Abstract
Natural selection should favour parents that are able to adjust their offspring's life-history strategy and resource allocation in response to changing environmental and social conditions. Pathogens impose particularly strong and variable selective pressure on host life histories, and parental genes will benefit if offspring are appropriately primed to meet the immunological challenges ahead. Here, we investigated transgenerational immune priming by examining reproductive resource allocation by female mice in response to direct infection with Babesia microti prior to pregnancy. Female mice previously infected with B. microti gained more weight over pregnancy, and spent more time nursing their offspring. These offspring generated an accelerated response to B. microti as adults, clearing the infection sooner and losing less weight as a result of infection. They also showed an altered hormonal response to novel social environments, decreasing instead of increasing testosterone production upon social housing. These results suggest that a dominance-resistance trade-off can be mediated by cues from the previous generation. We suggest that strategic maternal investment in response to an infection leads to increased disease resistance in the following generation. Offspring from previously infected mothers downregulate investment in acquisition of social dominance, which in natural systems would reduce access to mating opportunities. In doing so, however, they avoid the reduced disease resistance associated with increased testosterone and dominance. The benefits of accelerated clearance of infection and reduced weight loss during infection may outweigh costs associated with reduced social dominance in an environment where the risk of disease is high.
Keywords: dominance, hormones, immunocompetence, maternal effects, testosterone, trade-off
1. Introduction
Maternal effects occur when factors in the maternal environment cause phenotypic changes in offspring [1]. These effects are the phenotypic product of the interaction between the parental genotype and that genotype's environment as expressed in the next generation [2–4]. They can be viewed as manifestations of transgenerational phenotypic plasticity [5–8]. Where this plasticity increases fitness, maternal effects can be considered adaptive; adaptive maternal effects are thought to play an important role in shaping life history in many taxa [1].
The idea that the maternal environment can influence the development and hence the phenotype of offspring has attracted particular attention in studies of the impact of environmental challenges on human health. ‘Foetal programming’ as a result of maternal exposure to disease or malnutrition, for example, has been shown to have diverse consequences for the health of children and even grandchildren [9]. Such maternal effects in humans are typically thought of as being detrimental to offspring fitness, but in studies of other species there has been more focus on potentially adaptive priming of offspring [2,10]. If cues in the maternal environment predict the conditions likely to be experienced by the next generation, a mother's inclusive fitness can be increased if she can manipulate offspring phenotype appropriately. In many cases, it is hypothesized that maternal effects mediate life-history trade-offs in offspring involving investment in traits associated with reproductive success and survival [1]. However, the adaptive nature of maternal effects is still being actively debated. Some suggest that effects which are perceived to be adaptive may in fact simply be accidental products of gene–environment interactions with little impact on fitness [10–12].
The ability to resist or tolerate infection is crucial for animals exposed to pathogens, but it is also costly, and requires investment of resources that might otherwise be directed elsewhere [13]. Because immature animals do not have fully developed sensory and immune systems, parents often play a key role in the protection of offspring from infection. In species with parental care, protection can take several forms. Parents may directly reduce risk from pathogens through preening, nest-cleaning, etc. [14,15], and/or they may provide passive immunity by transferring antibodies to offspring in milk or via the yolk or placenta [16]. In addition, and of particular relevance here, parents may confer long-lasting protection by manipulating offspring phenotype according to the perceived risk of infection.
Transgenerational immune priming (TGIP) is a pathogen-mediated maternal effect that is known to occur in a wide range of taxa [9,17,18]. Maternal exposure to pathogens or antigens (e.g. bacterial polysaccharides) promotes offspring immunocompetence in species ranging from insects to birds and mammals [19–21]. The mechanisms that bring about TGIP are varied [9]. Some instances appear to involve optimization of maternal reproductive behaviour to benefit offspring (e.g. skewing laying order relative to offspring gender if one sex is more vulnerable to disease) [22]. Others involve direct transfer of antibodies or immunoglobulins through the milk or yolk, which can have long-term impacts on the development of the juvenile immune system, in addition to providing passive immunity [16,23]. A third group of mechanisms involves epigenetic effects, whereby maternal experience affects DNA expression, but not sequences, in offspring [24]. This may be achieved by the modulation of DNA methylation via changes in the exposure of offspring to androgens and other hormones [19,25,26], or through other biochemical mechanisms [27].
The potential for TGIP is of great interest in an evolutionary context because pathogens impose strong selective pressures on host life histories, forcing hosts to allocate resources to immune defence to the detriment of other life-history components [13,28,29]. For example, immunologically challenged female blue tits (Cyanistes caeruleus) and pied flycatchers (Ficedula hypoleuca) reduce the rate at which they feed offspring [30,31]. Other examples illustrate an opposite effect, i.e. increased investment in reproduction or growth can cause a reduction in immunocompetence. Female odour causes male mice to invest in secondary sexual characters, leading them subsequently to lose more weight fighting an experimental infection [32]. Individuals of many bird species that have larger broods show reduced immunocompetence [1]. Reproductive activity also reduces immunocompetence in fruit flies (Drosophila melanogaster) and damselflies (Matrona basilaris) [33,34].
The widespread evidence for a trade-off between reproductive traits and immunocompetence leads to clear predictions about the consequences of TGIP for offspring. If females can use environmental cues to anticipate pathogenic challenges for their offspring, and upregulate offspring immunocompetence as a result, we would expect to see the costs of this priming to impact negatively on traits associated with reproductive success [35]. In many species, trade-offs between survival and reproduction are manifest in impacts of changes in immunity on social and reproductive behaviour. There is a well-documented negative association between social dominance and disease resistance in mice [36–38]. Aggressive interactions among male mice determine social dominance, and hence access to mating opportunities, but increased aggression is costly and is associated with reduced resistance to disease. The trade-off between investment in social dominance and immunocompetence is thought to be regulated by androgens: increased testosterone production generally increases aggression, but also increases vulnerability to infection [39]. Where such a trade-off exists, we can predict that upregulation of offspring immunocompetence as a result of TGIP by mothers may lead to decreased circulating levels of testosterone and consequently reduced social dominance.
Although pathogens have been shown to affect reproductive investment by mothers, and subsequently the behaviour and life history of offspring, such changes do not necessarily represent adaptive maternal effects. In fact, there are three possible evolutionary explanations for pathogen-mediated changes in offspring: (i) they reflect adaptive manipulation beneficial to the pathogen [40–42], (ii) they are the inevitable side-effects of the cost of combating disease [43,44], or (iii) they are adaptive responses to infection by the host ensuring their own reproductive success and that of their offspring [1]. An example of a transgenerational effect that might benefit a parasite is seen in flea-infested great-tits (Parus major), which produce fledglings that disperse shorter distances than healthy birds, and are more likely to be recruited to the local population, providing more nearby future hosts for the parasite [45]. If this is adaptive manipulation of host offspring behaviour by the parasite, perhaps achieved via epigenetic modifications of host DNA [24], the phenotype of the parasite can be considered to extend (sensu Dawkins [46]) across two host generations. In contrast, however, mite-infected female lizards (Lacerta vivipara) display a parasite-mediated maternal effect that appears to be adaptive for the host. They produce offspring that develop faster, invest more in early reproduction and run faster: literally and figuratively living faster and dying younger in an environment when life-expectancy is reduced [47,48]. Such examples highlight the diverse nature of the possible evolutionary origins of transgenerational effects of pathogens. However, experimental studies that trace the impacts of infection across generations such that the likely consequences of observed changes for maternal and offspring fitness can be evaluated fully remain very scarce [1].
The cause of any responses to maternal infection observed in offspring is often attributed to whichever agent (host or pathogen) is intuitively thought to benefit. Because previous experimental studies of TGIP have usually examined offspring of females that were infected at the time of pregnancy/egg laying, responses that are adaptive for the pathogen are generally very plausible, since the pathogen is still an active agent at the moment the offspring are primed [10,49]. An alternative experimental approach is provided by tests of the downstream effects of TGIP in the offspring of females that have cleared infection prior to pregnancy/egg laying; such an approach allows us to focus more on changes in offspring traits that might plausibly be adaptive for the mother.
The environmental cues that trigger TGIP might be direct if the mother is infected prior to the birth of her offspring, but they might also be indirect. An important possible source of indirect information about infection risk in the environment comes from visual or olfactory cues associated with infected conspecifics [50]. In a previous experiment [51], we examined whether such indirect cues, when perceived by mothers, actually impact on offspring phenotype. We showed that housing pregnant female mice next to infected neighbours led to the production of offspring that were more resistant to disease and less aggressive as adults. This provided the first evidence for transgenerational regulation of immune response based on ambient social information. In addition, our findings supported the idea that mothers adaptively regulate the trade-off between social dominance and disease resistance in their offspring in response to variable infection risk.
In the current paper, we report on a second study designed to investigate transgenerational transmission of immunological information using the same experimental system as before. This time we examined the response in mothers and their offspring to direct evidence of infection risk in the maternal environment. We infected female mice with the protozoan blood parasite Babesia microti, and allowed the infection to clear prior to pregnancy. Thus, females in the experiment had direct information about infectious agents in the environment, and potentially had reduced resources owing to recent infection, but any priming of their offspring could only occur after infection had cleared, in the absence of the parasite. We examined how recent infection affects reproductive output and maternal behaviour, and how it affects the social behaviour and disease resistance of offspring as adults in later life. We predicted that we would see enhanced immunocompetence but reduced testosterone, aggression and social dominance in offspring of infected mothers.
2. Material and methods
(a). Methods overview
Female laboratory mice were either infected with the blood protozoan B. microti, or subjected to one of four control treatments. Babesia species are tick-borne haemoprotozoan parasites that infect virtually all mammalian species, with significant economic consequences in domestic animals and human health implications, particularly in the USA [52,53]. In mice, B. microti induces high but transient parasitaemias, which are quickly cleared (clearance beginning approximately 10 days after infection) [54]. This makes B. microti an ideal parasitic tool to investigate the effects of disease in neighbours over a limited period, such as during pregnancy. In natural infections, natural killer (NK) cells and macrophages act to limit the extent of parasitaemia [55] before a resolution stage begins, with parasitaemia levels peaking and then rapidly declining owing to the action of CD4+ T cells and IFN-γ [54]. Following primary infection, mice are protected against future infection by the action of CD4+ T cells and IFN-γ, with little or no requirement for B-cells or antibodies [56].
In our experiment, following clearance of infection (time to clearance was less than or equal to 14 days (see [51])), each female was co-housed with a sire male for 6 days. Mated females were then removed to single-housing cages until parturition. At 11 days old, each litter was reduced to four males. Females were removed from offspring at 24 days old. At 50 days old, approximately half of the offspring were re-housed with three novel males from the same treatment group. Continuous behavioural observations totalling 200 min per group were carried out, recording aggressive behaviour in the adult offspring. At 70 days old, the adult offspring were singly housed and infected with B. microti. The time course of the infection was monitored by blood smears until clearance. Blood was taken from females and offspring at various time points during the experiment and assayed for testosterone, corticosterone and total IgG.
(b). Detailed methods
(i). Mice and housing
A priori power tests were conducted to determine minimum sample sizes necessary to detect treatment effects of a magnitude that would be considered important, in order to minimize animal use. The subjects were 358 mice of the Bantam and Kingman: White (BKW) strain, which included 75 subject females (seven weeks old), 75 sire males (nine weeks old) and 208 male offspring from the subject females (from birth to 90 days of age). We kept subjects in standard polypropylene cages (48 × 15 × 13 cm: model M3, North Kent Plastics, UK) throughout. All cages contained wood shavings as a floor substrate, a cotton nestlet for bedding material and a cardboard tube. Subjects had ad libitum access to standard laboratory rodent food pellets and water. Room temperature was maintained between 20°C and 22°C and humidity between 45 and 55 per cent. All animals were maintained under a 12 L : 12 D reversed light : dark cycle with lights on at 20.00 h, and illuminated by a dim red light during the dark cycle to facilitate observations.
(ii). Maternal treatment phase
Seven week old females were randomly divided into five treatment groups of 15 individuals each. These were: (i) Babesia treatment: infection with 5 × 107 mouse red blood cells harbouring B. microti; (ii) sham Babesia: sham infection of B. microti, comprising only the vehicle used to suspend erythrocytes in treatment (i) (an infection vehicle control treatment); (iii) sheep red blood cell (SRBC) treatment: inoculation with 5 × 107 foreign SBRCs (an immune activity treatment); (iv) sham SRBC treatment: sham inoculation of SRBCs, comprising only the vehicle used to suspend the SRBCs (immune activity vehicle control treatment); and (v) complete control: no treatment. The treatment phase lasted 20 days, by which time the infection had peaked and been cleared in all of the infected females (Babesia treatment) (the infection monitoring method is explained in §2b(vii)).
(iii). Mating phase
The females were then introduced to the home cages of randomly allocated single-housed sire males, and kept in these pairs for 6 days. This method was used to minimize sire–female aggression, and maximize likelihood of impregnation [57]. After 6 days, females were removed from sire cages and housed singly to continue pregnancy and then give birth. They were then kept with full litters for 11 days.
(iv). Pre-weaning phase
At 11 days old, when pups could be reliably sexed by non-invasive means and individually marked, all were sexed and each litter reduced to four males (three males in the case of three litters that had only three males each). This was to enable manageable sample sizes for individual observations both at pre-weaning and in subsequent phases. Male pups were chosen because previous work has shown important dominance–resistance trade-offs in male mice [36–38]. A total of 208 male pups from 52 females were included in the adult offspring stages of the experiment. Thirteen females did not become pregnant in the mating phase, and a further 10 females did not have at least three sons at 11 days post-partum, but neither the likelihood of becoming pregnant nor litter sex ratio were affected by treatment (see §3). We marked the male pups in an individually distinctive pattern using black eyelash dye (Colorsport, Brodie and Stone plc, London, UK). At 24 days of age, all mothers were removed from litters, and pups (subject males) were left in their fraternal groups until 50 days old.
(v). Social grouping and single-housing phases
At 50 days old, all subject males were separated from their siblings. Approximately half of the subject males (100 mice) were randomly chosen and re-housed with three novel males from the same treatment group, with whom they were allowed to establish dominance hierarchies over 20 days. Continuous behavioural observations totalling 200 min (10 min d−1) per group were carried out. Various social interactions were recorded, including the number of attacks, mounts and aggressive allogrooms, in order to determine social rank within these groups. Individuals that displayed more aggressive behaviours were considered dominant in later analysis, and those that were more commonly the recipients of these behaviours were considered subordinates [36,58]. The remaining half (108 mice) was housed singly to act as a non-socialized treatment. At the end of this phase, all males were housed singly in new clean cages.
(vi). Offspring infection phase
At 70 days old, the adult offspring, all now housed singly, were injected with 1 × 107 mouse red blood cells infected with B. microti. The time course of the infection was closely monitored until clearance. No other infection or immune challenge was given to the adult offspring.
(vii). Technical procedures
All inoculations, infections and sham manipulations involved a single intra-peritoneal injection of 200 µl Hanks' solution, containing the appropriate inoculants. Mothers in treatment group E received no injection. All monitoring, sampling and handling for treatments A and C were repeated accordingly in sham treatments B, D and E. Sham infection and inoculation involved the introduction of Hanks' solution only. All females and subject male offspring were infected in a random order with 1 × 107 mouse red blood cells infected with B. microti. The King's 67 strain of B. microti was used throughout, and frozen stock was passaged five times in BKW mice before being used on stimulus or subject males.
To monitor B. microti infection, a peripheral tail vein was nicked and a single drop of blood transferred to a glass microscope slide every other day during the infection. The drop was immediately smeared to give a monolayer of erythrocytes, then fixed and stained. For staining, fixed slides were placed in a solution of one part Giemsa stain to three parts Sorenson's buffer for 40 min, before rinsing in Sorenson's buffer and drying in air. Larger blood samples (50 µl) were also collected in heparinized haematocrit tubes, on no more than three occasions per animal, and never twice within a two week period. Females were sampled at the start and at the end of the stimulus phase. Offspring had 50 µl blood samples taken at weaning, prior to and at the end of social grouping. An additional blood sample was taken from all animals during autopsy.
The larger blood samples were assayed for testosterone (males only), corticosterone and total IgG (used as a bystander measure of immunocompetence [36]) using kits or reagents supplied by IDS Ltd, Tyne and Wear, UK (testosterone); R & D Systems Europe Ltd, Abington, UK (corticosterone); and Universal Biological, Cambridge, UK (IgG). All plates were processed using Micro plate Manager v. 5.2. In all cases, blood samples were anonymized and analysed in a random order, so that it was not possible for investigators to know the treatment group or relatedness of individual samples. In a number of cases, limited serum volumes precluded reliable estimates for all three serum factors at all time points from certain individuals. As a consequence, sample sizes of some analyses vary.
(viii). Statistical analysis
Analysis was carried out using SPSS v. 15 (SPSS Inc. Chicago, IL, USA). Alpha was set at 0.05 and all tests were two-tailed. Where appropriate, values were nested within female (which was included as a random factor), or averaged per female to avoid within-litter pseudoreplication. Where appropriate, body mass was fitted as a covariate. In addition to sample size variation owing to blood sampling limitations, sample sizes vary among early phase analyses (for example, changes in female physiology over infection phase, compared with offspring measures) because not all females became pregnant or produced four sons.
3. Results
(a). Physiological responses to treatment in the female
Because no significant differences were detected among the four control treatments for the response variables analysed (electronic supplementary material, table S1), these were grouped as one treatment labelled ‘control treatments’ and compared against the Babesia-treated females.
Following infection, Babesia-treated females showed significantly higher levels of serum IgG throughout the experiment than control females (figure 1, repeated measures generalized linear model (GLM) on log10 (IgG): F3,105 = 39.983, p < 0.0001). There was no effect of infection on serum corticosterone (repeated measures GLM: F3,147 = 0.283, p = 0.838). Babesia-treated females lost significantly more weight over the period of infection than females in all other control groups (GLM: F1,72 = 7.517, p = 0.008), but the difference in weight at the time of mating was of marginal significance (GLM: F1,72 = 3.884, p = 0.053). Body weight at mating did not appear to have significant downstream effects on the likelihood of becoming pregnant (GLM: F1,72 = 1.272, p = 0.263) or on litter size (Pearson's correlation: n = 60, r = −0.203, p = 0.119).
Figure 1.
Female serum immunoglobulin, measured at the start of the experiment prior to treatment (time 1, day one), following clearance of infection and prior to mating (time 2, day 20), at the end of pregnancy (time 3, day 47) and upon weaning (time 4, day 60). Error bars represent ± 1 s.e. Filled circles with solid line, Babesia treatment; filled circles with dashed line, control treatments.
(b). Reproductive responses to treatment in the females
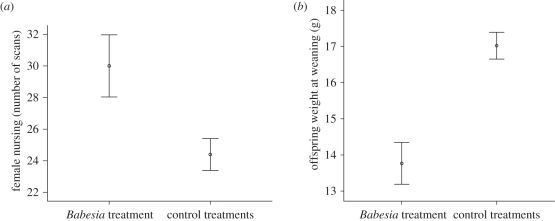
Infection with B. microti did not affect the likelihood of becoming pregnant (χ2-test: χ2 = 0.538, d.f. = 1, p = 0.970) or producing a live litter (χ2-test: χ2 = 0.397, d.f. = 1, p = 0.983). Nor did it affect the litter size (GLM: F1,60 = 0.031, p = 0.831) or the sex ratio of litters (GLM: F1,57 = 0.056, p = 0.813). However, the weight gain over pregnancy, and weight loss at parturition, were significantly affected by treatment. Babesia-infected females gained more weight during pregnancy (31.1 ± 0.56 g) and lost more at parturition (23.15 ± 0.61 g) than did control females (27.5 ± 0.63 and 20.9 ± 0.58 g, respectively), even when covariates were fitted to control for weight loss during infection and litter size (repeated measures GLM: F2,116 = 6.566, p = 0.002). Babesia-treated females then spent significantly more time nursing offspring than control females (figure 2a, GLM: F1,51 = 5.940, p = 0.018).
Figure 2.
Female nursing behaviour and offspring growth. (a) Total number of instantaneous scans in which females were seen nursing one or more pup. (b) Offspring weight at weaning (24 days of age). Error bars represent ± 1 s.e.
(c). Responses to treatment in the young offspring
It was not possible to look at the effects of treatment on the weight at birth in offspring, because interference with pups at this age carries too high a risk of infanticide. We detected no effect of maternal treatment on early pup growth (weight gain between day 11 and weaning; repeated measures GLM: F2,114 = 0.818, p = 0.444), but by weaning Babesia-treated pups were significantly lighter than control pups (figure 2b: GLM: F1,51 = 4.914, p = 0.031). This was particularly surprising as Babesia-treated pups spent significantly more time suckling (27.4 ± 0.83 scans compared with 22.62 ± 0.50 scans, GLM: F1,51 = 6.806, p = 0.012). The effect of treatment on weight was no longer evident by five weeks of age, or thereafter (repeated measures GLM: F3,159 = 0.149, p = 0.930).
Offspring from Babesia-treated females showed significantly higher levels of IgG at weaning at 24 days of age (1893 ± 307 mg l−1 compared with 1071.29 ± 147.6 mg l−1; GLM with log10 transformed data: F1,53 = 17.529, p < 0.001), a difference that persisted to seven weeks of age (Babesia: 2139 ± 239 mg l−1; control: 1450 ± 125 mg l−1; GLM with log10 transformed data: F1,53 = 9.612, p = 0.003). There was no effect of treatment on early offspring corticosterone or testosterone (repeated measures GLMs: corticosterone: F3,153 = 0.423, p = 0.737; testosterone: F3,159 = 0.647, p = 0.586).
(d). Responses to treatment in the adult offspring
Over the social grouping phase, when the adult males met non-sibling conspecifics for the first time, offspring from control treatments showed an increase in testosterone production, whereas Babesia-treated males showed a reduction (figure 3; GLM of change in serum testosterone (after minus before): F1,44 = 6.296, p = 0.016). There was no such effect on corticosterone or immunoglobulin over the novel social group period (GLM of change in corticosterone: F1,49 < 0.001, p = 0.993; change in IgG: F1,48 = 1.206, p = 0.278). Despite the effect on testosterone, there was no observable effect of treatment on social behaviour in adult offspring (GLM of square-root transformed total number of observed acts of aggression: F1,56 = 0.247, p = 0.622).
Figure 3.
Changes in offspring testosterone. Serum testosterone measured on introduction to (49 days old) and removal from (65 days of old) novel social group caging as adults. Error bars represent ± 1 s.e. Filled circles with solid line, Babesia treatment; filled circles with dashed line, control treatments.
(e). Effect of treatment on the response of adult offspring to infection
Offspring from Babesia-treated females had a significantly different response to B. microti infection as adults (repeated measures GLM: F5,255 = 3.314, p = 0.006) with a faster resolution phase of the infection profile (figure 4). The accelerated response to infection was reflected in Babesia-treated males losing less weight in the two week period after inoculation (1.57 ± 0.30 g lost by Babesia-treated males compared with 3.05 ± 0.22 g lost by controls; GLM: F1,48 = 6.181, p = 0.016). Interestingly, Babesia-treated males showed less of an increase in IgG over infection, even when early IgG levels were controlled for (Babesia: increase of 18 038 ± 2264 mg l−1; control: 23 826 ± 2071 mg l−1; repeated measures GLM with log10 transformed data: F1,51 = 6.197, p = 0.016).
Figure 4.
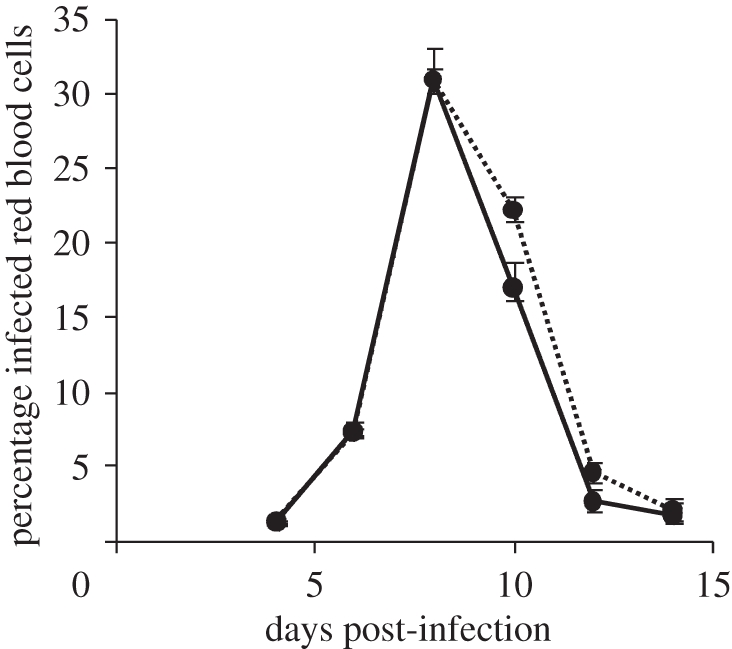
Offspring infection profiles. Percentage of red blood cells infected with Babesia microti at various time points following intraperitoneal injection with 1 × 107 infected red blood cells. Error bars represent ±1 s.e. Filled circles with solid line, Babesia treatment; filled circles with dashed line, control treatments.
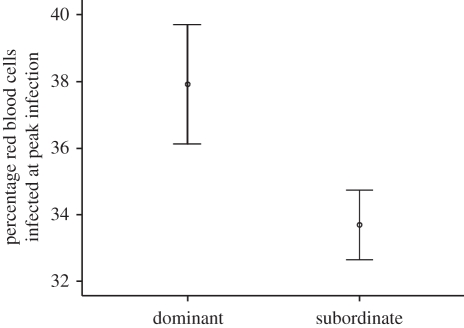
There was no overall effect of social grouping on infection profile (repeated measures GLM: F5,925 = 0.122, p = 0.988), but males that were grouped and displayed the most aggression in their groupings (dominants) experienced higher infection peaks (figure 5: GLM: F1,98 = 4.172, p = 0.044) than males that were less aggressive in groupings (subordinates). Treatment did not interact with the effect of social rank on peak infection (GLM: F1,96 = 0.004, p = 0.947). Individuals of different rank did not show significantly different patterns of testosterone production over their lifetimes (repeated measures GLM: F3,276 = 0.518, p = 0.671). No significant interaction between rank and treatment on testosterone production over an individual's lifetime was found (repeated measures GLM: F3,270 = 1.883, p = 0.133).
Figure 5.
The effect of social rank on infection peaks. Offspring grouped in cages of four were ranked as dominant (one per cage) or subordinate (three per cage) based on their aggressive behaviour. Graph shows subsequent infection levels at the peak of infection. Error bars represent ± 1 s.e.
4. Discussion
We examined how experience of recent infection affects reproductive output and maternal behaviour in female mice, and social behaviour and resistance to infection in their offspring in subsequent adult life. The results support the findings of our previously reported study [51] in which females were indirectly exposed to infection by co-housing with non-contagious conspecifics. In both experiments, females exposed to immunological cues had physiological responses to their exposure, and produced offspring with an accelerated response to infection as adults. Unlike in the earlier study, here the sons of the directly infected females did not display lower aggression as adults, but nevertheless differed in their hormonal response to novel social environments, decreasing instead of increasing testosterone production upon social housing. Testosterone in male adult mice is associated with social dominance, aggression, territory acquisition and maintenance, and consequently increased access to mating opportunities [59–61]. However, there is evidence that the benefits of dominance are counterbalanced by costs associated with reduced resistance to infection [59,62,63]. Other studies strongly implicate testosterone in the mediation of these dominance–resistance trade-offs [32,59,62,64].
We believe that the patterns of maternal investment in Babesia-treated females in our study could reflect immunocompetence-directed investment. Females may be specifically priming offspring for life in an environment in which the risk of infection is high [9]. Our results add to a growing body of evidence that suggests that effects of this sort occur across a wide range of taxa. Queen bumblebees (Bombus terrestris) injected with bacteria produce workers that show higher levels of antibacterial activity [65]. Immunized pied flycatchers (F. hypoleuca) produce offspring with elevated endogenous antibody production [66]. Offspring from mice infected with an intestinal nematode when pregnant are more likely to clear infection as adults [20,67].
Although we observed a difference in hormonal response to social challenge in the offspring of Babesia-challenged females, we did not observe a concomitant difference in levels of aggression. This may have been because levels of observed aggression were generally low in our experiment, and also because none of the male offspring in this study had ever been singly housed prior to social grouping (single housing increases subsequent territorial behaviours; see [58]). Despite the low levels of aggression recorded, it was possible to allocate ranks within each cage group, and higher-ranking males showed a downstream immunocompetence cost, experiencing higher levels of infected cells at peak infection. This further supports the conclusion that a dominance–resistance trade-off occurred in this experiment.
We chose to study the effects of maternal infection on male offspring and not on females because a dominance–immunocompetence trade-off is relatively well documented in male mice [36–38,59], and because the inclusion of offspring of both sexes was not feasible while maintaining sample sizes at the levels that were indicated to be required by a priori power analysis. While our results are consistent with the idea of adaptive maternal mediation of a trade-off in male offspring, it is difficult to predict what might occur if our experimental observations were repeated with female offspring. Maternal effects on life-history traits and behaviour in the female offspring of rodents are well documented [68,69], but selection pressures on males and females are often very different, and it is generally expected that if mothers have the ability to adjust investment differentially in sons and daughters, maternal effects will be offspring-sex-specific [4,22]. While the upregulation of immunocompetence in sons is predicted to result in androgen-mediated changes in social behaviour/dominance, trade-offs with other behavioural or life-history traits are plausible in daughters. Litter and offspring body size, and the timing of birth, are known to be the target of maternal effects in female rodents [68,69], and we might therefore expect maternally induced redirection of resources towards immune defence to come at the expense of reduced fertility and fecundity, or modified reproductive behaviour. Until appropriate experiments are conducted in mice and other species, however, discussion of such differential maternal effects on the sexes in response to the threat of disease remains speculative.
Babesia-treated females gained more weight during pregnancy, lost more weight at parturition and spent more time nursing their offspring. This may reflect an increased investment in the current litter, to the potential detriment of future reproduction. Iteroparous species, such as mice, usually do not put all their investment into one reproductive bout, because it may reduce future reproductive success and therefore overall lifetime fecundity [70,71]. However, if longevity is reduced by a parasite, such a shift in investment priorities may occur [72]. Immunologically challenged common eider ducks (Somateria mollissima) incubate eggs for longer, lose more weight in the process and display higher levels of parental care after hatching [73], findings similar to those seen here. Nematode-infected pregnant mice produce significantly and substantially larger litters [20], but subsequent litters are smaller [74]. However, the similarity of these patterns is somewhat complicated by the unexpected finding that Babesia-treated offspring gained less weight than control offspring during the pre-weaning period. It is conceivable that increased suckling observed among the offspring of dams that had experienced infection was driven by the offspring rather than by the mothers, and hence may have reflected the need to increase suckling time owing to potentially limited or lower quality milk production [75–77]. An earlier experiment by Barnard et al. [37] showed that suckling behaviour affected both social rank and immunity in mice, with aggressiveness inversely proportional to suckling, but, as in our study, it was not possible to determine whether this was offspring- or parent-driven. The mice in the Barnard et al. study [37] that suckled more actually gained less weight, suggesting that increased suckling time does not reflect increased milk consumption [76]. These mice then developed into lower ranking individuals, but with enhanced immunocompetence [37]. The same relationship between suckling and weight gain was found in previous studies on mice [78]. In our experiment, Babesia-treated sons also suckled more, gained less weight and then went on to respond differently to both social and immunological challenges; it is thus possible that suckling experience in infancy mediates immunological and social priming.
Our experiment appears to show a general increase in investment in the immune system in both mothers exposed to infection and their offspring. In our view, it is likely that such changes are adaptive for the mother and her offspring, equipping them better to face an environment in which the threat of infection is high. It is never possible, however, to exclude completely the chance that the responses seen are driven by adaptive manipulation of the host by the parasite, or indeed that the changes have no adaptive significance [24]. In our experiment, we allowed full clearance of infection before pregnancy, and it is unlikely that the direction of the responses observed in both females and offspring reflected increased fitness of B. microti. Instead, we believe that our findings support the conclusion that females invested in reproduction in such a way as to maximize offspring resistance at a time when the risk of disease was high.
We aimed to explore transgenerational effects of a parasitic infection in the mother, rather than foetal immunological responses to direct challenge. Therefore, it was important that offspring were not directly infected by vertical transmission [79]. Babesial agents have been shown on occasion to pass transplacentally from mother to offspring in utero [53,79]. For example, Babesia bovis in cattle, Babesia canis in dogs, Theileria equi (formerly Babesia equi) in horses and B. microti in humans have all been shown to pass through the placenta [53,79]. By infecting females prior to mating and ensuring that all infected females had cleared infection prior to mating, our experimental design was aimed at minimizing any risk of vertical transmission. We further validated that offspring were not infected by taking smears at weaning. Serum immunological constituents and response to infection later in life confirmed the absence of infection in newborn offspring (see §3).
Other than documented cases of vertical transmission, and foetal abortion owing to babesiosis during pregnancy [53], there is very little information in the literature regarding the impact of perinatal Babesia infection on offspring. More research has been done on the consequences of the similar malarial parasites, Plasmodium falciparum and Plasmodium vivax, which are very similar to Babesia spp., but which are a much more significant cause of serious disease and mortality in humans [80,81]. Research has shown that humans born to malarial mothers have lower birth weights owing to inter-uterine growth retardation. They also tend to be premature, and there is an associated increased risk of stillbirth (e.g. [82]). However, most of these studies of malaria investigate the implications of perinatal parasitaemia from a human health perspective. Very few offer insight into offspring life-history consequences of maternal infection (but see [81]), or into the potentially adaptive offspring disease resistance owing to maternal exposure prior to pregnancy. Here, we demonstrate a potentially adaptive life-history consequence for the offspring of infected mothers.
Following infection with B. microti, both females and their offspring showed elevated levels of IgG. In natural systems, Babesia species are transmitted into the host bloodstream by ticks [53], and IgG antibodies can block B. microti sporozoites from invading erythrocytes shortly following infection. If this fails, NK cells and macrophages act to limit the extent of parasitaemia [54]. Ultimately, a resolution stage begins, with parasitaemia levels peaking and then rapidly declining owing to the action of CD4+ T cells and IFN-γ [54,56]. While IgG is primarily involved in the early stages of infection, it is expected that circulating levels remain high following clearance, and as such are a useful bystander measure of immunocompetence [36]. IgG is also of relevance to this study because it can pass between rodent females and their offspring via the milk [83]. Therefore, the elevated levels seen in the offspring of Babesia-treated females could be a direct consequence of changes occurring in the maternal immune system as a consequence of infection.
As adults, Babesia-treated offspring showed a faster resolution phase in response to direct Babesia infection. They also showed a relative reduction in testosterone in response to a social challenge. Testosterone is known to interact directly and indirectly with CD4+ T cells (see [84] for a discussion of evidence of both up- and downregulation), which are important for the resolution phase of B. microti infection recovery [54]. The Babesia-treated offspring lost less weight during infection, indicating lower adverse effects from parasitism [85], possibly less pathology and hence lower intensity of disease. It is difficult to interpret the relatively low levels of IgG production during infection in this same treatment group. It may be that in Babesia-treated offspring, other immune cells (e.g. CD4+ T cells) and molecules were downregulated less by testosterone, and therefore the Babesia-treated offspring were less dependent on escalating production and systemic sustenance of IgG. Alternatively, the difference may simply reflect a faster return to normal levels of immune components in the Babesia-treated offspring, because they cleared the infection more efficiently.
In conclusion, our results support the existence of a trade-off between social dominance and disease resistance, and suggest that such a trade-off can be mediated by maternal experiences before pregnancy. While our findings do not perfectly mimic those found following an indirect challenge by co-housing pregnant females with infected neighbours [51], consistent trends have emerged. In both cases, maternal exposure led to physiological changes in the female, which ultimately filtered down, affecting the responses of offspring to social challenge (either behaviourally or physiologically) and to infection. The presence of Babesia in the maternal environment caused offspring to downregulate investment in dominance acquisition, which in natural systems, among wild animals, would probably have led to reduced access to mating opportunities [59–61]. In doing so, however, the offspring avoided the associated reduced resistance to infection that is typically associated with high dominance status [59,62,86]. In an environment in which the risk of parasitism is high, cues experienced by the mothers led to increased resistance in offspring. The results of our experiment emphasize the roles of both maternal effects and parasites, and the interplay between the two, in shaping life histories and disease susceptibility.
Acknowledgements
This work was made possible by the efforts and imagination of Chris Barnard, who sadly died in June 2007. He is greatly missed, and he continues to be an inspiration to us all. We thank H. Travis and A. Lowe for technical support. This work was supported by the BBSRC and the School of Biology, University of Nottingham.
References
- 1.Mousseau T. A., Fox C. W. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press [Google Scholar]
- 2.Lacey E. P. 1998. What is an adaptive environmentally induced parental effect? In Maternal effects as adaptations (eds Mousseau T. A., Fox C. W.). New York, NY: Oxford University Press [Google Scholar]
- 3.Taborsky B. 2006. Mothers determine offspring size in response to own juvenile growth conditions. Biol. Lett. 2, 225–228 10.1098/rsbl.2005.0422 (doi:10.1098/rsbl.2005.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438 10.1016/j.tree.2008.04.005 (doi:10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 5.Mousseau T. A., Dingle H. 1991. Maternal effects in insects: examples, constraints and geographic variation. In The unity of evolutionary biology (ed. Dudley E. C.). Portland, OR: Dioscorides Press [Google Scholar]
- 6.Anway M. D., Cupp A. S., Uzumcu M., Skinner M. K. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 10.1126/science.1108190 (doi:10.1126/science.1108190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anway M. D., Skinner M. K. 2006. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147, S43–S49 10.1210/en.2005-1058 (doi:10.1210/en.2005-1058) [DOI] [PubMed] [Google Scholar]
- 8.Sheriff M. J., Krebs C. J., Boonstraa R. 2010. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91, 2983–2994 10.1890/09-1108.1 (doi:10.1890/09-1108.1) [DOI] [PubMed] [Google Scholar]
- 9.Drake A. J., Liu L. 2010. Intergenerational transmission of programmed effects: public health consequences. Trends Endocrinol. Metabol. 21, 206–213 10.1016/j.tem.2009.11.006 (doi:10.1016/j.tem.2009.11.006) [DOI] [PubMed] [Google Scholar]
- 10.Marshall D. J., Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963 10.1111/j.2007.0030-1299.16203.x (doi:10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 11.Groothuis T. G., Eising C. M., Dijkstra C., Müller W. 2005. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 1, 78–81 10.1098/rsbl.2004.0233 (doi:10.1098/rsbl.2004.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groothuis T. G., Muller W., von Engelhardt N., Carere C., Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 13.Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 14.Mennerat A., Mirleau P., Blondel J., Perret P., Lambrechts M. M., Heeb P. 2009. Aromatic plants in nests of the blue tit Cyanistes caeruleus protect chicks from bacteria. Oecologia 161, 849–855 10.1007/s00442-009-1418-6 (doi:10.1007/s00442-009-1418-6) [DOI] [PubMed] [Google Scholar]
- 15.Bize P., Roulin A. 2009. Effects of common origin and common rearing environment on variance in ectoparasite load and phenotype of nestling alpine swifts. Evol. Biol. 36, 301–310 10.1007/s11692-009-9063-x (doi:10.1007/s11692-009-9063-x) [DOI] [Google Scholar]
- 16.Boulinier T., Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288 10.1016/j.tree.2007.12.006 (doi:10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 17.Agrawal A. A., Laforsch C., Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60–63 10.1038/43425 (doi:10.1038/43425) [DOI] [Google Scholar]
- 18.Gallizzi K., Guenon B., Richner H. 2008. Maternally transmitted parasite defence can be beneficial in the absence of parasites. Oikos 117, 223–230 10.1111/j.2007.0030-1299.16172.x (doi:10.1111/j.2007.0030-1299.16172.x) [DOI] [Google Scholar]
- 19.Grindstaff J. L., Hasselquist D., Nilsson J. A., Sandell M., Smith H. G., Stjernman M. 2006. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B 273, 2551–2557 10.1098/rspb.2006.3608 (doi:10.1098/rspb.2006.3608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristan D. M. 2004. Intestinal nematode infection affects host life history and offspring susceptibility to parasitism. J. Anim. Ecol. 73, 227–238 10.1111/j.0021-8790.2004.00794.x (doi:10.1111/j.0021-8790.2004.00794.x) [DOI] [Google Scholar]
- 21.Moret Y., Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168 10.1126/science.290.5494.1166 (doi:10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 22.Badyaev A. V., Hamstra T. L., Oh K. P., Seaman D. A. A. 2006. Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proc. Natl Acad. Sci. USA 103, 14 406–14 411 10.1073/pnas.0602452103 (doi:10.1073/pnas.0602452103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson L. A., Korotkova M., Lundin S., Haversen L., Silfverdal S.-A., Mattsby-Baltzer I., Strandvik B., Telemo E. 2003. The transfer of immunity from mother to child. Ann. N. Y. Acad. Sci. 987, 199–206 10.1111/j.1749-6632.2003.tb06049.x (doi:10.1111/j.1749-6632.2003.tb06049.x) [DOI] [PubMed] [Google Scholar]
- 24.Poulin R., Thomas F. 2008. Epigenetic effects of infection on the phenotype of host offspring: parasites reaching across host generations. Oikos 117, 331–335 10.1111/j.2007.0030-1299.16435.x (doi:10.1111/j.2007.0030-1299.16435.x) [DOI] [Google Scholar]
- 25.Clark M. M., Karpiuk P., Galef B. G. 1993. Hormonally mediated inheritance of acquired characteristics in Mongolian gerbils. Nature 364, 712. 10.1038/364712a0 (doi:10.1038/364712a0) [DOI] [PubMed] [Google Scholar]
- 26.Murgatroyd C., et al. 2009. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 12, 1559–1566 10.1038/nn.2436 (doi:10.1038/nn.2436) [DOI] [PubMed] [Google Scholar]
- 27.Jones P. A., Takai D. 2001. The role of DNA methylation in mammalian epigenetics. Science 293, 1068–1070 10.1126/science.1063852 (doi:10.1126/science.1063852) [DOI] [PubMed] [Google Scholar]
- 28.Hosken D. J. 2001. Sex and death: microevolutionary trade-offs between reproductive and immune investment in dung flies. Curr. Biol. 11, R379–R380 10.1016/S0960-9822(01)00211-1 (doi:10.1016/S0960-9822(01)00211-1) [DOI] [PubMed] [Google Scholar]
- 29.Dingemanse N. J., Edelaar P., Kempenaers B. 2010. Why is there variation in baseline glucocorticoid levels? Trends Ecol. Evol. 25, 261–262 10.1016/j.tree.2010.01.008 (doi:10.1016/j.tree.2010.01.008) [DOI] [PubMed] [Google Scholar]
- 30.Ilmonen P., Taarna T., Hasselquist D. 2000. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. Lond. B 267, 665–670 10.1098/rspb.2000.1053 (doi:10.1098/rspb.2000.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raberg L., Nilsson J. A., Ilmonen P., Stjernman M. 2000. The cost of an immune response: vaccination reduces parental effort. Ecol. Lett. 3, 382–386 10.1046/j.1461-0248.2000.00154.x (doi:10.1046/j.1461-0248.2000.00154.x) [DOI] [Google Scholar]
- 32.Zala S. M., Chan B. K., Bilbo S. D., Potts W. K., Nelson R. J., Penn D. J. 2008. Genetic resistance to infection influences a male's sexual attractiveness and modulation of testosterone. Brain Behav. Immun. 22, 381–387 10.1016/j.bbi.2007.09.003 (doi:10.1016/j.bbi.2007.09.003) [DOI] [PubMed] [Google Scholar]
- 33.McKean K. A., Nunny L. 2001. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 7904–7909 10.1073/pnas.131216398 (doi:10.1073/pnas.131216398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siva-Jothy M. T., Tsubaki Y., Hooper R. E. 1998. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiol. Entomol. 26, 1–5 [Google Scholar]
- 35.Kirsten T. B., Taricano M., Maiorka P. C., Palermo-Neto J., Bernardi M. M. 2010. Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation 2010, 240–251 10.1159/000290040 (doi:10.1159/000290040) [DOI] [PubMed] [Google Scholar]
- 36.Barnard C. J., Behnke J. M., Sewell J. 1996. Social status and resistance to disease in house mice (Mus musculus): status-related modulation of hormonal responses in relation to immunity costs in different social and physical environments. Ethology 102, 63–84 10.1111/j.1439-0310.1996.tb01104.x (doi:10.1111/j.1439-0310.1996.tb01104.x) [DOI] [Google Scholar]
- 37.Barnard C. J., Behnke J. M., Gage A. R., Brown H., Smithurst P. R. 1998. Maternal effects on the development of social rank and immunity trade-offs in male laboratory mice (Mus musculus). Proc. R. Soc. Lond. B 265, 2087–2093 10.1098/rspb.1998.0544 (doi:10.1098/rspb.1998.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnard C. J., Behnke J. M., Gage A. R., Brown H., Smithurst P. R. 1997. Immunity costs and behavioural modulation in male laboratory mice (Mus musculus) exposed to the odours of females. Physiol. Behav. 62, 857–866 10.1016/S0031-9384(97)00249-7 (doi:10.1016/S0031-9384(97)00249-7) [DOI] [PubMed] [Google Scholar]
- 39.Hamilton W. D., Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 40.Chadwick W., Little T. J. 2005. A parasite-mediated life-history shift in Daphnia magna. Proc. R. Soc. B 272, 505–509 10.1098/rspb.2004.2959 (doi:10.1098/rspb.2004.2959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly A., Hatcher M. J., Dunn A. M. 2003. The impact of a vertically transmitted microsporidian, Nosema granulosis on the fitness of its Gammarus duebeni host under stressful environmental conditions. Parasitology 126, 119–124 10.1017/S003118200200269X (doi:10.1017/S003118200200269X) [DOI] [PubMed] [Google Scholar]
- 42.Mautner S. I., Cook K. A., Forbes M. R., McCurdy D. G., Dunn A. M. 2007. Evidence for sex ratio distortion by a new microsporidian parasite of a Corophiid amphipod. Parasitology 134, 1567–1573 10.1017/S0031182007003034 (doi:10.1017/S0031182007003034) [DOI] [PubMed] [Google Scholar]
- 43.Whitaker S., Fair J. 2002. The costs of immunological challenge to developing mountain chickadees, Poecile gambeli, in the wild. Oikos 99, 161–165 10.1034/j.1600-0706.2002.990116.x (doi:10.1034/j.1600-0706.2002.990116.x) [DOI] [Google Scholar]
- 44.Hakkarainen H., Huhta E., Koskela E., Mappes T., Soveri T., Suorsa P. 2007. Eimeria-parasites are associated with a lowered mother's and offspring's body condition in island and mainland populations of the bank vole. Parasitology 134, 23–31 10.1017/S0031182006001120 (doi:10.1017/S0031182006001120) [DOI] [PubMed] [Google Scholar]
- 45.Heeb P., Werner I., Mateman A. C., Kolliker M., Brinkhof M. W. G., Lessells C. M., Richner H. 1999. Ectoparasite infestation and sex-biased local recruitment of hosts. Nature 400, 63–65 10.1038/21881 (doi:10.1038/21881) [DOI] [PubMed] [Google Scholar]
- 46.Dawkins R. 1982. The extended phenotype. Oxford, UK: Oxford University Press [Google Scholar]
- 47.Sorci G., Clobert J. 1995. Effects of maternal parasite load on offspring life-history traits in the common lizard (Lacerta vivipara). J. Evol. Biol. 8, 711–723 10.1046/j.1420-9101.1995.8060711.x (doi:10.1046/j.1420-9101.1995.8060711.x) [DOI] [Google Scholar]
- 48.Sorci G., Massot M., Clobert J. 1994. Maternal parasite load increases sprint speed and philopatry in female offspring of the common lizard. Am. Nat. 144, 153–164 10.1086/285666 (doi:10.1086/285666) [DOI] [Google Scholar]
- 49.Maizels R. M. 2010. Parasite immunomodulation and polymorphisms of the immune system. J. Biol. 8, 62. 10.1186/jbiol166 (doi:10.1186/jbiol166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kilpimaa J., Alatalo R. V., Siitari H. 2004. Trade-offs between sexual advertisement and immune function in the pied flycatcher (Ficedula hypoleuca). Proc. R. Soc. Lond. B 271, 245–250 10.1098/rspb.2003.2568 (doi:10.1098/rspb.2003.2568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curno O., Behnke J. M., McElligott A. G., Reader T., Barnard C. J. 2009. Mothers produce less aggressive sons with altered immunity when there is a threat of disease during pregnancy. Proc. R. Soc. B 276, 1047–1054 10.1098/rspb.2008.1612 (doi:10.1098/rspb.2008.1612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J. Y., et al. 2007. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J. Clin. Microbiol. 45, 2084–2087 10.1128/JCM.01334-06 (doi:10.1128/JCM.01334-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kjemtrup A. M., Conrad P. A. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30, 1323–1337 10.1016/S0020-7519(00)00137-5 (doi:10.1016/S0020-7519(00)00137-5) [DOI] [PubMed] [Google Scholar]
- 54.Homer M. J., Aguilar-Delfin I., Telford S. R., Krause P. J., Persing D. H. 2000. Babesiosis. Clin. Microbiol. Rev. 13, 451–469 10.1128/CMR.13.3.451-469.2000 (doi:10.1128/CMR.13.3.451-469.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogdan C. 2007. Oxidative burst without phagocytes: the role of respiratory proteins. Nat. Immunol. 8, 1029–1031 10.1038/ni1007-1029 (doi:10.1038/ni1007-1029) [DOI] [PubMed] [Google Scholar]
- 56.Igarashi I., et al. 1999. Roles of CD4+ T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect. Immun. 67, 4143–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koyama S. 2004. Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities. Horm. Behav. 46, 303–310 10.1016/j.yhbeh.2004.03.002 (doi:10.1016/j.yhbeh.2004.03.002) [DOI] [PubMed] [Google Scholar]
- 58.Van Loo P. L. P., Van Zutphen L. F. M., Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab. Anim. 37, 300–313 10.1258/002367703322389870 (doi:10.1258/002367703322389870) [DOI] [PubMed] [Google Scholar]
- 59.Barnard C. J., Behnke J. M., Sewell J. 1994. Social behaviour and susceptibility to infection in house mice (Mus musculus): effects of group size, aggressive behaviour and status-related hormonal responses prior to infection on resistance to Babesia microti. Parasitology 108, 487–496 10.1017/S0031182000077349 (doi:10.1017/S0031182000077349) [DOI] [PubMed] [Google Scholar]
- 60.Meagher S., Penn D. J., Potts W. K. 2000. Male-male competition magnifies inbreeding depression in wild house mice. Proc. Natl Acad. Sci. USA 97, 3324–3329 10.1073/pnas.060284797 (doi:10.1073/pnas.060284797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waterman J. M. 2007. Male mating strategies in rodents. In Rodent societies: an ecological and evolutionary perspective (eds Wolff J. O., Sherman P. W.), pp. 27–41 Chicago, IL: Chicago University Press [Google Scholar]
- 62.Whitacre C. C. 2001. Sex differences in autoimmune disease. Nat. Immunol. 2, 777–780 10.1038/ni0901-777 (doi:10.1038/ni0901-777) [DOI] [PubMed] [Google Scholar]
- 63.Zala S. M., Potts W. K., Penn D. J. 2008. Exposing males to female scent increases the cost of controlling Salmonella infection in wild house mice. Behav. Ecol. Sociobiol. 62, 895–900 10.1007/s00265-007-0513-0 (doi:10.1007/s00265-007-0513-0) [DOI] [Google Scholar]
- 64.Alexander J., Stimson W. H. 1988. Sex hormones and the course of parasitic infection. Parasitol. Today 4, 189–193 10.1016/0169-4758(88)90077-4 (doi:10.1016/0169-4758(88)90077-4) [DOI] [Google Scholar]
- 65.Sadd B. M., Kleinlogel Y., Schmid-Hempel R., Schmid-Hempel P. 2005. Trans-generational immune priming in a social insect. Biol. Lett. 1, 386–388 10.1098/rsbl.2005.0369 (doi:10.1098/rsbl.2005.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grindstaff J. L., Hasselquist D., Nilsson J.-A., Sandell M., Smith H. G., Stjernman M. 2006. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B 273, 2551–2557 10.1098/rspb.2006.3608 (doi:10.1098/rspb.2006.3608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kristan D. M. 2002. Maternal and direct effects of the intestinal nematode Heligmosomoides polygyrus on offspring growth and susceptibility to infection. J. Exp. Biol. 205, 3967–3977 [DOI] [PubMed] [Google Scholar]
- 68.Gotz A. A., Wolf M., Stefanski V. 2008. Psychosocial maternal stress during pregnancy: effects on reproduction for F0 and F1 generation laboratory rats. Physiol. Behav. 93, 1055–1060 10.1016/j.physbeh.2008.01.009 (doi:10.1016/j.physbeh.2008.01.009) [DOI] [PubMed] [Google Scholar]
- 69.Herrenkohl L. 1979. Prenatal stress reduces fertility and fecundity in female offspring. Science 206, 1097–1099 10.1126/science.573923 (doi:10.1126/science.573923) [DOI] [PubMed] [Google Scholar]
- 70.Adamo S. A. 1999. Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Anim. Behav. 57, 117–124 10.1006/anbe.1998.0999 (doi:10.1006/anbe.1998.0999) [DOI] [PubMed] [Google Scholar]
- 71.Williams G. C. 1966. Natural selection, the cost of reproduction and a refinement of Lack's principle. Am. Nat. 100, 687–690 10.1086/282461 (doi:10.1086/282461) [DOI] [Google Scholar]
- 72.Javois J., Tammaru T. 2004. Reproductive decisions are sensitive to cues of life expectancy: the case of a moth. Anim. Behav. 68, 249–255 10.1016/j.anbehav.2003.10.022 (doi:10.1016/j.anbehav.2003.10.022) [DOI] [Google Scholar]
- 73.Hanssen S. A. 2006. Costs of an immune challenge and terminal investment in a long-lived bird. Ecology 87, 2440–2446 10.1890/0012-9658(2006)87[2440:COAICA]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2440:COAICA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 74.Kristan D. M. 2002. Effects of intestinal nematodes during lactation: consequences for host morphology, physiology and offspring mass. J. Exp. Biol. 205, 3955–3965 [DOI] [PubMed] [Google Scholar]
- 75.Agrawal A. F., Brodie I. I. I. E. D., Brown J. 2001. Parent-offspring coadaptation and the dual genetic control of maternal care. Science 292, 1710–1712 10.1126/science.1059910 (doi:10.1126/science.1059910) [DOI] [PubMed] [Google Scholar]
- 76.Cameron E. Z. 1998. Is suckling behaviour a useful predictor of milk intake? A review. Anim. Behav. 56, 521–532 10.1006/anbe.1998.0793 (doi:10.1006/anbe.1998.0793) [DOI] [PubMed] [Google Scholar]
- 77.Hinde C. A., Johnstone R. A., Kilner R. M. 2010. Parent-offspring conflict and coadaptation. Science 327, 1373–1376 10.1126/science.1186056 (doi:10.1126/science.1186056) [DOI] [PubMed] [Google Scholar]
- 78.Mendl M., Paul E. S. 1990. Litter composition affects parental care, offspring growth and the development of aggressive behaviour in wild house mice. Behaviour 116, 91–107 [Google Scholar]
- 79.New D. L., Quinn J. B., Qureshi M. Z., Sigler S. J. 1997. Vertically transmitted babesiosis. J. Pediatr. 131, 163–164 10.1016/S0022-3476(97)70143-4 (doi:10.1016/S0022-3476(97)70143-4) [DOI] [PubMed] [Google Scholar]
- 80.Krause P. J., Daily J., Telford S. R., Vannier E., Lantos P., Spielman A. 2007. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol. 23, 605–610 10.1016/j.pt.2007.09.005 (doi:10.1016/j.pt.2007.09.005) [DOI] [PubMed] [Google Scholar]
- 81.Marzal A., Lope F. d, Navarro C., Møller A. P. 2005. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142, 541–545 10.1007/s00442-004-1757-2 (doi:10.1007/s00442-004-1757-2) [DOI] [PubMed] [Google Scholar]
- 82.Menendez C., Ordi J., Ismail M. R., Ventura P. J., Aponte J. J., Kahigwa E., Font F., Alonso P. L. 2000. The impact of placental malaria on gestational age and birth weight. J. Infect. Dis. 181, 1740–1745 10.1086/315449 (doi:10.1086/315449) [DOI] [PubMed] [Google Scholar]
- 83.Diaz R. L., Hoang L., Wang J., Vela J. L., Jenkins S., Aranda R., Martin M. G. 2004. Maternal adaptive immunity influences the intestinal microflora of suckling mice. J. Nutr. 134, 2359–2364 [DOI] [PubMed] [Google Scholar]
- 84.Roberts C. W., Walker W., Alexander J. 2001. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 14, 476–488 10.1128/CMR.14.3.476-488.2001 (doi:10.1128/CMR.14.3.476-488.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Persing D. H., Conrad P. A. 1995. Babesiosis: new insights from phylogenetic analysis. Infect. Agents Dis. 4, 182–195 [PubMed] [Google Scholar]
- 86.Barnard C. J., Behnke J. M., Sewell J. 1993. Social behaviour, stress and susceptibility to infection in house mice (Mus musculus): effects of duration of grouping and aggressive behaviour prior to infection on susceptibility to Babesia microti. Parasitology 107, 183–192 10.1017/S0031182000067299 (doi:10.1017/S0031182000067299) [DOI] [PubMed] [Google Scholar]