic animals genome sequencing and assembling, a series of publications and studies were published(Table 3), for bovine (Liu 2009; Elsik et al. 2009; Zimin et al. 2009), porcine (Wiedmann et al. 2008; Amaral et al. 2009; Ramos et al. 2009; Isom et al. 2010; Leifer et al. 2010), sheep (Archibald et al. 2010), horse (Bright et al. 2009; Coleman et al. 2010), chicken (Dalloul et al. 2010, Marklund and Carlborg 2010).
In the past, animal genome researches have effectively adapted to traditional breeding program objectives, i.e., genetic improvement of the livestock through marker assisted selection (MAS) and gene assisted selection (GAS) program. The MAS programs were successfully implemented during mid 1990s through global animal genome scan procedures and quantitative trait loci (QTL) mapping (for example cattle: Georges et al. 1995 and pig: Andersson et al. 1994). The GAS program was well implemented to animal genomes with identification of candidate genes and detection of casual quantitative trait nucleotides (QTN) discoveries (for example cattle: Grisart et al. 2001 and pig: Van Laere et al. 2003). In the past five years, HT-NGS technologies emerged as a potential research tool for the development of animal genomes research. Now, in this genomics era, the livestock researchers are working on effective implementation of genomics breeding for selection (GBS) in their traditional breeding program toward genetic improvement of economic traits.
In contrast to human, the progress to adopt the HT-NGS technologies in animal genome is at a lower pace, predominantly because of high cost and limited publically funded projects. However, continuous advancement of HT-NGS through the fast track human research and feasibility of genome sequencing for 1000 $, the pace will be geared up faster in the near future. The overall review on impact of current HT-NGS in animal genome is presented in Table 4.
Table 4.
An overview of impact HT NGS technology on domestic animals genome research
| Domestic animals | Description | References |
|---|---|---|
| Cow (Bos Taurus) | Construction of high-density bovine SNP arrays: paper addressed an economical, efficient, single step method for SNP discovery, validation and characterization that utilizes the HT-NGS sequencing generated reduced representation libraries (RRLs) from specified target populations. The developed strategy allowed simultaneous de novo assembling of high-quality SNPs and the population characterization of allele frequencies may be applied to any species with at least a partially sequenced genome | Van Tassell et al. 2008 |
| Whole genome sequencing of Fleckvieh breed: study generated 24 gigabases of sequence, mainly using 36-bp paired-end reads, resulting in an average 7.4-fold sequence depth and identified 2.44 million SNPs, 82% of which were previously unknown, and 115,000 small indels. | Eck et al. 2009 | |
| Assembling of Bos taurus genome: systematic three steps of bovine genome assembling were described. It is accomplished by first assembling of BACs in combination with the individual overlapping WGS reads, followed by assembly of only the WGS sequences. Secondly, both assemblies were combined to create a more complete genome representation that retained the high quality BAC-based local assembly information with gaps between BACs filled in with the WGS-only assembly, and finally the entire assembly was placed on chromosomes using the available map information. | Liu et al. 2009 | |
| Development of diagnostics test: Prospective of HT-NGS technology which may lead to develop specific and sensitive diagnostics test for M. bovis infection and eventually in eradication of tuberculosis from cattle populations were discussed. | MacHugh et al. 2009 | |
| Assembling of Bos taurus genome: upgrading of existing Bos taurus genome with excellent large-scale contiguity in which a large majority (approximately 91%) of the genome has been placed onto all 30 Bos taurus chromosomes and re-constructed a new cow-human synteny map and also identified for the first time a portion of the Bos taurus Y chromosome. | Zimin et al. 2009 | |
| Identification of the genetic disorder: using combined an array-based sequence capture and massively parallel sequencing approach causative mutation of Bovine Arachnomelia was identified in bovine sulfite oxidase (SUOX) gene. | Drögemüller et al. 2010 | |
| Within the host dissecting the FMD virus population: study compared the viral populations within two bovine epithelial samples (foot lesions) from a single animal with the inoculums used to initiate experimental infection using Genome Analyzer HT-NGS platform (Illumina). | Wright et al. 2011 | |
| Water buffalo (Bubalus bubalis) | A comprehensive review: on water buffalo genome: apart from genomic anatomy, mapping, prospects of whole genome sequencing and HT-NGS platforms were discussed. | Michelizzi et al 2010 |
| Application of Illumina BovineSNP50 BeadChip on water buffalo genome: Study effectively and successfully utilized the Illumina BovineSNP50 BeadChip on water buffalo genome and 54,001 fully scored and 6,711 partially scored SNPs. Study provides a solid foundation to further characterize the SNP evolutionary process, thus improving understanding of within- and between-species biodiversity, phylogenetics and adaption to environmental changes. | Michelizzi et al. 2011 | |
| Aurochs (Bos primigenius) | Genome sequencing of ancient DNA: Study performed the aurochs (Bos primigenius) genome (289.9 MB) and mitochondrial sequencing using both Sanger and Illumina HT-NGS platforms. Comparative genome analysis with bos taurus genome revealed high-confidence calls with no discrepancies. | Edwards et al. 2010 |
| Pig (Sus scrofa domesticus) | Porcine SNP discovery: using a combined reduced representation and HT-NGS approach, approximately 5 million sequence reads were collected and assembled into contigs having an overall observed depth of 7.65-fold coverage and 12.6 fold porcine SNP coverages. Study identified large number of SNP (115,572) with greater confidence and relatively high minor allele frequencies (MAF) in 47,830 contigs. | Wiedmann et al. 2008 |
| Porcine SNP discovery using the Illumina 1 G Genome Analyzer: identification large numbers of porcine SNPs (17,489) by creating strict rules for sequence selection, which simultaneously decreases sequence ambiguity, i.e., a higher sequence quality (SQ) threshold leads to more reliable identification of SNPs. | Amaral et al. 2009 | |
| Comparative analysis of four pig breeds by HT-NGS: study identified and assembled de novo over 372 k porcine SNPs and more than 549 K SNPs were used to design the Illumina Porcine SNP60K iSelect Beadchip. | Ramos et al. 2009 | |
| Prenatal transcriptomic profiling by HT-NGS: RNA sequencing of trophectoderm (TE) and embryonic disc (ED) of a single day 12 porcine embryo was performed using the Illumina NGS platform. Study confirms the presence of abundance of TE and ED specific genes in HT-NGS generated data. | Isom et al. 2010 | |
| Genome sequencing of classical swine fever (CSF) virus: five different strains of CSFV subgroup 2.3 were completely sequenced using these newly developed NGS protocols. | Leifer et al. 2010 | |
| Poultry (Gallus gallus) | Identification of SNPs using re-sequencing of limited coverage (~5X) chicken genome: On average, ~3.7 SNPs/kb were detected by re-sequencing of pooled DNA on 60 K SNP chip, with about 5% lower density on microchromosomes than on macrochromosomes. | Marklund and Carlborg 2010 |
| Genome assembly and analysis of domestic turkey: approximately 5x and 25x genome coverages of domestic turkey were generated through Roche FLX and Illumina HT-NGS platforms. A total of 28,261 scaffolds containing 917 Mb of sequence were also assigned to turkey chromosomes. | Dalloul et al. 2010 | |
| Horse (Equus ferus caballus) | HT-NGS database: Upgrading the equine functional annotation database for the emerging equine NGS database. | Bright et al. 2009 |
| Equine mRNA sequencing by Illumina NGS platform: RNA-seq from eight equine tissues generated 293 758 105 sequence tags of 35bases each, equalling 10.28 gbp of total sequence data. The tag alignments represent approximately 207× coverage of the equine mRNA transcriptome and confirmed transcriptional activity for roughly 90% of the protein-coding gene structures predicted by Ensembl and NCBI. Study utilized the Ensembl and NCBI annotation pipelines to combine the 75 116 RNA-seq-derived transcriptional units and generated a consensus equine protein-coding gene with a set of 20 302 defined loci. | Coleman et al. 2010 | |
| Sheep (Ovis aries) | The construction of a reference genome of sheep: Initiated by international Sheep Genomics Consortium, a draft reference genome of sheep was constructed utilizing NGS data together with Sanger sequencing data. | Archibald et al. 2010 |
| Giant Panda (Ailuropoda melanoleuca) | Classical example of De Novo sequencing: Using HT-NGS technology alone, study successfully generated and assembled a draft sequence of the giant panda genome with the assembled contigs of 2.25 gigabases (Gb) cover approximately 94% of the whole genome. | Li et al. 2010b |
| General review | A comprehensive review on domestic animal genomics: paper summarizes the proceedings of the USDA animal genomics workshop “Charting the road map for long term USDA efforts in agricultural animal genomics” in which future target goals such as: priorities for structural and functional genomics, bioinformatics resources in domestic animal genomics were discussed. | Green et al. 2007 |
| A comprehensive review on vertebrate experimental organisms: an overview of the current next-generation sequencing platforms and the newest computational tools for the analysis of next-generation sequencing data in the context of vertebrate model organism genetics were disccued. | Turner et al. 2009 | |
| A comprehensive review of HT-NGS technology applied to domestic animals: The review paper discussed the experimental design, RNA-seq, computational approach for whole genome sequence association studies, as opposed to classical SNP-based association, and implementing this new source of information into breeding programs. | Pérez-Enciso and Ferretti 2010 | |
| A comprehensive review on profiling of regulatory microRNA transcriptomes in various biological processes: review highlighted the application of miRNA, particularly in regular biological and pathological processes within cells and tissues and as a potential tool for cellular control of gene regulation studies in human and animal genome researches. | Shah et al. 2010 |
Concluding summary and future prospective
In the fast growing HT-NGS technologies, the main challenge is to cope with the analysis of vast production of sequencing database through advanced bioinformatics tools. As the year 2011 has been marked as the 10th anniversary of the first human genome sequencing, nucleic acid research (NAR) has recently published its 18th annual database issue (Volume 39, supplement 1 January 2011: http://nar.oxfordjournals.org/content/39/suppl_1) to dedicate 10 years achievements in genome sequencing and its future challenges ahead. The published issues comprised of 96 new online databases covering a variety of molecular biology data and 83 data resources that have previously been published in NAR or other journals, in total the database collection now includes 1330 data sources.
The availability of ultra-deep sequencing of genomic DNA will transform the medical (in analysis of the causes of disease, development of new drugs and diagnostics) and veterinary (genetic improvement of animal health and productivity) fields in the near future. Further, it may become a promising tool in the analysis of chromatin immunoprecipitation coupled to DNA microarray (ChIP-chip) or sequencing (ChIP-seq), RNA sequencing (RNA-seq), whole genome genotyping, de novo assembling and re-assembling of genome, genome wide structural variation, mutation detection and carrier screening, detection of inherited disorders and complex human diseases, DNA library preparation, paired ends and genomic captures, sequencing of mitochondrial genome and personal genomics etc. It is anticipated that HT-NGS technology, for clinical purposes in human medicine and implementation of genomic selection in farm animals breeding programs will probably be fully adopted in the next couple of decades. The recent technological advancements in HT-NGS analysis is not only setting the benchmark in the advancement in genomics researches but also in the fields of proteomics, other omics and cancer research at a unprecedented pace (e.g., DNA and protein microarrays, quantitative PCR, mass spectrometry and others). Although, the HT-NGS with short DNA sequence reads (25–50 bases) and moderate sequence reads (500 bases) have already found many potential applications, but for genomic sequencing and for analysis of the ever more important structural genetic variations in genomes, such as copy number variations, chromosomal translocations, inversions, large deletions, insertions and duplications, it would be a great advantage if sequence read length on the original single DNA molecule could be increased to several 1000 bases and more per second. With the progress of third NGS platform at tremendous pace, one can hope that the goal of determining a whole chromosome sequence from a single original DNA molecule or genome sequence for $1000 could be feasible soon in the near future.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Ahn SM, Kim TH, Lee S, Kim D, Ghang H, Kim DS, et al. The first Korean genome sequence and analysis: full genome sequencing for a socio-ethnic group. Genome Res. 2009;19:1622–1629. doi: 10.1101/gr.092197.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AJ, Megens HJ, Kerstens HH, Heuven HC, Dibbits B, Crooijmans RP, den Dunnen JT, Groenen MA. Application of massive parallel sequencing to whole genome SNP discovery in the porcine genome. BMC Genomics. 2009;10:374. doi: 10.1186/1471-2164-10-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstutz U, Andrey-Zürcher G, Suciu D, Jaggi R, Häberle J, Largiadèr CR. Sequence capture and next-generation resequencing of multiple tagged nucleic acid samples for mutation screening of urea cycle disorders. Clin Chem. 2011;57:102–111. doi: 10.1373/clinchem.2010.150706. [DOI] [PubMed] [Google Scholar]
- Andersson L, Haley CS, Ellegren H, Knott SA, et al. Genetic mapping of quantitative trait loci for growth and fatness in pigs. Science. 1994;263:1771–1774. doi: 10.1126/science.8134840. [DOI] [PubMed] [Google Scholar]
- Ansorge W, et al. A non-radioactive automated method for DNA sequence determination. J Biochem Biophys Methods. 1986;13:315–323. doi: 10.1016/0165-022X(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Ansorge W, et al. Automated DNA sequencing: ultrasensitive detection of fluorescent bands during electrophoresis. Nucleic Acids Res. 1987;15:4593–4602. doi: 10.1093/nar/15.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald AL, Cockett NE, Dalrymple BP, International Sheep Genomics Consortium et al. The sheep genome reference sequence: a work in progress. Anim Genet. 2010;41:449–453. doi: 10.1111/j.1365-2052.2010.02100.x. [DOI] [PubMed] [Google Scholar]
- Astier Y, Braha O, Bayley H. Toward Single Molecule DNA Sequencing: Direct Identification of Ribonucleoside and Deoxyribonucleoside 5’-Monophosphates by Using an Engineered Protein Nanopore Equipped with a Molecular Adapter. J Am Chem Soc. 2006;128:1705–1710. doi: 10.1021/ja057123+. [DOI] [PubMed] [Google Scholar]
- Bansal V. A statistical method for the detection of variants from next-generation resequencing of DNA pools. Bioinformatics. 2010;26:i318–i324. doi: 10.1093/bioinformatics/btq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashamboo A, Ledig S, Wieacker P, Achermann JC, McElreavey K. New technologies for the identification of novel genetic markers of disorders of sex development (DSD) Sex Dev. 2010;4:213–224. doi: 10.1159/000314917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011;3:65ra4. doi: 10.1126/scitranslmed.3001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaijee F, Pepper DJ, Pitman KT, Bell D. New developments in the molecular pathogenesis of head and neck tumors: a review of tumor-specific fusion oncogenes in mucoepidermoid carcinoma, adenoid cystic carcinoma, and NUT midline carcinoma. Ann Diagn Pathol. 2011;15:69–77. doi: 10.1016/j.anndiagpath.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Bormann G, Chung CA, Boyd VL, McKernan KJ, Fu Y, Monighetti C, Peckham HE, Barker M. Whole methylome analysis by ultra-deep sequencing using two-base encoding. PLoS One. 2010;5:e9320. doi: 10.1371/journal.pone.0009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne SJ, Sullivan LS, Koboldt DC, Ding L, et al. Identification of disease-causing mutations in autosomal dominant retinitis pigmentosa (adRP) using next-generation DNA sequencing. Invest Ophthalmol Vis Sci. 2011;52:494–503. doi: 10.1167/iovs.10-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braslavsky I, Hebert B, Kartalov E, Quake SR. Sequence information can be obtained from single DNA molecules. Proc Natl Acad Sci USA. 2003;100:3960–3964. doi: 10.1073/pnas.0230489100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright LA, Burgess SC, Chowdhary B, Swiderski CE, McCarthy FM. Structural and functional-annotation of an equine whole genome oligoarray. BMC Bioinforma. 2009;11:S8. doi: 10.1186/1471-2105-10-S11-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buermans HP, Ariyurek Y, van Ommen G, et al. New methods for next generation sequencing based microRNA expression profiling. BMC Genomics. 2010;11:716. doi: 10.1186/1471-2164-11-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DJ. Human Disease: Next-generation sequencing of the next generation. Nat Rev Genet. 2011;12:78–79. doi: 10.1038/nrg2943. [DOI] [PubMed] [Google Scholar]
- Burt D, (2006) The Chicken Genome. In: Vertebrate Genomes, Volff J-N (ed). Genome Dyn. Karger 2:123–137 [DOI] [PubMed]
- Chowdhary B, Raudsepp T (2006) The Horse Genome. In: Vertebrate Genomes, Volff J-N (ed). Genome Dyn. Karger 2:97–110 [DOI] [PubMed]
- Cockett N (2006) The Sheep Genome. In: Vertebrate Genomes, Volff J-N (ed). Genome Dyn. Karger, 2:79–85 [DOI] [PubMed]
- Coleman SJ, Zeng Z, Wang K, Luo S, Khrebtukova I, Mienaltowski MJ, Schroth GP, Liu J, MacLeod JN. Structural annotation of equine protein-coding genes determined by mRNA sequencing. Anim Genet. 2010;41:121–130. doi: 10.1111/j.1365-2052.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature. 2003;422:835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- Costa V, Gallo MA, Letizia F, Aprile M, Casamassimi A, Ciccodicola A. PPARG: Gene Expression Regulation and Next-Generation Sequencing for Unsolved Issues. PPAR Res. 2010;2010:409168. doi: 10.1155/2010/409168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, Angelini C, De Feis I, Ciccodicola A. Uncovering the complexity of transcriptomes with RNA-Seq. J Biomed Biotechnol. 2010;2010:853916. doi: 10.1155/2010/853916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul RA, Long JA, Zimin AV, Aslam L, Beal K, et al. Multi-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): genome assembly and analysis. PLoS Biol. 2010;8:e1000475. doi: 10.1371/journal.pbio.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dames S, Durtschi J, Geiersbach K, Stephens J, Voelkerding KV. Comparison of the Illumina Genome Analyzer and Roche 454 GS FLX for resequencing of hypertrophic cardiomyopathy-associated genes. J Biomol Tech. 2010;21:73–80. [PMC free article] [PubMed] [Google Scholar]
- Day-Williams AG, Zeggini E (2010) The effect of next-generation sequencing technology on complex trait research. Eur J Clin Invest. doi:10.1111/j.1365-2362.2010.02437.x [DOI] [PMC free article] [PubMed]
- Ding L, Wendl MC, Koboldt DC, Mardis ER. Analysis of next-generation genomic data in cancer: accomplishments and challenges. Hum Mol Genet. 2010;19:R188–R196. doi: 10.1093/hmg/ddq391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drögemüller C, Tetens J, Sigurdsson S, Gentile A, Testoni S, Lindblad-Toh K, Leeb T. Identification of the bovine Arachnomelia mutation by massively parallel sequencing implicates sulfite oxidase (SUOX) in bone development. PLoS Genet. 2010;6:e1001079. doi: 10.1371/journal.pgen.1001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin RM, Abecasis GR, Altshuler DL, The 1000 Genomes Project Consortium et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck SH, Benet-Pagès A, Flisikowski K, Meitinger T, Fries R, Strom TM. Whole genome sequencing of a single Bos taurus animal for single nucleotide polymorphism discovery. Genome Biol. 2009;10:R82. doi: 10.1186/gb-2009-10-8-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, et al. Automated DNA sequencing of the human HPRT locus. Genomics. 1990;6:593–608. doi: 10.1016/0888-7543(90)90493-E. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Magee DA, Park SD, McGettigan PA, et al. A complete mitochondrial genome sequence from a mesolithic wild aurochs (Bos primigenius) PLoS One. 2010;5:e9255. doi: 10.1371/journal.pone.0009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- Elsik CG, Tellam RL, et al. The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science. 2009;324:522–528. doi: 10.1126/science.1169588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard F, Zimmer R. Classification of ncRNAs using position and size information in deep sequencing data. Bioinformatics. 2010;26:i426–i432. doi: 10.1093/bioinformatics/btq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euskirchen GM, Rozowsky JS, Wei CL, et al. Mapping of transcription factor binding regions in mammalian cells by ChIP: comparison of array- and sequencing-based technologies. Genome Res. 2007;17:898–909. doi: 10.1101/gr.5583007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yoon JG, Li L, Yu W, Shao J, Hua D, Zheng S, Hood L, Goodlett DR, Foltz G, Lin B. The SOX2 response program in glioblastoma multiforme: an integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genomics. 2011;12:11. doi: 10.1186/1471-2164-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias-Hesson E, Erikson J, Atkins A, Shen P, Davis RW, Scharfe C, Pourmand N. Semi-automated library preparation for high-throughput DNA sequencing platforms. J Biomed Biotechnol. 2010;2010:617469. doi: 10.1155/2010/617469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouse SD, Nagarajan RP, Costello JF. Genome-scale DNA methylation analysis. Epigenomics. 2010;2:105–117. doi: 10.2217/epi.09.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F, André C, (2006) The Dog Genome. In: Vertebrate Genomes, Volff J-N (ed). Genome Dyn. Karger, 2:46–59 [DOI] [PubMed]
- Georges M, Nielsen D, Mackinnon M, Mishra A, Okimoto R, et al. Mapping Quantitative Trait Loci Controlling Milk Production in Dairy Cattle by Exploiting Progeny Testing. Genetics. 1995;139:907–920. doi: 10.1093/genetics/139.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R, Weinstock G, Rohrer G, Kappes S, Schook L, Skow L, Womack J (2002) Bovine genomic sequencing initiative Cattle-izing the human genome. Bovine sequencing white paper. pp: 1–12. http://www.genome.gov/pages/research/sequencing/seqproposals/bovineseq.pdf
- Green RD, Qureshi MA, Long JA, Burfening PJ, Hamernik DL. Identifying the Future Needs for Long-Term USDA Efforts in Agricultural Animal Genomics. Int J Biol Sci. 2007;3:185–191. doi: 10.7150/ijbs.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf WJ, Block SM. Single-molecule, motion-based DNA sequencing using RNA polymerase. Science. 2006;313:801. doi: 10.1126/science.1130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisart B, Coppieters W, Farnir F, Karim L, Ford C, et al. Positional candidate cloning of a QTL in dairy cattle: Identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res. 2001;12:222–231. doi: 10.1101/gr.224202. [DOI] [PubMed] [Google Scholar]
- Hackenberg M, Barturen G, Oliver JL, Smeth NG. A database for next-generation sequencing single-cytosine-resolution DNA methylation data. Nucleic Acids Res. 2011;39:D75–D79. doi: 10.1093/nar/gkq942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirasouliha I, Hormozdiari F, Alkan C, Kidd JM, Birol I, Eichler EE, Sahinalp SC. Detection and characterization of novel sequence insertions using paired-end next-generation sequencing. Bioinformatics. 2010;26:1277–1283. doi: 10.1093/bioinformatics/btq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TD, Buzby PR, Babcock H, Beer E, Bowers J, Braslavsky I, Causey M, et al. Single-molecule DNA sequencing of a viral genome. Science. 2008;320:106–109. doi: 10.1126/science.1150427. [DOI] [PubMed] [Google Scholar]
- Henn BM, Gravel S, Moreno-Estrada A, Acevedo-Acevedo S, Bustamante CD. Fine-scale population structure and the era of next-generation sequencing. Hum Mol Genet. 2010;19:R221–R226. doi: 10.1093/hmg/ddq403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestand MS, Klingenhoff A, Scherf M, Ariyurek Y, et al. Tissue-specific transcript annotation and expression profiling with complementary next-generation sequencing technologies. Nucleic Acids Res. 2010;38:e165. doi: 10.1093/nar/gkq602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier LW, Miller W, Birney E, Warren W, International Chicken Genome Sequencing Consortium et al. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Hong D, Park SS, Ju YS, Kim S, Shin JY, et al. TIARA: a database for accurate analysis of multiple personal genomes based on cross-technology. Nucleic Acids Res. 2011;39:D883–D888. doi: 10.1093/nar/gkq1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom SC, Spollen WG, Blake SM, Bauer BK, Springer GK, Prather RS. Transcriptional profiling of day 12 porcine embryonic disc and trophectoderm samples using ultra-deep sequencing technologies. Mol Reprod Dev. 2010;77:812–819. doi: 10.1002/mrd.21226. [DOI] [PubMed] [Google Scholar]
- Jex AR, Hall RS, Littlewood DT, Gasser RB. An integrated pipeline for next-generation sequencing and annotation of mitochondrial genomes. Nucleic Acids Res. 2010;38:522–533. doi: 10.1093/nar/gkp883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Bailey CP, Zhang S, Ladunga I. Probabilistic peak calling and controlling false discovery rate estimations in transcription factor binding site mapping from ChIP-seq. Methods Mol Biol. 2010;674:161–177. doi: 10.1007/978-1-60761-854-6_10. [DOI] [PubMed] [Google Scholar]
- Katsios C, Ziogas DE, Liakakos T, Zoras O, Roukos DH (2010) Translating Cancer Genomes Sequencing Revolution into Surgical Oncology Practice. J Surg Res. doi:10.1016/j.jss.2010.10.038 [DOI] [PubMed]
- Kedes L, Liu E, Jongeneel CV, Sutton G. Judging the Archon Genomics X PRIZE for whole human genome sequencing. Nature Genet. 2011;43:175. doi: 10.1038/ng0311-175. [DOI] [PubMed] [Google Scholar]
- Kim JI, Ju YS, Park H, Kim S, Lee S, Yi JH, Mudge J, Miller NA, et al. A highly annotated whole-genome sequence of a Korean individual. Nature. 2009;460:1011–1015. doi: 10.1038/nature08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley CB. Identification of causal sequence variants of disease in the next generation sequencing era. Methods Mol Biol. 2011;700:37–46. doi: 10.1007/978-1-61737-954-3_3. [DOI] [PubMed] [Google Scholar]
- Kuhlenbäumer G, Hullmann J, Appenzellerm S. Novel genomic techniques open new avenues in the analysis of monogenic disorders. Hum Mutat. 2011;32:144–151. doi: 10.1002/humu.21400. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LW, Zhang S, Etheridge A, Ma L, Martin D, Galas D, Wang K. Complexity of the microRNA repertoire revealed by next-generation sequencing. RNA. 2010;16:2170–2180. doi: 10.1261/rna.2225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer I, Hoffmann B, Höper D, Bruun Rasmussen T, Blome S, Strebelow G, Höreth-Böntgen D, Staubach C, Beer M. Molecular epidemiology of current classical swine fever virus isolates of wild boar in Germany. J Gen Virol. 2010;91:2687–2697. doi: 10.1099/vir.0.023200-0. [DOI] [PubMed] [Google Scholar]
- Levene MJ, Korlach J, Turner SW, Foquet M, Craighead HG, Webb WW. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–686. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
- Li R, Zhu H, Ruan J, Qian W, Fang X, et al. de novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20:265–272. doi: 10.1101/gr.097261.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Fan W, Tian G, Zhu H, He L, Cai J, et al. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463:311–317. doi: 10.1038/nature08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind C, Ferriola D, Mackiewicz K, Heron S, Rogers M, et al. Next-generation sequencing: the solution for high-resolution, unambiguous human leukocyte antigen typing. Hum Immunol. 2010;71:1033–1042. doi: 10.1016/j.humimm.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- Liu GE. Applications and case studies of the next-generation sequencing technologies in food, nutrition and agriculture. Recent Pat Food Nutr Agric. 2009;1:75–79. doi: 10.2174/1876142910901010075. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin X, Henry Song X, et al. Bos taurus genome assembly. BMC Genomics. 2009;10:180. doi: 10.1186/1471-2164-10-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Reid JG, Gonzaga-Jauregui C, Rio Deiros D, Chen DC, et al. Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N Engl J Med. 2010;362:1181–1191. doi: 10.1056/NEJMoa0908094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHugh DE, Gormley E, Park SD, Browne JA, Taraktsoglou M, O'Farrelly C, Meade KG. Gene expression profiling of the host response to Mycobacterium bovis infection in cattle. Transbound Emerg Dis. 2009;56:204–214. doi: 10.1111/j.1865-1682.2009.01082.x. [DOI] [PubMed] [Google Scholar]
- Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- Mardis ER. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008;3:133–141. doi: 10.1016/j.tig.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Mardis ER. New strategies and emerging technologies for massively parallel sequencing: applications in medical research. Genome Med. 2009;1:40. doi: 10.1186/gm40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER. The $1,000 genome, the $100,000 analysis? Genome Med. 2010;2:84. doi: 10.1186/gm205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER. A dacade’s perspective on DNA sequencing technology. Nature. 2011;470:198–203. doi: 10.1038/nature09796. [DOI] [PubMed] [Google Scholar]
- Maher CA, Kumar-Sinha C, Cao X, Kalyana-Sundaram S, et al. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerat S, Bähler J. RNA-seq: from technology to biology. Cell Mol Life Sci. 2010;67:569–579. doi: 10.1007/s00018-009-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S, Carlborg O. SNP detection and prediction of variability between chicken lines using genome resequencing of DNA pools. BMC Genomics. 2010;11:665. doi: 10.1186/1471-2164-11-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci USA. 1977;74:560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DJ, Orpana AK, Sotiriou C, Joensuu H, Stephens PJ, et al. Use of cancer-specific genomic rearrangements to quantify disease burden in plasma from patients with solid tumors. Genes Chromosomes Cancer. 2010;49:1062–1069. doi: 10.1002/gcc.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComish BJ, Hills SF, Biggs PJ, Penny D. Index-free de novo assembly and deconvolution of mixed mitochondrial genomes. Genome Biol Evol. 2010;2:410–424. doi: 10.1093/gbe/evq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan KJ, Peckham HE, Costa GL, McLaughlin SF, Fu Y, et al. Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two-base encoding. Genome Res. 2009;19:1527–1541. doi: 10.1101/gr.091868.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies- the next generation. Nat Rev Genet. 2010;1:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11:685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- Michelizzi VN, Dodson MV, Pan Z, Amaral ME, Michal JJ, McLean DJ, Womack JE, Jiang Z. Water buffalo genome science comes of age. Int J Biol Sci. 2010;6:333–349. doi: 10.7150/ijbs.6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelizzi VN, Wu X, Dodson MV, Michal JJ, Zambrano-Varon J, McLean DJ, Jiang Z. A Global View of 54,001 Single Nucleotide Polymorphisms (SNPs) on the Illumina BovineSNP50 BeadChip and their transferability to Water Buffalo. Int J Biol Sci. 2011;7:18–27. doi: 10.7150/ijbs.7.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RD, et al. Digital genotyping and haplotyping with polymerase colonies. Proc Natl Acad Sci USA. 2003;100:5926–5931. doi: 10.1073/pnas.0936399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G (1965) Cramming more components onto integrated circuits. Electronics 38
- Morin RD, Zhao Y, Prabhu AL, Dhalla N, McDonald H, Pandoh P, Tam A, Zeng T, Hirst M, Marra M. Preparation and analysis of microRNA libraries using the Illumina massively parallel sequencing technology. Methods Mol Biol. 2010;650:173–199. doi: 10.1007/978-1-60761-769-3_14. [DOI] [PubMed] [Google Scholar]
- Mote B, Rothschild M (2006) Cracking the genomic piggy bank: Identifying secrets of the pig genome. In: Vertebrate Genomes, Volff J-N (ed). Genome Dyn. Karger, 2:86–96 [DOI] [PubMed]
- Murphy W (2006) The Feline Genome. In: Vertebrate Genomes, Volff JN (ed). Genome Dyn. Karger, 2:60–68 [DOI] [PubMed]
- Nagalakshmi U, Waern K, Snyder M. RNA-Seq: a method for comprehensive transcriptome analysis. Curr Protoc Mol Biol. 2010;Chapter 4(Unit 4.11):1–13. doi: 10.1002/0471142727.mb0411s89. [DOI] [PubMed] [Google Scholar]
- Nagarajan N, Pop M. Sequencing and genome assembly using next-generation technologies. Methods Mol Biol. 2010;673:1–17. doi: 10.1007/978-1-60761-842-3_1. [DOI] [PubMed] [Google Scholar]
- Neff T, Armstrong SA. Chromatin maps, histone modifications and leukemia. Leukemia. 2009;23:1243–1251. doi: 10.1038/leu.2009.40. [DOI] [PubMed] [Google Scholar]
- Nyren, et al. Solid phase DNA minisequencing by an enzymatic luminometric inorganic pyrophosphate detection assay. Anal Biochem. 1993;208:171–175. doi: 10.1006/abio.1993.1024. [DOI] [PubMed] [Google Scholar]
- Nyren P. The history of pyrosequencing. Methods Mol Biol. 2007;373:1–14. doi: 10.1385/1-59745-377-3:1. [DOI] [PubMed] [Google Scholar]
- Ozsolak F, Kapranov P, Foissac S, Kim SW, Fishilevich E, Monaghan AP, John B, Milos PM. Comprehensive polyadenylation site maps in yeast and human reveal pervasive alternative polyadenylation. Cell. 2010;143:1018–1029. doi: 10.1016/j.cell.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F, Milos PM. Transcriptome profiling using single-molecule direct RNA sequencing. Methods Mol Biol. 2011;733:51–61. doi: 10.1007/978-1-61779-089-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Enciso M, Ferretti L. Massive parallel sequencing in animal genetics: wherefroms and wheretos. Anim Genet. 2010;41:561–569. doi: 10.1111/j.1365-2052.2010.02057.x. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Hainaut P. Next-generation sequencing: emerging lessons on the origins of human cancer. Curr Opin Oncol. 2011;23:62–68. doi: 10.1097/CCO.0b013e3283414d00. [DOI] [PubMed] [Google Scholar]
- Pontius JU, Mullikin JC, Smith DR, Lindblad-Tohet K, Team AS, et al. Initial sequence and comparative analysis of the cat genome. Genome Res. 2007;17:1675–1689. doi: 10.1101/gr.6380007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool R, Waddell K (2002) Exploring horizons for domestic animal genomics. Workshop summary. National Academic Press, Constitution Avenue, NW, Washington, D.C. (www.nap.edu). [PubMed]
- Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkarev D, Neff NF, Quake SR. Single-molecule sequencing of an individual human genome. Nat Biotechnol. 2009;27:847–850. doi: 10.1038/nbt.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AM, Crooijmans RP, Affara NA, Amaral AJ, Archibald AL, et al. Design of a high density SNP genotyping assay in the pig using SNPs identified and characterized by next generation sequencing technology. PLoS One. 2009;4:e6524. doi: 10.1371/journal.pone.0006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsingh G, Koboldt DC, Trissal M, Chiappinelli KB, et al. Complete characterization of the microRNAome in a patient with acute myeloid leukemia. Blood. 2010;116:5316–5326. doi: 10.1182/blood-2010-05-285395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond FL, Whittaker J, Jenkins L, Lench N, Chitty LS. Molecular prenatal diagnosis: the impact of modern technologies. Prenat Diagn. 2010;30:674–681. doi: 10.1002/pd.2575. [DOI] [PubMed] [Google Scholar]
- Robertson G, Hirst M, Bainbridge M, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- Rohrer G, Beever JE, Rothschild MF, Schook L, Gibbs R, Weinstock G (2002) Porcine genomic sequencing initiative. Porcine sequencing white paper. pp: 1–10. http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/PorcineSEQ021203.pdf
- Ronaghi M, Karamohamed S, Pettersson B, Uhlen M, Nyren P. Real-time DNA sequencing using detection of pyrophosphate release. Anal Biochem. 1996;242:84–89. doi: 10.1006/abio.1996.0432. [DOI] [PubMed] [Google Scholar]
- Ronaghi M, Uhlen M, Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- Rusk N. Cheap third-generation sequencing. Nat Methods. 2009;6:244–245. doi: 10.1038/nmeth0409-244a. [DOI] [PubMed] [Google Scholar]
- Sanger F. The Croonian Lecture, 1975: Nucleotide Sequences in DNA, B191 Proc. London: Royal Soc; 1975. [DOI] [PubMed] [Google Scholar]
- Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94:441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Turner S, Kasarskis A. A window into third-generation sequencing. Hum Mol Genet. 2010;19:R227–R240. doi: 10.1093/hmg/ddq416. [DOI] [PubMed] [Google Scholar]
- Schuster SC, et al. Method of the year, next-generation DNA sequencing: Functional genomics and medical applications, Nat. Methods. 2008;5:11–21. [Google Scholar]
- Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- Senapathy P, Bhasi A, Mattox J, Dhandapany PS, Sadayappan S. Targeted genome-wide enrichment of functional regions. PLoS One. 2010;5:e11138. doi: 10.1371/journal.pone.0011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Meese E, Blin N. Profiling of regulatory microRNA transcriptomes in various biological processes. a review. J Appl Genet. 2010;51:501–507. doi: 10.1007/BF03208880. [DOI] [PubMed] [Google Scholar]
- Shapiro B, Hofreiter M. Analysis of ancient human genomes: using next generation sequencing, 20-fold coverage of the genome of a 4,000-year-old human from Greenland has been obtained. BioEssays. 2010;32:388–391. doi: 10.1002/bies.201000026. [DOI] [PubMed] [Google Scholar]
- Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, et al. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309:1728–1732. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Hardy J, Traynor BJ, Houlden H. Towards a complete resolution of the genetic architecture of disease. Trends Genet. 2010;26:438–442. doi: 10.1016/j.tig.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, et al. Fluorescence detection in automated DNA sequence analysis. Nature. 1986;321:674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Soroka M, Burzynski A. Complete sequences of maternally inherited mitochondrial genomes in mussels Unio pictorum (Bivalvia, Unionidae) J Appl Genet. 2010;51:469–476. doi: 10.1007/BF03208876. [DOI] [PubMed] [Google Scholar]
- Sutton G, Liu E, and Jongeneel CV. Kedes L (2011) Archon genomics X PRIZE validation protocol. Nature Preceedings (http://precedings.nature.com/documents/5731/version/1). [DOI] [PubMed]
- Tawfik DS, Griffiths AD. Man-made cell-like compartments for molecular evolution. Nature Biotech. 1998;16:652–656. doi: 10.1038/nbt0798-652. [DOI] [PubMed] [Google Scholar]
- Turner DJ, Keane TM, Sudbery I, Adams DJ. Next-generation sequencing of vertebrate experimental organisms. Mamm Genome. 2009;20:327–338. doi: 10.1007/s00335-009-9187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell CP, Smith TPL, Matukumalli LK, Taylor JF, Schnabel RD, et al. SNP discovery and allele frequency estimation by deep sequencing of reduced representation libraries. Nat Methods. 2008;5:247–252. doi: 10.1038/nmeth.1185. [DOI] [PubMed] [Google Scholar]
- Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, Archibald AL, Haley CS, Buys N, Tally M, Andersson G, Georges M, Andersson L. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature. 2003;425:832–836. doi: 10.1038/nature02064. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vermeer S, Hoischen A, Meijer RP, Gilissen C, Neveling K, et al. Targeted next-generation sequencing of a 12.5 Mb homozygous region reveals ANO10 mutations in patients with autosomal-recessive cerebellar ataxia. Am J Hum Genet. 2010;87:813–819. doi: 10.1016/j.ajhg.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkerding KV, Dames S, Durtschi JD. Next generation sequencing for clinical diagnostics-principles and application to targeted resequencing for hypertrophic cardiomyopathy: a paper from the 2009 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2010;12:539–551. doi: 10.2353/jmoldx.2010.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CM, Giulotto E, Sigurdsson S, Zoli M, Gnerre S, et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science. 2009;326:865–867. doi: 10.1126/science.1178158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Lee MK, Casadei S, Thornton AM, Stray SM, Pennil C, Nord AS, Mandell JB, Swisher EM, King MC. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci USA. 2010;107:12629–12633. doi: 10.1073/pnas.1007983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang W, Li R, Li Y, Tian G, Goodman L, Fan W, Zhang J, et al. The diploid genome sequence of an Asian individual. Nature. 2008;456:60–65. doi: 10.1038/nature07484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Lin FM, Chang WC, Lin KY, Huang HD, Lin NS. miRExpress: analyzing high-throughput sequencing data for profiling microRNA expression. BMC Bioinforma. 2009;10:328. doi: 10.1186/1471-2105-10-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T. Next generation sequencing in functional genomics. Brief Bioinform. 2010;11:499–511. doi: 10.1093/bib/bbq018. [DOI] [PubMed] [Google Scholar]
- Wernersson R, Schierup MH, Jørgensen FG, Gorodkin J, Panitz F, et al. Pigs in sequence space: A 0.66× coverage pig genome survey based on shotgun sequencing. BMC Genomics. 2005;6:70. doi: 10.1186/1471-2164-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- Whiteford N, Skelly T, Curtis C, Ritchie ME, Löhr A, Zaranek AW, Abnizova I, Brown C. Swift: primary data analysis for the Illumina Solexa sequencing platform. Bioinformatics. 2009;25:2194–2199. doi: 10.1093/bioinformatics/btp383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann RT, Smith TP, Nonneman DJ. SNP discovery in swine by reduced representation and high throughput pyrosequencing. BMC Genet. 2008;9:81. doi: 10.1186/1471-2156-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack J. The Bovine Genome. In: Vertebrate Genomes, Volff JN (ed). Genome Dyn. Karger. 2006;2:69–78. doi: 10.1159/000095095. [DOI] [PubMed] [Google Scholar]
- Wright CF, Morelli MJ, Thébaud G, Knowles NJ, Herzyk P, Paton DJ, Haydon DT, King DP. Beyond the consensus: dissecting within-host viral population diversity of foot-and-mouth disease virus using next-generation genome sequencing. J Virol. 2011;85:2266–2275. doi: 10.1128/JVI.01396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R, Kim TM, Park PJ. Detecting structural variations in the human genome using next generation sequencing. Brief Funct Genomics. 2010;9:405–415. doi: 10.1093/bfgp/elq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamey G. Scientists unveil first draft of human genome. BMJ. 2000;321:7. doi: 10.1136/bmj.321.7252.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Shao P, Zhou H, Chen YQ, Qu LH. deepBase: a database for deeply annotating and mining deep sequencing data. Nucleic Acids Res. 2011;38:D123–D130. doi: 10.1093/nar/gkp943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin AV, Delcher AL, Florea L, Kelley DR, et al. A whole-genome assembly of the domestic cow. Bos Taurus Genome Biol. 2009;10:R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Warren ST. Neurogenetics: advancing the "next-generation" of brain research. Neuron. 2010;68:165–173. doi: 10.1016/j.neuron.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


