Abstract
To efficiently provide an animal with relevant information, the design of its visual system should reflect the distribution of natural signals and the animal's tasks. In many behavioural contexts, however, we know comparatively little about the moment-to-moment information-processing challenges animals face in their daily lives. In predator avoidance, for instance, we lack an accurate description of the natural signal stream and its value for risk assessment throughout the prey's defensive behaviour. We characterized the visual signals generated by real, potentially predatory events by video-recording bird approaches towards an Uca vomeris colony. Using four synchronized cameras allowed us to simultaneously monitor predator avoidance responses of crabs. We reconstructed the signals generated by dangerous and non-dangerous flying animals, identified the cues that triggered escape responses and compared them with those triggering responses to dummy predators. Fiddler crabs responded to a combination of multiple visual cues (including retinal speed, elevation and visual flicker) that reflect the visual signatures of distinct bird and insect behaviours. This allowed crabs to discriminate between dangerous and non-dangerous events. The results demonstrate the importance of measuring natural sensory signatures of biologically relevant events in order to understand biological information processing and its effects on behavioural organization.
Keywords: fiddler crabs, Uca vomeris, predator avoidance, vision, natural visual signals
1. Introduction
Brains and sensory systems are costly to maintain [1], and are thus not adapted to extract all possible information from the environment, but to analyse the particular stimuli that are available and behaviourally relevant to an animal in its natural habitat. Several studies have examined neuronal responses to natural visual stimuli in the laboratory in recent years (e.g. [2–9]). We learned from these studies that coding properties of neurons can differ dramatically in response to natural compared with artificial stimuli. While these findings argue for the need to use natural and ecologically relevant stimuli in neurophysiological experiments, they expose how ignorant we are about visual signal processing under natural conditions. This is largely because it is extremely difficult to measure the sensory signal stream experienced by freely behaving animals in their natural environment and to quantify the information that the animals can potentially extract from it. For vision, for instance, this requires detailed knowledge of an animal's visual system, the ability to follow gaze in a freely moving animal, an understanding of the tasks the animal has to solve, and a way of simultaneously recording visual signals and behaviour under natural conditions.
(a). Predator avoidance in fiddler crabs
In this respect, fiddler crabs—and in particular their predator avoidance responses—offer unique opportunities. The crabs are prey to a large number of avian predators hunting with a wide variety of techniques [10–13]. They detect these predators using exclusively visual cues [14,15]. In the open mudflats they inhabit, the crabs are forced to take shelter from a passing bird as often as every 2 or 3 min, which must exert a very high selective pressure for efficient anti-predator strategies. The crabs' burrow-centred lifestyle makes it possible to record the complete behavioural repertoire of many animals over extended periods of time with a stationary video camera. Our detailed knowledge of the crabs' sampling array and visual topography [16–18], combined with the fact that they do not make directed eye movements, allows us to quantify the exact visual information available to every individual crab at any point in time (reviewed by Zeil & Hemmi [19]).
Owing to the comparatively poor resolving power of their eyes, the information available to crabs at the time of escape from a predator is extremely limited [18,20–22]. Furthermore, their closely set eyes prevent them from using binocular stereopsis to gain distance information at the distances relevant in predator avoidance (e.g. [19,23–25]). This allows us to examine their anti-predator behaviour using small dummies that the crabs cannot distinguish from real predators. Most of our current knowledge about the visual cues guiding crab escape responses is based on their responses to such dummies [14–16,26–28]. When approached by a predator (dummy or real) fiddler crabs initiate a multi-staged escape response [20,28]. When first detecting a potential threat, the crabs cease any activity and remain still. They then initiate a sudden and fast home run towards their burrow, at the entrance of which they usually remain for a while before finally descending into the burrow. This response cascade can be interrupted at any time to limit the costs of potential false alarms.
Fiddler crabs are thus challenged to limit their responses to the most dangerous events in their environment, to avoid false alarms and to make life or death decisions with incomplete information that does not correlate strongly with real risk. The first step when trying to spot a large or flying predator in a flat environment is to only treat signals above the horizon as potential threats [16,25,29–31]. But while this strategy prevents the crabs from treating conspecifics as predators, it still leaves a large number of ambiguous signals to identify as either harmless or threatening. One possible solution is that the crabs' response criteria reflect the differences in the statistical properties of dangerous and harmless events. In the absence of distance information, other crab species have been shown (under varying experimental conditions) to respond to a number of visual features of dangerous events. For instance, looming cues seem to play a role in response to directly approaching objects and in the laboratory (e.g. [26,32]). Under more natural conditions, however, fiddler crabs (Uca vomeris) base most of their initial escape decisions on a criterion related to retinal speed [14,15]. While retinal speed provides a sensitive early warning system, it is not strictly related to predation risk. The retinal speed of a directly approaching object is smaller than that of an object that passes by, and crabs therefore respond later to dummy predators that approach more directly. Other factors influencing escape responses in dummy predation experiments [14,15] are retinal size, elevation and the direction of motion. These additional cues might help alleviate the costs of false alarms, provided they better reflect the signal differences between dangerous and harmless natural events.
(b). Natural visual cues
Although some of the visual cues that trigger predator avoidance in fiddler crabs are known, it remains unclear to what extent they reflect the signals normally encountered by crabs when confronted with natural predators. The visual signature of a flying bird seen against a dynamic mangrove background or against the blue sky is likely to be very different from that of a black dummy approaching in a straight line. The dominant natural predators of fiddler crabs on the mudflats of northeastern Australia, for instance, are gull-billed terns Gelochelidon nilotica. These relatively small birds regularly scan the mudflats, flying into the wind a few metres above ground at a speed of about 3 m s–1. Whenever a tern spots a crab without refuge, it extends its wings to brake in midair and dives down in an attempt to catch the prey [10]. The retinal image of a real tern thus constantly changes shape (through the beating of its wings), direction, speed and contrast, depending on such flight manoeuvres.
Not all birds are predators and not all movement in the sky signals danger. While birds and mammals can, to a certain degree, recognize and distinguish the shape or visual details of predators and non-predators [33–39], many small prey animals do not have these cues available under natural circumstances. As we will see, at the time at which fiddler crabs react to approaching birds, the apparent size of the predator is between 0.5° and 2° (see also [15]). The bird is therefore seen by only one or two ommatidia, making shape recognition virtually impossible. Nonetheless, crabs must have ways of discriminating the sensory signatures of harmless events from those generated by predators in order to keep the number of false alarms low. This means that we can expect sensory and neural filters tuned to discriminate approaching terns from those that are departing, from other birds (such as kites and eagles) that do not hunt fiddler crabs, from small but close insects or from mangrove leaves carried past by the wind. The question thus becomes ‘what properties do these sensory and neural filters need to have?’
The aim of this study is to characterize the visual signal distributions generated by natural events from the perspective of freely behaving crabs. Using four synchronized cameras, we recorded events from the viewpoint of fiddler crabs in the field, while at the same time monitoring the crabs' responses. We examine how the differences in visual signatures relate to escape decisions, which allows us to predict the filter parameters that enable crabs to discriminate between harmless and dangerous events in their world.
2. Material and methods
(a). Animals and apparatus
Experiments were conducted in April 2008 with U. vomeris (McNeill) (Ocypodidae: Brachyura: Decapoda) on intertidal mudflats near Cungulla (19°24′ S, 147°6′ E), south of Townsville, Queensland, Australia. Four camcorders (Panasonic NV-GS300) were mounted on two vertical steel poles that were placed 192 cm apart on the mudflat (figure 1). The two upper cameras (crab cameras) were mounted at a height of 160 cm above ground level and arranged such that they recorded the activity of crabs on two adjacent patches of mudflat, each 1 m2 in size. The lower cameras (bird cameras) were fitted with wide-angle lenses (Raynox Pro semi-fish-eye conversion lens 0.3×), mounted as close to the ground as possible (13 cm above ground level) and tilted upwards at an angle of approximately 30° to monitor bird approaches from crab perspective in a visual field of about 50° elevation and 80° azimuth.
Figure 1.
The synchronized four-camera set-up. The two lower cameras were fitted with fish-eye lenses to observe a field of view of approximately 50° by 80° each. The top cameras pointed down to observe crab behaviour on the mudflat. Sample images are shown next to each camera.
Both bird cameras were directed northwards along the beach, into the direction from which most terns approached. Through a central custom-made controller box, all four cameras received a common audio signal. This signal consisted of four tones that alternated in a pre-programmed, pseudo-random sequence, which later allowed us to timestamp individual frames recorded by the four cameras to within 1 ms. The set-up was observed from at least 10 m away and all bird movements were noted to ensure that in the subsequent analysis crab responses to birds outside the cameras' field of view were not mistaken for responses to birds recorded by the cameras.
(b). Video analysis
We recorded a total of 17 experimental sessions (80–90 min each) over 8 days and finally analysed the session with the highest number of valid approaches. From this session, we analysed a total of 37 sequences during which one or more approaching animals were visible. In these 37 sequences, we recorded the activity of a total of 62 animals (14 terns, 20 kites, 20 insects and eight others, including eagles, crows, herons and gulls) and responses of 10 crabs. The videos were digitized and calibrated using a checkerboard test pattern to correct for optical and perspective distortions, and to determine camera position relative to the ground [40]. We determined bird movements with 20 ms precision and crab responses with 200 ms precision, following the procedures developed by Hemmi [14,15]. We analysed bird behaviour by under-sampling at 200 ms, with, for instance, ‘speed’ for a certain 200 ms interval to be the maximum value of the last ten 20 ms intervals. We quantified bird movements by measuring their elevation, and their horizontal and vertical angular speed and acceleration. For technical reasons, we were unable to collect useful information on the angular size of birds. We filmed with wide-angle lenses and for most of the time birds were only a few pixels wide with low contrast. Bird signals often disappeared in the image background noise, requiring motion analysis across a sequence of images to separate them from the background and identify their position, because a moving noisy dot is much easier to see than a stationary noisy dot. To define the signal at the level of a single photoreceptor, we calculated average pixel differences in a window of 3 × 3 pixels around a bird's position and then calculated flicker (temporal local contrast change) at any given time as the maximum signal change (average pixel difference) that had occurred at a bird's current position during the preceding 200 ms. More details of the analysis procedure and the selection of trials can be found in the electronic supplementary material.
(c). Signal description and descriptive statistics
Traditional experimental stimuli in the context of predator avoidance are usually designed in a way that makes the parameter of interest progressively more threatening. A dummy predator, for instance, is moved towards a colony of crabs until all crabs disappear down their burrow (e.g. [14,15]), making it possible to evaluate responses as a function of the distance, speed, retinal size or any other relevant parameter. Responses can then be analysed, for instance, in the form of a survival curve showing the number of animals that have not responded to a stimulus at a particular time or distance (e.g. [28]). For a more threatening stimulus, curves will be shifted towards earlier times and longer distances. Natural visual stimuli, on the other hand, are rarely monotonous. The three parameters we examine here in detail—elevation, retinal speed and flicker—usually increase and decrease multiple times during each approach. A cumulative analysis such as a survival curve or a linear model including the maximum ‘threat’ experienced so far is therefore not appropriate in this case.
To examine the relevant cues available to crabs on a moment-by-moment basis, we analysed response probability as a function of maximum elevation, retinal speed and flicker experienced in the preceding 200 ms.1 This time window corresponds with behaviourally and physiologically determined response latencies in the crab Chasmagnathus [32], and was used in all dummy studies involving fiddler crabs.
We describe differences in visual signals between four classes of events (terns, kites, insects and commuters) in example paths and histograms displaying for each parameter the observation probability 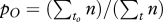 , where t denotes all 200 ms time frames, to all time frames in which a certain parameter value was observed and n the number of active crabs in that time frame. An active crab is defined as any crab more than 5 cm away from its burrow; this ensures that a home-run response can actually be identified. In other words, for a crab that is away from its burrow while a bird is present somewhere, this is the probability to observe this particular condition. The total sum of all observation probabilities of a certain parameter therefore equals 100 per cent. The crabs' responses are described by their response probability
, where t denotes all 200 ms time frames, to all time frames in which a certain parameter value was observed and n the number of active crabs in that time frame. An active crab is defined as any crab more than 5 cm away from its burrow; this ensures that a home-run response can actually be identified. In other words, for a crab that is away from its burrow while a bird is present somewhere, this is the probability to observe this particular condition. The total sum of all observation probabilities of a certain parameter therefore equals 100 per cent. The crabs' responses are described by their response probability 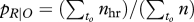 , with to and n as above, and nhr the number of crabs that responded by starting a home run in the following frame. By this definition, response likelihood to a certain speed, for instance, is independent of the actual distribution of speeds. That is to say, it is the likelihood of a response given that the speed has been observed. These probabilities therefore do not add up to 100 per cent, but each individual one could theoretically reach 100 per cent, provided all crabs had always reacted to the parameter value when they observed it.
, with to and n as above, and nhr the number of crabs that responded by starting a home run in the following frame. By this definition, response likelihood to a certain speed, for instance, is independent of the actual distribution of speeds. That is to say, it is the likelihood of a response given that the speed has been observed. These probabilities therefore do not add up to 100 per cent, but each individual one could theoretically reach 100 per cent, provided all crabs had always reacted to the parameter value when they observed it.
(d). Statistical model of predator avoidance decisions
Individual moment-to-moment decisions (to start a home run or not to start a home run) for a total of 12 962 time intervals fulfilling the above criteria (including 137 responses and 12 825 non-responses) were evaluated in a generalized linear mixed model (GLMM) [41] in R v. 2.9 (using the glmer function of the lme4 package). Insect approaches were excluded from this analysis. We took into account individual variance between crabs (crab identity) and between birds (bird identity) by treating them as random factors. The GLMM used logit as a link function. The final model was selected by sequentially fitting parameters of interest and including only those parameters that reached significance at a 5 per cent level when added to the final model.
3. Results
During our monitoring period, the crabs experienced a high level of activity by predatory birds and harmless animals flying above the mudflat. Even though our cameras monitored only about one quarter of all possible approach directions, birds were visible in at least one of the two cameras during 16 per cent of the 80 min we examined. This high exposure rate stresses the importance of an effective strategy to distinguish harmless from dangerous events. The crabs appear to be able to do this to some degree: although terns (the actual predators) only contributed 16 per cent of valid analysis frames, they elicited 34 per cent of the responses (table 1). Surprisingly, the crabs we observed in this study only descended into their burrows following 14 out of the 152 home runs. The following analysis therefore only examines the second stage of the escape response (the home run).
Table 1.
Recording times and elicited responses. ‘Time recorded’ is time during which the animal was recorded in at least one observation camera, and ‘valid frames’ the sum of all frames multiplied by the number of crabs active (greater than 5 cm away from burrow) in the frame. After excluding insects, these are the 12 962 frames evaluated in the GLMM (table 2). Note that although terns only make up 16% of all observation time, they elicited 34% of all responses.
| type (no.) | time recorded | valid frames | home runs | burrow descents |
|---|---|---|---|---|
| terns (14) | 3 min, 08 s (25%) | 2119 (16%) | 51 (34%) | 6 |
| kites (20) | 7 min, 01 s (57%) | 9002 (66%) | 66 (43%) | 6 |
| insects (20) | 43 s (6%) | 581 (4%) | 15 (10%) | 0 |
| commuters (8) | 1 min, 32 s (12%) | 1841 (14%) | 20 (13%) | 2 |
| total | 12 min, 24 s | 13 543 | 152 | 14 |
(a). Different flying animals produce different visual signatures
We first explore differences in visual signatures for four common groups of flying organisms crabs were exposed to. We present example flight paths for each group in figure 2 and summarize the statistics of all available data in figure 3, in apparent units as seen by crabs (e.g. retinal speed in ommatidia s−1) rather than absolute units (e.g. real speed in m s–1).
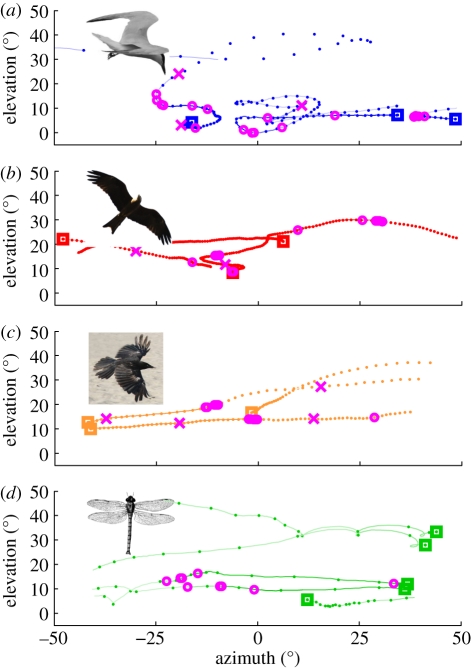
Figure 2.
Typical example flight paths, as seen from crab perspective, of several (a) terns, (b) kites, (c) commuters and (d) insects. Squares mark the start of each path, and dots indicate position every 200 ms. Individual paths were off-set horizontally for visibility. Crab responses are shown as magenta circles for home runs and crosses for burrow entries. If several responses occurred at the same time, circles were slightly offset for clarity. The saturation of the traces indicates the percentage of crabs that are still active on the surface: no line between points indicates that all previously active crabs have run home. In concurrent tracks, response markers are shown on both tracks.
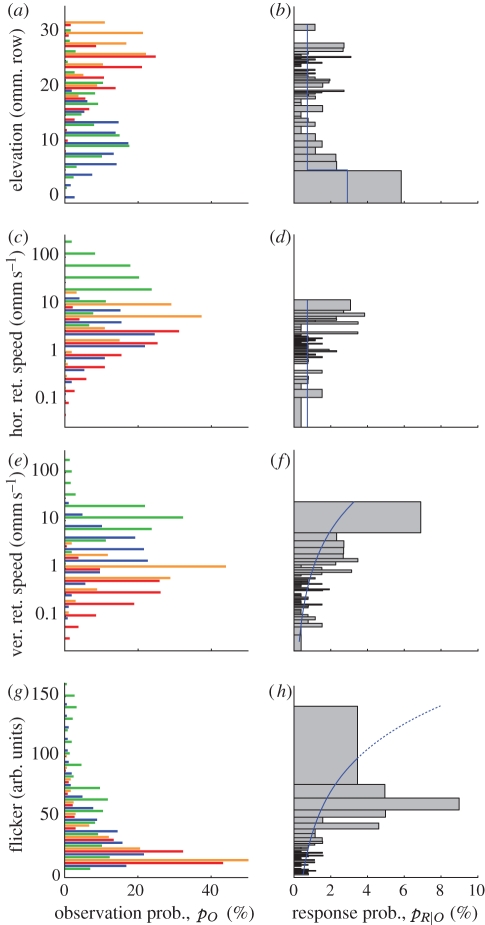
Figure 3.
Statistics of bird approaches and crab responses. (a,c,e,g) Observation probability and (b,d,f,h) response probability are shown for (a,b) elevation, (c,d) horizontal and (e,f) vertical retinal speed, and (g,h) flicker. Elevation and retinal speed take into account crab eye optics and sampling array, and are expressed in ommatidial rows (a,b) and ommatidia per second (c–f). Histogram bins in b,d,f,h have been adjusted to include approximately the same number of observations in each bin (259 ± 4), i.e. wider bars indicate sparser sampling and lower numbers in a,c,e,g. This has been done to make each bar equally reliable. Blue lines are predictions from the GLMM (table 2), assuming mean values for all parameters except the one examined in a given panel. Owing to the correlation between parameters, these predictions will generally underestimate the response probabilities shown in the histograms. Predictions for high flicker values are based on a small number of observations (indicated by the dotted blue line). Blue bars, terns; red bars, kites; orange bars, commuters; green bars, insects.
Terns usually fly across the mudflat in low, relatively straight paths (e.g. [10]), which typically generate a wide range of apparent speeds (figure 2a: blue dots every 200 ms), frequent sharp turns and—when compared with the other groups—a large amount of vertical retinal motion. To the human observer, one of the most conspicuous features of a tern's approach is the strong flicker signal it creates owing to the interplay of the bright upper side and shaded underside of its beating wings. A typical and frequent manoeuvre can be observed in the path in the middle of figure 2a. The tern entered the camera's field of view from the right (blue square). It continued to the left at medium apparent speed and then swooped down in an attempt to catch a crab far away from the recording site. A large number of crabs responded to the swoop, including six running home within the recording area (indicated by magenta circles). During the swoop, retinal elevation decreased rapidly to almost 0°. Note that the swoop is preceded by a sudden horizontal deceleration and vertical acceleration. The spreading of the wings that initiates this change of direction also causes a large and conspicuous flicker signal.
Kites (e.g. Brahmini kites Haliastur indus) are frequently seen soaring above the edges of the mudflats in search of food. Fiddler crabs, however, are not part of their diet and should therefore ideally ignore them. Figure 2b shows three typical examples of flight paths of soaring kites that clearly differ in several aspects from those of terns. Flight paths are quite straight, with few sharp turns. Retinal speeds are generally low (less than 5° s–1), and the birds are generally seen at comparatively high elevations between 10° and 30°. Large flicker signals are only created on the rare occasions when kites are seen against a cloud, where their brown plumage suddenly creates a much larger contrast than against the blue sky.
Many bird species (here referred to summarily as commuters), including eagles, herons, pelicans, crows and oystercatchers, feed over the open ocean or at the water's edge. On their way, they pass over fiddler crab colonies, but none of these birds aerially hunt fiddler crabs. Typical flight paths (figure 2c) are very straight, generating medium to large, mostly horizontally dominated retinal speeds (here between 5 and 10° s–1), and only occasional flicker signals through wingbeats or a change in background intensity. The flight paths are thus quite similar to those of kites, albeit generally faster.
Occasionally, small nearby insects such as flies, butterflies and dragonflies also evoke predator avoidance responses. Example paths of several dragonflies (figure 2d) demonstrate the main signal characteristics generated by flying insects as they are seen by crabs. First, encounters are brief. All but one of the dragonflies in these examples passed the camera's field of view within 2–3 s. Because they are very close, however, insects are seen at a wide range of elevations, generate higher retinal speeds than any bird and create strong flicker signals, owing to reflections on the animals' shiny exoskeletons.
Figure 3a,c,e,g summarize observation probability of elevation, horizontal and vertical retinal speed and flicker for all four groups of flying animals. To simulate as closely as possible the sensory input crabs experience, we calculated stimulus elevation as the ommatidial row on the crabs' eyes and retinal speed in ommatidia per second (using data from [18]).
Observed elevation (figure 3a) falls into two main groups. Terns and insects (blue and green bars) are most commonly observed by ommatidial rows 0–20 (where 0 looks directly at the horizon), while kites and commuters (red and orange bars) generally fly at higher altitudes and are predominantly seen above ommatidial row 15. The overlap between these two groups is quite substantial, but kites and commuters are practically never seen below row 10. The distributions of vertical and horizontal retinal speeds (figure 3c,e) are very similar, with medium speeds being most common and most measurements between 0.1 and 30 ommatidia per second. It is worth noting that the actual vertical retinal speeds (in degrees per second) are much lower than horizontal speeds. They are transformed into similar ranges owing to the higher vertical resolution of the fiddler crab eye [18]. The only major exceptions are horizontal retinal speeds generated by insects that can reach several hundred facets per second. The three bird groups produced almost identical distributions of horizontal retinal speeds. In a vertical direction, however, terns move on average faster than kites and commuters. The distribution of flicker values, finally, is similar in all four groups (figure 3g), with low values dominating the distributions. Most large flicker values, however, are produced by terns and insects.
(b). Can crabs use the differences in visual signatures to detect threat?
The results in figure 3 show that different birds do generate different statistical distributions of visual signatures. They are similar enough, however, that distinguishing them would require an animal to observe a bird for a certain period of time. To investigate how effective certain stimulus conditions are in eliciting home-run responses, we calculated the probability that a certain parameter reading will elicit a response (§2; figure 3b,d,f,g). Based purely on these probabilities, it is impossible to isolate which response criteria are actually being used by the crabs and whether the crabs are able to differentiate between real threats and harmless events. The main problem is that all parameters are strongly correlated. A fast, dark bird against a bright background, for example, necessarily also creates large flicker signals. Similarly, a close bird will create larger retinal speeds on average and be seen at higher elevations than a more distant one. Furthermore, behaviours like the swooping of terns are accompanied by distinct sets of correlated parameters. To isolate and identify the parameters that most parsimoniously predict crab responses, a GLMM (R) was applied to individual crab decisions (response/non-response) within each 200 ms time frame. Tested parameters included elevation, retinal speeds (in ommatidia per second, logarithmically transformed) and flicker. We also tested retinal horizontal and vertical acceleration, calculated as the difference between current and last speed measurement in our GLMM, but neither had a significant effect (p = 0.20 and p = 0.14, respectively). As the effect of elevation appears to be a step function (figure 3b), it was included as a two-level factor with ‘horizon level’ defined as any elevation at or below ommatidial row 5. While a linear or polynomial fit would also have been possible, we believe that a step function provides a more biologically plausible way to model the effect of retinal elevation. Two-way interactions were examined, but no interactions achieved significance. Owing to the close distance they are recorded at, insect observations unfortunately had to be excluded from the following statistical analysis because the viewpoints of our bird cameras were different from those of the observed crabs, leading to a large parallax error in estimated elevation.
The predictions of the final GLMM model are shown in figure 3b,d,f,h (blue lines) together with the response probability to different elevations, horizontal and vertical retinal speeds and flicker (grey bars). Model predictions for a certain parameter (e.g. elevation) are calculated with all other variables set at their respective means (table 2). At elevations around the horizon, crabs are more likely to respond to a signal than at higher elevations (figure 3b; χ2 = 15.0, p < 0.001). If elevation is also added to the model as a variable after accounting for this effect, there is a slight trend for higher response probabilities at higher elevations (χ2 = 2.67, p = 0.10). High horizontal retinal speeds appear to be linked to higher response probabilities, but this effect is not significant after accounting for the other variables (figure 3d; χ2 = 2.82, p = 0.093), suggesting that this effect is based on the correlation between parameters. High vertical retinal speeds, on the other hand, increase response probabilities significantly (figure 3f; χ2 = 14.6, p < 0.001). Finally, the most significant predictor of high response probabilities is pronounced flicker (figure 3h; χ2 = 29.3, p ≪0.001). After accounting for these variables, there is no difference in response probabilities between different types of bird (χ2 = 1.46, p = 0.48), suggesting that the three parameters elevation, vertical retinal speed and flicker are sufficient to explain the crabs' differential response to terns, kites and commuters (table 1). In other words, they respond more often to terns, because these birds create a faster signal with a higher degree of flickering closer to the visual horizon. It should be noted that slight changes in the parameters of the statistical model (for instance, the elevation cut-off or the selection of included frames) can naturally influence the quantitative results of the model. The qualitative conclusions, however, are not affected by these changes (i.e. elevation, vertical retinal speed and flicker remain the strongest predictors of responses).
Table 2.
Results of the generalized linear mixed model analysis (GLMM; n = 12 962; random model: crab identity + bird identity). The model predicts the logarithm of the odds ratio; response probabilities are therefore predicted by p = (1 + Σiaiei)−1.
| fixed effects (xi) | effect (ai) | d.f. | χ2 | p |
|---|---|---|---|---|
| intercept | −4.99 | 1 | <1×10−15 | |
| horizon level (row ≤ 5) | 1.40 | 1 | 15.0 | <1×10−3 |
| flicker | 0.0202 | 1 | 29.3 | <1×10−7 |
| vert. ret. speed (omm s−1) | 0.841 | 1 | 14.6 | <1×10−3 |
| hor. ret. speed (omm s−1) | — | 1 | 2.82 | 0.093 |
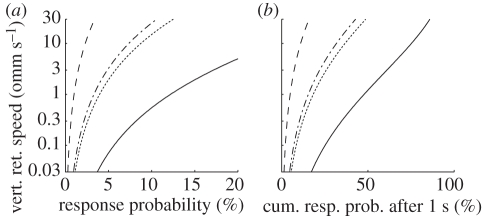
Although the response probabilities we documented may seem low, it is important to note that they are calculated and predicted for any 200 ms interval. For instance, only a fast object at the horizon that creates large flicker will trigger a response in the next 200 ms interval with more than 10 per cent probability (figure 4a, solid line). However, whenever an event is experienced for a longer period of time without signals decreasing in intensity, probabilities will accumulate (figure 4b). If the same strong stimulus (fast moving plus strong flicker at the horizon) is observed for just 1 s, the model predicts a response likelihood of over 80 per cent.
Figure 4.
(a) Predicted response probabilities in a 200 ms interval after 200 ms of observation as a function of vertical retinal speed for both levels of the factor ‘horizon’ and two values of flicker. H +/F80, for instance (black line) indicates the response probability for strongly flickering objects at the horizon. (b) Accumulated response probabilities after observing the same stimulus for 1 s. Note the difference in horizontal axis scales between (a) and (b). Dashed lines, H−/F20; dotted lines, H+/F20; dashed-dotted lines, H−/F80; solid line, H+/F80.
4. Discussion
In this study, we characterized the visual signals that a group of fiddler crabs experienced in the presence of natural predators on a typical day in the field and related them to the visual cues that are known to elicit predator avoidance responses in fiddler crabs. We have shown that different types of birds produce systematically different visual signals, the statistical properties of which are in principle sufficient to allow crabs to discriminate between threatening and harmless events. As we have seen, the crabs did so to a certain degree. They responded to terns, their real predators, more than twice as often as expected from their observation frequency.
(a). Natural visual triggers of escape responses
The three main signal characteristics that predicted the timing of home runs were elevation, vertical retinal speed and flicker. The predictive effect of elevation (highest response probabilities at the horizon) was unexpected, as only an occurrence within 5° of the horizon increased response probability. Any bird approaching at constant height progressively rises in elevation and high elevation should therefore be a robust indicator of predation risk. Dummy experiments have also confirmed that fiddler crabs are sensitive to the elevation of a stimulus [15], responding more strongly to dummies that appeared at a higher elevation. However, the signal statistics of real birds demonstrate that crabs should clearly avoid responding to most stimuli they see above 20° of elevation (as many of them are caused by harmless birds), unless other criteria suggest a real threat. At the horizon, in contrast, almost all observed birds are terns. In combination with a flickering and/or a fast-moving signal, low retinal elevations are therefore a good indicator of a real predator. This does not necessarily indicate a true imminent threat; in fact, most flickering signals at very low elevations are probably relatively distant terns. The fact that the crabs react so strongly to flicker thus suggests that, overall, false alarms in response to distant terns are less costly than those in response to the ever-present kites and commuters. One reason could be that terns even at a distance are likely to become a threat in the near future.
In dummy experiments, fiddler crab predator avoidance decisions are clearly influenced by retinal speed [15]. Both in response to an artificial looming stimulus [15] (figure 4) and in response to natural events, crabs are much more sensitive to vertical than to horizontal retinal speed. In fact, horizontal retinal speed had no significant predictive effect in this study. This may be because large horizontal speeds in natural signals are correlated with both high vertical speeds (r = 0.25) and large flicker signals (r = 0.34). The influence of high horizontal speeds might therefore be masked by these two effects.
Strong flicker appears to have the most consistent effect identified in this study. In many cases, flicker is caused by the wingbeats of birds (especially terns) or by high-contrast birds flying at high speeds. Flicker therefore includes, to some degree, the effects of speed, but carries additional information about objects that are stationary on the retina. One large advantage of flicker as a response criterion is that it can be evaluated from the input of just a single ommatidium, whereas speed, when evaluated by motion detectors, can only be detected by correlating the photoreceptor signals between at least two ommatidia. Acceptance functions of ommatidia in the dorsal visual field of fiddler crabs are narrow (to increase contrast sensitivity) and sparsely distributed above 10° of elevation [18]. Small objects such as distant birds will therefore rarely be seen by two neighbouring ommatidia and evaluation by motion detectors will be unreliable. In contrast, flicker can be detected in the time course of individual photoreceptor signals.
A possible alternative explanation for the effect of flicker considers the fact that strong flicker can only be created by a high-contrast object. It could be argued that the crabs might simply not be able to perceive birds at lower contrasts and in fact respond as soon as they detect a signal. However, a number of responses were elicited by low-contrast signals. Furthermore, dummy experiments show that even in the presence of a strong visual signal, for instance high retinal speed [15] or a strong flicker signal [42], crabs are still sensitive to other risk factors, such as the distance to their burrow. When the animals are closer to their burrow, they respond later (e.g. [15,28]), an adjustment that would not be possible if a response occurred immediately after detection.
Angular size and looming have previously been shown to affect the timing of predator avoidance responses [15,26,32]. It seems highly unlikely, however, that these cues are directly available to fiddler crabs in a natural situation when they first decide whether to run towards home. Most birds change apparent shape and size constantly through wingbeat and changes in orientation. We were thus unable to accurately measure angular size in our video recordings. Moreover, at the time the home run is initiated, the apparent size of dummies [14,15,28] and of birds (this study) is between 0.5° and 2°. Flying predators are thus seen by only one or two ommatidia when crabs respond, which makes it very unlikely that they use retinal size or looming as a decision criterion at this early stage. However, a bird of larger apparent size will present a signal of higher contrast to photoreceptors and therefore create larger flicker if it is moving fast or if it beats its wings. Effects of retinal size and looming measured by previous experiments might therefore have been caused by the effect of flicker. Looming signals might play a role in response to large walking birds, which were not examined in this study, or in cluttered environments, where predators can be much closer and therefore appear larger when they are first detected. Looming and retinal size might also come into play after the home run, when the crabs have to decide whether or not to descend into their burrow. At this stage, potential predators are much closer and present more risk-correlated information to the crabs [28].
(b). After the home run
Following the home run, fiddler crabs only rarely descended straight down into their burrow. Instead, they usually remained at the entrance, and only went underground when the threat persisted and the risk increased. During the 37 natural predator approaches we analysed, we observed a total of 152 home runs, but only on 14 occasions did crabs go underground. We have previously shown that this strategy allows fiddler crabs to stay in relative safety—as the actual burrow descent only takes a fraction of a second—while collecting as much information as possible about the approaching threat [20,28]. The additional information gathered during this time allows crabs to respond to more risk-related cues such as distance, elevation, angular size and/or looming [28], and is probably also the reason why the underground response can habituate more strongly to repeated, but harmless stimuli than the home run [27].
In experiments with bird dummies, where the threat often approaches very directly and very fast, crabs are often found to descend into their burrow only after the danger has actually passed (J. M. Hemmi, J. Smolka & J. Zeil 2008, personal observation). It appears that the crabs use the opportunity to enter their burrow for other reasons, for instance to replenish their water supply, before heading out on their next excursion, and thereby prolonging the time they can spend on the surface without having to return from a distance. The low rate of responses in this study might thus indicate that in the presence of an unreliable, unpredictable signal, the crabs avoid descending into their burrow as long as they cannot be certain about the status of the potential predator. In other words, while dummies have to clearly and visibly move away from the crabs before they will enter their burrow, natural predators rarely do that. They might not provide sufficient cues to the crabs to convince them of their departure.
(c). Conclusions
To our knowledge, this study is the first to examine the exact visual stimuli prey animals experience in the field when responding to potential predators. Our analysis of natural visual stimuli suggests that different species of potential and real aerial predators produce sufficiently distinct and statistically different visual signatures to potentially allow fiddler crabs to discriminate between them. The crabs' response criteria reflect these properties: in the natural setting of these prey animals, we identified flicker as an important, but hitherto unrecognized visual cue that might provide the crabs with a very early, though unreliable, escape criterion.
Acknowledgements
We would like to thank Mark Snowball, Robert Parker and the RSBS workshop for the help in the construction of the synchronized camera set-up; Martin How and Waltraud Pix for their help in the field; and Eric Warrant for many helpful comments on the manuscript. J.S. was funded through an Australian National University PhD scholarship and an International Postgraduate Research Scholarship. Additional funding was provided by the ARC Center of Excellence in Vision Science, the Center for Visual Sciences and an ARC Discovery Grant to J.M.H. and J.Z.
Endnote
Note that this latency is calculated not from the response frame (where movement towards the burrow is first observed; §2), but from the decision frame (1 frame per 200 ms before the start of the response).
References
- 1.Niven J. E., Laughlin S. B. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 10.1242/jeb.017574 (doi:10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 2.Baddeley R., Abbott L. F., Booth M. C., Sengpiel F., Freeman T., Wakeman E. A., Rolls E. T. 1997. Responses of neurons in primary and inferior temporal visual cortices to natural scenes. Proc. R. Soc. Lond. B 264, 1775–1783 10.1098/rspb.1997.0246 (doi:10.1098/rspb.1997.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeddeker N., Lindemann J. P., Egelhaaf M., Zeil J. 2005. Responses of blowfly motion-sensitive neurons to reconstructed optic flow along outdoor flight paths. J. Comp. Physiol. A 191, 1143–1155 10.1007/s00359-005-0038-9 (doi:10.1007/s00359-005-0038-9) [DOI] [PubMed] [Google Scholar]
- 4.David S. V., Vinje W. E., Gallant J. L. 2004. Natural stimulus statistics alter the receptive field structure of V1 neurons. J. Neurosci. 24, 6991–7006 10.1523/jneurosci.1422-04.2004 (doi:10.1523/jneurosci.1422-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hateren J. H., Kern R., Schwerdtfeger G., Egelhaaf M. 2005. Function and coding in the blowfly H1 neuron during naturalistic optic flow. J. Neurosci. 25, 4343–4352 10.1523/jneurosci.0616-05.2005 (doi:10.1523/jneurosci.0616-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kern R., van Hateren J. H., Michaelis C., Lindemann J. P., Egelhaaf M. 2005. Function of a fly motion-sensitive neuron matches eye movements during free flight. PLoS Biol. 3, e171. 10.1371/journal.pbio.0030171 (doi:10.1371/journal.pbio.0030171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkworth R. S. A., Mah E. L., Gray J. P., O'Carroll D. C. 2008. Photoreceptor processing improves salience facilitating small target detection in cluttered scenes. J. Vis. 8, 1–17 10.1167/8.11.8 (doi:10.1167/8.11.8) [DOI] [PubMed] [Google Scholar]
- 8.Römer H. 1998. Strategies for hearing in noise: peripheral control over auditory sensitivity in the bushcricket Sciarasaga quadrata (Austrosaginae: Tettigoniidae). J. Exp. Biol. 201, 1023–1033 [DOI] [PubMed] [Google Scholar]
- 9.Lewen G. D., Bialek W., van Steveninck R. R. D. 2001. Neural coding of naturalistic motion stimuli. Netw. Comput. Neural Syst. 12, 317–329 [PubMed] [Google Scholar]
- 10.Land M. F. 1999. The roles of head movements in the search and capture strategy of a tern (Aves, Laridae). J. Comp. Physiol. A 184, 265–272 10.1007/s003590050324 (doi:10.1007/s003590050324) [DOI] [Google Scholar]
- 11.Zwarts L. 1985. The winter exploitation of fiddler cabs Uca tangeri by waders in Guinea-Bissau. Ardea 73, 3–12 [Google Scholar]
- 12.Ens B. J., Klaassen M., Zwarts L. 1993. Flocking and feeding in the fiddler crab (Uca tangeri): prey availability as risk-taking behavior. J. Sea Res. 31, 477–494 [Google Scholar]
- 13.Iribarne O. O., Martinez M. M. 1999. Predation on the southwestern Atlantic fiddler crab (Uca uruguayensis) by migratory shorebirds (Pluvialis dominica, P. squatarola, Arenaria interpres, and Numenius phaeopus). Estuaries 22, 47–54 10.2307/1352926 (doi:10.2307/1352926) [DOI] [Google Scholar]
- 14.Hemmi J. M. 2005. Predator avoidance in fiddler crabs. I. Escape decisions in relation to the risk of predation. Anim. Behav. 69, 603–614 10.1016/j.anbehav.2004.06.018 (doi:10.1016/j.anbehav.2004.06.018) [DOI] [Google Scholar]
- 15.Hemmi J. M. 2005. Predator avoidance in fiddler crabs. II. The visual cues. Anim. Behav. 69, 615–625 10.1016/j.anbehav.2004.06.019 (doi:10.1016/j.anbehav.2004.06.019) [DOI] [Google Scholar]
- 16.Land M. F., Layne J. E. 1995. The visual control of behavior in fiddler crabs. I. Resolution, thresholds and the role of the horizon. J. Comp. Physiol. A 177, 81–90 10.1007/BF00243400 (doi:10.1007/BF00243400) [DOI] [Google Scholar]
- 17.Zeil J., Al-Mutairi M. M. 1996. The variation of resolution and of ommatidial dimensions in the compound eyes of the fiddler crab Uca lactea annulipes (Ocypodidae, Brachyura, Decapoda). J. Exp. Biol. 199, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 18.Smolka J., Hemmi J. M. 2009. Topography of vision and behaviour. J. Exp. Biol. 212, 3522–3532 10.1242/jeb.032359 (doi:10.1242/jeb.032359) [DOI] [PubMed] [Google Scholar]
- 19.Zeil J., Hemmi J. M. 2006. The visual ecology of fiddler crabs. J. Comp. Physiol. A 192, 1–25 10.1007/s00359-005-0048-7 (doi:10.1007/s00359-005-0048-7) [DOI] [PubMed] [Google Scholar]
- 20.Hemmi J. M., Zeil J. 2005. Animals as prey: perceptual limitations and behavioural options. Mar. Ecol. Prog. Ser. 287, 274–278 [Google Scholar]
- 21.Land M. F., Layne J. E. 1995. The visual control of behavior in fiddler crabs. II. Tracking control systems in courtship and defense. J. Comp. Physiol. A 177, 91–103 10.1007/BF00243401 (doi:10.1007/BF00243401) [DOI] [Google Scholar]
- 22.Zeil J., Zanker J. M. 1997. A glimpse into crabworld. Vis. Res. 37, 3417–3426 10.1016/S0042-6989(97)00106-5 (doi:10.1016/S0042-6989(97)00106-5) [DOI] [PubMed] [Google Scholar]
- 23.Collett T. S., Harkness L. I. K. 1982. Depth vision in animals. In Analysis of visual behaviour (eds Ingle D. J., Goodale M. A., Mansfield R. J. W.), pp. 111–176 Cambridge, MA: MIT Press [Google Scholar]
- 24.Smolka J. 2009. Sampling visual space: topography, colour vision and visually guided predator avoidance in fiddler crabs (Uca vomeris). PhD thesis, The Australian National University, Canberra, Australia [Google Scholar]
- 25.Layne J. E. 1998. Retinal location is the key to identifying predators in fiddler crabs (Uca pugilator). J. Exp. Biol. 201, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 26.Nalbach H. O. 1990. Visually elicited escape in crabs. In Frontiers in crustacean neurobiology (eds Wiese K., Krent W. D., Tautz J., Reichert H., Mulloney B.), pp. 165–172 Basel, Switzerland: Birkhäuser Verlag [Google Scholar]
- 27.Hemmi J. M., Merkle T. 2009. High stimulus specificity characterizes anti-predator habituation under natural conditions. Proc. R. Soc. B 276, 4381–4388 10.1098/rspb.2009.1452 (doi:10.1098/rspb.2009.1452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemmi J. M., Pfeil A. 2010. A multi-stage anti-predator response increases information on predation risk. J. Exp. Biol. 213, 1484–1489 10.1242/jeb.039925 (doi:10.1242/jeb.039925) [DOI] [PubMed] [Google Scholar]
- 29.Layne J., Land M., Zeil J. 1997. Fiddler crabs use the visual horizon to distinguish predators from conspecifics: a review of the evidence. J. Mar. Biol. Assoc. UK 77, 43–54 10.1017/S0025315400033774 (doi:10.1017/S0025315400033774) [DOI] [Google Scholar]
- 30.Zeil J., Nalbach G., Nalbach H. O. 1986. Eyes, eye stalks and the visual world of semi-terrestrial crabs. J. Comp. Physiol. A 159, 801–811 10.1007/BF00603733 (doi:10.1007/BF00603733) [DOI] [Google Scholar]
- 31.Zeil J., Nalbach G., Nalbach H. O. 1989. Spatial vision in a flat world: optical and neural adaptations in arthropods. In Neurobiology of sensory systems (eds Singh R. N., Strausfeld N. J.), pp. 123–137 New York, NY: Plenum [Google Scholar]
- 32.Oliva D., Medan V., Tomsic D. 2007. Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathus granulatus (Decapoda: Grapsidae). J. Exp. Biol. 210, 865–880 10.1242/jeb.02707 (doi:10.1242/jeb.02707) [DOI] [PubMed] [Google Scholar]
- 33.Hinde R. A. 1954. Factors governing the changes in strength of a partially inborn response, as shown by the mobbing behaviour of the chaffinch (Fringilla coelebs). I. The nature of the response, and an examination of its course. Proc. R. Soc. Lond. B 142, 306–331 10.1098/rspb.1954.0028 (doi:10.1098/rspb.1954.0028) [DOI] [PubMed] [Google Scholar]
- 34.Curio E. 1975. The functional organization of anti-predator behaviour in the pied flycatcher: a study of avian visual perception. Anim. Behav. 23, 1–115 10.1016/0003-3472(75)90056-1 (doi:10.1016/0003-3472(75)90056-1) [DOI] [PubMed] [Google Scholar]
- 35.Robinson S. R. 1980. Antipredator behaviour and predator recognition in Belding's ground squirrels. Anim. Behav. 28, 840–852 10.1016/S0003-3472(80)80144-8 (doi:10.1016/S0003-3472(80)80144-8) [DOI] [Google Scholar]
- 36.Kerlinger P., Lehrer P. H. 1982. Owl recognition and anti-predator behaviour of sharp-shinned hawks. Z. Tierpsych. 58, 163–173 10.1111/j.1439-0310.1982.tb00314.x (doi:10.1111/j.1439-0310.1982.tb00314.x) [DOI] [Google Scholar]
- 37.Evans C. S., Macedonia J. M., Marler P. 1993. Effects of apparent size and speed on the response of chickens, Gallus gallus, to computer-generated simulations of aerial predators. Anim. Behav. 46, 1–11 10.1006/anbe.1993.1156 (doi:10.1006/anbe.1993.1156) [DOI] [Google Scholar]
- 38.Hanson M. T., Coss R. G. 1997. Age differences in the response of California ground squirrels (Spermophilus beecheyi) to avian and mammalian predators. J. Comp. Psych. 111, 174–184 10.1046/j.1439-0310.2001.00659.x (doi:10.1046/j.1439-0310.2001.00659.x) [DOI] [PubMed] [Google Scholar]
- 39.Curio E. 1993. Proximate and developmental aspects of antipredator behavior. In Advances in the study of behavior vol. 22 (eds Slater P., Milinski M., Snowdon C., Rosenblatt J.), pp. 135–238 San Diego, CA: Academic Press [Google Scholar]
- 40.Bouguet J. Y. 2005. Camera calibration toolbox for Matlab. See http://www.vision.caltech.edu/bouguetj
- 41.Breslow N. E., Clayton D. G. 1993. Approximate inference in generalized linear mixed models. J. Am. Stat. Assoc. 88, 9–25 10.2307/2290687 (doi:10.2307/2290687) [DOI] [Google Scholar]
- 42.Smolka J., Hemmi J. M. In preparation Flicker as a visual clue for fiddler crab predator avoidance. [Google Scholar]