Abstract
No other theme in animal biology seems to be more central than the concept of employing strategies to survive and successfully reproduce. In nature, controlling or avoiding pathogens and parasites is an essential fitness strategy because of the ever-present disease-causing organisms. The disease-control strategies discussed here are: physical avoidance and removal of pathogens and parasites; quarantine or peripheralization of conspecifics that could be carrying potential pathogens; herbal medicine, animal style, to prevent or treat an infection; potentiation of the immune system; and care of sick or injured group members. These strategies are seen as also encompassing the pillars of human medicine: (i) quarantine; (ii) immune-boosting vaccinations; (iii) use of medicinal products; and (iv) caring or nursing. In contrast to animals, in humans, the disease-control strategies have been consolidated into a consistent and extensive medical system. A hypothesis that explains some of this difference between animals and humans is that humans are sick more often than animals. This increase in sickness in humans leading to an extensive, cognitively driven medical system is attributed to an evolutionary dietary transition from mostly natural vegetation to a meat-based diet, with an increase in health-eroding free radicals and a dietary reduction of free-radical-scavenging antioxidants.
Keywords: behavioural defences, parasites, medicine, herbal medicine, animal behaviour
1. Introduction
Probably, no other theme in animal biology is more central than the concept that animals employ various strategies, or coping mechanisms, which enable them to survive into reproductive adulthood and assure survival of their offspring. The fact that animals must avoid predators and acquire resources in order to survive to reproductive age and successfully rear young ones shapes various aspects of social, feeding and reproductive behaviour. The existence of disease-causing viruses, bacteria and parasites represents a major force shaping behaviour that is, arguably, as profound as the forces having to do with predator avoidance or resource utilization.
Because the domestic animals all around us tend to live in clean environments, and are vaccinated against diseases and medically treated when sick, it is easy to forget that their ancestors evolved and thrived in natural environments with an array of pathogens and parasites long before these protective measures were available. Survival of animals in nature, and indeed survival of ancient humans prior to the advent of modern medicine, reflects the efficacy of various behavioural strategies used to avoid, control or rid the body of life-threatening pathogens and parasites.
Previously, I have categorized five types of behaviours used for disease control by animals: avoidance of pathogens and parasites, controlled exposure to pathogens to potentiate the immune system, the behaviour of animals when they get sick (sickness behaviour) to facilitate the febrile response in combating illness, helping sick group mates, and sexual selection of mating partners with the genetic endowment for resistance to parasites [1]. The universal occurrence of sickness behaviour (anorexia, depression and inactivity) when vertebrates become ill with virtually any febrile disease is discussed elsewhere [2,3], and will not be dealt with here. This paper will also not address the currently active field of sexual selection for disease resistance, which has become quite complex over the past one or two decades (e.g. [4,5]).
The specific behaviours to be discussed under separate headings in this discourse are: (i) ways in which animals physically avoid or remove pathogens or parasites from their bodies; (ii) quarantine, animal style; (iii) herbal medicine in animals; (iv) facilitating immunological competence by exposure to small samples of potentially infectious pathogens; and (v) the behaviour of helping sick or injured group mates.
An underlying assumption in animals is that these behavioural patterns for disease-control are not cognitively implemented; animals cannot see, and usually would not feel, the bacteria, viruses or parasites involved. Consequently, these behaviours must have a strong genetic or evolved basis. Also, the concept of disease avoidance does not imply that individuals remain disease-free any more than predator avoidance implies that animals never succumb to predation. Potentially harmful bacteria, viruses and parasites are commonly carried at a level that does not cause any noticeable decrement in health, but might cause active disease when there are extreme demands on an animal's resources such as during a food shortage; clearly, animals living in nature do sometimes succumb to disease and parasitism.
An important point to be made is that in the species studied, only one or two of the disease-control strategies is typically apparent. The examples chosen are to illustrate the variety of species and the different diseases that are apparently controlled by these strategies.
One of the most pervasive developments over the course of human history is the practice of medicine; no animal has the variety, or degree, of disease-control involvement seen in modern-day humans. Whether the practitioner was a devoted Neolithic mother caring for a sick child, or a traditional healer in an ancient Egyptian pharaoh's court administering a medicinal herb to relieve gastrointestinal pain, the roots of modern medicine go back to our earliest human ancestors. The title of the widely acclaimed book by medical historian, Roy Porter [6], The greatest benefit to mankind: a medical history of humanity, encapsulates the pivotal role of medicine in human history. Consequently, I will introduce the parallels between strategies of avoiding or dealing with disease in animals and humans much as behaviourists study the parallels in animals and humans with regard to tool use and language development.
The pillars of human medicine, as one could recognize them today are: (i) quarantine of potentially infectious conspecifics; (ii) medication to treat or prevent infections from pathogens; (iii) potentiation of the immune system, primarily through vaccinations; and (iv) caring or nursing of sick or injured group members. By discussing both the animal and human sides of the pillars of medicine, some useful perspectives may emerge, again, not unlike that seen in discussing the parallels in tool use and language development in humans and animals.
2. Natural defence strategies in animals against pathogens and parasites
(a). Strategy 1. Avoiding and removing pathogens and parasites
The variety of behavioural patterns involved in avoidance of disease constitutes the most pervasive aspect of disease and parasite control in animals and includes components of feeding, eliminative grooming, social, sexual, maternal and sleeping behaviours. Only some examples will be cited here. Additional examples are reviewed in a number of publications elsewhere [1,7–9].
(i). Grooming to remove ticks, lice and fleas
The pelage and plumage of animals serve as a home for a variety of parasitic arthropods. Although mammals and birds can, and usually do, carry a few ectoparasites with no effects on health or fitness, parasites have the capacity to reproduce exponentially and, without defensive mechanisms, animals could be overcome by ectoparasites. Mammals and birds have developed behavioural means to remove ectoparasites from their pelage or plumage by grooming or preening [1,9].
Some of the earliest observations were on mice where it was found that grooming could reduce a louse population to a fraction of that of controls [10]. Fleas are much more mobile than lice and, of course, less easily removed by grooming. However, a thorough groomer like a cat can control a flea infestation, and in a study of cats, grooming reduced flea numbers by one-half [11]. Among ungulates, the most compelling evidence for the removal of ectoparasites by grooming comes from a study on impala, an African antelope of medium size, where grooming reduced adult tick numbers to one-twentieth that of controls [12].
(ii). Fly-repelling behaviour
Virtually, all people are familiar with human fly-repelling behaviours by our own swatting of mosquitoes. While we may not be aware of the significant loss of blood that an animal in nature may experience from biting flies, we are familiar with the diseases that are transmitted by flying insects. Animals have a variety of fly-repelling behaviours, including ear twitching, head-tossing, leg stamping, muzzle flicking, muscle twitching and tail switching [13–17]. When biting fly intensity is high, fly-repelling behaviour increases and those that engage in the most fly-repelling have fewer biting flies around them.
Some of the larger animals, such as elephants, cannot be as nimble as the smaller ones in repelling flies. For the highly intelligent elephant, the way around this is to fabricate a tool from a branch to use as a fly switch (figure 1). The switch does, in fact, reduce the numbers of biting flies around them [18], and when the branch they find is too long or bushy, they modify it to a suitable size [19].
Figure 1.
Fly switching by captive elephants, as in wild elephants, increases when flies are present and the behaviour significantly reduces the number of blood-sucking flies [18]. (Photo by the author.)
(iii). Feeding and eliminative behaviours to avoid parasites and pathogens
Intestinal parasites characteristically lay eggs which pass out with the faeces; when the eggs hatch into larvae, after a couple days, they are then infectious. Animals have a variety of behaviours that relate to both feeding and elimination that reduce their exposure to infectious stages of intestinal parasites. Avoiding grazing on forage adjacent to recently dropped faeces has been documented in sheep [20], horses [21] and cattle [22].
While avoidance of faeces is an effective way of reducing intestinal parasite larvae, selective defaecation is another. This is particularly true of animals that spend time around one particular den or rest area, such as felids and canids that practice strict den sanitation. Owners of pet dogs take advantage of this den-sanitation behaviour in housetraining their dogs [23]. Young carnivores, however, are born without the mobility to eliminate away from the den, and would be at risk of re-infesting themselves with intestinal parasites, were it not for their mother's vigilance; mother dogs and cats quickly consume faecal deposits of the newborn, keeping the den clean. This behaviour does not expose the mother to more intestinal parasites because she picks up the stools immediately, before parasite eggs can hatch into infectious larvae [23].
One aspect of feeding behaviour that would seem to put carnivores and omnivores at risk for pathogens is feeding upon the carcass of a dead animal. Most carnivores or omnivores will, in fact, scavenge upon the carcass of a member of another species that it runs across. Even if the dead animal had died of an infectious disease, the animal feeding upon it is not likely to contract the disease, because in most instances, pathogens are species-specific. If, however, the carcass is that of a conspecific that has recently died, there is a chance that the animal would contract the disease which initially killed the conspecific [1]. It turns out that animals will virtually never feed on the carcass of a recently dead conspecific, a well-documented phenomenon referred to as the cannibalism taboo [24,25].
(iv). Licking: use of the medicine cabinet in the mouth
A source of systemic infection is through untreated open wounds that animals may have acquired from fights or in confronting prey. As an adaptation to this potential risk, animals of several species readily lick their wounds and liberally apply a coat of saliva on the wound. Saliva contains a surprising number of antibacterial and wound-healing substances including lysozyme, lactoferrin, leucocytes, lactoperoxidase and immunoglobins [26,27], along with epidermal growth factors [28,29]. When used together with the simple tongue washing of areas to be cleaned, saliva is a readily available, all-purpose medicinal ointment. As an indication of the effectiveness of this treatment, saliva of dogs was found to be bactericidal to both Escherichia coli and Streptococcus canis that are common wound contaminants [30].
The predisposition of animals to lick body parts is one of the reasons we rarely hear about devastating sexually transmitted diseases in animals in nature. Male rats, like dogs and cats, compulsively lick the penis after copulation [31]. Rat saliva is effective in killing two pathogens implicated in rodent genital infections (Mycoplasma pulmonis and Pasteurella pneumotropica) [32]. Penis licking not only cleanses the penis physically, but the antibacterial saliva also protects the male from contracting venereal diseases from the female they have just mated and from passing such infections onto females they subsequently mate.
One more example serves to illustrate the value of the portable medicine cabinet, ready for immediate use; this is an integral aspect of good mothering. Consider the plight of newborn in nature in a dirty den, used season after season, where adults are tracking in all sorts of bacteria from the outside world. The lactating mother drags her nipples over all this filth going to and from the litter. Newborn have an undeveloped immune system, lacking the protective intestinal bacterial flora of adults, and have intestinal epithelium that is permeable to bacteria for the first 48–72 h [33]. As a protective measure, mothers generously lick the nipples just before the newborn start to suckle, applying a salivary wash that is bactericidal for common disease-causing pathogens [30].
Here is one final word about the medicinal effects of saliva. Most of the information comes from studies on human saliva as an aspect of dental medicine to understand more about the presence or absence of cavities. In contrast to the intuitive meaning of the expression ‘go lick your wounds’, actual wound licking is not mentioned in treatises on management of even minor wounds. The current meaning is something like taking time to recover from a bad experience. One would expect that ancestral Homo sapiens, without antibiotic ointments around, would have made frequent use of this type of disease prevention. The wise matriarch of a family may well have instructed a wounded family member to ‘go lick your wounds’.
The general strategy of physically avoiding and removing pathogens and parasites discussed above is an ongoing area of research that is generally supported by empirical data with appropriate control groups. The other disease-control strategies, discussed below, are derived more from naturalistic and opportunistic observations and, while necessary to fully understand disease-control systems in nature, do not tend to lend themselves to the experimental paradigm.
(b). Strategy 2. Quarantine, animal style
In the animal world, one would expect to see a type of quarantine, or active repelling of strange conspecifics, when a close encounter would possibly transmit strange pathogens to the residents. This is a function of territorial defense that is usually not mentioned along with protection of mates, nesting sites and food resources. In primates, for example, peripheralization of the stranger may include being repeatedly threatened, but with no physical contact [34,35]. Freeland mentions that this type of enforced peripheralization could provide behavioural and nutritional stress which could make the intruder visibly sick, if it is harbouring a latent infection. In such cases, the intruder may not be allowed into the group, or may die [35].
Another type of quarantine for disease control that is not necessarily appreciated by either animal behaviourists or veterinarians is seen in the occasional cannibalism of newborn in species that give birth to multiple young, like dogs and cats. Although newborn animals are mostly resistant to indigenous pathogens by virtue of antibodies in colostrum, the occasional newborn may become sick if it does not receive sufficient colostrum or suffers from inadequate genetically acquired resistance. If allowed to continue as part of the litter, the sick neonate could act as a reservoir for multiplication of the pathogen to sufficient numbers to overwhelm the nascent immunity of the littermates. A mother, by reacting to the early signs of a sick infant, such as inactivity and hypothermia, and disposing of the sick infant, may well save the rest of the litter [23]. Not surprisingly, laboratory investigators and animal breeders have long recognized that it is often the sick infants that are most likely to be cannibalized [36].
(c). Strategy 3. Use of medicinal herbs: animals practise medicine
Here is a disease-control strategy that is rather limited among animal species, but where studied or reported is quite convincing. Two general types of herbal medicine are seen: the prophylactic mode to prevent damaging infections, usually from parasites; and the therapeutic mode to actively treat and remove a pathogen, usually a bacteria. In animals, the prophylactic mode is by far the most frequently seen and documented by data-based empirical studies [37].
As a way of introducing this strategy, reflect on the importance of dog owners having their pets checked for intestinal parasites and, if infected, treating with an appropriate anthelmintic medication. Now consider the wild canid, constantly exposed to worm-infected faecal droppings from other canids, but without any powerful medication to avoid an excessive parasite load. The apparent medication in nature for this disease challenge is still seen by multitudes of dog and cat owners as a reflection of a behaviour inherited from their wild ancestors, when their animals go out and munch on a bit of grass (figure 2). Assuming that the function of grass or plant eating in pet dogs and cats is intestinal parasite control, then this is the most common example of herbal medicine, animal style, that people see. Plant eating, as a regular prophylactic means of clearing out parasites, is the predecessor of anthelmintic medical treatment.
Figure 2.
Dogs, like cats, regularly eat plants, especially grass. Contrary to common beliefs the behaviour is not typically preceded by signs of illness nor followed by vomiting; the dogs are usually normal in behaviour, as reported by the owners. Plant eating would appear to be a behaviour inherited from wild ancestors that, in nature, had a role in maintaining low intestinal parasite loads [38]. Photo courtesy of Sheila D'Arpino, of her dog Cinco.
Dogs, like wild canids, would not be expected to know whether or not they are infected with worms. Plant material has been found in 2–4% of scats and stomach content samples of wolves and cougars, revealing that they regularly consume non-digestible plants [39–42]. The famous wolf biologist, Murie, described seeing leaves of grass associated with expelled intestinal worms in wolf scats [43].
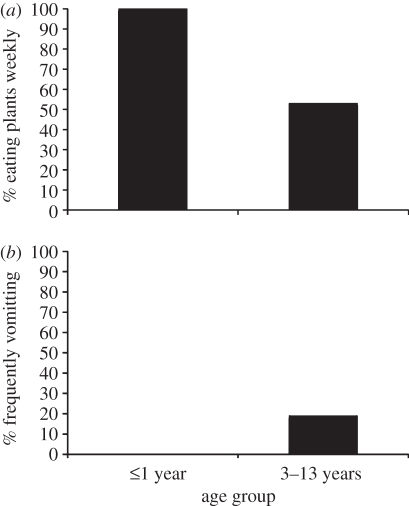
Looking for evidence that plant eating in dogs evolved as a means of intestinal parasite control, rather than reflecting a dietary deficiency or a way of inducing vomiting in sick dogs, as commonly believed, a broad ranging web-based survey of thousands of dog owners was posted. It was found that only 9 per cent of the plant-eating dogs regularly appeared ill prior to eating plants and only 22 per cent regularly vomited afterwards; the great majority appeared normal [38]. An important finding was that dogs under 1 year of age ate plants much more frequently than the older dogs and were even less likely to appear ill beforehand or vomit afterwards (figure 3). In nature, the young are most vulnerable to intestinal parasites, having not developed some adult-like immunity to the worms and being more vulnerable to the loss of blood from parasites.
Figure 3.
A comparison of plant eating by dogs up to 1 year of age and 3–13 years of age. (a) The younger dogs eat plants more frequently. (b) Not only do younger dogs appear ill beforehand less frequently, but they also rarely vomit afterwards. These differences reflect an apparent innate developmental adaptation to the young being more vulnerable to the costs of intestinal parasites and eating the parasite-purging plants more frequently [38].
While pet dogs offer the most familiar example of this type of disease control, a parallel in chimpanzees, that swallow whole leaves as a means of intestinal parasite control, is the most well-studied by direct observation. Wild chimpanzees eat leaves from a variety of plants which pass through the intestinal tract undigested. In some instances, the plant material increases intestinal motility that then purges the intestinal tract of nematodes [44]. Sometimes the leaves are even seen associated with expelled worms, just as Murie reported seeing in wolf scats [43].
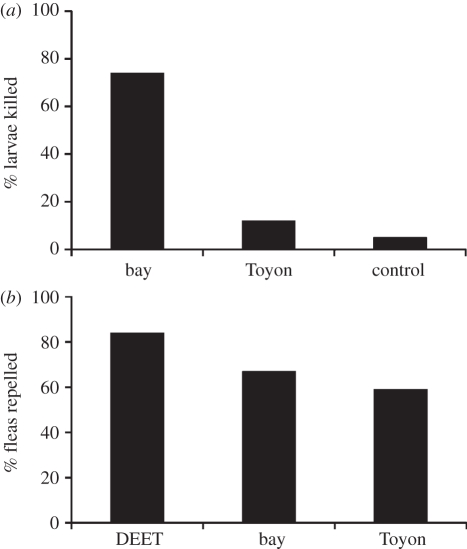
Another, frequently occurring, but more obscure, example of prophylactic herbal medicine is seen in the northern California dusky-footed wood rats that bring fresh bay leaves into their sleeping nests with the apparent effect of controlling for an over abundance of fleas. One in vitro study with flea larvae found that torn bay leaves killed 75 per cent of the larvae, compared with almost none by control plants (figure 4) [45]. A second in vitro study, testing the effect of bay leaf extract in repelling adult fleas, found that the bay leaf extract repelled fleas 80 per cent as effectively as the powerful repellant N,N-diethyl-m-toluamide (DEET) (figure 4; A. Alvarado & B. L. Hart 2005, unpublished data). This nest fumigation study in wood rats is a mammalian counterpoint to a similar phenomenon in starlings where plants are woven into the nests when young starlings are present. The plants, which like bay leaves are quite aromatic, retarded the hatching of louse eggs and inhibited growth of nest-borne bacteria [46,47].
Figure 4.
In vitro experiments on the effects of bay leaves, which are brought into the nests of dusky-footed wood rats, in controlling fleas by killing the larvae or repelling adult fleas. (a) Effects of incubation of torn bay leaves, and leaves from control plants, in killing flea larvae. Bay leaves killed significantly more larvae than control plants [45]. (b) Effects of extracts of bay leaves, compared with the commercial repellant, DEET, and control plant, Toyon, in repelling adult fleas. Bay leaf extract did not differ significantly from DEET in repelling fleas, whereas Toyon did significantly repel fewer fleas than DEET (A. Alvarado & B. L. Hart 2005, unpublished data).
The therapeutic use of medicinal plants, more akin to what we think of with regard to traditional human herbal medicine, is relatively rare in the animal world. The most convincing example in animals involves chimpanzees. An apparently ill chimpanzee was seen extracting, and chewing upon, the bitter pith of a Vernonia sp. plant that has known antimicrobial effects [48].
An interesting side in this section is that information on the specific constituents of plant parts that convey the therapeutic effects has come from work on animal models, albeit in the laboratory rather than in nature, where various medicinal herbs have been shown to have one or more of the following properties: anti-inflammatory, antimicrobial, immunomodulatory and analgesic [37]. The medicinal plant parts usually have a bitter or astringent marker taste indicating efficacy. The therapeutic use of such herbs, seen in the chimpanzee example above, implies that the sick individual experiences illness or pain and subsequently seeks a biologically effective, usually bitter-tasting, medicinal product: the ‘bitter pill’ in nature.
(d). Strategy 4. Immunization, animal style
In the current climate of health management of both people and domestic animals, we tend to think of man-made vaccines as providing a basis for immunity for common diseases. But, of course, long before the advent of vaccines, animals and humans acquired immunity to the pathogens in their immediate environment. The passive antibodies against local pathogens, provided to the young through maternal colostrum, gradually disappear. Subsequently, self-sustaining antibodies are developed through exposure to potentially pathogenic organisms in small doses sufficient to evoke antibody production without causing disease [49]. The immune system is ‘personalized’ according to the particular environment. Because much of the exposure to sensitizing doses for antibody production is incidental to interacting with conspecifics, particular behaviours related to the acquisition of immunity in animals are a bit difficult to discern.
However, potentiation of the immune system, which is so important to survival, is a lifetime, ongoing process and one would expect behaviours to be selected that provide sensitizing doses to new pathogens when the environment changes a bit. In the discussion above of the quarantine of would-be intruders that may be carrying strange pathogens into a closely knit group, the peripheralization of strangers can also have an immune-potentiating effect. The enforced space barrier between residents and the stranger allows the residents to experience a small immune-sensitizing dose of foreign pathogens from the stranger through faecal droppings and shared water resources. Similarly, the stranger, that may be eventually allowed into the group, becomes gradually immune to germs of the residents [1].
Newborn of primate groups must acquire immunity to the group's potential pathogens, and the more quickly the young of the species mature, the more quickly this immune development must occur. The behaviour of mothers in some early maturing species in intentionally passing their infants around to group members, as cited by Freeland [35], is an example of immune system priming because species with a longer maturation stage tend not to engage regularly in such behaviour.
The weanling young of carnivores are likewise up against an environment full of strange potential pathogens. A system that evidently meets this demand is seen when mothers bring back kills that the young consume. While providing the post-weaning bridge to hunting, her behaviour of dragging the kill over a bit of the filth and germs on the floor of the den, picking up samples of local micro-organisms, helps sensitize the immune system of her young [1]. As with quarantine, immune-priming behaviours that play a role in disease protection can also be important for introducing the young to real food, protection of food resources and maintaining good nesting sites.
(e). Strategy 5. Caring of the sick and injured
This strategy enjoys rather widespread taxonomic representation, undoubtedly because caring for sick or injured group members can be critical in helping a functional member of the group, or a close relative, recover. All the examples come from opportunistic observations by seasoned field investigators.
A particularly detailed account of caring and support was recorded in a group of dwarf mongooses. The normal behaviour of mongooses is to forage for insects during the day and then overnight in a termite mound, moving the next day to a new foraging site and different termite mound at nightfall. On this occasion, a male member of the investigator's study group was bitten by a snake so severely that the skin over the inner side of the left leg was torn off. The injured animal was immediately surrounded by other group members and they went, with the injured animal, into the nearest termite mound and remained inside for the rest of the day and night, and did not move on the next day. Group members frequently groomed the injured one who was also provided with food from group members [50].
Some other noteworthy examples, illustrating the species diversity of this strategy, are a wounded lioness in the Serengeti, which was unable to hunt and was provided food by other members of the pride for nine months [51]. In foxes, an ill female was provisioned by a male partner and another fox provisioned by a sister [52].
A detailed account of caring was documented by Huffman & Seifu [48] of the same sick female referred to previously that selected and chewed on the pith of a Vernonia sp. stalk. Her companions slowed their movement so that the ill female could follow and took care of her young one when she was resting. In the morning, when the sick female did not leave her bed, another female built a bed next to her and lay there for some time.
Of all non-human animals, the helping of disabled conspecifics is particularly evident in African elephants, and here there are many accounts by seasoned field investigators [53,54]. Typical of such accounts is a description of a young immobilized elephant that was ‘lifted repeatedly on the tusks of the older cows, until, after two hours, she was able to stand and was marched off into the forest’ [55].
A recent observation involved GPS radio-tagged animals, field observations and photo documentation. The investigators were able to follow reactions of elephants to a matriarch, Eleanor, who had been severely injured in a fall and was seen with a swollen trunk. The staggering, severely injured elephant was approached by another female who lifted and pushed her, repeatedly, in an attempt to get her to walk [56].
Further observations relating to the severely injured matriarch, Eleanor, referred to above, introduced a syndrome in elephants directly related to helping disabled conspecifics, which is described as ‘grieving’ and ‘standing vigilant’ over the body of a recently deceased conspecific. Eleanor was so disabled that she could not get up and died the next day. On the day of her death, a family member spent 7 h in the vicinity of the corpse. During that time, a female from another family hesitantly approached Eleanor's body, extended her trunk, sniffed the body and then touched it, as if to confirm that she was dead. During the 5 days after her death, the body was visited not only by family members, but also elephants from three other families (figure 5). This description of grieving, or standing vigilant over a recently deceased family member is not unlike those of many other accounts in the literature and recently reviewed [53,54].
Figure 5.
Example of social-empathic behaviour in elephants showing the grieving-like, or standing-vigilant behaviour over a recently deceased elephant. In this instance, prior to the severely injured matriarch's death, repeated attempts were made to help her get up. After her death her body was visited on a daily basis by different elephants [56]. (Photo courtesy of Shivani Bhalla—Save the Elephants.)
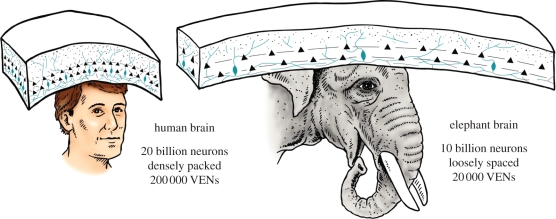
Helping of sick or injured conspecifics, and standing vigilant over deceased group members, are referred to as empathic or social-empathic behaviours, and elephants clearly stand out in this aspect of disease-related behaviours. A new development in the biological understanding of social-empathic behaviours is the discovery, in the elephant, of unique, large, spindle-shaped neurons in layer 4 of the cerebral cortex, referred to as von Economo neurons (VENs) that are believed to be involved in the mediation of social-empathic behaviours. The neurons were first described for humans, but recent work reveals their presence in elephants as well cetaceans and great apes [57,58]. Elephants have proportionately more VENs than chimpanzees, but proportionately the same as humans [57]. Figure 6 portrays the position of VENs in humans and elephants. The predisposition of elephants to readily engage in human-like, social-empathic caring and grieving adds a biological underpinning of this disease-related behaviour, shared especially with humans.
Figure 6.
A diagrammatic illustration of the unique von Economo neurons (VENs), in the human and elephant cerebral cortex. The information-processing pyramidal neurons, profiled here as black triangles are layers 3 and 5. The blue (grey) spindle-shaped VENs, which are much less dense, are believed to mediate social-empathic behaviours, such as caring and grieving over deceased conspecifics. Illustration by the author is based on published data [57,58].
In summary of this section, the disease-control strategies discussed here range from those that are supported by numerous data-based empirical studies, especially in the physical-avoidance behaviours, to those that are opportunistic, but self-evident with regard to function and adaptive value. No species comes across as regularly engaging in behaviours from all of the categories. Rather, different disease-control behaviours are evident in different species depending upon the disease risks to which they are primarily exposed in their particular environments. The level of cognitive ability does not, at this time, come across as a deciding factor in the degree to which disease-control strategies are implemented.
3. The pillars of medicine: parallels with animals
The four pillars of human medicine, as proposed here, are extensions of some of the defences against pathogens and parasites discussed above. As with comparisons in language and tool use between animals and humans, the differences in the practice of medicine clearly reflect differences with regard to cognitive and linguistic ability. I will argue, however, that another difference is that humans get sick more often than animals and therefore implement the strategies for dealing with sickness more often and more extensively. This latter hypothesis will be discussed after a brief introduction of the pillars of medicine in humans and the counterparts in animals.
(a). Pillar 1: quarantine
In today's world, quarantine is a rather drastic step in human disease control. However, it is considered from time-to-time as a means to limit some diseases that represent a risk to large populations when vaccinations are not successful. The historic treatment of individuals with leprosy and the classical tuberculosis sanitariums are familiar examples. Quarantine is more common in the husbandry of domesticated animals. Well-known is the quarantine of pets being introduced to certain countries. The killing of diseased animals, or those that might be carriers of disease, is a form of quarantine. The consistency with which territorial animals attempt to exclude or peripheralize strange conspecifics that may be carrying alien pathogens is an obvious parallel to quarantine from the animal world.
(b). Pillar 2: medication
A contrast with the prophylactic use of disease-controlling plants by animals, such as eating of plants for control of intestinal parasites or using bay leaves to fumigate nests for fleas, is that in humans, the use is largely therapeutic, to treat an illness. However, the parallel between the use of medicinal plant products in animals and the use of medications by humans is that medicinal herbs are the forerunners of most modern pharmaceuticals, and still constitute the basis for medication in most of the world's population which is little influenced by Western medicine [59–61].
This parallel is underscored by finding signs of herbal medicine in humans going back several thousand years. Analyses of the possessions found on the 5300 year-old ‘iceman’ discovered in 1991 in the Italian Alps included pieces of birch fungus, presumably used as a laxative and antibiotic [62]. Even earlier indications of the use of medicinal herbs by hominids come from excavations of a 60 000 year-old encampment of Neanderthals in the Shanidar Cave in Kurdistan, where analysis of pollen samples from the floor of the cave revealed the presence of plants which could have been used for medicinal purposes. The putative medicinal plants included species of the genera with known medicinal qualities, especially anti-inflammatory effects (Achillea, and Althaea) and the familiar stimulant, Ephedra [63].
Indications of the use of medicinal herbs by hominids in prehistoric times raises the question of whether herbal medicine is the result of learning that a certain plant part is effective for treating a certain malady, or a result of natural selection for a behavioural predisposition to seek out and use plant parts with particular physical or chemosensory markers of efficacy. Examination of the predictions and requirements of both the learned and evolutionary explanations for the origin of herbal medicine points primarily to an evolutionary process which was expanded upon by learning and social transmission [37]. The cornerstone of this explanation is that individuals suffering from acute illness or inflammatory conditions are drawn to bitter or astringent plant parts that are likely to have anti-inflammatory, antimicrobial, immunomodulatory and/or analgesic effects that may be effective in a non-specific manner. The evolutionary perspective explains why ineffective plant parts are maintained in the repertoire of putative medicinal herbs [37].
(c). Pillar 3: immunization
As discussed above, long before the recent advent of vaccines, immunity to local pathogens in both animals and humans was acquired through exposure to potentially pathogenic organisms sufficient to evoke antibody production but in doses that did not cause disease [49]. The advent of vaccines was obviously a reflection of the cognitive prowess and language ability of humans. Interestingly, prior to vaccines, any predisposition of humans to ‘intentionally’ expose vulnerable family or group members to small, immune-sensitizing doses of foreign pathogens, as alluded to in the discussion of animals, seems not to be profiled, but certainly would be expected.
(d). Pillar 4: nursing and caring
Along with medication, caring is a highly visible aspect of human healthcare that hardly needs any elaboration. Such caring ranges from the attention a parent bestows on a sick child to the organized nursing care seen in hospitals of all types around the world. A related aspect to this pillar is the ceremonial grieving that occurs when the patient that was cared for does not recover.
The parallels in the animal world are evident from opportunistic observations which include, dwarf mongooses, chimpanzees and elephants. In elephants, the phenomenon of grieving over a conspecific who failed to recover from an illness or injury is particularly evident. As mentioned previously in §2e, the prominence of cortical VENs in both humans and elephants, that are believed to subserve social-empathic behaviours such as caring and grieving over deceased conspecifics, invites a direct comparison of elephants with humans in the biological mediation of this behaviour.
4. Do humans get sick more often than animals and hence engage more often in disease-control behaviours?
A hypothesis put forth in this discourse is that humans get sick more often than animals and, correspondingly, typically engage in medical practices more frequently than animals. There is relatively little direct evidence that might firmly refute or confirm this hypothesis. One approach is examining skeletal remains for evidence of diseases which can leave tell-tale signs such as periostitis, osteitis, osteomyelitis, tuberculosis, yaws, syphilis, leprosy and smallpox in different stages of human evolution. In support of the perspective presented here, skeletal remains of the earliest hunter–gatherers show fewer signs of infection than those of early farmers that had a more meat-based diet [64].
Another approach to gathering such evidence is to compare the frequency of acute sicknesses such as influenza, upper respiratory infections and gastrointestinal disorders in domestic animals (which are very visible to their owners) to the frequency of similar sicknesses in the owners. As a first step in this direction, questions were inserted into ongoing web-based surveys directed to dog owners, who were asked about the number of times in the past year their dog had such an acute illness and how often they, the owners, had an acute illness. A comparison of 2473 pairs of dogs and their owners found that dogs were about 50 per cent less likely to have had two or more acute illnesses in the previous year than their human family members (p < 0.01, preliminary data).
Assuming that humans do get sick more often, I am postulating that this is at least partially reflective of the transition in ancient humans to a meat-eating lifestyle that brought with it a much greater production of health-eroding free radicals and correspondingly, the predisposition to more sickness.
(a). The dietary shift to meat-eating
The transition to a meat-eating diet is viewed as starting with the Early Stone Age, about 2 Myr ago where, in the African location of hominid evolution, the environment was changing to a much more arid climate with shrinking tropical forests [65]. Foraging for tropical, energy-rich fruits, nuts and vegetables became very competitive, giving more agile forest-dwelling species an advantage over resident hominids, while the drier savannah offered a generous source of large mammals as an alternative food supply with abundant protein and energy-rich lipids [66].
The evidence of a shift to meat-eating in the hominid diet comes from several lines of recent research in addition to the indications from tooth wear, hunting tool scrapings on bones of prey and linking stone tool implements with hunting [66,67]. There were several anatomical adaptations for endurance running that would have been essential in allowing early Homo erectus to hunt large prey such as antelope, including longer legs with spring-like tendons for a long stride, a plantar arch and small toes for a fast push off, wider joints in the ankles and knees to prevent skeletal damage when the feet hit the ground, massive gluteal muscles that stabilize posture while running, and smaller, easier to swing, arms for improved postural stability [68,69]. In the Early Stone Age, even prior to the development of stone- or bone-tipped spears and arrows, endurance running would have enabled early, physically fit, swift-footed people to run down ungulate prey to exhaustion.
Evidence for a transition to a meat-eating diet also comes from studies showing in humans that the intestinal tract has a proportional increase in the meat-digesting small intestine over the plant-digesting, large intestine, caecum and colon of plant-eating primates [70,71]. A biochemical adaptation is the evolution of meat-adaptive genes in humans for the metabolic processing of fat and meat by-products, without producing dangerously high cholesterol levels [72]. Modern hunter–gatherers are considered the best available surrogate of Stone Age societies, and a recent and comprehensive review of published dietary habits of 225 hunter–gatherer societies revealed an average of about 60 per cent total energy intake from wild ungulates [73]. With the transition to a meat-based diet, a protein- and energy-demanding brain could rapidly enlarge, evolutionarily [74].
The meat-eating perspective is linked to the advent of controlled fire in preparing food at the same time that meat-eating was becoming routine. Cooking does not usually enhance the nutrition of plant foods, but does make the nutrients in meat much more available than in raw meat [75]. Hence, the widespread use of controlled fire and cooking would be an important adaptation related to the transition to a meat-based diet. Another aspect of food preparation, presumably related to meat-eating, is the advent of using spices. These spices, especially the hot ones, by virtue of antibacterial effects, not only preserve meat, but also reduce gastrointestinal upsets from eating spoiled meat [76].
(b). Illness-related consequences of a meat-based diet
In ancestral modern humans, the remarkably large brain that consumes 20 per cent of the body's energy [77], and the energy-demanding lifestyle for endurance running in bringing down large mammals, would have resulted in much greater overall energy expenditure in H. erectus and H. sapiens compared with hominids living the vegetarian lifestyle [78–80].
The utilization of energy produces free radicals in proportion to the energy used and, along with endogenous antioxidants, exogenous antioxidants play a major role in scavenging these health-eroding, free radicals [81–87]. The diets of early hominids that were dependent upon plant-based foods included the woody parts of plants such as leaves, stems, bark and roots, which are especially high in antioxidants [88,89]. However, with the transition to a meat-based diet, the intake of plant-based antioxidants decreased, exacerbating the problems arising from an increased production of health-eroding free radicals. As time went on, humans started to explore farming, selecting against bitter-tasting plants with antioxidants [88,89]. Thus, once farming began, humans would have become even more likely to get sick, as indicated by the tell-tale skeletal remains alluded to above [64].
With a large brain and a lifestyle that resulted in increased production of health-eroding free radicals, and a reduction in health-promoting antioxidants from natural vegetation, H. sapiens would have had an increased likelihood of becoming sick as well as suffering longer from illness and debilitating injuries. Thus, the stage was set for an emphasis on the pillars of medicine, especially in caring for one's own health and younger kin when sick and the therapeutic use of herbal medicines.
One challenge to the hypothesis relating the likelihood of illness to a meat-based diet, is how one deals with animals that are carnivorous but do not appear to be prone to illness in the sense laid out here for humans. Carnivores have a long evolutionary history with meat-based diets, and while biochemical information bearing on the issue is absent, they undoubtedly have evolved biochemical mechanisms for dealing with the metabolism of meat products, such as adequate amounts of endogenous antioxidants. Also, carnivores have a much smaller brain than humans, producing fewer damaging free radicals through the oxidation of glucose. And, in contrast to humans, where a longer lifespan opens the door for more cumulative exposure to free-radical damage and infectious disease, carnivores have a much shorter lifespan leaving them less opportunity to acquire life-threatening ailments.
5. Conclusions
Animals have evolved an array of behavioural strategies that enable them to live in an environment teeming with health-threatening pathogens and parasites. The most important and wide-ranging strategy is physical avoidance and removal of health-threatening parasites and pathogens. The efficacy of this strategy, seen in grooming, feeding, eliminative and licking behaviours, is revealed by numerous data-based empirical studies.
Other strategies are involved in the quarantine or peripheralization of strangers which minimizes disease exposure, the prophylactic use of herbal medicine, especially in dealing with parasites, potentiation of the immune system through controlled exposure to sensitizing doses of potential pathogens, and caring of sick or injured group mates. The latter is especially noteworthy in elephants and humans, where the cerebral cortical VENs provide a conceptual link to humans with regard to the mediation of this disease-related strategy.
To profile the parallels between the disease-control strategies in animals and those in humans, I have outlined four basic pillars of human medicine: quarantine, medication, immunization and caring. While, as mentioned, representations of each pillar can be found in one animal species or another, the integration and extensive development of these pillars seem to be uniquely human. Among the reasons for this is an increased predisposition of humans to get sick as a reflection of the consequences of production of health-eroding free radicals stemming from a transition to a meat-based diet, the evolution of a large energy-guzzling brain, the energetic demands of hunting and the concomitant reduced consumption of plant-based antioxidants.
A logical question to ask is why humans did not evolve the necessary endogenous antioxidants to deal with the surge in free radicals as the meat-eating carnivores undoubtedly have, and along the lines of the evolution of meat-adaptive genes for metabolic processing of meat by-products in humans, without producing dangerously high levels of cholesterol [72]. One should keep in mind that the essentials of the pillars of medicine were already in place in ancestral hominids, and expansion of the human brain, with immense cognitive ability, was dramatically rapid in evolutionary time [80,90]. Consequently, an increase in sickness, reflecting the increase in disease-producing free radicals from the meat-intense diet, arguably would have immediately evoked life-saving disease-control behaviours, especially herbal medicine and caring, before natural selection could operate to lead to the survival of just individuals with a suite of protective biochemical changes for processing excessive free radicals. This disease-control behavioural solution has apparently worked, and the practice of medicine has become a mainstay of human civilization.
One final point is that if, in fact, the emergence of H. sapiens was accompanied by an increased predisposition towards illness, then the ability to communicate about the pillars of medicine could have contributed an important driving force to the evolution of the biological adaptations necessary for human language capability. This conjecture becomes more plausible because of the immediate fitness value of intra-group communication, such as instructions on how to find and use medicinal herbs, administer caring and guard against exposure to strangers. Interestingly, the evolutionary emergence of the human form of the FOXP2 gene controlling neural mechanisms involved in human language [91], as well as the modern vocal tract structure necessary for speaking [92,93] appears to have occurred 100 000 years ago or less, while the expansion of the brain to the modern size was about 200 000 years ago, before the biological adaptations necessary for modern human language.
The behavioural strategies involved in the avoidance and resolution of sickness and disease will be ongoing processes that continue to be defining elements in understanding the similarities and differences between us and our animal companions living under natural conditions with ever-present pathogen and parasite challenges to health and well-being.
Acknowledgements
I particularly appreciate the useful comments from my colleagues, Prof. Lynette Hart (Veterinary Medicine), Richard Coss (Psychology) and Lynne Isbell (Anthropology) of U. Davis as well as an anonymous reviewer and, especially, the editor of this special issue. Research cited in this review by the author was supported by grants from the US National Institutes of Health and the National Science Foundation. Preparation of the review was supported by the UC Davis Centre for Companion Animal Health (allocation no. 03-65-F).
References
- 1.Hart B. L. 1990. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294 10.1016/S0149-7634(05)80038-7 (doi:10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 2.Hart B. L. 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 10.1016/S0149-7634(88)80004-6 (doi:10.1016/S0149-7634(88)80004-6) [DOI] [PubMed] [Google Scholar]
- 3.Hart B. L. Beyond fever: comparative perspectives on sickness behavior. In Encyclopedia of animal behavior, vol. 1 (eds Breed M., Moore J.), pp. 205–210 Oxford, UK: Academic Press [Google Scholar]
- 4.Reid J. M., Arcese P., Cassidy A. L. E. V., Marr A. B., Smith N. M., Keller L. F. 2005. Hamilton and Zuk meet heterozygosity? Song repertoire size indicates inbreeding and immunity in song sparrows (Melospiza melodia). Proc. R. Soc. B 272, 481–487 10.1098/rspb.2004.2983 (doi:10.1098/rspb.2004.2983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mays H. L., Jr, Albrecht T., Liu M., Hill G. E. 2008. Female choice for genetic complementarity in birds: a review. Genetica 134, 147–158 10.1007/s10709-007-9219-5 (doi:10.1007/s10709-007-9219-5) [DOI] [PubMed] [Google Scholar]
- 6.Porter R. 1997. The greatest benefit to mankind: a medical history of humanity. New York, NY: W. W. Norton [Google Scholar]
- 7.Hart B. L. 1992. Behavioral adaptations to parasites: an ethological approach. J. Parasitol. 78, 256–265 10.2307/3283472 (doi:10.2307/3283472) [DOI] [PubMed] [Google Scholar]
- 8.Hart B. L. 1994. Behavioral defense against parasites: interaction with parasite invasiveness. Parasitology 109, 5139–5151 10.1017/S0031182000085140 (doi:10.1017/S0031182000085140) [DOI] [PubMed] [Google Scholar]
- 9.Hart B. L. 1997. Behavioural defence. In Host–parasite evolution: general principles and avian models (eds Clayton D. H., Moore J.), pp. 59–77 Oxford, UK: Oxford University Press [Google Scholar]
- 10.Murray M. D. 1987. Effects of host grooming on louse populations. Parasitology 3, 276–278 10.1016/0169-4758(87)90105-0 (doi:10.1016/0169-4758(87)90105-0) [DOI] [PubMed] [Google Scholar]
- 11.Eckstein R. A., Hart B. L. 2000. Grooming and control of fleas in cats. Appl. Anim. Behav. Sci. 68, 141–150 10.1016/S0168-1591(00)00095-2 (doi:10.1016/S0168-1591(00)00095-2) [DOI] [PubMed] [Google Scholar]
- 12.Mooring M. S., McKenzie A. A., Hart B. L. 1996. Grooming in impala: role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiol. Behav. 59, 965–971 10.1016/0031-9384(95)02186-8 (doi:10.1016/0031-9384(95)02186-8) [DOI] [PubMed] [Google Scholar]
- 13.Espmark Y. 1961. Behavior of reindeer attacked by nostril and grub flies. Zool. Rev. 2, 28–37 [Google Scholar]
- 14.Harvey T. L., Launchbaugh J. L. 1982. Effect of horn flies on behavior of cattle. J. Econ. Entomol. 75, 25–27 [Google Scholar]
- 15.Muller M. J., Murray M. D. 1977. Blood sucking flies feeding on sheep in Eastern Australia. Aust. J. Zool. 25, 75–85 10.1071/ZO9770075 (doi:10.1071/ZO9770075) [DOI] [Google Scholar]
- 16.Okumura T. 1977. The relationship of attacking fly abundance to behavioral responses of grazing cattle. Jpn. J. Appl. Entomol. Zool. 21, 119–122 10.1303/jjaez.21.119 (doi:10.1303/jjaez.21.119) [DOI] [Google Scholar]
- 17.Harris J. A., Hillerton J. E., Morant S. V. 1987. Effect on milk production of controlling muscid flies, and reducing fly-avoidance behaviour, by use of Fenvalerate ear tags during the dry period. J. Dairy Res. 54, 165–171 10.1017/S0022029900025309 (doi:10.1017/S0022029900025309) [DOI] [PubMed] [Google Scholar]
- 18.Hart B. L., Hart L. A. 1994. Fly switching by Asian elephants: tool use to control parasites. Anim. Behav. 48, 35–45 10.1006/anbe.1994.1209 (doi:10.1006/anbe.1994.1209) [DOI] [Google Scholar]
- 19.Hart B. L., Hart L. A., McCoy M., Sarath C. R. 2001. Cognitive behavior in Asian elephants: use and modification of branches for fly switching. Anim. Behav. 62, 839–847 10.1006/anbe.2001.1815 (doi:10.1006/anbe.2001.1815) [DOI] [Google Scholar]
- 20.Taylor R. J. 1954. Grazing behavior and helmintic disease. Br. J. Anim. Behav. 2, 61–62 10.1016/S0950-5601(54)80033-5 (doi:10.1016/S0950-5601(54)80033-5) [DOI] [Google Scholar]
- 21.Odberg F. O., Francis-Smith K. 1977. Studies on the formation of ungrazed eliminative areas in fields used by horses. Appl. Anim. Ethol. 3, 27–34 10.1016/0304-3762(77)90068-2 (doi:10.1016/0304-3762(77)90068-2) [DOI] [Google Scholar]
- 22.Michel J. F. 1955. Parasitological significance of bovine grazing behaviour. Nature 175, 1088–1089 10.1038/1751088a0 (doi:10.1038/1751088a0) [DOI] [Google Scholar]
- 23.Hart B. L., Hart L. A., Bain M. J. 2006. Canine and feline behavior therapy, 2nd edn Ames, IA: Blackwell [Google Scholar]
- 24.Fox L. R. 1975. Cannibalism in natural populations. Ann. Rev. Ecol. Syst. 6, 719–728 10.1146/annurev.es.06.110175.000511 (doi:10.1146/annurev.es.06.110175.000511) [DOI] [Google Scholar]
- 25.Carr W. J., Hirsch J. T., Campellone B. L., Marasco E. 1979. Some determinants of a natural food aversion in Norway rats. J. Comp. Physiol. Psychol. 93, 899–906 10.1037/h0077612 (doi:10.1037/h0077612) [DOI] [Google Scholar]
- 26.Bowen W. H. 1974. Defense mechanisms in the mouth and their possible role in the prevention of dental caries: a review. J. Oral Pathol. 3, 266–278 10.1111/j.1600-0714.1974.tb01721.x (doi:10.1111/j.1600-0714.1974.tb01721.x) [DOI] [PubMed] [Google Scholar]
- 27.Mandel J. D. 1987. The functions of saliva. J. Dent. Res. 66, 623–627 [DOI] [PubMed] [Google Scholar]
- 28.Dagogo-Jack S., Atkinson S., Kendall-Taylor P. 1985. Homologous radioimmunology for epidermal growth factor in human saliva. J. Immunoassay 6, 125–136 10.1080/01971528508063025 (doi:10.1080/01971528508063025) [DOI] [PubMed] [Google Scholar]
- 29.Petrides P. E., Bfhlen P., Shivley J. E. 1984. Chemical characterization of the two forms of epidermal growth factor in murine saliva. Biochem. Biophys. Res. Commun. 125, 218–228 10.1016/S0006-291X(84)80357-5 (doi:10.1016/S0006-291X(84)80357-5) [DOI] [PubMed] [Google Scholar]
- 30.Hart B. L., Powell K. 1990. Antibacterial properties of saliva: role in maternal periparturient grooming and in licking wounds. Physiol. Behav. 48, 383–386 10.1016/0031-9384(90)90332-X (doi:10.1016/0031-9384(90)90332-X) [DOI] [PubMed] [Google Scholar]
- 31.Sachs B. D., Barfield R. J. 1977. Functional analysis of masculine copulatory behavior in the rat. In Advances in the study of behavior, vol. 7 (eds Rosenblatt J. S., Shaw E., Beer C. G.), pp. 91–156 New York, NY: Academic Press [Google Scholar]
- 32.Hart B. L., Korinek E., Brennan P. 1987. Postcopulatory genital grooming in male rats: prevention of sexually transmitted infections. Physiol. Behav. 41, 321–325 10.1016/0031-9384(87)90395-7 (doi:10.1016/0031-9384(87)90395-7) [DOI] [PubMed] [Google Scholar]
- 33.Greene C. E. 1984. Clinical microbiology and infectious diseases of the dog and cat. Philadelphia, PA: Lea and Febiger [Google Scholar]
- 34.Altmann S. A., Altmann J. 1970. Baboon ecology: African field research. Chicago, IL: University of Chicago Press [Google Scholar]
- 35.Freeland W. J. 1976. Pathogens and the evolution of primate sociality. Biotropica 8, 12–24 10.2307/2387816 (doi:10.2307/2387816) [DOI] [Google Scholar]
- 36.Harkness J. E., Wagner J. E. 1989. The biology and medicine of rabbits and rodents. Philadelphia, PA: Lea and Febiger [Google Scholar]
- 37.Hart B. L. 2005. The evolution of herbal medicine: behavioural perspectives. Anim. Behav. 70, 973–989 10.1016/j.anbehav.2005.03.005 (doi:10.1016/j.anbehav.2005.03.005) [DOI] [Google Scholar]
- 38.Sueda K. L. C., Hart B. L., Cliff K. D. 2008. Characterisation of plant eating in dogs. Appl. Anim. Behav. Sci. 111, 120–132 10.1016/j.applanim.2007.05.018 (doi:10.1016/j.applanim.2007.05.018) [DOI] [Google Scholar]
- 39.Andersone Z. 1998. Summer nutrition of wolf (Canis lupus) in the Slitere Nature Reserve, Latvia. Proc. Latvian Acad. Sci. 52, 79–80 [Google Scholar]
- 40.Andersone Z., Ozolins J. 2004. Food habits of wolves Canis lupus in Latvia. Acta Theriol. 49, 357–367 10.1007/BF03192534 (doi:10.1007/BF03192534) [DOI] [Google Scholar]
- 41.Papageorgiou N., Vlachos C., Sfougaris A., Tsachalidis E. 1994. Status and diet of wolves in Greece. Acta Theriol. 39, 411–416 [Google Scholar]
- 42.Stahler D. R., Smith D. W., Guernsey D. S. 2006. Foraging and feeding ecology of the grey wolf (Canis lupus): lessons from Yellowstone National Park, Wyoming, USA. J. Nutr. 136, S1923–S1926 [DOI] [PubMed] [Google Scholar]
- 43.Murie A. The wolves of Mount McKinley. 1944. Fauna of the National Parks, No. 5. Washington, DC: US Government Printing Office. (Reprinted by University of Washington Press 1985.) See http://www.cr.nps.gov/history/online_books/fauna5/fauna.htm . [Google Scholar]
- 44.Huffman M. A., Caton J. M. 2001. Self-induced increase of gut motility and the control of parasitic infections in wild chimpanzees. Internat. J. Primatol. 22, 329–346 10.1023/A:1010734310002 (doi:10.1023/A:1010734310002) [DOI] [Google Scholar]
- 45.Hemmes R. B., Alvarado A., Hart B. L. 2002. Use of California bay foliage by wood rats for possible fumigation of nest-borne ectoparasites. Behav. Ecol. 13, 381–385 10.1093/beheco/13.3.381 (doi:10.1093/beheco/13.3.381) [DOI] [Google Scholar]
- 46.Clark L., Mason J. R. 1985. Use of nest material as insecticidal and anti-pathogenic agents by the European starling. Oecologia 67, 169–276 10.1007/BF00384280 (doi:10.1007/BF00384280) [DOI] [PubMed] [Google Scholar]
- 47.Clark L., Mason J. R. 1988. Effect of biologically active plants used as nest material and the derived benefit to starling nestlings. Oecologia 77, 174–180 10.1007/BF00379183 (doi:10.1007/BF00379183) [DOI] [PubMed] [Google Scholar]
- 48.Huffman M. A., Seifu M. 1989. Observations on the illness and consumption of a possibly medicinal plant, Vernonia amygdalina (Del.), by a wild chimpanzee in the Mahale Mountains National Park, Tanzania. Primates 30, 51–63 10.1007/BF02381210 (doi:10.1007/BF02381210) [DOI] [Google Scholar]
- 49.Burnet M., White E. O. 1972. Natural history of infectious disease, 4th edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 50.Rasa O. A. E. 1983. A case of invalid care in wild dwarf mongooses. Zeits. Tierpsychol. 62, 235–240 10.1111/j.1439-0310.1983.tb02153.x (doi:10.1111/j.1439-0310.1983.tb02153.x) [DOI] [PubMed] [Google Scholar]
- 51.Schaller G. B. 1972. The Serengeti lion: a study of predator prey relations. Chicago, IL: University of Chicago Press [Google Scholar]
- 52.MacDonald D. W. 1987. Running with the fox. London, UK: Unwin Hyman [Google Scholar]
- 53.Hart B. L., Hart L. A., Pinter-Wolman N. 2008. Large brains and cognition: where do elephants fit in? Neurosci. Biobehav. Rev. 32, 86–98 10.1016/j.neubiorev.2007.05.012 (doi:10.1016/j.neubiorev.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 54.Bates L. A., Lee P. C., Njiraini N., Poole J. H., Sayialel S., Moss C. J., Byrne R. W. 2008. Do elephants show empathy? J. Consciousness Stud. 15, 204–225 [Google Scholar]
- 55.Harthoorn A. M. 1970. The flying syringe. Ten years of immobilizing wild animals in Africa, p. 205 London, UK: Geoffrey Books [Google Scholar]
- 56.Douglas-Hamilton I., Bhalla S., Wittemyer G., Vollrath F. 2006. Behavioural reactions of elephants towards a dying and deceased matriarch. Appl. Anim. Behav. Sci. 100, 87–102 10.1016/j.applanim.2006.04.014 (doi:10.1016/j.applanim.2006.04.014) [DOI] [Google Scholar]
- 57.Hakeem A. Y., Sherwood C. C., Bonar C. J., Butti C., Hof P. R., Allman J. M. 2009. Von Economo neurons in the elephant brain. Anat. Rec. 292, 242–248 10.1002/ar.20829 (doi:10.1002/ar.20829) [DOI] [PubMed] [Google Scholar]
- 58.Butti C., Sherwood C. C., Hakeem A. Y., Allman J. M., Hof P. R. 2009. Total number of and volume of von Economo neurons in the cerebral cortex of cetaceans. J. Comp. Neurol. 515, 243–259 10.1002/cne.22055 (doi:10.1002/cne.22055) [DOI] [PubMed] [Google Scholar]
- 59.Farnsworth N. R., Akerele O., Bingel A. S., Soejarto D. D., Guo Z. 1985. Medicinal plants in therapy. Bull. World Health Organ. 43, 965–981 [PMC free article] [PubMed] [Google Scholar]
- 60.Blumenthal M., Brinckman J., Goldberg A. 2000. Herbal medicine: Expanded Commission E Monographs. Austin, TX: American Botanical Council [Google Scholar]
- 61.Chevallier A. 2000. Encyclopedia of herbal medicine, 2nd edn. New York, NY: DK Press [Google Scholar]
- 62.Capasso L. 1998. 5300 years ago the Ice Man used natural laxatives and antibiotics. Lancet 352, 1864. 10.1016/S0140-6736(05)79939-6 (doi:10.1016/S0140-6736(05)79939-6) [DOI] [PubMed] [Google Scholar]
- 63.Solecki M. 1971. Shanidar: the first flower people. New York, NY: Alfred A. Knopf [Google Scholar]
- 64.Cohen M. 1989. Health and rise of civilization. New Haven, CT: Yale University Press [Google Scholar]
- 65.deMenocal P. B. 1995. Plio-Pleistocene African climate. Science 270, 53–59 10.1126/science.270.5233.53 (doi:10.1126/science.270.5233.53) [DOI] [PubMed] [Google Scholar]
- 66.Dominguez-Rodrigo M. 2002. Hunting and scavenging by early humans: the state of the debate. J. World Prehistory 16, 1–54 10.1023/A:1014507129795 (doi:10.1023/A:1014507129795) [DOI] [Google Scholar]
- 67.De Heinzelin J., Clark J. D., White R. D., Hart W., Renne P., Wolde Gabriel G., Beyne Y., Vrba E. 1999. Environment and behavior of 2.5-million-year-old Bouri hominids. Science 284, 625–629 10.1126/science.284.5414.625 (doi:10.1126/science.284.5414.625) [DOI] [PubMed] [Google Scholar]
- 68.Bramble D. M., Lieberman D. E. 2004. Endurance running and the evolution of Homo. Nature 432, 345–352 10.1038/nature03052 (doi:10.1038/nature03052) [DOI] [PubMed] [Google Scholar]
- 69.Lieberman D. E., Raichlen D. A., Pontzer H., Bramble D. M., Cutright-Smith E. 2006. The human gluteus maximus and its role in running. J. Exp. Biol. 209, 2143–2155 10.1242/jeb.02255 (doi:10.1242/jeb.02255) [DOI] [PubMed] [Google Scholar]
- 70.Milton K. 1999. A hypothesis to explain the role of meat-eating in human evolution. Evol. Anthropol. 8, 11–21 (doi:10.1002/(SICI)1520-6505(1999)8:1<11::AID-EVAN6>3.0.CO;2-M) [DOI] [Google Scholar]
- 71.Milton K. 1999. Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition 15, 488–498 10.1016/S0899-9007(99)00078-7 (doi:10.1016/S0899-9007(99)00078-7) [DOI] [PubMed] [Google Scholar]
- 72.Finch C. E., Stanford C. B. 2004. Meat-adaptive genes and the evolution of slower aging in humans. Q. Rev. Biol. 79, 3–50 10.1086/381662 (doi:10.1086/381662) [DOI] [PubMed] [Google Scholar]
- 73.Cordain L., Eaton S. B., Miller J. B., Mann N., Hill K. 2002. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Europ. J. Clin. Nutr. 56, S42–S52 10.1038/sj.ejcn.1601353 (doi:10.1038/sj.ejcn.1601353) [DOI] [PubMed] [Google Scholar]
- 74.Mann F. D. 1998. Animal fat and cholesterol may have helped primitive man evolve a large brain. Perspect. Biol. Med. 41, 417–419 [DOI] [PubMed] [Google Scholar]
- 75.Wrangham R. 2009. Catching fire: how cooking made us human. New York, NY: Basic Books [Google Scholar]
- 76.Billing J., Sherman P. W. 1998. Antimicrobial functions of spices: why some like it hot. Q. Rev. Biol. 73, 4–38 [DOI] [PubMed] [Google Scholar]
- 77.Aiello L. C., Wheeler P. 1995. The expensive-tissue hypothesis. Curr. Anthropol. 36, 199–221 10.1086/204350 (doi:10.1086/204350) [DOI] [Google Scholar]
- 78.Kaplan H., Hill K., Lancaster J., Hurtado A. M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [DOI] [Google Scholar]
- 79.Foley R. A., Lee P. C. 1991. Ecology and energetics of encephalization in hominid evolution. Phil. Trans. R. Soc. Lond. B 334, 223–232 10.1098/rstb.1991.0111 (doi:10.1098/rstb.1991.0111) [DOI] [PubMed] [Google Scholar]
- 80.Kappelman J. 1996. The evolution of body mass and relative brain size in fossil hominids. J. Hum. Evol. 30, 243–276 10.1006/jhev.1996.0021 (doi:10.1006/jhev.1996.0021) [DOI] [Google Scholar]
- 81.Gutteridge J. M., Halliwell B. 1994. Antioxidants in nutrition, health and disease. Oxford, UK: Oxford University Press [Google Scholar]
- 82.Hiramatsu M., Yoshikawa T., Inoue M. (eds) 1997. Food and free radicals. New York, NY: Plenum Press [Google Scholar]
- 83.Kubow S. 1998. The influence of oxidative stress and antioxidant supplementation on lipoprotein metabolism. Rec. Devel. Lipid Res. 2, 81–99 [Google Scholar]
- 84.Machlin L. J., Bendich A. 1987. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 4, 441–445 [PubMed] [Google Scholar]
- 85.Proteggente A. R., Pannala A. S., Paganga G., Buren L. V., Wagner E., Wiseman S., Put F. V. D., Dacombe C., Rice-Evans C. A. 2002. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radical Res. 36, 217–233 10.1080/10715760290006484 (doi:10.1080/10715760290006484) [DOI] [PubMed] [Google Scholar]
- 86.Szeto Y. T., Tomlinson B., Benzie F. F. 2002. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br. J. Nutr. 87, 55–59 10.1079/BJN2001483 (doi:10.1079/BJN2001483) [DOI] [PubMed] [Google Scholar]
- 87.Halvorsen B. L., et al. 2002. A systematic screening of total antioxidants in dietary plants. J. Nutr. 132, 461–471 [DOI] [PubMed] [Google Scholar]
- 88.Johns T. 1990. With bitter herbs they shall eat it. Tucson, AZ: University of Arizona Press [Google Scholar]
- 89.Johns T. 1999. The chemical ecology of human ingestive behaviors. Ann. Rev. Anthropol. 28, 27–50 10.1146/annurev.anthro.28.1.27 (doi:10.1146/annurev.anthro.28.1.27) [DOI] [Google Scholar]
- 90.Broadhurst C. L., Wang Y., Crawford M. A., Cunnane S. C., Parkington J. E., Schmidt W. 2002. Brain-specific lipids from marine, lacustrine or terrestrial food resources: potential impact on early African Homo sapiens. Comp. Biochem. Physiol. B 131, 653–673 10.1016/S1096-4959(02)00002-7 (doi:10.1016/S1096-4959(02)00002-7) [DOI] [PubMed] [Google Scholar]
- 91.Enard W., Przeworski M., Fisher S. E., Lai C. S. L., Wlebe V., Kitano T., Monaco A. P., Paabo S. 2002. Molecular evolution of FOXP2, a gene involved in speech and language. Nature 418, 869–872 10.1038/nature01025 (doi:10.1038/nature01025) [DOI] [PubMed] [Google Scholar]
- 92.Lieberman P. 2007. The evolution of human speech. Cur. Anthropol. 48, 39–66 10.1086/509092 (doi:10.1086/509092) [DOI] [Google Scholar]
- 93.Lieberman P. 2011. The evolution of the human head. Cambridge, MA: Harvard University Press [Google Scholar]