Abstract
This paper describes a novel archaeological resource—preserved epiphytes on the timber structure of vernacular buildings—used, to our knowledge, for the first time to quantify a loss of biodiversity between pre-industrial and post-industrial landscapes. By matching the confirmed occurrence of epiphyte species for the pre-industrial period, with a statistical likelihood for their absence in the present-day landscape (post-1960), we robustly identified species that have been extirpated across three contrasting regions in southern England. First, the scale of biodiversity loss observed—up to 80 per cent of epiphytes—severely challenges biodiversity targets and environmental baselines that have been developed using reference points in the post-industrial period. Second, we examined sensitivity in the present-day distribution of extirpated species, explained by three environmental drivers: (i) pollution regime, (ii) extent of ancient woodland, and (iii) climatic setting. Results point to an interacting effect between the pollution regime (sulphur dioxide) and changed woodland structure, leading to distinctive regional signatures in biodiversity loss.
Keywords: ancient woodland, biodiversity loss, epiphyte, lichen, pollution, sulphur dioxide
1. Introduction
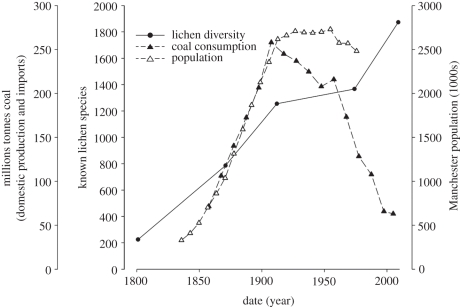
The process of industrialization involves an unprecedented human modification of the environment and associated biodiversity loss [1–3]. There is, therefore, significant concern over the pathway of economic convergence between developing nations and today's industrialized and wealthier states [4–6]. Comparatively less concern has been directed towards the historic consequences of industrialization for biodiversity in temperate Europe and North America, perhaps because these impacts are often hidden from the scrutiny of objective analysis. Historic effects of the industrial revolution from the mid-eighteenth century outpaced the collection of high-resolution biodiversity and distributional data (figure 1). These effects also predated by a century or more the science of ecology and the establishment of long-term datasets [8,9], and the emergence of a social conservation ethic [10,11]. The environmental and conservation policy of industrialized temperate regions are therefore vulnerable to ‘shifting baselines’ (sensu [12]), in which regional biodiversity status, as a reference point for measuring species loss and protection, may be normalized with respect to the post-industrial landscape.
Figure 1.
Comparing biodiversity knowledge, as the known lichen species in Britain [7] (filled circles), with indicators for the process of industrialization: domestic coal consumption (filled triangles; compiled by the former Department for Business, Enterprise and Regulatory Reform), and urbanization, i.e. the population of Manchester (open trianlges; compiled by Greater Manchester Metropolitan Council). Biodiversity knowledge accumulated throughout the period of industrialization and post-industrialization; by the time industrialization has been fully effected (peak coal and patterns of demographic change sustained), the known number of lichen species is only 63% complete relative to 2009. Considering the additional sampling effort required to generate high-resolution biogeographic information, and the pervasive effects of industrialization from the late eighteenth century, pre-industrial patterns of lichen epiphyte diversity are unknown.
The Industrial Revolution began in England in the mid-eighteenth century, and spread quickly throughout Western Europe and North America [13]. This paper focuses on one of the first regions to be impacted by industrialization—southern England—drawing on a novel archaeological resource to examine biodiversity shifts across the boundary of industrialization. This new evidence allows us to critically reappraise our baseline expectations for biodiversity conservation. We use epiphytic lichens preserved on the timbers of vernacular buildings (fifteenth–eighteenth centuries) to reconstruct species distributions for the pre-industrial landscape, and we infer the degree of change in epiphyte species richness compared with a period post-1960. Epiphytes include well-developed bioindicators for a range of industrial pollutants [14–17]; they are sensitive to changes in woodland habitat quality emerging from landscape management [18–20] as well as spatial–temporal climatic variation [21–23]. Additionally, epiphytes play a role in ecosystem function, including cycling of limiting nutrients [24] and as a focal node in forest food webs [25–27]. Focusing on the epiphytes of southern England provides an important regional context; it represents a historic template of social and economic change (industrialization) which is expanding across a majority of terrestrial ecosystems. Shifts in epiphyte biodiversity across the English industrial boundary, therefore, provide a long-term context for biodiversity drivers operating globally in the present-day (cf. [28]). Detecting historic impacts on epiphyte diversity will also provide a qualitative model for previous shifts in ecosystem function and services [29]. Our aims were: (i) to quantify the epiphyte shift in species richness between the pre- and post-industrial periods, and (ii) to infer the drivers of this change, and to set the relative importance of these drivers within a landscape context.
2. Methods
(a). Field sampling
We surveyed timber structures of low-status vernacular buildings for the presence of bark and epiphytes in three villages across lowland England (cf. figure 2): Coggeshall (Essex: 51.8717° N, 0.6913° E), Downton (Wiltshire: 50.993° N, 1.743° W) and Stogursey (Somerset: 51.1807° N, 3.1413° W). The villages (study sites) were selected in order to provide regional contrasts, in terms of climatic setting, pollution regime and landscape history. Preserved bark in vernacular buildings tends to be accessible and untreated in roofs and loft spaces [30–32], and sampling was targeted to buildings within each study site, with original roof elements remaining from the eighteenth century or earlier. Dating of timber structures was estimated to century based on the style of construction (e.g. [33]) and in consultation with local buildings specialists (e.g. [34]).
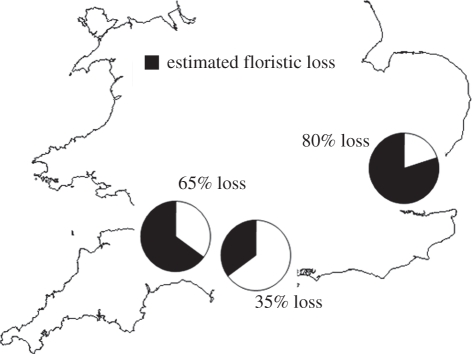
Figure 2.
The distribution of study sites in southern England, with regional estimates of floristic loss (block segments of circles) for lichen epiphytes, compared between the pre- and post-industrial periods. Calculations of loss were based on the confirmed historic occurrence of species, compared with their expected absence derived using spatial distributions in the post-industrial landscape.
All visible and untreated timber elements were examined for the presence of bark. Where necessary, bark samples were returned to the laboratory at the Royal Botanic Garden Edinburgh for identification of preserved epiphytes using standard microscopic and chemical methods [35,36]. The species of tree for each bark sample was recorded.
(b). Time-series distributional change
We aimed to compare the confirmed occurrence of a species in the pre-industrial landscape with a statistical estimate (likelihood) of its presence–absence in the post-industrial landscape. For lichen species recorded on historic bark samples from across the three study sites, we scored their presence–absence in the modern landscape for the 10 km grid squares, which matched these localities (cf. figure 2). Analysis was based on lichen mapping data collated since 1960, and archived by the British Lichen Society (University of Bradford). These datapoints for each of the three study sites (‘centroid’) were accompanied by a measure of the lichen species' occurrence in consecutively sized buffers, positioned at 10 km intervals, up to a maximum buffer of 50 km (electronic supplementary material, appendix S1). Presence–absence in centroid grid squares was treated as a response variable, and the proportion of grid squares occupied within each of the differently sized buffers (spatial density of confirmed occurrences) was treated as explanatory variables. These response and explanatory data were combined into a single dataset, combining all species across the three study sites. The order of samples in the dataset was randomized with respect to species and study site, and the matrix was split into 10 subsets.
Using k-fold cross-validation, nine subsets were used to build a generalized linear model (GLM) with a logit-link function (implemented using the base package in R [37]). The GLM was used to predict the likelihood of presence–absence in the subset of samples excluded from model development. Predictive capacity was estimated for the combined results of k-fold validation (all independent predictions) as the area under the receiver-operating characteristic curve (area under curve, AUC) [38–40]. The optimum predictive model was used to calculate an AUC threshold [39]. This threshold is positioned at the model likelihood value that maximizes the sum of sensitivity (true positive predictions, as a proportion of observed presences) and specificity (true negative predictions, as a proportion of observed absences). On this basis, comparing the confirmed historic occurrence of a species to its predicted likelihood of occurrence (based on present-day spatial density) yields four outcomes:
— historic absent, present-day expected occurrence,
— historic absent, present-day expected absence,
— historic occurrence, present-day expected occurrence, and
— historic occurrence, present-day expected absence.
Outcomes 1 and 2 are invalidated by the need to prove a negative—it is impossible to demonstrate the absence of a species from the historic landscape, based on its non-occurrence in the timber samples. However, outcomes 3 and 4 are tractable—for example, we can definitively confirm the presence of a species within the historic landscape, and we can match this against the statistical likelihood that the same species is present or absent based on its post-1960 spatial distribution. Where a species occurs in the historic landscape, and is not expected to occur within an equivalent centroid square in the modern landscape (likelihood values < AUC threshold), we can reasonably infer species extirpation—a functional shift in its spatial distribution and abundance, compared between the pre- and post-industrial time periods. Additionally, we performed a sensitivity analysis to test the robustness of our optimum AUC decision threshold, comparing the estimated magnitude of species extirpations for a range of contrasting threshold values. If the optimum threshold is robust, we would expect little change in the outcome across a range of thresholds either side of our optimum.
This statistical comparative approach, based on a species' wider distribution pattern (i.e. within a 30 km buffer), was preferred to a field search of the landscape immediately surrounding each study site, and/or the direct comparison with presence–absence in the centroid grid square. It is extremely difficult, if not impossible, to definitively confirm the absence of species based on the field survey [41], perhaps especially so for diminutive lichen species. However, the method we used is founded on a massive combined sampling effort by members of the British Lichen Society, over a 50 year period (greater than 1 million records). Consequently, while it may be impossible to unequivocally confirm a species' absence from a given 10 km grid square, species' wider distributions are reliably known. On this basis, we can show with a degree of statistical certainty that a particular study site lies outside a species' current geographical range, in an area where an occurrence would be anomalous. Accordingly, the occurrence on an historic building timber (our sample of which undoubtedly represents a vanishingly small proportion of historically available habitat) might be taken as strong evidence for a distributional shift: the change from a state in which that species was historically more abundant. Alternatively, presence–absence data for a single location (e.g. the 10 km grid square containing a study site) could be directly compared with the historic record. In addition to the problems outlined above, this direct comparison would sacrifice important information on the ‘likelihood’ that a species might be sampled into a vernacular building—we would simply know that a species occurs within a 10 km square, without any wider distributional context, including a cross-scale link to local abundance (cf. [42–45]). The direct comparison of presence–absence would, therefore, fail to resolve situations where a species' sampled occurrence from the historic landscape is statistically anomalous and biologically interesting, being in an area that lies outside its known present-day distribution.
(c). Environmental drivers of change
For those species which we calculated to have undergone extirpation, we used their spatial distribution in the present-day landscape to estimate sensitivity to three landscape-scale environmental drivers: climatic setting, pollution regime, and spatial–temporal woodland structure. First, we summarized the present-day spatial distribution of extirpated species, by calculating their density within buffers that were spaced equidistantly across the British Isles, and including the landscape surrounding the three study sites (n = 63; the electronic supplementary material, appendix S2). The size of buffers (30 km) matched the scale used to optimally discriminate species presence–absence (see §2b, above). The spatial density was standardized between 0 and 1 for individual species, and formed the response in an ordination analysis by non-metric multi-dimensional scaling (NMDS). Ordination was based on a Sørenson distance matrix with NMDS implemented using 75 runs with observed data, and 100 randomized trials to assess significance (500 interations per run). Analysis was implemented in PC-ORD v. 4.41, with default values for the remaining parameters, and allowing a solution of up to six dimensions [46]. An optimum significant solution (i.e. number of dimensions) was selected to minimize latent stress (S) and maximize stability.
Second, we compared NMDS axis scores—summarizing patterns in the spatial density of extirpated species—to putative environmental drivers. Modelled pollution data were derived as 3-year average values for the period 2004–2006, at a 5 km scale downloaded from publically available datasets [47]. Three measures of urban and industrial pollutants were used: sulphur dioxide (SO2) concentration (µg kg−1 at 1013 hPa and 25°C), deposition of nitrogen oxide (NOx) (kg N ha−1yr−1) and acid deposition (keq ha−1 yr−1), and three measures associated with eutrophication from intensive agriculture: ammonia (NH3) concentration (µg kg−1 at 1013 hPa and 25°C), and deposition of reduced nitrogen (NHx) (kg N ha−1 yr−1), along with total-nitrogen (N) (kg N ha−1 yr−1). Pollution data were summarized using principal components analysis (PCA), used to reduce multiple collinear variables to a series of summary axes. PCA was implemented using CANOCO v. 4.5 [48], with the values for contrasting variables standardized and centred [48,49]. PCA analysis was used to select two important variables in explaining regional pollution trends, but which were contrasting and weakly co-varying (see the electronic supplementary material, appendix S3): SO2 and NHx.
Climate data at a 5 km grid-square resolution were derived from UK Met Office modelled datasets [50]: estimated monthly and annual climatic averages for average, maximum and minimum monthly temperatures (°C) and precipitation (mm). Estimated climate data were verified against averages based on point data for the period 1961–2000 at 540 and 4400 monitoring stations across Britain, for temperature and precipitation, respectively. As with the pollution data, climate data were summarized using PCA (see the electronic supplementary material, appendix S4); we selected two summary climate gradients, which were weakly correlated and described contrasting aspects of the regional climatic setting: mean annual temperature (°C) and annual precipitation (mm).
Values of woodland continuity were derived from the ancient woodland inventory (AWI: [51–53]). This included only class 1a and 2a woodlands, comprising natural and semi-natural woodland, with a continuous record of woodland cover since the mid-nineteenth century (‘ancient woodland’). Sites that have been cut-over and replanted represent a break in continuity for tree-dwelling epiphytes, and were excluded. We calculated the per cent land area occupied by ancient woodland.
Values for the putative explanatory environmental variables were averaged for the 63 buffers (cf. electronic supplementary material, appendix S2) using spatial analyst tools in ArcMap v. 9.1 [54]. The values for SO2 and NHx, mean annual temperature (°C) and annual precipitation (mm), and class 1 and 2a ancient woodland extent were compared using ordinary linear regression with NMDS axes. A Bonferroni correction was used to control for multiple tests and adjust the type I error (α = 0.05). This allowed us to select the variables that best explained present-day patterns in the spatial density of extirpated species; we then tested the status of the three study sites, with respect to these selected environmental variables. We bootstrapped the values for environmental variables (10 000 resamples), using resampling with replacement, where the size of the resample is n = 63. For each resampled set, we calculated the 95% confidence intervals. These intervals were then used to test the difference between the environmental settings of our three study sites, relative to the bootstrap confidence intervals for the median of the 30 km buffers positioned across Britain.
3. Results
Analysis of the timber artefacts yielded archaeological records for 56 lichen species (see the electronic supplementary material, appendix S5); of these, three species recorded from pre-industrial Essex are now extinct across the East of England: Bacidia laurocerasi, Bacidia rubella and Chaenothecopsis nigra [36]. Analysis of timber tree species showed that elm (Ulmus spp.) is regionally important as a building material, especially in Somerset [31], and we therefore excluded one lichen species from analysis (Caloplaca luteoalba), whose post-1960 distribution has been influenced by the spread of Dutch elm disease [36,55]. Present-day species occurrence in a centroid grid square was strongly predicted by surrounding spatial density at buffers ranging in size from 10 to 50 km (table 1). We selected the buffer with the highest AUC score (30 km) to generate estimates of a species' presence–absence based on its landscape-scale distribution. It was therefore possible to statistically isolate species with a confirmed occurrence in the historic landscape, but which are no longer expected to occur in the post-industrial landscape: i.e. present-day spatial density below an AUC threshold value (figure 2). Study sites from each of the three regions show a decline in epiphyte species richness, in the order: Essex (80%) > Somerset (65%) > Wiltshire (35%). This decision-making framework is robust to shifts in the AUC-threshold, with consistent estimates for the magnitude of extirpation across a wide range of values (electronic supplementary material, appendix S6). This emphasizes the conservative estimate of loss provided by our method. For example, even with an extremely weak decision threshold (skewed towards false presences), e.g. restricting an expected present-day absence until there is ≤0.5 per cent likelihood of occurrence, the measure of species loss remains relatively high: Essex (57%) > Somerset (45%) > Wiltshire (14%).
Table 1.
Diagnostic tests for the optimisation of a GLM, explaining species presence–absence for a 10 km grid square (centroid), based on the density of confirmed species occurrences at contrasting-scales within the surrounding landscape (cf. electronic supplementary material, appendix S1). (AIC, Akaike information criterion.)
| scale of analysis (size of buffer; km) | model diagnostics |
k-fold cross-validation |
||
|---|---|---|---|---|
| AIC | χ2-p | AUC | AUC threshold | |
| 10 | 54.56 | <0.0001 | 0.957 ± 0.016 | 0.45 |
| 20 | 63.54 | <0.0001 | 0.950 ± 0.016 | 0.43 |
| 30 | 56.94 | <0.0001 | 0.959 ± 0.012 | 0.41 |
| 40 | 55.76 | <0.0001 | 0.958 ± 0.013 | 0.35 |
| 50 | 57.313 | <0.0001 | 0.958 ± 0.013 | 0.38 |
Analysis by PCA for our two multi-variate environmental drivers—pollution regime and climatic setting—was successful in reducing these complex gradients to summary variables. The PCA for climate demonstrated that individual temperature and pollution variables tended to be inter-correlated, but that temperature and precipitation explain contrasting aspects of variation in British climate space (see the electronic supplementary material, appendix S4). Thus, mean annual temperature (°C) and annual precipitation (mm) were selected as summary climatic gradients. For pollution, SO2 and NHx were the least correlated variables, and were associated with contrasting regimes in pollution space (see the electronic supplementary material, appendix S3). Concentration of SO2 was correlated with mean annual temperature (r61 = 0.611, p < 0.0001) and annual precipitation (r61 = −0.66, p < 0.0001), with no significant correlation between NHx and climatic variables, or between extent of ancient woodland and any climatic or pollution variables. However, because these variables were regressed individually against NMDS axes (see below), the correlation structure did not influence the subsequent identification of explanatory variables.
The selected climate and pollution variables—along with extent of ancient woodland—formed the basis for explaining environmental sensitivity of extirpated species. NMDS ordination summarizing the present-day distribution/abundance of extirpated species resulted in an optimum two-dimensional solution, with stress = 8.313, instability < 0.00001 (p < 0.01). Axis one explained 78.4 per cent of variation in the original distance matrix, and was significantly explained by SO2 pollution, while axis two explained 12.1 per cent of variation in the original distance matrix, and was significantly explained by the extent of ancient woodland (table 2).
Table 2.
Diagnostic tests for the comparison of environmental variables (30 km buffers), with NMDS axis scores used to summarize the present-day British distribution (spatial density of occurrence) for extirpated species (d.f. = 61). (Using a Bonferonni correction for multiple tests, results are significant when p < 0.01 (indicated in bold).)
| explanatory variables | NMDS axis 1 |
NMDS axis 2 |
||
|---|---|---|---|---|
| r2 | p | r2 | p | |
| mean temperature | 0.0075 | 0.4671 | 0.0585 | 0.0314 |
| mean precipitation | 0.0821 | 0.0130 | 0.0018 | 0.2952 |
| SO2 | 0.244 | 0.000023 | 0.0147 | 0.7486 |
| NHx | 0.006 | 0.4304 | 0.01752 | 0.1519 |
| ancient woodland | 0.0904 | 0.0125 | 0.1487 | 0.00164 |
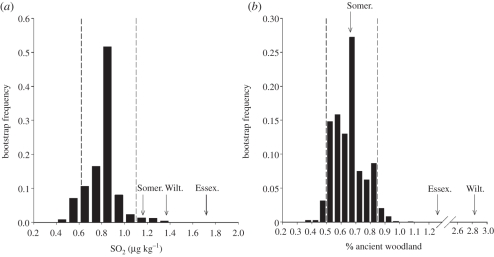
Bootstrap sampling which was used to compare the environmental status of the study sites, to values averaged for 30 km buffers from across Britain, showed that the regional setting with the greatest loss of epiphyte biodiversity (Essex) was characterized by significantly high SO2 pollution—with pollution for study sites in Wiltshire and Somerset lower, though also outside the 95% bootstrap intervals (figure 3a). However, the bootstrap sampling for ancient woodland indicated that the Wiltshire study site was characterized by significantly more ancient woodland than generally observed for British 30 km buffers, along with the Essex site, while the Somerset study site had an approximately median value for ancient woodland cover (figure 3b).
Figure 3.
Bootstrapped values for environmental variables (30 km buffers) selected to explain the spatial density of extirpated species in the present-day landscape (cf. table 2): (a) SO2 and (b) per cent cover of ancient woodland. Values derived for each of the three study sites (cf. figure 2) are indicated on the graphs. Dashed lines show the 95% bootstrap confidence intervals.
4. Discussion
The first goal of this study was to use a novel archaeological resource, lichens on historic timbers, to estimate the magnitude of biodiversity loss for epiphytes between the pre- and post-industrial periods. The fact that this resource exists has been demonstrated previously [30,32], and it can be confidently assumed that preserved lichens provide representative subsamples of epiphyte communities that existed at the historic time-point a tree was harvested and included within the built structure [31]. However, the methodology described here is subject to several caveats. First, we make the important assumption that the epiphyte species preserved on historic timbers are sampled from the local environment surrounding a village. The timbers included in this analysis date from between the fifteenth and eighteenth centuries. Although documentary evidence for the source of timbers used in vernacular architecture is generally lacking [56], most materials—including timbers—used to construct low-status buildings in this period are considered to have come from the local environment (M. Bridge 2010, personal communication). Documented instances of long-distance transport of timber for building are exceptional [57], and we assume that large timbers were not commonly traded during the pre-industrial period.
Second, with only three study sites, it was necessary to amalgamate the estimate of threshold presence–absence for all species combined. Our results depend upon a generic AUC threshold value, relevant to those lichen species recorded from historic timbers. In reality, the likelihood of occurrence for a species, predicted based on its spatial density, may be subject to interspecific variation controlled by life-history traits such as dispersal limitation and habitat specialization [58,59]. However, GLMs were highly significant across the range of spatial-scales, with AUC values indicating strong predictive ability. This can be explained by a pattern of spatial aggregation for each of our target lichen species (with present-day distributions skewed away from the study sites), underpinning the utility of this method as a statistically robust way of estimating a species' presence–absence when combined with a heavily sampled floristic dataset. While it may be very difficult to predict the presence–absence of a species for an individual grid square, the general distribution of lichens in the British Isles is reliably known, and estimates based on these spatially aggregated larger-scale patterns provide a robust method for comparing confirmed historic presences with post-industrial distributions.
On the basis of statistical likelihoods for present-day absence, we estimated that greater than or equal to 80 per cent of pre-industrial epiphyte diversity may have become locally extinct from the landscape in the southeast of England. This figure varied regionally in our study between 80 and 35 per cent, and these estimates of loss bring into sharp question assessments of biodiversity status developed using post-industrial data. The importance of this result is highlighted by the debate surrounding the environmental Kuznets curve (EKC) hypothesis [60,61]. Based on inter-region comparison, the EKC predicts that beyond a threshold level of per capita wealth, the threat to habitats and/or species decreases as socio-economic forces undergo ‘ecological modernization’ and become environmentally progressive [62–65]. Notwithstanding criticism levelled at the socio-economic basis of the EKC [61,66,67], our study highlights a further critical flaw: correlating biodiversity threat with per capita wealth for a single time-period hides the fact that economically developed regions may have suffered an historic massive loss of species in attaining wealth, therefore invalidating straightforward spatial comparisons. This is an example of the wider problem of shifting base-lines [12]: i.e. the elements of biodiversity that are used to inform conservation policy may be a recalcitrant subset of those that existed during a pre-industrial period, and, given as a baseline, extant species will be relatively easily protected for those economies that have already industrialized.
Our second goal was to infer the possible drivers of species extirpation. Pollution is expected to be the most significant large-scale driver of biodiversity change between the pre- and post-industrial periods [68], though the transition also encompasses the end of the Little Ice Age [69,70] and a period of changed countryside management, including commercial planting into ancient and traditionally managed woodlands [71,72]. Individual variables included within measures of climatic setting or pollution regime often covary across a range of scales, and it can be difficult to use regression techniques to isolate the effect of many competing and correlated explanatory variables. To overcome this problem, we reduced each suit of potential explanatory variables, describing pollution and climate, to a set of weakly correlated variables. We tested these against the present-day distribution of those species, which had been extirpated, to identify a signature for species sensitivity. However, we assumed that spatial patterns in SO2 averaged for the period 2004–2006 are highly correlated with the spatial pattern that existed during the most severe period of SO2 pollution, even though absolute values have since declined to a point allowing early signs of lichen recovery [68,73]. This is supported by the highly significant correlation between SO2 concentrations for 1987, and those for the period 2004–2006 (see the electronic supplementary material, appendix S3). On this basis, post-1960 lichen distribution patterns that continue to reflect the severe effects of SO2 pollution can be modelled against present-day concentrations of SO2.
The spatial distribution of extirpated species pointed to a sensitivity to SO2 pollution, implying the strong impact of this pollutant on epiphytes between the pre- and post-industrial periods. The target region inferred to have lost the most species—Essex—had outlying values at the extreme upper-end of SO2 pollution. This is entirely consistent with previous work demonstrating the severe damaging effect of SO2 on lichen distributions in Britain [14,15], though subject to the emerging importance of N-pollution in modifying lichen epiphyte distribution/abundance [16,74]. This result also suggests that the loss of species richness should not be alternatively interpreted as temporal species turnover, causing an ‘apparent decline’: i.e. our interpretation is supported by the fact that patterns in lichen species richness show a general decline from a relatively clean-air environment (e.g. rural northern Britain) towards the industrialized regions of England [68,75]. However, our results also point to the potential interaction between pollution impacts and habitat quality (extent of ancient woodland). This interaction may modify the direct effect of pollution, leading to regional variability in biodiversity loss (see also [17,76]). Thus, Wiltshire suffered a greater SO2 pollution load than Somerset, but with fewer extirpated species. This might be explained by the buffering effect of ancient woodland in low to moderate pollution regimes (Wiltshire has high values for ancient woodland, including the neighbouring Hampshire New Forest), scenarios where local habitat quality is expected to offset the negative effect of pollution on epiphytes.
In summary, we have provided a novel quantification for the extent of epiphyte floristic loss between the pre- and post-industrial periods, for a region that went through an early period of industrialization. Our evidence using epiphytes provides high-resolution taxonomic data that extends ‘long-term’ ecological datasets by several centuries (e.g. [8]). It adds an archaeological perspective to palaeoecological studies that have strongly argued for a longer-term framework when developing biodiversity ‘baselines’ and conservation strategy [77–79]. Drawing on the sensitivity of lichen epiphytes to environmental setting, our results invoke a multiplicity of factors underlying landscape variation in species extirpation during industrialization—pollution regime, in combination with changes in habitat quality accompanying an intensification of rural land-use. The magnitude of biodiversity loss provides an important context when directing concern towards developing countries, which may occupy regions of high global diversity. We question whether, to maintain parity when seeking to mitigate biodiversity impacts in other nations (tropical developing countries), and when faced with massive regional biodiversity loss suffered during the English industrial revolution, it should be necessary to reconsider UK baselines for biodiversity conservation and environmental recovery with respect to pre-industrial conditions.
Acknowledgements
We thank The Leverhulme Trust for providing a research grant to undertake this work. We thank archaeological specialists who gave freely of their time and knowledge including Richard Harris, Joe Thompson, Roger Champion, Dorothy Treasure, Nigel Walker, John Dallimore, Isabel Richardson, David Andrews, Brenda Watkin, Malcolm Loveday, Nick Joyce, John Letts, Paula Harber and the many generous homeowners who provided us with access to their timber-framed houses. We thank the Editors (Professors Cuthill and Long), and three anonymous reviewers for comments that improved the submitted manuscript.
References
- 1.Ehrlich P. R. 1994. Energy use and biodiversity loss. Phil. Trans. R. Soc. Lond. B 344, 99–104 10.1098/rstb.1994.0057 (doi:10.1098/rstb.1994.0057) [DOI] [Google Scholar]
- 2.Steffen W., Crutzen P. J., McNeill J. R. 2007. The anthropocene: are humans now overwhelming the great forces of nature? Ambio 36, 614–621 10.1579/0044-7447(2007)36[614:TAAHNO]2.0.CO;2 (doi:10.1579/0044-7447(2007)36[614:TAAHNO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 3.Vitousek P. M., Mooney H. A., Luchenco J., Melillo J. M. 1997. Human domination of Earth's ecosystems. Science 277, 494–499 10.1126/science.277.5325.494 (doi:10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 4.Gardner T. A., Barlow J., Chazdon R., Ewers R. M., Harvey C. A., Peres C. A., Sodhi N. S. 2009. Prospects for tropical forest diversity in a human modified world. Ecol. Lett. 12, 561–582 10.1111/j.1461-0248.2009.01294.x (doi:10.1111/j.1461-0248.2009.01294.x) [DOI] [PubMed] [Google Scholar]
- 5.Jha S., Bawa K. S. 2006. Population growth, human development, and deforestation in biodiversity hotspots. Conserv. Biol. 20, 906–912 10.1111/j.1523-1739.2006.00398.x (doi:10.1111/j.1523-1739.2006.00398.x) [DOI] [PubMed] [Google Scholar]
- 6.Lawn P. 2008. Macroeconomic policy, growth, and biodiversity conservation. Conserv. Biol. 22, 1418–1423 10.1111/j.1523-1739.2008.01092.x (doi:10.1111/j.1523-1739.2008.01092.x) [DOI] [PubMed] [Google Scholar]
- 7.Hawksworth D. L., Coppins B. J., Rose F. 1974. Changes in the British lichen flora. In The changing flora and fauna of Britain (ed. Hawksworth D. L.), pp. 47–78 London, UK: Academic Press [Google Scholar]
- 8.Magurran A. E., Baillie S. R., Buckland S. T., Dick J. M., Elston D. A., Scott E. M., Smith R. I., Somerfield P. J., Watt A. D. 2010. Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol. Evol. 25, 574–582 10.1016/j.tree.2010.06.016 (doi:10.1016/j.tree.2010.06.016) [DOI] [PubMed] [Google Scholar]
- 9.Tansley A. G. 1947. The early history of modern plant ecology in Britain. J. Ecol. 35, 130–137 10.2307/2256503 (doi:10.2307/2256503) [DOI] [Google Scholar]
- 10.Evans D. 1992. A history of nature conservation in Britain. London, UK: Routledge [Google Scholar]
- 11.Nicholson E. M. 1970. The environmental revolution: a guide for the new masters of the World. London, UK: Hodder & Staughton [Google Scholar]
- 12.Pauly D. 1995. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430. 10.1016/S0169-5347(00)89171-5 (doi:10.1016/S0169-5347(00)89171-5) [DOI] [PubMed] [Google Scholar]
- 13.Mathias P. 2001. The first industrial nation: the economic history of Britain, 1700–1914. London, UK: Routledge [Google Scholar]
- 14.Hawksworth D. L., Rose F. 1970. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 227, 145–148 10.1038/227145a0 (doi:10.1038/227145a0) [DOI] [PubMed] [Google Scholar]
- 15.Hawksworth D. L., Rose F. 1976. Lichens as pollution monitors. London, UK: Edward Arnold [Google Scholar]
- 16.Van Herk C. M. 1999. Mapping of ammonia pollution with epiphytic lichens in the Netherlands. Lichenologist 31, 9–20 [Google Scholar]
- 17.Wolseley P. A., James P. W., Theobald M. R., Sutton M. A. 2006. Detecting changes in atmospheric lichen communities at sites affected by atmospheric ammonia from agricultural sources. Lichenologist 38, 161–176 10.1017/S0024282905005487 (doi:10.1017/S0024282905005487) [DOI] [Google Scholar]
- 18.Kuusinen M., Siitonen J. 1998. Epiphytic lichen diversity in old-growth and managed Picea abies stands in southern Finland. J. Veg. Sci. 9, 283–292 10.2307/3237127 (doi:10.2307/3237127) [DOI] [Google Scholar]
- 19.Lesica P., McCune B., Cooper S. V., Hong W. S. 1991. Differences in lichen and bryophyte communities between old-growth and managed second-growth forests in the Swan Valley, Montana. Can. J. Bot. 69, 1745–1755 10.1139/b91-222 (doi:10.1139/b91-222) [DOI] [Google Scholar]
- 20.Möning C., Werth S., Dziock F., Bassler C., Bradtka J., Hothorn T., Muller J. 2009. Lichen diversity in temperate montane forests is influenced by forest structure more than climate. Forest Ecol. Manage. 258, 745–751 10.1016/j.foreco.2009.05.015 (doi:10.1016/j.foreco.2009.05.015) [DOI] [Google Scholar]
- 21.Ellis C. J., Coppins B. J., Dawson T. P., Seaward M. R. D. 2007. Response of British lichens to climate change scenarios: trends and uncertainties in the projected impact for contrasting biogeographic groups. Biol. Conserv. 140, 217–235 10.1016/j.biocon.2007.08.016 (doi:10.1016/j.biocon.2007.08.016) [DOI] [Google Scholar]
- 22.Giordani P., Incerti G. 2008. The influence of climate on the distribution of lichens: a case study in a borderline area (Liguria, NW Italy). Plant Ecol. 195, 257–272 10.1007/s11258-007-9324-7 (doi:10.1007/s11258-007-9324-7) [DOI] [Google Scholar]
- 23.Van Herk C. M., Aptroot A., Van Dobben H. F. 2002. Long-term monitoring in the Netherlands suggests that lichens respond to global warming. Lichenologist 34, 141–154 10.1006/lich.2002.0378 (doi:10.1006/lich.2002.0378) [DOI] [Google Scholar]
- 24.Knops J. M. H., Nash T. H., Boucher V. L., Schlesinger W. H. 1991. Mineral recycling and epiphytic lichens: implications at the ecosystem level. Lichenologist 23, 309–321 10.1017/S0024282991000452 (doi:10.1017/S0024282991000452) [DOI] [Google Scholar]
- 25.Gunnarsson B., Hake M., Hultengren S. 2004. A functional relationship between species richness of spiders and lichens in spruce. Biodivers. Conserv. 13, 685–693 10.1023/B:BIOC.0000011720.18889.f7 (doi:10.1023/B:BIOC.0000011720.18889.f7) [DOI] [Google Scholar]
- 26.Pettersson R. B., Ball J. P., Renhorn K.-E., Esseen P. A., Sjöberg K. 1995. Invertebrate communities in boreal forest canopies as influenced by forestry and lichens with implications for passerine birds. Biol. Conserv. 74, 57–63 10.1016/0006-3207(95)00015-V (doi:10.1016/0006-3207(95)00015-V) [DOI] [Google Scholar]
- 27.Stubbs C. S. 1989. Patterns of distribution and abundance of corticolous lichens and their invertebrate associates on Quercus rubra in Maine. Bryologist 92, 453–460 10.2307/3243665 (doi:10.2307/3243665) [DOI] [Google Scholar]
- 28.Sala O. E., et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 10.1126/science.287.5459.1770 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 29.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute [Google Scholar]
- 30.Coppins B. J., Rose F., Tittensor R. M. 1985. Lichens from a 16th century Sussex cottage. Lichenologist 17, 297–298 10.1017/S0024282985000391 (doi:10.1017/S0024282985000391) [DOI] [Google Scholar]
- 31.Yahr R., Coppins B. J., Ellis C. J. In press Preserved epiphytes as an archaeological resource in pre-industrial vernacular buildings. J. Archaeol. Sci. 10.1016/j.jas.2010.12.012 (doi:10.1016/j.jas.2010.12.012) [DOI] [Google Scholar]
- 32.Yahr R., Ellis C. J. 2009. Lichens in the attic. Building Conserv. Directory 2009, 13–14 [Google Scholar]
- 33.Hewett C. A. 1980. English historic carpentry. Sussex, NJ: Phillimore & Co [Google Scholar]
- 34.Penoyre J. 2005. Traditional houses of Somerset. Tiverton, UK: Somerset Books [Google Scholar]
- 35.Orange A., James P. W., White F. J. 2001. Microchemical methods for the identification of lichens. London, UK: British Lichen Society [Google Scholar]
- 36.Smith C. W., Aptroot A., Coppins B. J., Fletcher A., Gilbert O. L., James P. W., Wolseley P. A. 2009. The lichens of Great Britain and Ireland. London, UK: British Lichen Society [Google Scholar]
- 37.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 38.Fielding A. H., Bell J. F. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49 10.1017/S0376892997000088 (doi:10.1017/S0376892997000088) [DOI] [Google Scholar]
- 39.Manel S., Williams H. C., Ormerod S. J. 2001. Evaluating presence–absence models in ecology: the need to account for prevalence. J. Appl. Ecol. 38, 921–931 10.1046/j.1365-2664.2001.00647.x (doi:10.1046/j.1365-2664.2001.00647.x) [DOI] [Google Scholar]
- 40.Swets J. A. 1988. Measuring the accuracy of diagnostic systems. Science 240, 1285–1293 10.1126/science.3287615 (doi:10.1126/science.3287615) [DOI] [PubMed] [Google Scholar]
- 41.MacKenzie D. I. 2005. Was it there? Dealing with imperfect detection for species presence/absence data. Aust. N. Z. J. Stat. 47, 65–74 10.1111/j.1467-842X.2005.00372.x (doi:10.1111/j.1467-842X.2005.00372.x) [DOI] [Google Scholar]
- 42.Brown J. H. 1984. On the relationship between the distribution and abundance of species. Am. Nat. 124, 255–279 10.1086/284267 (doi:10.1086/284267) [DOI] [Google Scholar]
- 43.Gaston K. 1996. The multiple forms of the interspecific abundance-distribution relationship. Oikos 76, 211–220 10.2307/3546192 (doi:10.2307/3546192) [DOI] [Google Scholar]
- 44.Gaston K., Blackburn T. M. 2000. Pattern and process in macroecology. Oxford, UK: Blackwell Science [Google Scholar]
- 45.Kunin W. E. 1998. Extrapolating species abundance across spatial scales. Science 281, 1513–1515 10.1126/science.281.5382.1513 (doi:10.1126/science.281.5382.1513) [DOI] [PubMed] [Google Scholar]
- 46.McCune B., Mefford M. J. 1999. PC-Ord v. 4.41, multivariate analysis of ecological data. Oregon, OR: MjM Software [Google Scholar]
- 47.CEH 2010. UK air pollution information system. See http://www.apis.ac.uk/
- 48.Ter Braak C. J. F., Šmilauer P. 2002. CANOCO 4.5: software for canonical community ordination. Ithaca, NY: Microcomputer Power [Google Scholar]
- 49.Lepš J., Šmilauer P. 2003. Multivariate analysis of ecological data using CANOCO. Cambridge, UK: Cambridge University Press [Google Scholar]
- 50.Perry M. C., Hollis D. M. 2005. The generation of monthly gridded datasets for a range of climate variables over the UK. J. Climatol. 25, 1041–1054 10.1002/joc.1161 (doi:10.1002/joc.1161) [DOI] [Google Scholar]
- 51.Roberts A. J., Russell C., Walker G. J., Kirby K. J. 1992. Regional variation in the origin, extent and composition of Scottish woodland. Botanical J. Scot. 46, 167–189 10.1080/03746600508684786 (doi:10.1080/03746600508684786) [DOI] [Google Scholar]
- 52.Spencer J., Kirby K. 1992. An inventory of ancient woodland for England and Wales. Biol. Conserv. 62, 77–93 10.1016/0006-3207(92)90929-H (doi:10.1016/0006-3207(92)90929-H) [DOI] [Google Scholar]
- 53.Walker G. J., Kirby K. J. 1989. Inventories of ancient, long-established and semi-natural woodland for Scotland. Peterborough, UK: Nature Conservancy Council; Research and Survey in Nature Conservation No. 22 [Google Scholar]
- 54.ESRI 2005. ArcMap v. 9.1. Redlands, CA: ESRI [Google Scholar]
- 55.Watson M. F., Hawksworth D. L., Rose F. 1988. Lichens on elms in the British Isles and the effects of Dutch elm disease on their status. Lichenologist 20, 327–352 10.1017/S0024282988000441 (doi:10.1017/S0024282988000441) [DOI] [Google Scholar]
- 56.Bridge M. 2000. Can vernacular architecture be used to indicate the source of oak within Britain? Vernacular Archit. 31, 67–72 [Google Scholar]
- 57.Rackham O. 1980. Ancient woodland. London, UK: Edward Arnold [Google Scholar]
- 58.Hedenas H., Ericson L. 2008. Species occurrences at stand level cannot be understood without considering the landscape context: cyanolichens on aspen in boreal Sweden. Biol. Conserv. 141, 710–718 10.1016/j.biocon.2007.12.019 (doi:10.1016/j.biocon.2007.12.019) [DOI] [Google Scholar]
- 59.Löbel S., Snäll T., Rydin H. 2006. Species richness patterns and metapopulation processes: evidence from epiphyte communities in boreo-nemoral forests. Ecography 29, 169–182 10.1111/j.2006.0906-7590.04348.x (doi:10.1111/j.2006.0906-7590.04348.x) [DOI] [Google Scholar]
- 60.IBRD 1992. World development report 1992: development and the environment. New York, NY: Oxford University Press [Google Scholar]
- 61.Stern D. I., Common M. S., Barbier E. B. 1996. Economic growth and environmental degradation: the environmental Kuznets curve and sustainable development. World Dev. 24, 1151–1160 10.1016/0305-750X(96)00032-0 (doi:10.1016/0305-750X(96)00032-0) [DOI] [Google Scholar]
- 62.Ehrhardt-Martinez K., Crenshaw E. M., Jenkins J. C. 2002. Deforestation and the environmental Kuznets curve: a cross-national investigation of intervening mechanisms. Soc. Sci. Q. 83, 226–243 10.1111/1540-6237.00080 (doi:10.1111/1540-6237.00080) [DOI] [Google Scholar]
- 63.Hoffman J. P. 2004. Social and environmental influences on endangered species: a cross-national study. Soc. Perspect. 47, 79–107 10.1525/sop.2004.47.1.79 (doi:10.1525/sop.2004.47.1.79) [DOI] [Google Scholar]
- 64.Mather A. S., Needle C. L., Fairbairn J. 1999. Environmental Kuznets curves and forest trends. Geography 84, 55–65 [Google Scholar]
- 65.McPherson M. A., Nieswiadomy M. L. 2005. Environmental Kuznets curve: threatened species and spatial effects. Ecol. Econom. 55, 395–407 10.1016/j.ecolecon.2004.12.004 (doi:10.1016/j.ecolecon.2004.12.004) [DOI] [Google Scholar]
- 66.Mills J. H., Waite T. A. 2009. Economic prosperity, biodiversity conservation, and the environmental Kuznets curve. Ecol. Econom. 68, 2087–2095 10.1016/j.ecolecon.2009.01.017 (doi:10.1016/j.ecolecon.2009.01.017) [DOI] [Google Scholar]
- 67.Stern D. I. 2004. The rise and fall of the environmental Kuznets curve. World Dev. 32, 1419–1439 10.1016/j.worlddev.2004.03.004 (doi:10.1016/j.worlddev.2004.03.004) [DOI] [Google Scholar]
- 68.Coppins B. J., Hawksworth D. L., Rose F. 2001. Lichens. In The changing wildlife of Great Britain and Ireland (ed. Hawksworth D. L.), pp. 126–147 London, UK: Taylor and Francis [Google Scholar]
- 69.Bradley R. S. 1985. Quaternary paleoclimatology. London, UK: Unwin Hymen [Google Scholar]
- 70.Lamb H. H. 1977. Climate, present, past and future. Volume II, climatic history and the future. London, UK: Methuen [Google Scholar]
- 71.James N. D. G. 1981. A history of English forestry. Oxford, UK: Basil Blackwell [Google Scholar]
- 72.Rackham O. 1976. Trees and woodland in the British landscape. London, UK: Dent & Sons [Google Scholar]
- 73.Seaward M. R. D. 1998. Time-space analysis of the British lichen flora, with particular reference to air quality surveys. Folia Cryptogamica Estonica 32, 85–96 [Google Scholar]
- 74.Van Herk C. M. 2001. Bark pH and susceptibility to toxic air pollutants as independent causes of change in epiphytic lichen composition in space and time. Lichenologist 33, 419–441 10.1006/lich.2001.0337 (doi:10.1006/lich.2001.0337) [DOI] [Google Scholar]
- 75.Ellis C. J., Coppins B. J. 2009. Quantifying the role of multiple landscape-scale drivers controlling epiphyte composition and richness in a conservation priority habitat (juniper scrub). Biol. Conserv. 142, 1291–1301 10.1016/j.biocon.2009.01.036 (doi:10.1016/j.biocon.2009.01.036) [DOI] [Google Scholar]
- 76.Ellis C. J., Coppins B. J. 2010. Integrating multiple landscape-scale drivers in the lichen epiphyte response: climatic setting, pollution regime, and woodland spatial–temporal structure. Divers. Distrib. 16, 43–52 10.1111/j.1472-4642.2009.00624.x (doi:10.1111/j.1472-4642.2009.00624.x) [DOI] [Google Scholar]
- 77.Froyd C. A., Willis K. J. 2008. Emerging issues in biodiversity and conservation management: the need for a palaeoecological perspective. Q. Sci. Rev. 27, 1723–1732 10.1016/j.quascirev.2008.06.006 (doi:10.1016/j.quascirev.2008.06.006) [DOI] [Google Scholar]
- 78.Swetnam T. W., Allen C. D., Betancourt J. L. 1999. Applied historical ecology: using the past to manage for the future. Ecol. Appl. 9, 1189–1206 10.1890/1051-0761(1999)009[1189:AHEUTP]2.0.CO;2 (doi:10.1890/1051-0761(1999)009[1189:AHEUTP]2.0.CO;2) [DOI] [Google Scholar]
- 79.Willis K. J., Gillson L., Brncic T. M., Figueroa-Rangel B. L. 2005. Providing baselines for biodiversity measurement. Trends Ecol. Evol. 20, 107–108 10.1016/j.tree.2004.12.003 (doi:10.1016/j.tree.2004.12.003) [DOI] [PubMed] [Google Scholar]