Abstract
A wide range of biological processes exhibit circadian rhythm, enabling plants to adapt to the environmental day–night cycle. This rhythm is generated by the so-called ‘circadian clock’. Although a number of genetic approaches have identified >25 clock-associated genes involved in the Arabidopsis clock mechanism, the molecular functions of a large part of these genes are not known. Recent comprehensive studies have revealed the molecular functions of several key clock-associated proteins. This progress has provided mechanistic insights into how key clock-associated proteins are integrated, and may help in understanding the essence of the clock's molecular mechanisms.
Keywords: Arabidopsis thaliana, Circadian clock, Genetic circuit, Protein function
Introduction
Circadian rhythm is the temporal oscillation of genetic, metabolic and physiological processes based on the 24 h cycle, allowing organisms to anticipate day–night changes in the environment (Bunning 1967, Pittendrigh 1993). A wide variety of organisms from cyanobacteria to mammals display circadian rhythms at the level of metabolism, physiology and behavior under conditions in which there are no external time cues, indicating that these rhythms are driven by an endogenous timekeeping mechanism, the so-called ‘circadian clock’.
The circadian clock in Arabidopsis plants regulates a number of biological processes, such as rhythmic leaf movement (Bunning 1967, Millar et al. 1995), petal opening (Bunning 1967), the elongation rate of stems, hypocotyls and roots (Lecharny 1985, Dowson-Day and Millar 1999, Nozue et al. 2007, Yazdanbakhsh et al. 2011), circumnutation of stems (Niinuma et al. 2005), central and secondary metabolite biosynthesis (Warren and Wilkins 1961, Kolosova et al. 2001, Blasing et al. 2005, Fukushima et al. 2009), hormone biosynthesis and responses (Thain et al. 2004, Covington and Harmer 2007, Covington et al. 2008, Michael et al. 2008, Mizuno and Yamashino 2008), water stress responses (Fowler et al. 2005, Bieniawska et al. 2008, Kidokoro et al. 2009, Legnaioli et al. 2009, Nakamichi et al. 2009), stomatal opening (Holmes 1986, Somers et al. 1998), Ca2+ concentrations in certain cellular compartments (Johnson et al. 1995, Xu et al. 2007), water uptake (Takase et al. 2011), seed dormancy (Penfield and Hall 2009) and defence against pathogens (W. Wang et al. 2011). In addition, the clock is used in some plants to measure the environmental photoperiod to induce inflorescence meristems (Bunning 1967), so that flowering occurs during the correct season (photoperiodic flowering) (Ganner and Allard 1920). These phenomena coordinately contribute to fitness (or adaptive advantage) in 24 h day–night cycles (Green et al. 2002, Dodd et al. 2005, Yerushalmi et al. 2011).
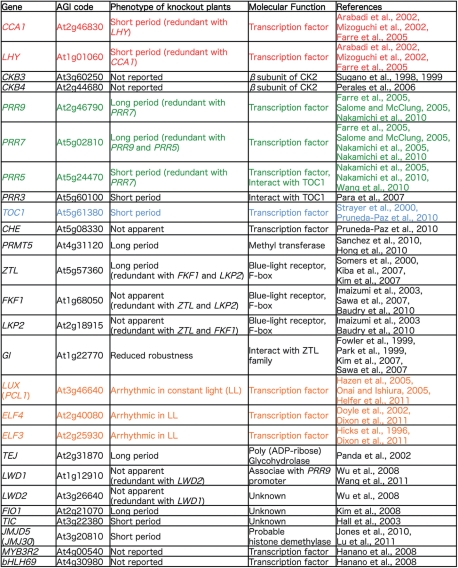
Mutant screening and genetic mapping–cloning approaches have been taken in Arabidopsis in order to understand the molecular mechanisms of the plant clock (Millar et al. 1995). At least 25 genes associated with clock function have been identified by classical genetics strategies, as well as by reverse genetics (Fig. 1). A number of recent studies have revealed the molecular functions of clock-associated proteins, which have long been undetermined. These findings provide us for the first time with enough information to understand how and when clock-associated proteins act in the circadian clock. It is now apparent that these genes interact to form a ‘genetic circuit’ which underlies the 24 h endogenous cycle. In this review, recent studies on the temporal and functional characterization of clock-associated proteins are summarized, followed by a discussion of how these proteins are integrated into the genetic circuit in the clock.
Fig. 1.
List of genes involved in the Arabidopsis circadian clock. Genes colored in red act in the morning (‘early shift’), green-colored genes are active from early daytime until midnight, and those in light blue and orange act during the night.
Clock-associated genes in Arabidopsis
Identification of clock genes began with traditional genetic approaches in the 1990s. Several key genes, including TIMING OF CAB EXPRESSION 1 (TOC1), ZEITLUPE (ZTL), TEJ, TIME FOR COFFEE (TIC), LUXARRHYTHMO [LUX or PHYTOCLOCK 1 (PCL1)], FIONA1 (FIO1) and PROTEIN ARGININE METHYL TRANSFERASE 5 (PRMT5), were isolated via large-scale screening experiments using gene promoters controlled under the circadian clock (e.g. chlorophyll a/b-binding protein, Cab) (Somers et al. 2000, Strayer et al. 2000, Panda et al. 2002, Hall et al. 2003, Hazen et al. 2005b, Onai and Ishiura 2005, Kim et al. 2008, Hong et al. 2010, Sanchez et al. 2010). Furthermore, the screening of mutants impaired in biological processes regulated by the circadian clock, such as photoperiodic flowering and hypocotyl elongation, led to the isolation of EARLY FLOWERING 3 (ELF3), ELF4, GIGANTEA (GI) and LATE ELONGATED HYPOCOTYL (LHY) (Schaffer et al. 1998, Fowler et al. 1999, Park et al. 1999, Hicks et al. 2001, Doyle et al. 2002).
Several approaches have been used to identify other key clock-associated genes. CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and CCA1 HIKING EXPEDITION (CHE) were identified by isolating proteins that bind to rhythmic gene promoters (Wang et al. 1997, Wang and Tobin 1998, Pruneda-Paz et al. 2009). Reverse genetics approaches have identified four TOC1 homologs, PSEUDO-RESPONSE REGULATOR 9 (PRR9), PRR7, PRR5 and PRR3, as components of the clock (Matsushika et al. 2000, Eriksson et al. 2003, Michael et al. 2003, Yamamoto et al. 2003, Para et al. 2007). Functional redundancies among genes showing high sequence similarities are often observed in Arabidopsis, which may make further discovery of novel clock-associated genes by a classical genetic approach technically challenging. For example, independent large-scale mutagenesis screenings identified allelic mutants, suggesting that these screenings were saturated (Onai et al. 2004, Hazen et al. 2005a). Biochemical and reverse genetic approaches, however, have identified new genes. Chemical genetics is emerging as a method which can also transcend the problem of functional redundancy by targeting specific receptor–ligand or other small molecule interactions.
MYB transcription factors take the ‘early shift’
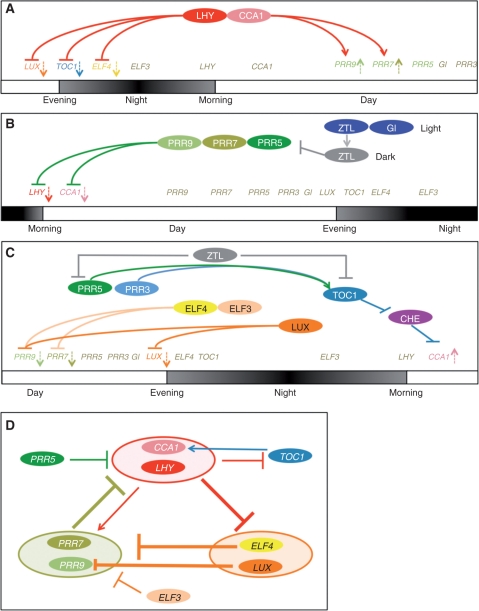
CCA1 and LHY (CCA1/LHY) are the closest paralogs of a single MYB transcription factor expressed with a morning acrophase, indicating that these genes take the ‘early shift’ in clock regulation, corresponding to early morning duty in a shift-work labor system (Fig. 2A) (Wang et al. 1997, Schaffer et al. 1998, Wang and Tobin 1998, Perales and Mas 2007). Overexpression of CCA1 or LHY abolishes the rhythms of clock output genes under constant light conditions (Schaffer et al. 1998, Wang and Tobin 1998). Single mutations in the cca1 or lhy loci have a short period phenotype, and cca1 lhy double mutants have a shorter period than either single mutation alone, indicating that CCA1/LHY are redundant but that both are required for proper clock function (Green and Tobin 1999, Alabadi et al. 2002, Mizoguchi et al. 2002). CCA1/LHY bind to a CCA1-binding site (AACAATCT or AAAAATCT) and to an evening element (AAAATATCT) (Wang et al. 1997, Alabadi et al. 2001). They repress the transcription of TOC1, ELF4 and LUX (Alabadi et al. 2001, Hazen et al. 2005b, Perales and Mas 2007, Li et al. 2011), but activate the transcription of PRR9 and PRR7 through these binding sites (Farre et al. 2005) (Fig. 2A).
Fig. 2.
Timetable for actions of clock-associated proteins. Genes colored in red act in the morning (‘early shift’), green-colored genes are active from early daytime until midnight, and light blue and orange genes act during the night. (A) In the morning, LHY and CCA1 proteins repress the evening-phase genes LUX, TOC1 and ELF4, and activate the day-phase genes PRR9 and PRR7. (B) From early daytime until midnight, PRR9, PRR7 and PRR5 repress the morning-phase genes CCA1 and LHY. Blue light enhances interaction of ZTL and GI. In the dark, the ZTL–GI complex is decoupled, allowing ZTL to promote the degradation of PRR5. (C) In the evening, LUX represses PRR9 and LUX expression, and ELF4 and ELF3 repress PRR9 and PRR7. PRR5 enhances nuclear localization of TOC1, and PRR3 stabilizes TOC1 in the evening. On the other hand, ZTL promotes the degradation of PRR5 and TOC1 at night (in darkness). TOC1 acts to activate CCA1 expression by antagonizing CHE on the CCA1 promoter. (D) Genetic circuit in the clock. CCA1 and LHY activate PRR9 and PRR7, and repress TOC1, ELF4 and LUX. ELF4, LUX and ELF3 repress PRR9 and PRR7. PRR9, PRR7 and PRR5 repress CCA1 and LHY, whereas TOC1 activates CCA1. These interactions illustrate the ‘genetic circuit’, which underlies the 24 h endogenous cycle.
CCA1 function is subject to post-translational modification. CCA1 is phosphorylated by the protein kinase CASEIN KINASE 2 (CK2), which is required for the formation of a DNA–protein complex containing CCA1 (Sugano et al. 1998, Sugano et al. 1999). Overexpressors of the CKB3 regulatory subunit of CK2 (Sugano et al. 1998), or CKB4 (Perales et al. 2006), which exhibits higher CK2 activity, display a short-period phenotype. On the other hand, overexpression of a mutant CCA1 which cannot be phosphorylated by CK2 does not result in a hypermorphic phenotype (Daniel et al. 2004), suggesting that CCA1 phosphorylation by CK2 is necessary for its function in the Arabidopsis clock. However, in rice it is unlikely that CK2 is involved in the clock mechanism. This difference was partly attributed to the lack of a serine residue, which is a CK2 target site, in OsLHY (a CCA1 ortholog), implying the divergence of post-translational regulation of CCA1 and LHY in higher plants during evolution (Ogiso et al. 2010).
Among the seven close homologs of CCA1/LHY in Arabidopsis, EARLY-PHYTOCHROME-RESPONSIVE 1 or REVEILLE 7 (RVE7) (Kuno et al. 2003), CIRCADIAN 1 or RVE2 (Zhang et al. 2007), and RVE1 (Rawat et al. 2009) have been implicated in the output function of the clock. Recently, however, it was shown that one member of the homolog set, RVE8 or LHY-CCA1-LIKE 5, is involved in the clock by directly activating the expression of both TOC1 and PRR5 (Farinas and Mas 2011, Rawat et al. 2011).
PRR9, PRR7 and PRR5 take the ‘day and swing shifts’
PRR9, PRR7 and PRR5 (PRRs) are sequentially expressed from early daytime until around midnight, corresponding to the ‘day shift’ and ‘swing shift’ in this metaphorical shift-work system (Farre and Kay 2007, Ito et al. 2007b, Kiba et al. 2007, Fujiwara et al. 2008, Nakamichi et al. 2010) (Fig. 2B). They possess a pseudo-receiver (PR) domain at their N-termini, and a CONSTANS, CONSTANS-LIKE1 and TOC1 (CCT) motif at their C-termini (Makino et al. 2000, Matsushika et al. 2000, Strayer et al. 2000).
Genetic studies have shown that PRR9, PRR7 and PRR5 function redundantly and/or synergistically within the clock mechanism (Farre et al. 2005, Nakamichi et al. 2005, Salome and McClung 2005). Given that expression of CCA1 and LHY is decreased in PRR7 or PRR5 overexpression lines (Sato et al. 2002, Farre and Kay 2007), and increased in prr9 prr7, prr7 prr5 and prr9 prr7 prr5 mutants (Farre et al. 2005, Nakamichi et al. 2005, Salome et al. 2010), these PRR genes are likely to be negative regulators of CCA1 and LHY. A recent study demonstrated that these PRR proteins associate with CCA1 and LHY promoters in vivo, and repress these genes from early daytime until midnight (Nakamichi et al. 2010) (Fig. 2B). Each PRR protein works at a specific time; PRR9 functions during early daytime, PRR7 is active from early daytime until midnight, and PRR5 works from noon until midnight. PRRs seem to act as active transcriptional repressors, because the repression motif, which confers transcriptional repressor activity on the yeast GAL4 DNA-binding domain, is present in the PRRs (Nakamichi et al. 2010). However, it is not yet known how the PRRs are recruited to the CCA1 and LHY promoter regions. Given that the CCT motif of CONSTANS is involved in interactions with the target DNA (Wenkel et al. 2006, Tiwari et al. 2010), it is possible that the CCT motif of PRRs is also involved in interactions with target DNA regions. In addition, PRR proteins participate in post-translational regulation. PRR5 interacts with TOC1 through its PR domains, and enhances phosphorylation and nuclear localization of TOC1 (Wang et al. 2010) (Fig. 2C).
‘The graveyard shift’: TOC1, ELF3, ELF4 and LUX
The toc1-1 mutant was the very first Arabidopsis clock mutant found, identified because of its short-period phenotype (Millar et al. 1995, Strayer et al. 2000). TOC1 proteins are expressed during the night, the time of the ‘graveyard shift’ (Makino et al. 2000, Matsushika et al. 2000, Strayer et al. 2000). Reduction of TOC1 expression by RNA interference (RNAi) shortens the period length, whereas increased TOC1 expression under the control of its own promoter results in a lengthened period (Mas et al. 2003a), suggesting that both the timing and level of TOC1 expression are crucial for maintaining a proper period length. TOC1 is thus subjected to multiple layers of regulation. In addition to the transcriptional and post-transcriptional regulation of TOC1 described in earlier sections (Fig. 2A, C), TOC1 is targeted for degradation by ZTL family proteins (Fig. 2C) (Mas et al. 2003b, Kim et al. 2007, Baudry et al. 2010). On the other hand, TOC1 is stabilized by PRR3 via protein–protein interactions (Fig. 2C) (Para et al. 2007).
The exact molecular function of TOC1 within the circadian clock remained elusive for a long time, but recent studies indicate that TOC1 is a transcriptional regulator. TOC1 activates CCA1 expression by antagonizing the action of CHE, a repressor of CCA1 (Pruneda-Paz et al. 2009) (Fig. 2C). CHE is a TCP (for TEOSINTE BRANCHED1, CYCLOIDEA and PCF) transcription factor. Other TCPs also have the ability to bind to TOC1 (Giraud et al. 2010), implying that TOC1 activates CCA1 expression through its binding to TCPs.
ELF3, ELF4 and LUX are essential for sustaining the circadian rhythm under constant light conditions, since mutations in each gene result in arrhythmia (Hicks et al. 1996, Doyle et al. 2002, Hazen et al. 2005b, Onai and Ishiura 2005). ELF3, ELF4 and LUX are expressed from evening until midnight. All three genes are required for full CCA1 and LHY expression, as evidenced by decreased expression of CCA1 and LHY in elf3, elf4 and lux mutants (Doyle et al. 2002, Hazen et al. 2005b, Kikis et al. 2005, Onai and Ishiura 2005). ELF3, ELF4 and LUX encode structurally distinct proteins: ELF3 encodes a putative transcriptional regulator, ELF4 encodes a protein with unknown function, and LUX encodes a GARP-type MYB transcription factor. Recent studies indicate that ELF3 and ELF4 are transcriptional repressors of PRR9 and PRR7, and that LUX is a night-time repressor of PRR9 (Dixon et al. 2011, Helfer et al. 2011) (Fig. 2C). LUX also directly represses its own expression by binding to the LUX promoter, forming a negative feedback loop (Helfer et al. 2011).
The ZTL–GI complex as a light sensor
ZTL is the best characterized factor involved in post-translational regulation of clock-associated proteins. ZTL protein contains an F-box domain, which is a component of the Skp/Cullin/F-box E3 ubiquitin ligases acting in the proteasome-dependent protein degradation pathway. In addition, this protein possesses an N-terminal LOV (light, oxygen, voltage-dependent) domain that perceives blue light, suggesting that ZTL functions as a blue light-regulated F-box protein. Indeed, ZTL targets TOC1 and PRR5 for degradation in the absence of blue light (Mas et al. 2003b, Kiba et al. 2007). There are two ZTL homologs in Arabidopsis: FLAVIN BINDING, KELCH REPEAT, F-BOX1 (FKF1) (Nelson et al. 2000, Imaizumi et al. 2003, Sawa et al. 2007) and LOV KELCH PROTEIN2 (LKP2) (Schultz et al. 2001). A recent study indicated that all members of the ZTL protein family are engaged in PRR5 and TOC1 degradation (Baudry et al. 2010).
Recently, Kim et al. (2007) demonstrated that ZTL oscillation (low in the morning; high in the evening) is established and sustained by blue light-enhanced interaction with GI, which is a factor responsible for the robustness of the rhythm, but with no known functional domain (Kim et al. 2007). The blue light-activated ZTL forms a complex with GI, resulting in stabilization of ZTL. In darkness, the complex is attenuated, thereby destabilizing ZTL. Because the timing of GI expression is determined by the clock, complex formation is also under clock control (David et al. 2006). The external light conditions and internal circadian clock together confer fine-tuned rhythms to ZTL, leading to robust TOC1 and PRR5 oscillations (Kim et al. 2007) (Fig. 2B, C).
Other, but indispensable, factors
There are some clock-associated proteins which are known to be involved in rhythmic control, but whose specific molecular function remains unknown. For example, TEJ (TEJ means ‘bright’ in Sanskrit) encodes a poly(ADP-ribose) glycohydrolase, which is involved in poly(ADP-ribosyl)ation of proteins (Panda et al. 2002). A tej mutation lengthens the circadian period. TEJ targets have not been identified.
TIC was named for the evidence that the gene is required for maintaining metabolic rhythm from mid- to late night when human activity often gets a boost from caffeine coffee (Hall et al. 2003). TIC encodes a nuclear-localized protein with probable ATP/GTP-binding site A motifs (P-loop) (Ding et al. 2007). Interestingly, the abundance and cellular localization of TIC are not under clock control, implying that some unknown factor or factors restricts the activity of TIC during the night (Ding et al. 2007).
A mutation in FIO1 (fiona means ‘flowering’ in Korean) results in longer circadian periods (Kim et al. 2008). FIO1 encodes a nuclear-localized protein with a DUF890 domain, which is found in the methyltransferase superfamily, though the precise function of FIO1 remains to be elucidated.
LIGHT-REGULATED WD1 (LWD1) and its closest homolog, LWD2, encode WD repeat-containing proteins (Wu et al. 2008). The lwd1 lwd2 double mutant has a short-period phenotype. Recently, it was shown that LWD1 associates with the promoter region of PRR9 to activate PRR9 expression (Y. Wang et al. 2011).
Two transcription factors, MYB3R2 and bHLH69A, were identified through systematic screening in which transcription factors were tested for their potential to alter circadian rhythms (Hanano et al. 2008). Although circadian periods were lengthened both in MYB3R2 and bHLH69A overexpression lines, the direct target of MYB3R2 and bHLH69 has not been identified.
A jumonji-C (JmjC) domain-containing protein (JMJD5 = JMJ30) gene, encoding a possible histone demethylase, was identified as a clock component (Jones et al. 2010, Lu et al. 2011). Mutations in JMJD5 result in shortened circadian periods, and the mutation enhances the effect of a toc1 mutation, suggesting that JMJD5 interacts synergistically with TOC1 (Jones et al. 2010).
Recently, PRMT5, which is involved in site-specific alternative splicing, was implicated in the clock mechanism (Hong et al. 2010, Sanchez et al. 2010). PRMT5 acts by dimethylating Sm proteins which participate in pre-mRNA splicing. The loss-of-function prmt5 mutation results in a long-period phenotype. Splicing of the third intron of PRR9 is impaired in prmt5, and the prr9 prr7 double mutation is epistatic to prmt5, suggesting that PRMT5 controls the period length in part by regulating PRR9 splicing.
Genetic circuit in the clock
Based on our current knowledge of the regulation and molecular functions of clock-associated genes, a ‘genetic circuit’ model has been proposed (Helfer et al. 2011). Three classes of repressors constitute the genetic circuit: (i) morning-phase proteins CCA1 and LHY repress ELF4 and LUX; (ii) evening-phase proteins ELF4 and LUX repress PRR9 and PRR7; and (iii) midday-phase proteins PRR9 and PRR7 repress CCA1 and LHY. Although there is no experimental evidence to show that the dynamics of the proposed circuit is responsible for clock function, a synthetic genetic circuit, known as ‘the repressilator’, which is a cyclic negative feedback loop composed of three transcriptional repressor genes, can produce an oscillating pattern (Elowitz and Leibler 2000). The sustainable oscillation in ‘the repressilator’ is dependent on similar decay rates of protein and mRNA, and large amounts of protein at its peak level. Oscillation patterns can also be generated in networks containing an activator and a repressor, or an odd number of repressors over three, if stochastic characters are negligible in the networks. Such theoretical approaches help to understand the molecular basis and dynamics of the clock.
Perspectives
Recent progress in genome research indicates that clock-associated genes identified in Arabidopsis are mostly conserved among angiosperms (Song et al. 2010). Furthermore, evidence is accumulating that these genes are orthologs of corresponding genes in Arabidopsis, suggesting that the proposed molecular clock mechanism is conserved among angiosperms [ELF3, GI and LHY in duckweed (Miwa et al. 2006, Serikawa et al. 2008), LHY and TOC1 in poplar (Ibanez et al. 2010)], including agriculturally important plants [ELF4 and GI in pea (Hecht et al. 2007, Liew et al. 2009), and GI and LHY in rice (Ogiso et al. 2010, Izawa et al. 2011)]. A key trait in crops under clock control is photoperiodic flowering, a critical aspect of crop production that has been selected during domestication. For example, photoperiod-insensitive wheat varieties predominate in relatively warm regions where wheat needs to flower and mature before the onset of high summer temperatures. A genetic locus responsible for advancing the heading date of such varieties is present at the upstream region of the PRR7 homolog (Ppd-D1a), which causes misexpression of this gene (Beales et al. 2007). On the other hand, mutations in a PRR7 homolog (Ppd-H1) were found as the genetic loci which delay the heading date of spring-sown barley (Turner et al. 2005). The ppd-H1 varieties have an extended vegetative growth phase and ultimately higher yields in Western Europe and North America. Quantitative trait loci for flowering time overlap with PRR7 homologs in rice and Brassica rapa (Murakami et al. 2005, Lou et al. 2011). Furthermore, GI and ELF4 orthologs were identified as the genetic loci that alter photoperiodic flowering in pea (Hecht et al. 2007, Liew et al. 2009). Therefore, studies of the Arabidopsis circadian clock should enable us to understand how plants generate responses to photoperiod, and thus to induce flowering, which will ultimately be a significant boon for agriculture.
Thanks to recent studies, the molecular functions and functional timing (duration) of many Arabidopsis clock-associated proteins have been determined, which has enabled us to propose a ‘genetic circuit’ model. Although it is widely accepted that the genetic circuit plays an important role in the clock system, whether or not the genetic circuit alone drives these rhythms is an open question. Since the transcriptional process is generally stochastic and depends on temperature and metabolic conditions (Raser and O'Shea 2005), and the circadian period is constant over a wide temperature range (known as temperature compensation), a genetic circuit alone does not meet the theoretical requirements for clock function. Recently, a post-translational (or non-transcriptional) oscillation was detected in cyanobacteria and the unicellular green alga Ostreococcus tauri (Nakajima et al. 2005, Tomita et al. 2005, O'Neill et al. 2011), which also possess transcription-based feedback loops (Kitayama et al. 2008, Corellou et al. 2009). The post-translational oscillators are coupled with transcription-based feedback loops in cyanobacteria and the green algae under physiological conditions (Kitayama et al. 2008, O'Neill et al. 2011). Interestingly, the cyanobacterial post-translational oscillator is sufficient to drive circadian rhythm by itself under extremely poor metabolic conditions and throughout a wide temperature range, illustrating the resilient nature of circadian periodicity. Compensations in period length for temperature fluctuations and metabolic changes are embedded in the post-translational oscillator (Nakajima et al. 2005, Tomita et al. 2005, Ito et al. 2007a). Whether the post-translational oscillator functions in higher plants is an open and interesting question that needs to be addressed before we can understand how the circadian clock evolved, and exactly how it functions in controlling the plant circadian clock. It is likely that answers to these questions will provide insights into many of the critical features of plant behavior.
Funding
Preparation of this review was supported by the Special Postdoctoral Researcher's Program; Yokohama Institute Director's Discretionary Fund; Technology Transfer Office Fund, from RIKEN.
Acknowledgments
Special thanks go to Drs. Takatoshi Kiba (RIKEN Plant Science Center) and Hiroshi Ito (Ochanomizu University) for critical reading of the manuscript.
Glossary
Abbreviations
- bHLH
basic helix–loop–helix
- CCA1
CIRCADIAN CLOCK-ASSOCIATED 1
- CCT
CONSTANS, CONSTANS-LIKE1 and TOC1
- CHE
CCA1 HIKING EXPEDITION
- CK2
CASEIN KINASE 2
- CKB
CASEIN KINASE β SUBUNIT
- ELF3
EARLY FLOWERING 3
- ELF4
EARLY FLOWERING 4
- FIO1
FIONA1
- FKF1
FLAVIN BINDING, KELCH REPEAT, F-BOX1
- GI
GIGANTEA
- JMJ
JUMONJI
- LHY
LATE ELONGATED HYPOCOTYL
- LKP2
LOV KELCH PROTEIN2
- LOV
light, oxygen, voltage
- LUX
LUXARRHYTHMO
- LWD
LIGHT-REGULATED WD
- PCL1
PHYTOCLOCK 1
- PR
Pseudo-receiver
- PRMT5
PROTEIN ARGININE METHYL TRANSFERASE 5
- PRR
PSEUDO-RESPONSE REGULATOR
- RVE
REVEILLE
- TCP
TEOSINTE BRANCHED1, CYCLOIDEA and PCF
- TIC
TIME FOR COFFEE
- TOC1
TIMING OF CAB EXPRESSION 1
- ZTL
ZEITLUPE.
References
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293:880–883. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- Alabadi D, Yanovsky MJ, Mas P, Harmer SL, Kay SA. Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr. Biol. 2002;12:757–761. doi: 10.1016/s0960-9822(02)00815-1. [DOI] [PubMed] [Google Scholar]
- Baudry A, Ito S, Song YH, Strait AA, Kiba T, Lu S, et al. F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell. 2010;22:606–622. doi: 10.1105/tpc.109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.) Theor. Appl. Genet. 2007;115:721–733. doi: 10.1007/s00122-007-0603-4. [DOI] [PubMed] [Google Scholar]
- Bieniawska Z, Espinoza C, Schlereth A, Sulpice R, Hincha DK, Hannah MA. Disruption of the Arabidopsis circadian clock is responsible for extensive variation in the cold-responsive transcriptome. Plant Physiol. 2008;147:263–279. doi: 10.1104/pp.108.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, et al. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell. 2005;17:3257–3281. doi: 10.1105/tpc.105.035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning E. The Physiological Clock. New York: The Heidelberg Science Library, Springer-Verlag; 1967. [Google Scholar]
- Corellou F, Schwartz C, Motta JP, Djouani-Tahri el B, Sanchez F, Bouget FY. Clocks in the green lineage: comparative functional analysis of the circadian architecture of the picoeukaryote ostreococcus. Plant Cell. 2009;21:3436–3449. doi: 10.1105/tpc.109.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington MF, Harmer SL. The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol. 2007;5:e222. doi: 10.1371/journal.pbio.0050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008;9:R130. doi: 10.1186/gb-2008-9-8-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel X, Sugano S, Tobin EM. CK2 phosphorylation of CCA1 is necessary for its circadian oscillator function in Arabidopsis. Proc. Natl Acad. Sci. USA. 2004;101:3292–3297. doi: 10.1073/pnas.0400163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David KM, Armbruster U, Tama N, Putterill J. Arabidopsis GIGANTEA protein is post-transcriptionally regulated by light and dark. FEBS Lett. 2006;580:1193–1197. doi: 10.1016/j.febslet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ding Z, Millar AJ, Davis AM, Davis SJ. TIME FOR COFFEE encodes a nuclear regulator in the Arabidopsis thaliana circadian clock. Plant Cell. 2007;19:1522–1536. doi: 10.1105/tpc.106.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Knox K, Kozma-Bognar L, Southern MM, Pokhilko A, Millar AJ. Temporal repression of core circadian genes is mediated through EARLY FLOWERING 3 in Arabidopsis. Curr. Biol. 2011;21:120–125. doi: 10.1016/j.cub.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Dowson-Day MJ, Millar AJ. Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J. 1999;17:63–71. doi: 10.1046/j.1365-313x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma-Bognar L, Nagy F, et al. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature. 2002;419:74–77. doi: 10.1038/nature00954. [DOI] [PubMed] [Google Scholar]
- Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- Eriksson ME, Hanano S, Southern MM, Hall A, Millar AJ. Response regulator homologues have complementary, light-dependent functions in the Arabidopsis circadian clock. Planta. 2003;218:159–162. doi: 10.1007/s00425-003-1106-4. [DOI] [PubMed] [Google Scholar]
- Farinas B, Mas P. Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J. 2011;66:318–329. doi: 10.1111/j.1365-313X.2011.04484.x. [DOI] [PubMed] [Google Scholar]
- Farre EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 2005;15:47–54. doi: 10.1016/j.cub.2004.12.067. [DOI] [PubMed] [Google Scholar]
- Farre EM, Kay SA. PRR7 protein levels are regulated by light and the circadian clock in Arabidopsis. Plant J. 2007;52:548–560. doi: 10.1111/j.1365-313X.2007.03258.x. [DOI] [PubMed] [Google Scholar]
- Fowler SG, Cook D, Thomashow MF. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, et al. GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Wang L, Han L, Suh SS, Salome PA, McClung CR, et al. Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins. J. Biol. Chem. 2008;283:23073–23083. doi: 10.1074/jbc.M803471200. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Kusano M, Nakamichi N, Kobayashi M, Hayashi N, Sakakibara H, et al. Impact of clock-associated Arabidopsis pseudo-response regulators in metabolic coordination. Proc. Natl Acad. Sci. USA. 2009;106:7251–7256. doi: 10.1073/pnas.0900952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner WW, Allard HA. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J. Agr. Res. 1920;18:553–606. [Google Scholar]
- Giraud E, Ng S, Carrie C, Duncan O, Low J, Lee CP, et al. TCP transcription factors link the regulation of genes encoding mitochondrial proteins with the circadian clock in Arabidopsis thaliana. Plant Cell. 2010;22:3921–3934. doi: 10.1105/tpc.110.074518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Tingay S, Wang ZY, Tobin EM. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 2002;129:576–584. doi: 10.1104/pp.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Tobin EM. Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc. Natl Acad. Sci. USA. 1999;96:4176–4179. doi: 10.1073/pnas.96.7.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Bastow RM, Davis SJ, Hanano S, McWatters HG, Hibberd V, et al. The TIME FOR COFFEE gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell. 2003;15:2719–2729. doi: 10.1105/tpc.013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S, Stracke R, Jakoby M, Merkle T, Domagalska MA, Weisshaar B, et al. A systematic survey in Arabidopsis thaliana of transcription factors that modulate circadian parameters. BMC Genomics. 2008;9:182. doi: 10.1186/1471-2164-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SP, Borevitz JO, Harmon FG, Pruneda-Paz JL, Schultz TF, Yanovsky MJ, et al. Rapid array mapping of circadian clock and developmental mutations in Arabidopsis. Plant Physiol. 2005a;138:990–997. doi: 10.1104/pp.105.061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc. Natl Acad. Sci. USA. 2005b;102:10387–10392. doi: 10.1073/pnas.0503029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht V, Knowles CL, Vander Schoor JK, Liew LC, Jones SE, Lambert MJ, et al. Pea LATE BLOOMER1 is a GIGANTEA ortholog with roles in photoperiodic flowering, deetiolation, and transcriptional regulation of circadian clock gene homologs. Plant Physiol. 2007;144:648–661. doi: 10.1104/pp.107.096818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer A, Nusinow DA, Chow BY, Gehrke AR, Bulyk ML, Kay SA. LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. Curr. Biol. 2011;21:126–133. doi: 10.1016/j.cub.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Albertson TM, Wagner DR. EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell. 2001;13:1281–1292. doi: 10.1105/tpc.13.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Millar AJ, Carre IA, Somers DE, Straume M, Meeks-Wagner DR, et al. Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science. 1996;274:790–792. doi: 10.1126/science.274.5288.790. [DOI] [PubMed] [Google Scholar]
- Holmes MG, Klein WH. Photocontrol of dark circadian rhythms in stomata of Phaseolus vulgaris L. Plant Physiol. 1986;82:28–33. doi: 10.1104/pp.82.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Song HR, Lutz K, Kerstetter RA, Michael TP, McClung CR. Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA. 2010;107:21211–21216. doi: 10.1073/pnas.1011987107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C, Kozarewa I, Johansson M, Ogren E, Rohde A, Eriksson ME. Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiol. 2010;153:1823–1833. doi: 10.1104/pp.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature. 2003;426:302–306. doi: 10.1038/nature02090. [DOI] [PubMed] [Google Scholar]
- Ito H, Kageyama H, Mutsuda M, Nakajima M, Oyama T, Kondo T. Autonomous synchronization of the circadian KaiC phosphorylation rhythm. Nat. Struct. Mol. Biol. 2007a;14:1084–1088. doi: 10.1038/nsmb1312. [DOI] [PubMed] [Google Scholar]
- Ito S, Nakamichi N, Kiba T, Yamashino T, Mizuno T. Rhythmic and light-inducible appearance of clock-associated pseudo-response regulator protein PRR9 through programmed degradation in the dark in Arabidopsis thaliana. Plant Cell Physiol. 2007b;48:1644–1651. doi: 10.1093/pcp/pcm122. [DOI] [PubMed] [Google Scholar]
- Izawa T, Mihara M, Suzuki Y, Gupta M, Itoh H, Nagasno AJ, et al. Os-GIGANTEA confers robust diurnal rhythms on the global transcriptome of rice in the field. Plant Cell. 2011;23:1741–1755. doi: 10.1105/tpc.111.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, et al. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- Jones MA, Covington MF, Ditacchio L, Vollmers C, Panda S, Harmer SL. Jumonji domain protein JMJD5 functions in both the plant and human circadian systems. Proc. Natl Acad. Sci. USA. 2010;107:21623–21628. doi: 10.1073/pnas.1014204108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Henriques R, Sakakibara H, Chua NH. Targeted degradation of PSEUDO-RESPONSE REGULATOR5 by an SCFZTL complex regulates clock function and photomorphogenesis in Arabidopsis thaliana. Plant Cell. 2007;19:2516–2530. doi: 10.1105/tpc.107.053033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro S, Maruyama K, Nakashima K, Imura Y, Narusaka Y, Shinwari ZK, et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009;151:2046–2057. doi: 10.1104/pp.109.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikis EA, Khanna R, Quail PH. ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J. 2005;44:300–313. doi: 10.1111/j.1365-313X.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim Y, Yeom M, Kim JH, Nam HG. FIONA1 is essential for regulating period length in the Arabidopsis circadian clock. Plant Cell. 2008;20:307–319. doi: 10.1105/tpc.107.055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han L, et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature. 2007;449:356–360. doi: 10.1038/nature06132. [DOI] [PubMed] [Google Scholar]
- Kitayama Y, Nishiwaki T, Terauchi K, Kondo T. Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev. 2008;22:1513–1521. doi: 10.1101/gad.1661808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova N, Gorenstein N, Kish CM, Dudareva N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell. 2001;13:2333–2347. doi: 10.1105/tpc.010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno N, Moller SG, Shinomura T, Xu X, Chua NH, Furuya M. The novel MYB protein EARLY-PHYTOCHROME-RESPONSIVE1 is a component of a slave circadian oscillator in Arabidopsis. Plant Cell. 2003;15:2476–2488. doi: 10.1105/tpc.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecharny A, Schwall M, Wagner E. Stem extension rate in light-grown plants. Plant Physiol. 1985;79:625–629. doi: 10.1104/pp.79.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnaioli T, Cuevas J, Mas P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 2009;28:3745–3757. doi: 10.1038/emboj.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Siddiqui H, Teng Y, Lin R, Wan XY, Li J, et al. Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat. Cell Biol. 2011;13:616–622. doi: 10.1038/ncb2219. [DOI] [PubMed] [Google Scholar]
- Liew LC, Hecht V, Laurie RE, Knowles CL, Vander Schoor JK, Macknight RC, et al. DIE NEUTRALIS and LATE BLOOMER 1 contribute to regulation of the pea circadian clock. Plant Cell. 2009;21:3198–3211. doi: 10.1105/tpc.109.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou P, Xie Q, Xu X, Edwards CE, Brock MT, Weinig C, et al. Genetic architecture of the circadian clock and flowering time in Brassica rapa. Theor. Appl. Genet. 2011;123:397–409. doi: 10.1007/s00122-011-1592-x. [DOI] [PubMed] [Google Scholar]
- Lu SX, Knowles SM, Webb CJ, Celaya RB, Cha C, Siu JP, et al. The JmjC domain-containing protein JMJ30 regulates period length in the Arabidopsis circadian clock. Plant Physiol. 2011;115:906–915. doi: 10.1104/pp.110.167015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Kiba T, Imamura A, Hanaki N, Nakamura A, Suzuki T, et al. Genes encoding pseudo-response regulators: insight into His-to-Asp phosphorelay and circadian rhythm in Arabidopsis thaliana. Plant Cell Physiol. 2000;41:791–803. doi: 10.1093/pcp/41.6.791. [DOI] [PubMed] [Google Scholar]
- Mas P, Alabadi D, Yanovsky MJ, Oyama T, Kay SA. Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell. 2003a;15:223–236. doi: 10.1105/tpc.006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas P, Kim WY, Somers DE, Kay SA. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature. 2003b;426:567–570. doi: 10.1038/nature02163. [DOI] [PubMed] [Google Scholar]
- Matsushika A, Makino S, Kojima M, Mizuno T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant Cell Physiol. 2000;41:1002–1012. doi: 10.1093/pcp/pcd043. [DOI] [PubMed] [Google Scholar]
- Michael TP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, et al. A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol. 2008;6:e225. doi: 10.1371/journal.pbio.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Salome PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, et al. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science. 2003;302:1049–1053. doi: 10.1126/science.1082971. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Carre IA, Strayer CA, Chua NH, Kay SA. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science. 1995;267:1161–1163. doi: 10.1126/science.7855595. [DOI] [PubMed] [Google Scholar]
- Miwa K, Serikawa M, Suzuki S, Kondo T, Oyama T. Conserved expression profiles of circadian clock-related genes in two Lemna species showing long-day and short-day photoperiodic flowering responses. Plant Cell Physiol. 2006;47:601–612. doi: 10.1093/pcp/pcj027. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, et al. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell. 2002;2:629–641. doi: 10.1016/s1534-5807(02)00170-3. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yamashino T. Comparative transcriptome of diurnally oscillating genes and hormone-responsive genes in Arabidopsis thaliana: insight into circadian clock-controlled daily responses to common ambient stresses in plants. Plant Cell Physiol. 2008;49:481–487. doi: 10.1093/pcp/pcn008. [DOI] [PubMed] [Google Scholar]
- Murakami M, Matsushika A, Ashikari M, Yamashino T, Mizuno T. Circadian-associated rice pseudo response regulators (OsPRRs): insight into the control of flowering time. Biosci. Biotechnol. Biochem. 2005;69:410–414. doi: 10.1271/bbb.69.410. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kiba T, Henriques R, Mizuno T, Chua NH, Sakakibara H. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell. 2010;22:594–605. doi: 10.1105/tpc.109.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T. PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol. 2005;46:686–698. doi: 10.1093/pcp/pci086. [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kusano M, Fukushima A, Kita M, Ito S, Yamashino T, et al. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant Cell Physiol. 2009;50:447–462. doi: 10.1093/pcp/pcp004. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell. 2000;101:331–340. doi: 10.1016/s0092-8674(00)80842-9. [DOI] [PubMed] [Google Scholar]
- Niinuma K, Someya N, Kimura M, Yamaguchi I, Hamamoto H. Circadian rhythm of circumnutation in inflorescence stems of Arabidopsis. Plant Cell Physiol. 2005;46:1423–1427. doi: 10.1093/pcp/pci127. [DOI] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, et al. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, et al. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso E, Takahashi Y, Sasaki T, Yano M, Izawa T. The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol. 2010;152:808–820. doi: 10.1104/pp.109.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai K, Ishiura M. PHYTOCLOCK 1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells. 2005;10:963–972. doi: 10.1111/j.1365-2443.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- Onai K, Okamoto K, Nishimoto H, Morioka C, Hirano M, Kami-Ike N, et al. Large-scale screening of Arabidopsis circadian clock mutants by a high-throughput real-time bioluminescence monitoring system. Plant J. 2004;40:1–11. doi: 10.1111/j.1365-313X.2004.02191.x. [DOI] [PubMed] [Google Scholar]
- Panda S, Poirier GG, Kay SA. tej defines a role for poly(ADP-ribosyl)ation in establishing period length of the arabidopsis circadian oscillator. Dev. Cell. 2002;3:51–61. doi: 10.1016/s1534-5807(02)00200-9. [DOI] [PubMed] [Google Scholar]
- Para A, Farre EM, Imaizumi T, Pruneda-Paz JL, Harmon FG, Kay SA. PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell. 2007;19:3462–3473. doi: 10.1105/tpc.107.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, et al. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science. 1999;285:1579–1582. doi: 10.1126/science.285.5433.1579. [DOI] [PubMed] [Google Scholar]
- Penfield S, Hall A. A role for multiple circadian clock genes in the response to signals that break seed dormancy in Arabidopsis. Plant Cell. 2009;21:1722–1732. doi: 10.1105/tpc.108.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales M, Mas P. A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell. 2007;19:2111–2123. doi: 10.1105/tpc.107.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales M, Portoles S, Mas P. The proteasome-dependent degradation of CKB4 is regulated by the Arabidopsis biological clock. Plant J. 2006;46:849–860. doi: 10.1111/j.1365-313X.2006.02744.x. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Pruneda-Paz JL, Breton G, Para A, Kay SA. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science. 2009;323:1481–1485. doi: 10.1126/science.1167206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK. Noise in gene expression: origins, consequences, and control. Science. 2005;309:2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng Y, Andersson CR, et al. REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc. Natl Acad. Sci. USA. 2009;106:16883–16888. doi: 10.1073/pnas.0813035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat R, Takahashi N, Hsu PY, Jones MA, Schwartz J, Salemi MR, et al. REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 2011;7:e1001350. doi: 10.1371/journal.pgen.1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome PA, McClung CR. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell. 2005;17:791–803. doi: 10.1105/tpc.104.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome PA, Weigel D, McClung CR. The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. Plant Cell. 2010;22:3650–3661. doi: 10.1105/tpc.110.079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SE, Petrillo E, Beckwith EJ, Zhang X, Rugnone ML, Hernando CE, et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature. 2010;468:112–116. doi: 10.1038/nature09470. [DOI] [PubMed] [Google Scholar]
- Sato E, Nakamichi N, Yamashino T, Mizuno T. Aberrant expression of the Arabidopsis circadian-regulated APRR5 gene belonging to the APRR1/TOC1 quintet results in early flowering and hypersensitiveness to light in early photomorphogenesis. Plant Cell Physiol. 2002;43:1374–1385. doi: 10.1093/pcp/pcf166. [DOI] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carre IA, et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- Schultz TF, Kiyosue T, Yanovsky M, Wada M, Kay SA. A role for LKP2 in the circadian clock of Arabidopsis. Plant Cell. 2001;13:2659–2670. doi: 10.1105/tpc.010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikawa M, Miwa K, Kondo T, Oyama T. Functional conservation of clock-related genes in flowering plants: overexpression and RNA interference analyses of the circadian rhythm in the monocotyledon Lemna gibba. Plant Physiol. 2008;146:1952–1963. doi: 10.1104/pp.107.114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Schultz TF, Milnamow M, Kay SA. ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell. 2000;101:319–329. doi: 10.1016/s0092-8674(00)80841-7. [DOI] [PubMed] [Google Scholar]
- Somers DE, Webb AA, Pearson M, Kay SA. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- Song YH, Ito S, Imaizumi T. Similarities in the circadian clock and photoperiodism in plants. Curr. Opin. Plant Biol. 2010;13:594–603. doi: 10.1016/j.pbi.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Mas P, et al. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science. 2000;289:768–771. doi: 10.1126/science.289.5480.768. [DOI] [PubMed] [Google Scholar]
- Sugano S, Andronis C, Green RM, Wang ZY, Tobin EM. Protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein. Proc. Natl Acad. Sci. USA. 1998;95:11020–11025. doi: 10.1073/pnas.95.18.11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano S, Andronis C, Ong MS, Green RM, Tobin EM. The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc. Natl Acad. Sci. USA. 1999;96:12362–12366. doi: 10.1073/pnas.96.22.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase T, Ishikawa H, Murakami H, Kikuchi J, Sato-Nara K, Suzuki H. The circadian clock modulates water dynamics and aquaporin expression in Arabidopsis roots. Plant Cell Physiol. 2011;52:373–383. doi: 10.1093/pcp/pcq198. [DOI] [PubMed] [Google Scholar]
- Thain SC, Vandenbussche F, Laarhoven LJ, Dowson-Day MJ, Wang ZY, Tobin EM, et al. Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol. 2004;136:3751–3761. doi: 10.1104/pp.104.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Shen Y, Chang HC, Hou Y, Harris A, Ma SF, et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010;187:57–66. doi: 10.1111/j.1469-8137.2010.03251.x. [DOI] [PubMed] [Google Scholar]
- Tomita J, Nakajima M, Kondo T, Iwasaki H. No transcription–translation feedback in circadian rhythm of KaiC phosphorylation. Science. 2005;307:251–254. doi: 10.1126/science.1102540. [DOI] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science. 2005;310:1031–1034. doi: 10.1126/science.1117619. [DOI] [PubMed] [Google Scholar]
- Wang L, Fujiwara S, Somers DE. PRR5 regulates phosphorylation, nuclear import and subnuclear localization of TOC1 in the Arabidopsis circadian clock. EMBO J. 2010;29:1903–1915. doi: 10.1038/emboj.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Barnaby JY, Tada Y, Li H, Tor M, Caldelari D, et al. Timing of plant immune responses by a central circadian regulator. Nature. 2011;470:110–114. doi: 10.1038/nature09766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu JF, Nakamichi N, Sakakibara H, Nam HG, Wu SH. LIGHT-REGULATED WD1 and PSEUDO-RESPONSE REGULATOR9 form a positive feedback regulatory loop in the Arabidopsis circadian clock. Plant Cell. 2011;23:486–498. doi: 10.1105/tpc.110.081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- Warren DM, Wilkins MB. An endogenous rhythm in the rate of dark-fixation of carbon dioxide in leaves of Bryophyllum fedtschenkoi. Nature. 1961;191:686–688. [Google Scholar]
- Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, et al. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell. 2006;18:2971–2984. doi: 10.1105/tpc.106.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JF, Wang Y, Wu SH. Two new clock proteins, LWD1 and LWD2, regulate Arabidopsis photoperiodic flowering. Plant Physiol. 2008;148:948–959. doi: 10.1104/pp.108.124917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hotta CT, Dodd AN, Love J, Sharrock R, Lee YW, et al. Distinct light and clock modulation of cytosolic free Ca2+ oscillations and rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 promoter activity in Arabidopsis. Plant Cell. 2007;19:3474–3490. doi: 10.1105/tpc.106.046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Sato E, Shimizu T, Nakamich N, Sato S, Kato T, et al. Comparative genetic studies on the APRR5 and APRR7 genes belonging to the APRR1/TOC1 quintet implicated in circadian rhythm, control of flowering time, and early photomorphogenesis. Plant Cell Physiol. 2003;44:1119–1130. doi: 10.1093/pcp/pcg148. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh N, Sulpice R, Graf A, Stitt M, Fisahn J. Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 2011;34:877–894. doi: 10.1111/j.1365-3040.2011.02286.x. [DOI] [PubMed] [Google Scholar]
- Yerushalmi S, Yakir E, Green RM. Circadian clocks and adaptation in Arabidopsis. Mol. Ecol. 2011;20:1155–1165. doi: 10.1111/j.1365-294X.2010.04962.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Wang ZY, Chen Z, Gu H, Qu LJ. Constitutive expression of CIR1 (RVE2) affects several circadian-regulated processes and seed germination in Arabidopsis. Plant J. 2007;51:512–525. doi: 10.1111/j.1365-313X.2007.03156.x. [DOI] [PubMed] [Google Scholar]