Abstract
GABAergic neurons of the medial septum of the basal forebrain make up a substantial portion of the septo-hippocampal pathway fibers, and are known to modulate hippocampal amino acid neurotransmission and support cognitive function. Importantly, these neurons are also implicated in age-related cognitive decline. Hypothalamic orexin/hypocretin neurons innervate and modulate the activity of these basal forebrain neurons and also provide direct inputs to the hippocampus. However, the precise role of orexin inputs in modulating hippocampal amino acid neurotransmission—as well as how these interactions are altered in aging—has not been defined. Here, orexin A (OxA) was administered to CA1 and the medial septum of young (3–4 months) and aged (27–29 months) Fisher 344 Brown Norway rats, and hippocampal GABA and glutamate efflux was analyzed by in vivo microdialysis. Following CA1 infusion of OxA, extracellular GABA and glutamate efflux was increased, but the magnitude of orexin-mediated efflux was not altered as a function of age. However, medial septum infusion of OxA did not impact hippocampal efflux in young rats, while aged rats exhibited a significant enhancement in GABA and glutamate efflux compared to young counterparts. Furthermore, immunohistochemical characterization of the medial septum revealed a significant decrease in parvalbumin (PV)-positive cell bodies in aged animals, and a significant reduction in orexin fiber innervation to the remaining GABAergic cells within the septum, while orexin innervation to the hippocampus was unaltered by the aging process. These findings indicate that: 1) OxA directly modulates hippocampal amino acid neurotransmission in young animals, 2) Aged animals show enhanced responsivity to exogenous OxA activation of the septo-hippocampal pathway, and, 3) Aged animals undergo an intrinsic reduction in medial septum PV-immunoreactivity, and a decrease in orexin innervation to remaining septal PV neurons. Alterations in orexin regulation of septo-hippocampal activity may contribute to age-related dysfunctions in arousal, learning and memory.
Keywords: Aging, hippocampus, hypocretin, microdialysis, orexin, medial septum
The hippocampal formation is crucially involved the execution of learning and memory processes. Alterations in hippocampal function and anatomy are likely contributors to age-related cognitive dysfunction (Rosenzweig and Barnes, 2003). In human patients, normal aging is associated with significant volumetric reductions in the hippocampal formation (Du et al., 2001), and rodent studies indicate population specific neuronal loss in animal models of aging (Shetty and Turner, 1998, Vela et al., 2003, Stanley and Shetty, 2004, Gavilán et al., 2007, Stanley et al., 2011).
While numerous studies have examined the effects of aging on intra-hippocampal function, alterations in extra-hippocampal input may also contribute to aging-induced hippocampal dysfunction. The septo-hippocampal pathway is comprised of cholinergic, GABAergic and glutamatergic projections from the medial septum of the basal forebrain to the hippocampus, (Frotscher and Leranth, 1985, Freund and Antal, 1988, Leranth and Frotscher, 1989, Sotty et al., 2003), and is instrumental to learning and memory as well as the maintenance of sleep-wake cycles (Lee et al., 1994, Bassant et al., 1995). Moreover, the medial septum is classically viewed as the hippocampal theta rhythm generator (Buzsaki, 2002). Theta activity is a 4–10 Hz rhythmic neuronal oscillation that occurs in the hippocampus during exploratory behavior and REM sleep, and is important for encoding of information by hippocampal place cells in learning and memory processes (Winson, 1978, Buzsaki, 2005).
Appropriately, lesion of the medial septum or septo-hippocampal fibers results in impaired performance on hippocampally mediated tasks (Becker et al., 1980, Hagan et al., 1988, Numan and Quaranta, 1990) and reductions in hippocampal theta power (Green and Arduini, 1954, Yoder and Pang, 2005). Many of the effects observed following disruption of the septo-hippocampal transduction are similar to age-related alterations in cognitive and homeostatic processes. For instance, alongside decreased performance on cognitive tasks, aging is also associated with alterations in rhythmic sleep-wake cycles and reductions in hippocampal theta power (Lamour et al., 1989, Abe and Toyosawa, 1999). Sleep cycles in the elderly are characterized by an increase in fragmented daytime naps, earlier times of sleep onset, and alterations in the timing and frequency of nighttime rapid eye movement (REM) and slow wave sleep (Miles and Dement, 1980, Bliwise, 1993, Dijk et al., 1999) compared to young counterparts.
Age-related alterations in homeostatic mechanisms, such as the disturbance in states of arousal and sleep are suggestive of hypothalamic dysfunction. Indeed, numerous studies have indicated a significant reduction in the pro-arousal peptide orexin (hypocretin) over the aging spectrum (Porkka-Heiskanen et al., 2004, Zhang et al., 2005, Brownell and Conti, 2010, Kessler et al., 2011). Orexin cell bodies are localized to the perifornical lateral hypothalamus yet influence a vast number of homeostatic and physiological behaviors, such as feeding, attention, arousal, addiction and cognition (de Lecea et al., 1998, Sakurai et al., 1998, Date et al., 1999, Jaeger et al., 2002, Telegdy and Adamik, 2002, Deadwyler et al., 2007, Aston-Jones et al., 2010, Sakurai et al., 2010), through widespread neuronal projections to regions that mediate these phenomena (Peyron et al., 1998, Date et al., 1999, Nambu et al., 1999).
Both cholinergic and GABAergic cells of the medial septum receive a dense afferent input from hypothalamic orexin projections (Eggermann et al., 2001, Wu et al., 2002, Wu et al., 2004), and orexin modulation of these projection cells is speculated to modulate arousal and hippocampal theta rhythm (España et al., 2001, Gerashchenko et al., 2001). While direct orexin projections to the hippocampus are not as robust as those to medial septum, previous studies support a significant role for orexin in performance on tasks of hippocampal-dependent cognition (Akbari et al., 2006, Akbari et al., 2007, Akbari et al., 2008) and the induction of long term potentiation [LTP;(Selbach et al., 2004, Selbach et al., 2010, Akbari et al., 2011)]. Orexin may control hippocampal neurotransmission through direct as well as transsynaptic modulation of various pathways, including the septo-hippocampal pathway, and these effects are largely undefined. Furthermore, age-related alterations in the orexin system may contribute to hippocampal dysfunction via both direct and transsynaptic mechanisms.
These studies were designed to examine the anatomical and neurochemical impact of aging on orexin modulation of hippocampal function, both directly, and by way of the medial septum, using immunohistochemistry and in vivo microdialysis. The hypothalamic neuropeptide orexin A (OxA) was infused into either CA1 or the medial septum while simultaneously measuring extracellular levels of hippocampal glutamate and GABA efflux in young and old rats. Moreover, orexin innervation of CA1 and the medial septum was assessed as a function of age in order to determine the impact of orexin modulation of hippocampal function over the course of normal aging.
Experimental procedures
Animals
All animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina. Young (3–5 month) and aged (26–30 month) male, Fisher 344 Brown Norway F1 hybrid rats (National Institute of Aging breeding colony; Baltimore, MD, USA) were fed standard rat chow ad libitum and kept at a 12:12 light-dark cycle in a climate controlled facility. All in vivo experiments were conducted during the light cycle.
In vivo microdialysis
Under sodium pentobarbital anesthesia (60–70 mg/kg) all rats received unilateral implantation of guide cannulae (Bioanalytical Systems, Inc. (BAS); West Lafayette, IN, USA) in the caudal hippocampus in the following coordinates relative to Bregma: Young – anterior −5.2 mm, lateral +3.8 mm at 10° angle, ventral −3.6 mm; Aged – anterior −5.6 mm, lateral +4.0 mm at 10° angle, ventral −3.8 mm. A subset of rats received a second guide cannulae in the medial septum at the following coordinates relative to Bregma: Young, – anterior +0.2 mm, lateral +1.0 mm at 8° angle, ventral −5.5 mm; Aged, – anterior +0.2 mm, lateral +1.0 mm at 8° angle, ventral −5.6 mm. After a two day recovery period following stereotaxic surgery, rats were habituated to the microdialysis bowls for three consecutive days prior to the onset of microdialysis sampling. On the morning of each dialysis session, stylets were removed and replaced with probes (BAS, 30kDa cutoff) extending 2 mm beyond the ventral tip of guide cannulas. Probes were continuously perfused at 2 μl/min with artificial cerebrospinal fluid (aCSF; [in mM] NaCl 150, KCl 3.0, CaCl2 1.7, MgCl2 0.9, d-glucose 4.9, pH 6.9). Neostigmine (50 nM) was included in hippocampal aCSF (Fadel et al., 2005). Microdialysate collection began 3 hr after probe insertion and consisted of 11 collections in 15 minute intervals. During collections 5 through 8 the microdialysis inlet line in either the medial septum or hippocampus was switched to an aCSF solution containing either vehicle (aCSF), low orexin A (OxA; 0.1 μM; Bachem Americas, Inc.; Torrance, CA; product No. H-4172) or high OxA (10 μM). Most animals were tested under all three conditions. Dialysates were stored at −80°C until analysis for amino acids could be carried out by liquid chromatography. At the conclusion of dialysis sessions animals were sacrificed, and brains were removed. Probe placement was assessed using an acetylcholinesterase background stain. Animals with probe tracts outside of the target region were excluded from results.
Microdialysis samples were analyzed by liquid chromatography with electrochemical detection (Fadel et al., 2005, Reznikov et al., 2007). After pre-column o-phthaldidialdehyde/beta-mercaptoethanol derivatization, glutamate and GABA were separated on a Unijet microbore 3 μm CI8 column (BAS) using a mobile phase consisting of 0.1 M NaH2PO and 28.5% methanol (pH 6.4) and detected at a glassy carbon electrode (+700 mV). Amino acid quantification was accomplished by comparison of peak areas with a daily three-point standard curve defining the expected range of glutamate and GABA values. Following quantification of glutamate and GABA, basal values of neurotransmitter release were defined by averaging values during pre-drug collections (microdialysis fractions 1–4). Values were then expressed as percent baseline to account for variability in basal efflux across sessions and between subjects. Microdialysis data were uncorrected for probe recovery.
Immunohistochemistry
All tissue was processed according to previously described protocols (Frederick-Duus et al., 2007, Reznikov et al., 2008). Briefly, a separate group of experimentally-naïve rats were deeply anesthetized using isoflurane and transcardially perfused with 0.1M phosphate buffered saline and 4% paraformaldehyde. Whole brains were removed and post fixed overnight followed by cryoprotection in a 30% sucrose/0.1 M phosphate buffer solution. Tissue was coronally sectioned (45 μm thickness) on a cryostat using a 1:5 serial sectioning method (yielding 5 sets of tissue with adjacent sections 225 μm apart).
Free floating medial septum or hippocampal sections were incubated with a rabbit anti-OxA antibody (1:1000; Calbiochem; Darmstadt, Germany; product No. PC 362) for 24 hours at room temperature (RT). This step was followed by incubation in biotinylated donkey anti-rabbit secondary (Jackson ImmunoReasearch Inc.; product No. 7111-065-152) for 1.5 hours at RT, and horseradish peroxidase conjugated streptavidin (1:1,600; Jackson ImmunoResearch Laboratories Inc.; product No. 016-030-084) for 1 hour at RT. Immunoreactivity was developed using nickel sulfate-cobalt chloride intensified diaminobenzidine with hydrogen peroxide, yielding blue-black immunopositive cells. Tissue was then incubated in mouse anti-GAD67, mouse anti-Ca2+/calmodulin-dependent protein kinase II (CaMKII; 1:750; Upstate; Lake Placid, NY; Product No. 05-532), or mouse anti-PV overnight at RT followed by incubation in unlabeled donkey anti-mouse (1:200; Jackson ImmunoResearch Inc.; product No. 715-005-150; 2 hrs RT) and mouse peroxidase anti-peroxidase (1:500; Covance; Berkeley, CA; product Nos. SMI-450L; 1.5 hrs RT). Nickel-cobalt was omitted to yield a brown immunopositive cytoplasmic staining for PV+, CaMKII+ and GAD67+ cells.
Cell counts
Using Neurolucida software (MicroBrightField Inc.; Williston, VT), medial septum neurons and appositional counts were confined to an approximately 5 mm2 area and counted in 3 serial sections [approximately 0.7–0.2 mm anterior to Bregma (Paxinos and Watson, 1998)] and cell counts conducted at 20x magnification. Results were averaged to form a representative sample of all cell bodies and appositions within a single slice and a total of 8 young and 8 aged rats were included in data sets. Medial septum appositions were counted by randomly selecting 10 PV neurons positively labeled for the brown cytoplasmic stain at 10x magnification in an unfocused plane in order to obscure fiber apposition labeling, and refocusing at 40x to count apparent appositions of OxA fibers onto selected cells. For hippocampal studies of orexin appositions onto pyramidal cells and interneurons, CaMKII counts were conducted similar to medial septum counts, while GAD67+ neurons were categorized by layer [stratum oriens (SO), stratum pyramidale (SP), stratum radiatum (SR), stratum lacunosum moleculare (SLM)]. Sections containing dorsal hippocampus [approximately 3.14-3.6 mm caudal to Bregma, (Paxinos and Watson, 1998)] were selected for analysis, and counts were restricted to CA1b and CA1c. Each selected neuron was analyzed through the entire z-axis of the cytoplasmic labeling to determine the number of orexin appositions onto medial septum and hippocampal neurons. A point was counted as an apparent apposition if the overlapping brown cytoplasmic stain and black fiber labeling were in the same field of focus.
Statistical analysis
All cell counts were analyzed using an independent samples t-test for significant main effects. All in vivo microdialysis data were expressed as a percentage of mean baseline values for each animal. Because some animals were not able to complete all 3 microdialysis sessions in which the different doses of OxA were tested (due to issues such as bleeding in the vicinity of the probe tip during microdialysis sampling) data were analyzed using 2 separate ANOVAs—a TIME × DOSE repeated measures ANOVA for each age group, followed by a one way ANOVA post hoc on the means contributing to significance, and a TIME × AGE repeated measures ANOVA for each dose, with an independent samples t-test post hoc on the means contributing to the significance of the ANOVAs. Significant main effects were defined by P < 0.05 and all statistical analysis was performed in SPSS for windows (V.17.0, SPSS Inc.; Chicago, IL).
Results
Orexin innervation to CA1 is unaltered by aging
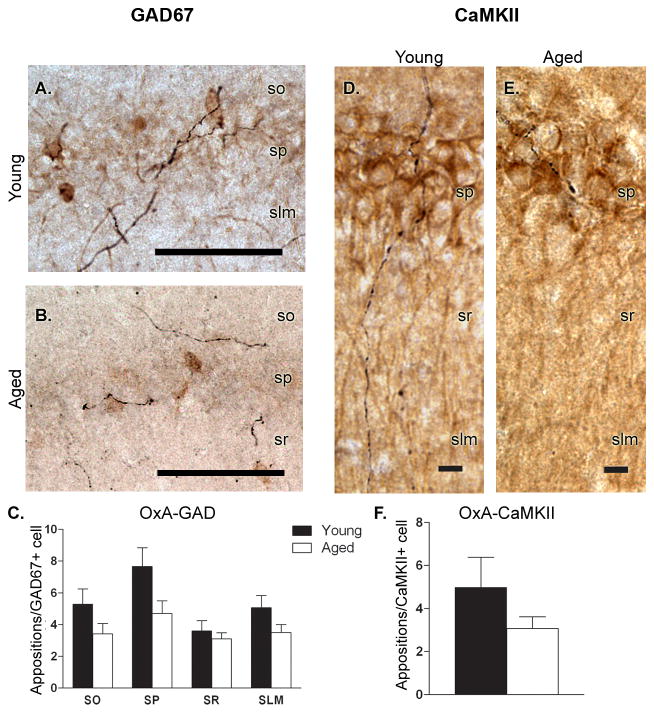
Double-labeled immunohistochemical techniques were employed to investigate orexin innervation of the two major cell types in CA1; GAD67+ cells, which encompass all subtypes of GABAergic inhibitory interneurons in this area, and CaMKII+ excitatory pyramidal cells (Fig. 1). Appositional counts for each marker revealed a decrease in OxA fiber innervation in the aged population that did not reach levels of statistical significance (Fig. 1). Specifically, GAD67 neurons in the pyramidal layer of aged rats displayed a trend for a decrease (t13 = 1.985, P = 0.069) with young animals receiving approximately 7.6 ± 1.2 orexin appositions/interneuron compared to the 4.9 ± 0.8 orexin appositions/neuron observed in aged rats. All other layers failed to exhibit any trends (P > 0.1) for reduced orexin innervation to interneurons within that lamina.
Figure 1. Orexin innervation of CA1 is unaltered as a function of age.
A,B) Double-label immunohistochemistry reveals orexin fibers (black) spanning all layers of the hippocampus and making apparent appositional contacts with GAD67+ interneurons (brown cell bodies) in both young (A) and aged (B) rats. C) A trend toward reduced orexin innervation of GAD67+ interneurons in stratum pyramidale (SP) was observed in aged rats, while interneurons in the remaining lamina were unaffected by age. D,E) Double-label immunohistochemistry for orexin fibers (black) and CaMKII+ pyramidal cells (brown) in young (D) and aged (E) rats. F) Orexin innervation of CaMKII+ pyramidal cells was unaffected by age. n = 8 young, 7 aged (SO-stratum oriens; SP- stratum pyramidale; SR-stratum radiatum; SLM-stratum lacunosum moleculare). Scale bars = approximately 100 μm (A,B) and 10 μm (D,E).
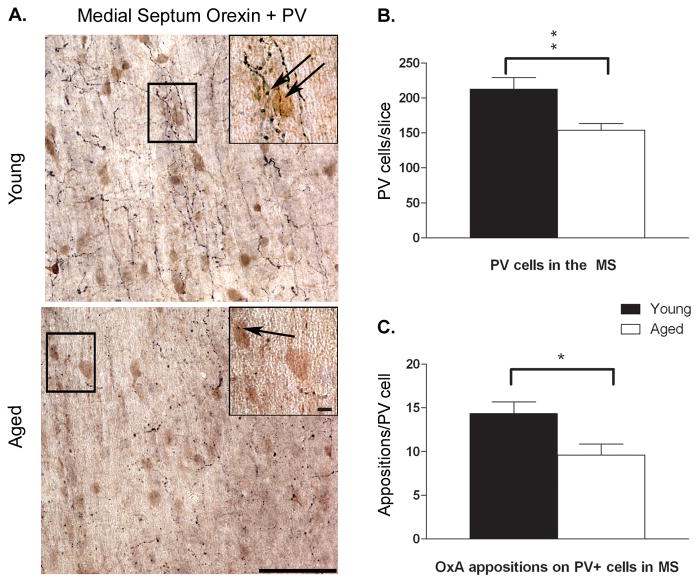
Numbers, and orexin innervation, of medial septum PV-IR cells is decreased in aging
Parvalbumin (PV) is a calcium-binding protein that marks GABAergic septohippocampal projection neurons. Double labeled immunohistochemical techniques were utilized to determine the effects of aging on the overall number of PV-IR, GABAergic projection cells, as well as orexin innervation to PV-containing neurons in the medial septum (Fig. 2A). Analysis of PV+ cells indicated a significant decrease (t14 = 3.125, P = 0.007) in immunoreactivity in aged rats (Fig. 2B), with young rats averaging 213 ± 16 cells per slice compared to the aged rat average of 154 ± 9 cells per slice. In addition to this decrease in the number of PV-IR neurons, aged animals showed significant reductions in apparent appositions formed by OxA varicosities onto PV- immunopositive medial septum cells (Fig. 2C; t14 = 2.587, P = 0.022). Young rats received approximately 14.4 ± 1.4 orexin appositions per PV+ cell versus the 9.6 ± 1.2 orexin appositions per PV+ cell observed in aged rats.
Figure 2. The number of parvalbumin-positive cells and orexin appositions in medial septum decreases as a function of age.
A) Double-label immunohistochemistry for orexin fiber innervation (black fibers) of PV+ neurons (brown cells) in the medial septum in young (top) and aged (bottom) tissue. B,C) PV+ cells were significantly reduced with aging process, as were orexin appositions onto PV+ cells (indicated black arrows in panel A). Black boxes in figure A indicate areas corresponding to inset images. Scale bar = approximately 100 μm. * P < 0.05, ** P < 0.01; n = 8 young, 8 aged.
Direct infusion of OxA differentially affects GABA efflux across the aging spectrum
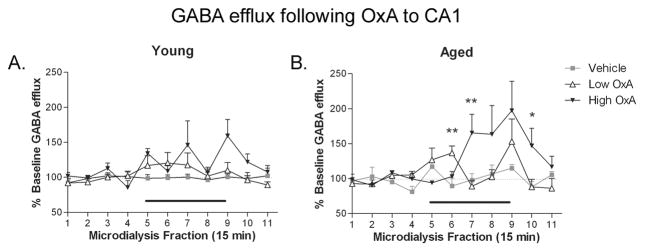
Local infusion of exogenous OxA via reverse microdialysis increased hippocampal GABA release in CA1 in both young and aged rats (Fig. 3). At both ages, DOSE × TIME ANOVAs revealed a significant effect of TIME (young: F(10,250) = 3.428, P = 0.001; aged: F(10,210) = 4.024 P ≤ 0.0001), indicating that hippocampal GABA was changing over the course of the dialysis session. A DOSE × TIME effect was evident in the aged group (Fig. 3B; F(20,210) = 2.245; P = 0.002) with collections 6, 7 and 10 exhibiting significant increases in GABA efflux (F(2,24) ≥5.685; P ≤ 0.039). Additionally, a strong trend for a DOSE × TIME interaction was observed in the young group (Fig. 3A; F(20,250) = 1.527; P = 0.073), but the oscillatory effects of the 10 μM dose of OxA on GABAergic efflux, which were not observed in aged rats, likely prevented this effect from reaching statistical significance.
Figure 3. Effects of hippocampal OxA administration on local GABA efflux.
CA1 infusion of OxA via reverse microdialysis (black bar over collections 5–8) dose-dependently increased GABA efflux in aged rats (B), while young rats (A) displayed only a dose-dependent trend (P = 0.073) for increased GABA efflux. Importantly, OxA-mediated GABA efflux following CA1 infusion was not altered as a function of age. * P < 0.05 vs. vehicle; ** P ≤ 0.01 vs. vehicle; n = 8–11 (young), 7–9 (aged).
TIME × AGE ANOVAs comparing OxA doses indicated an effect of TIME in both low and high OxA groups (low OxA: F(20,250) = 1.527, P = 0.073; high OxA: F(10,140) = 4.679, P ≤ 0.0001), while no TIME × AGE effects were observed. This finding indicates that both groups maintained similar responsivity to exogenous OxA following direct infusion into CA1.
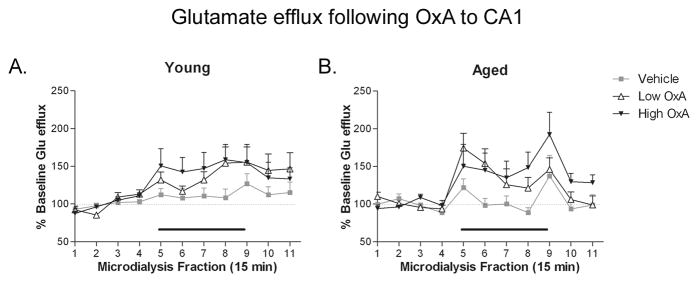
Direct CA1 infusion of OxA modulates glutamate efflux regardless of age
Infusion of exogenous OxA increased hippocampal glutamate release upon direct infusion into CA1 in both young and aged rats (Fig. 4). At both ages, DOSE × TIME ANOVAs revealed a significant effect of TIME (young: F(10,250) = 8.719, P ≤ 0.0001; aged F(10,210) = 6.146; P ≤ 0.0001), indicating that hippocampal glutamate efflux was changing over the course of the dialysis session. Conversely, no significance was found in DOSE × TIME analysis. Analysis of TIME × AGE ANOVAs revealed a significant effect of TIME at all three doses (vehicle: F(10,130) = 2.136, P = 0.026; low OxA: F(10,180) = 6.249, P ≤ 0.0001; high OxA: F(10,150) = 6.366, P ≤ 0.0001), indicating alterations in glutamate efflux over the duration of the dialysis session at all doses. Additionally, a TIME × AGE interaction was observed in the low OxA group (Fig. 4D; F(10,182) = 3.293; P < 0.0001), but post hoc analysis failed to reveal the point(s) of significance in the mean(s) contributing to the significance of the ANOVA.
Figure 4. Effects of hippocampal OxA administration on local glutamate efflux.
CA1 infusion of OxA via reverse microdialysis (black bar over collections 5–8) to young (A) and aged (B) rats produced a general trend for increased glutamate efflux, but this effect did not achieve statistical significance. The magnitude of OxA induced glutamate efflux was not altered as a function of age. n = 8–11 (young), 7–9 (aged).
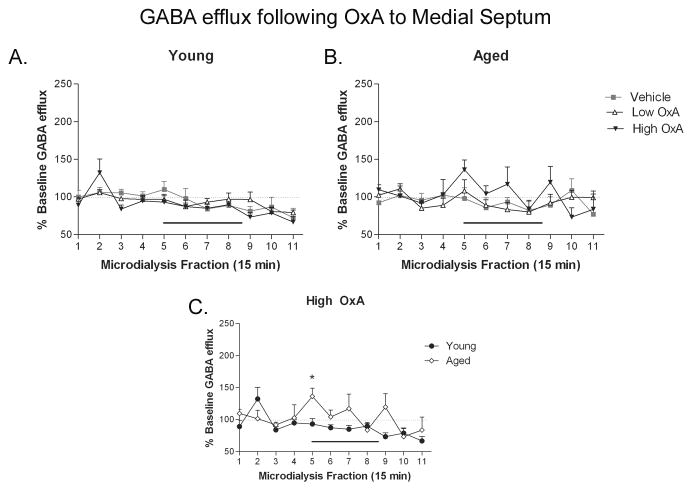
Medial septum OxA infusion increases hippocampal GABA in aged, but not young rats
Septal infusion of OxA produced differential effects on hippocampal GABA efflux as a function of age. At both ages, DOSE × TIME ANOVAs revealed a significant effect of TIME (young: F(10,160) = 6.502, P ≤ 0.0001; aged F(10,210) = 2.168; P = 0.021), indicating that GABA efflux was changing over the course of the dialysis session (Fig. 5). The changes observed in young animals (Fig. 5A) were likely the result of the steady depression of extracellular GABA efflux over the duration of the dialysis session, which was unaffected by OxA infusion. Conversely, in aged animals (Fig. 5B), the 10 μM OxA dose elicited an oscillatory GABA pattern similar to that observed in the young group following CA1 infusion of OxA (Fig. 5A). This oscillatory pattern started at the onset of infusion and continued across the duration of the dialysis session. Comparison of the high dose of OxA in young and aged groups (Fig. 5C) revealed a significant TIME × AGE interaction (F(10,130) = 2.032; P = 0.035). Post hoc evaluation of the points contributing to the significance of the ANOVA indicated a significant increase in GABA efflux in the aged group at the initial onset of OxA infusion (collection 5; t13 = 2.697; P = 0.018).
Figure 5. Delivery of OxA to medial septum differentially affects CA1 GABA efflux in young and aged rats.
OxA infusion (black bar over collections 5–8) into the medial septum via reverse microdialysis in young (A) and aged (B) rats did not alter hippocampal GABA efflux. C) However, infusion of the high (10 μM) dose of OxA yielded a significantly greater degree of GABA efflux in aged (open diamonds, ◇) compared to young rats (closed circles, ●) at the onset of OxA infusion (collection 5). * P < 0.05; n = 6–7 (young), 8 (aged).
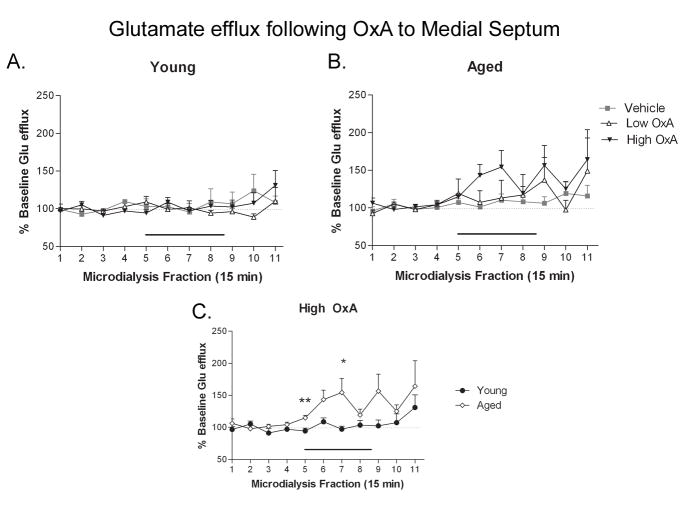
Medial septum OxA infusion increases hippocampal glutamate in aged, but not young rats
Septal infusion of OxA produced alterations in hippocampal glutamate efflux as a function of age. Medial septum infusion of OxA had no effect on hippocampal glutamate release in the young group (Fig. 6A). Conversely, the 2-way TIME × DOSE ANOVA revealed a significant effect of TIME in the aged population (Fig. 6B; F(10,220) = 3.144; P = 0.001), indicating differential glutamate efflux as a function of age. Analysis of the dose-specific effects of medial septum OxA infusion on hippocampal glutamate using a TIME × AGE ANOVA showed a significant effect of TIME (F(10,140) = 2.811; P =0.003) at the high dose (Fig. 6C).
Figure 6. Delivery of OxA to medial septum differentially affects CA1 glutamate efflux in young and aged rats.
OxA infusion (black bar over collections 5–8) into the medial septum via reverse microdialysis in young (A) and aged (B) rats did not alter hippocampal glutamate efflux. C) Infusion of the high (10 μM) dose of OxA generated a significantly greater degree of glutamate efflux in aged (open diamonds, ◇) compared to young rats (closed circles, ●) during collections 5 and 7. * P < 0.05, ** P < 0.01. n = 6–7 (young), 8 (aged).
Although the ANOVA yielded no signs of a TIME × AGE interaction at the high dose of OxA (F(10,140) = 1.092; P =0.373), a trend for a main effect of AGE was observed (F(1,14) = 4.388; P = 0.055). 10 μM OxA to the medial septum induced significant increases in hippocampal glutamate efflux in aged rats (Fig. 6C; t14 ≥ 2.261, P ≤ 0.04) starting at the initial onset of OxA application and continuing throughout the microdialysis session.
Basal efflux
Aged animals exhibited significant decreases in basal hippocampal GABA efflux (aged, 0.032 ± 0.005 μM vs. young, 0.081 ± 0.013 μM; t38 = 3.477; P = 0.001; data not shown). Aging was not associated with differences in basal hippocampal glutamate levels (0.592 ± 0.085 μM and 0.555 ± 0.067 μM in young and aged rats, respectively).
Discussion
We found that aging is associated with a reduction in PV projection cells of the septo-hippocampal pathway and a further reduction in orexin innervation to the remaining GABAergic projection neurons of the medial septum. Moreover, glutamate and GABA efflux were enhanced by medial septum administration of OxA in aged rats compared to young rats, while local infusion of the peptide was unaltered as a function of age. Taken together, these data implicate alterations in orexin signaling as a contributor toward age-related hippocampal dysfunction.
The orexin system is crucial for regulating vigilance (Nishino, 2007) and cognitive performance on attentional tasks (Boschen et al., 2009, Fadel and Burk, 2010). While the role of orexins in the hippocampal formation remains to be fully defined, prior work suggests a modulatory influence of this neuropeptide on hippocampal synaptic plasticity. In a study conducted by Selbach et al. (2004) bath application of OxA induced stimulus-independent LTP of Schaffer collateral synapses (LTPox) that required activation of glutamatergic and GABAergic receptors. Accordingly, in vivo electrophysiological recordings have demonstrated an enhancement of LTP in the dentate gyrus following OxA treatment (Wayner et al., 2004), which can be abolished by pretreatment with the orexin 1 receptor (Ox1R) antagonist, SB-334867 (Wayner et al., 2004, Akbari et al., 2011). Furthermore, blockade of Ox1R signaling in the hippocampus results in impaired performance on tests of spatial learning and memory and passive avoidance (Akbari et al., 2006, Akbari et al., 2007, Akbari et al., 2008). These studies suggest that direct (local) modulation of hippocampal function by orexin is primarily mediated by Ox1R, and the alterations in orexin regulation of hippocampal glutamate and GABA release that we observed in aged animals are likely to have functional behavioral correlates.
While local infusion of orexin tended to increase GABA and glutamate efflux in area CA1, we did not observe any effect of medial septum administration of OxA on hippocampal amino acid efflux. Importantly, this finding does not imply that OxA does not activate the GABAergic component of the septo-hippocampal pathway. Prior work has shown that OxA directly activates PV cells in septal slices (Wu et al., 2002), and hippocampal theta rhythms are completely ablated following Ox2R-saporin lesions of the medial septum (Gerashchenko et al., 2001), observations which predict that medial septum orexin administration should increase septo-hippocampal GABA release. However, GABAergic projections from the medial septum tend to synapse preferentially on GABAergic interneurons in the hippocampus (Freund, 1992). Thus, orexin activation of septo-hippocampal GABAergic neurons may produce a “zero-sum” net effect on hippocampal GABA efflux in young animals as measured by in vivo microdialysis. In this case, the significant increase in hippocampal GABA efflux following medial septum OxA application in old rats may reflect the significant age-related reduction in hippocampal GABAergic interneurons (Stanley et al., 2011).
An interesting oscillatory fluctuation in GABA release was noted in young animals following exposure to the 10 μM OxA dose directly into CA1. While the relatively poor temporal resolution of microdialysis negates the correlation of this phenomenon with better-characterized electrophysiological oscillations (e.g. theta or delta rhythms), it may reflect a type of long-term phase-locking neuronal firing pattern. Interestingly, the aging hippocampus was devoid of this oscillatory GABAergic pattern elicited by high OxA infusion. This could be the result of neuronal loss in both regions since the medial septum and hippocampus are reciprocally connected via the septo-hippocampal axis (Toth and Freund, 1992, Gulyas et al., 2003). It has also been suggested that age-related decreases in rhythmic bursting activity by septohippocampal neurons may stem from the loss of external synchronizing inputs (Apartis et al., 2000). The functional contribution of orexin to such as phenomenon is unestablished but may be significant given its role in arousal and cognitive function. GABAergic interneurons in stratum oriens (SO) project both locally within the hippocampal formation, and send inhibitory long-range efferents to the basal forebrain where they synapse preferentially on GABAergic neurons (Toth et al., 1993). In a recent study from our lab, SO interneurons were found to be particularly vulnerable to aging-induced neurodegeneration, with losses of 20% relative to young animals (Stanley et al., 2011). Thus, the significant reduction in both PV-containing medial septum projection cells and SO interneurons likely disrupts normal connectivity of the septo-hippocampal axis.
While the oscillatory producing effects of hippocampal OxA peptide infusion were absent in old rats, the magnitude of orexin-mediated GABA and glutamate efflux was unaffected as a function of age. This finding was unexpected due to the reports of decreased Ox1- and Ox2R mRNA in the hippocampus of old C57BL/6 mice (Terao et al., 2002), which would predict reductions in orexin-mediated hippocampal neurotransmitter efflux. Alongside reductions in orexin receptor mRNA, stereological assessment of orexin neurons conducted in our laboratory in the same strain and age of rats have indicated a greater than 40% reduction in orexin neuron number (Kessler et al., 2011). The similarity in magnitude between the loss of orexin neurons and the reduction in innervation of the medial septum reported here suggests a lack of compensatory sprouting from residual orexin neurons that project to this region. This finding is reinforced by reports of reduced peptide expression, as well as neuron and fiber immunoreactivity across the course of normal aging (Porkka-Heiskanen et al., 2004, Zhang et al., 2005, Brownell and Conti, 2010). Within the current studies, orexin fiber innervation to the medial septum was reduced in aged rats, while orexin innervation to the hippocampus was largely unaffected by the aging process. Anatomical analysis of orexin populations has indicated dense interconnectivity between the basal forebrain and hypothalamus, while orexin fibers directed to the hippocampus are much less dense (Peyron et al., 1998, Date et al., 1999, Nambu et al., 1999). Regardless of the degree of fiber innervation, infusion of OxA directly into CA1 effectively altered extracellular hippocampal amino acid concentrations, providing a functional implication for a pathway that is largely undefined.
The significant enhancement in extracellular amino acid efflux following OxA activation of the septo-hippocampal pathway in aged rats is likely a reflection of anatomical changes in both the medial septum and hippocampus described above. A decrease in both PV-projection cells in the medial septum (Han et al., 2002), and CA1 interneurons has been a well-documented aspect of normal aging (Shetty and Turner, 1998, Vela et al., 2003, Stanley and Shetty, 2004, Stanley et al., 2011). Since the net effect of PV projections of the septo-hippocampal pathway is to disinhibit the hippocampus (Freund and Antal, 1988, Toth et al., 1997, Wu et al., 2002), reductions in both GABAergic medial septum projection cells and hippocampal targets (hippocampal interneurons) may result in loss of disinhibition, and cause overall increases in inhibition. Moreover, GABAergic neurons of the medial septum project locally (Borhegyi et al., 2004) and modulate the firing of cholinergic cells. Since cholinergic cells of the medial septum project to hippocampal interneurons and pyramidal cells indiscriminately (Frotscher and Leranth, 1985, Leranth and Frotscher, 1989), the projections of the septo-hippocampal pathway that mediate both excitation and inhibition of the hippocampus are left unchecked.
Importantly, in the current study, basal levels of extracellular GABA are significantly reduced in aged rats. Thus, even when OxA produced maximal effects of GABA release in aged animals, the concentration of extracellular GABA did not exceed basal levels of young animals. Basal levels of glutamate, however, remained constant over the aging spectrum. Therefore, the increase in glutamate efflux observed following OxA to the medial septum of aged animals reflects an overall trend toward enhancement of excitatory neurotransmission.
The septo-hippocampal pathway is instrumental in normal learning and memory processes, and in the maintenance of normal sleep-wake cycles. Disruptions in cognition, hippocampal theta frequency (Lamour et al., 1989, Abe and Toyosawa, 1999), and sleep-wake cycles (Miles and Dement, 1980, Bliwise, 1993, Dijk et al., 1999) are evident over the course of normal aging, possibly on account of septo-hippocampal pathway disruption. Our data provide neurochemical evidence for both a direct and transsynaptic role in the modulation of hippocampal neurochemistry by the hypothalamic neuropeptide OxA.
Conclusion
Collectively, these studies show a pharmacologically-induced increase in hippocampal GABA and glutamate in young rats by the neuropeptide OxA through direct, but not transsynaptic mechanisms. Moreover, within aged animals, this orexin mediated hippocampal neurotransmission is significantly enhanced following OxA to the medial septum, presumably through alterations in GABAergic cells within the septo-hippocampal pathway and CA1. These data implicate a dysregulation in orexin modulation of GABA and glutamate tone in the aged hippocampus as a potential contributor to age-related disruptions in sleep-wake patterns and hippocampal theta rhythms. These mechanisms may serve as contributing factors in the manifestation of age-related deficits in cognitive and homeostatic processes.
Highlights.
Orexin/hypocretin inputs to the medial septum and hippocampus modulate behavioral arousal and performance on hippocampal-dependent cognitive tasks
Aging is associated with reduced orexin/hypocretin innervation of medial septum and hippocampus
Aged rats showed alterations in orexin/hypocretin modulation of hippocampal glutamate and GABA efflux
Changes in orexin/hypocretin signaling may contribute to age-related impairments in arousal and memory consolidation
Acknowledgments
This work was supported in part by National Institutes of Health R01AG030646 (JF).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- CA1
cornu ammonis 1
- CaMKII
calcium calmodulin kinase
- GABA
gamma-aminobutyric acid
- GAD67
glutamic acid decarboxylase 67
- LTP
long term potentiation
- Ox1R
orexin 1 receptor
- Ox2R
orexin 2 receptor
- OxA
orexin A
- PV
parvalbumin
- REM
rapid eye movement
- RT
room temperature
- SLM
stratum lacunosum moleculare
- SO
stratum oriens
- SP
stratum pyramidale
- SR
stratum radiatum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Toyosawa K. Age-Related Changes in Rat Hippocampal Theta Rhythms: A Difference between Type 1 and Type 2 Theta. J Vet Med Sci. 1999;61:543–548. doi: 10.1292/jvms.61.543. [DOI] [PubMed] [Google Scholar]
- Akbari E, Motamedi F, Davoodi FG, Noorbakhshnia M, Ghanbarian E. Orexin-1 receptor mediates long-term potentiation in the dentate gyrus area of freely moving rats. Behav Brain Res. 2011;216:375–380. doi: 10.1016/j.bbr.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Akbari E, Motamedi F, Naghdi N, Noorbakhshnia M. The effect of antagonization of orexin 1 receptors in CA1 and dentate gyrus regions on memory processing in passive avoidance task. Behav Brain Res. 2008;187:172–177. doi: 10.1016/j.bbr.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Akbari E, Naghdi N, Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav Brain Res. 2006;173:47–52. doi: 10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Akbari E, Naghdi N, Motamedi F. The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides. 2007;28:650–656. doi: 10.1016/j.peptides.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Apartis E, Poindessous-Jazat F, Epelbaum J, Bassant MH. Age-related changes in rhythmically bursting activity in the medial septum of rats. Brain Res. 2000;876:37–47. doi: 10.1016/s0006-8993(00)02571-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassant MH, Apartis E, Jazat-Poindessous FR, Wiley RG, Lamour YA. Selective immunolesion of the basal forebrain cholinergic neurons: effects on hippocampal activity during sleep and wakefulness in the rat. Neurodegeneration. 1995;4:61–70. doi: 10.1006/neur.1995.0007. [DOI] [PubMed] [Google Scholar]
- Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Res. 1980;200:307–320. doi: 10.1016/0006-8993(80)90922-1. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Varga V, Szilagyi N, Fabo D, Freund TF. Phase Segregation of Medial Septal GABAergic Neurons during Hippocampal Theta Activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen K, Fadel J, Burk J. Psychopharmacology. 2. Vol. 206. Springer; Berlin/Heidelberg: 2009. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats; pp. 205–213. [DOI] [PubMed] [Google Scholar]
- Brownell SE, Conti B. Age- and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neuroscience Letters. 2010;472:29–32. doi: 10.1016/j.neulet.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Freund TF. GABAergic septal and serotonergic median raphe afferents preferentially innervate inhibitory interneurons in the hippocampus and dentate gyrus. Epilepsy Res Suppl. 1992;7:79–91. [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Gavilán MP, Revilla E, Pintado C, Castaño A, Vizuete ML, Moreno-Gonzálezn I, Baglietto-Vargas D, Sánchez-Varo R, Vitorica J, Gutiérrez A, Ruano D. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem. 2007;103:984–996. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Salin-Pascual R, Shiromani PJ. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913:106–115. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal elecetrical activity in arousal. Journal of Neurophysiology. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Salamone JD, Simpson J, Iversen SD, Morris RG. Place navigation in rats is impaired by lesions of medial septum and diagonal band but not nucleus basalis magnocellularis. Behav Brain Res. 1988;27:9–20. doi: 10.1016/0166-4328(88)90105-2. [DOI] [PubMed] [Google Scholar]
- Han SH, McCool BA, Murchison D, Nahm SS, Parrish AR, Griffith WH. Single-cell RT-PCR detects shifts in mRNA expression profiles of basal forebrain neurons during aging. Brain Res Mol Brain Res. 2002;98:67–80. doi: 10.1016/s0169-328x(01)00322-9. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Farr SA, Banks WA, Morley JE. Effects of orexin-A on memory processing. Peptides. 2002;23:1683–1688. doi: 10.1016/s0196-9781(02)00110-9. [DOI] [PubMed] [Google Scholar]
- Kessler BA, Stanley EM, Frederick-Duus D, Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience. 2011;178:82–88. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour Y, Bassant MH, Robert A, Joly M. Septo-hippocampal neurons in the aged rat: relation between their electrophysiological and pharmacological properties and behavioral performances. Neurobiol Aging. 1989;10:181–186. doi: 10.1016/0197-4580(89)90028-6. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. Organization of the septal region in the rat brain: cholinergic-GABAergic interconnections and the termination of hippocampo-septal fibers. J Comp Neurol. 1989;289:304–314. doi: 10.1002/cne.902890210. [DOI] [PubMed] [Google Scholar]
- Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:1–220. [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Nishino S. The hypothalamic peptidergic system, hypocretin/orexin and vigilance control. Neuropeptides. 2007;41:117–133. doi: 10.1016/j.npep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Numan R, Quaranta JR., Jr Effects of medial septal lesions on operant delayed alternation in rats. Brain Res. 1990;531:232–241. doi: 10.1016/0006-8993(90)90779-b. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Alanko L, Kalinchuk A, Heiskanen S, Stenberg D. The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiology of Aging. 2004;25:231–238. doi: 10.1016/s0197-4580(03)00043-5. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Progress in Neurobiology. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, Haas HL. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiologica. 2010;198:277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- Selbach O, Doreulee N, Bohla C, Eriksson KS, Sergeeva OA, Poelchen W, Brown RE, Haas HL. Orexins/hypocretins cause sharp wave- and theta-related synaptic plasticity in the hippocampus via glutamatergic, gabaergic, noradrenergic, and cholinergic signaling. Neuroscience. 2004;127:519–528. doi: 10.1016/j.neuroscience.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Hippocampal interneurons expressing glutamic acid decarboxylase and calcium-binding proteins decrease with aging in Fischer 344 rats. J Comp Neurol. 1998;394:252–269. [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. The Journal of Physiology. 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J Neurochem. 2004;89:204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2010.12.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Adamik A. The action of orexin A on passive avoidance learning. Involvement of transmitters. Regul Pept. 2002;104:105–110. doi: 10.1016/s0167-0115(01)00341-x. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Morairty S, Freund YR, Kilduff TS. Age-related decline in hypocretin (orexin) receptor 2 messenger RNA levels in the mouse brain. Neuroscience Letters. 2002;332:190–194. doi: 10.1016/s0304-3940(02)00953-9. [DOI] [PubMed] [Google Scholar]
- Toth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J Neurosci. 1993;13:3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. The Journal of Physiology. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Vitorica J, Ruano D. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J Neurochem. 2003;85:368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci. 2002;22:7754–7765. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Pang KCH. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- Zhang J-H, Sampogna S, Morales FR, Chase MH. Age-related changes of hypocretin in basal forebrain of guinea pig. Peptides. 2005;26:2590–2596. doi: 10.1016/j.peptides.2005.05.003. [DOI] [PubMed] [Google Scholar]