Abstract
Evolutionary processes can interact with the mechanisms of steroid hormone action to drive interspecific variation in behavioural output, yet the exact nature of these interactions is poorly understood. To investigate this issue, we compare the endocrine machinery underlying the winner effect (an ability to increase winning behaviour in response to past victories) in two closely related species of Peromyscus mice. Typically, after winning a fight, California mice (Peromyscus californicus) experience a testosterone (T) surge that helps enhance their future winning behaviour, whereas white-footed mice (Peromyscus leucopus) experience neither a T surge nor a change in subsequent winning behaviour. However, our results indicate that when the post-victory T response of male white-footed mice is phenotypically engineered to resemble that of California mice, individuals are capable of developing a strong and lasting winner effect. Moreover, this ‘induced’ winner effect in white-footed mice qualitatively matches the winner effect that develops naturally in California mice. Taken together, these findings suggest that white-footed mice have the physiological machinery necessary to form a robust winner effect comparable to that formed by California mice, but are unable to endogenously activate this machinery after achieving winning experiences. We speculate that evolutionary processes, like selection, operate on the physiological substrates that govern post-victory T release to guide divergence in the winner effect between these two species.
Keywords: social behaviour, winner effect, testosterone, behavioural evolution, the challenge hypothesis, Peromyscus mice
1. Introduction
Steroid hormones secreted from the gonads and other peripheral tissues mediate many adaptive behavioural traits, such as mating, parental effort and territoriality [1]. Although this means that evolutionary processes interact with the mechanisms of steroid action to shape species differences in behaviour, the exact nature of these interactions is currently under debate. For example, evolutionary forces, like selection, may drive behavioural variation by modifying different components of a steroid signalling system independent of each other [2]. While most research has sought to address this issue by manipulating baseline steroid levels and then documenting the behavioural effect [3–5], only a limited number of studies have manipulated pulsatile steroid release and examined its impact on behaviour.
The winner effect is defined as an increased ability to win fights following the acquisition of prior victories [6]. Studies suggest that this ability to modify one's antagonistic behaviour in response to social experience is adaptive, as it facilitates the formation of social hierarchies [7] that are crucial for optimizing one's own fitness [8,9]. This idea is further supported by the fact that the presence and magnitude of the winner effect varies tremendously between species [6]. For example, an experiment in Peromyscus mice revealed that accruing three separate winning experiences induced a strong and lasting winner effect in the territorial California mouse (Peromyscus californicus), yet induced a weaker (non-significant) winner effect in the closely related and less territorial white-footed mouse (Peromyscus leucopus) [10–13]. Taken together, this body of research implies that evolutionary processes, including forces like natural or sexual selection, may drive interspecific variation in the winner effect by operating on this trait's underlying mechanisms. However, remarkably little is known about how the ability to form and express a winner effect is evolutionarily ‘gained’ or ‘lost’.
Androgen hormone action is the main endocrine process currently thought to mediate the winner effect [14,15]. In Peromyscus rodents, for example, male California mice experience a temporary testosterone (T) surge roughly 40–45 min after a fight [16], and this T response helps increase future winning behaviour [15]. Other work in California mice indicates that the brain's sensitivity to androgen hormones similarly contributes to victory-induced plasticity in winning ability [17]. By contrast, T does not increase in male white-footed mice in response to winning a fight, which corresponds to the species' inability to form a robust winner effect [10,11]. Research indicates that the ability to both increase androgens after a fight and detect androgens in neural target tissues is subject to selection and thus varies among different sexes and species [18,19]. For example, the ‘challenge hypothesis’ predicts that social traits, like mating strategy and male–male competitiveness, are related to whether individuals are able to increase androgens after aggressive disputes [20–22]. Because California mice are monogamous, paternal and territorial [23,24] and white-footed mice are promiscuous, non-paternal and less territorial [25,26], it is possible that selection acted on endogenous systems of androgen action to shape the differences in the winner effect. In particular, we hypothesize that selection acts on either the ability to mount a T response after achieving a victory, the brain's ability to respond to this T surge, or both of these processes.
We tested here whether the absence of a post-victory T response in male white-footed mice accounts for this species' inability to form a strong winner effect like its congener, the California mouse. Thus, this study represents a first step in elucidating the ways in which evolutionary processes might have interacted with the androgen signalling system to drive divergence in the ability to form and express a winner effect. All mice used in this experiment were raised and tested in the same environmental (laboratory) conditions, suggesting that differences between species have a genetic component. The experiment's immediate goals were to (i) confirm that winning experience does not induce a T pulse in male white-footed mice, and (ii) evaluate whether administration of a post-victory T pulse is sufficient to induce a robust winner effect in white-footed mice. To address the first goal, we measured T levels from different white-footed mice at various time points after a single victory. Although prior studies show that T does not change in white-footed mice 45 min after a win [10,11], a more detailed analysis to rule out the possibility that winning increases T at a different post-encounter time point has not been conducted. To address the second goal, we phenotypically engineered the T response of male white-footed mice to mimic that of California mice (i.e. T increases 40–45 min after a win [27]) and then measured whether these manipulated individuals formed a winner effect. We hypothesized that if male white-footed mice formed the winner effect in response to such hormonal manipulation, then this species must have at least some of the physiological machinery necessary to form a winner effect and that selection acted, at least in part, on the mechanisms of T release to drive divergence in this behavioural trait. On the other hand, if white-footed mice failed to form a robust winner effect after post-encounter androgen treatment, then selection probably shapes species differences in the winner effect by acting on multiple or other physiological factors that regulate this phenomenon.
2. Methods
(a). Animals
White-footed and California mice were obtained from two species-specific colonies at the University of Wisconsin–Madison. In each colony, mice were housed in same sex groups of two to three per standard cage (white-footed mouse cage: 28 × 18 × 12 cm; California mouse cage: 48 × 27 × 16 cm) and provided mouse chow and water ad libitum. Colony rooms were kept on a 14 L : 10 D light cycle. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Animals, and the University of Wisconsin–Madison Institutional Animal Use and Care Committee approved the research described here.
One week before each experiment began, mice were transported from the colony to a separate room used for behavioural testing (14 L : 10 D light cycle). Behavioural manipulations always occurred under dim red light at least 1 h after the onset of the dark cycle.
(b). Experiment 1: post-victory androgen responsiveness of white-footed mice
We first tested whether male white-footed mice experience an endogenous surge of plasma T after winning a single fight. On day 1, 54 male mice were each paired with a single female and placed in a standard cage with food and water provided ad libitum. On day 11, resident males were randomly assigned to one of the six treatment groups that were different in terms of the time blood was collected after a single winning experience (n = 9 per group; first five groups: 5, 30, 45, 60 or 1440 min). In the sixth group, mice were handled in lieu of winning (no-fight controls [10–13]) and then immediately euthanized for measurement of a species-specific T baseline. Also on day 11, each pair was moved from its standard cage into its own polycarbonate (transparent) observation cage (30 × 50 × 30 cm) that was lined with aspen bedding and contained food, water and a nest-box. On day 13, the actual winning or handling experience took place. Aggressive encounters were staged using a resident–intruder paradigm [10–13] in which females were removed from the observation cage; opaque dividers were inserted into observation cages so that residents were isolated on one side of the cage; intruders were placed in the vacant side of the observation cage; residents and intruders were given a 2 min acclimation; and dividers were removed to allow mice to freely interact for 10 min. To ensure that residents won these encounters, intruders (n = 54) were smaller, sexually inexperienced and unfamiliar with the contest area. An experimenter watched each encounter in real time and determined which individuals won. A winner was defined as the mouse that directed at least three consecutive attacks towards the opponent that, in turn, elicited losing or submissive behaviour ([10–13]; definitions of winning versus losing behaviour below). Detailed behavioural data were not collected from either residents or intruders during these encounters.
Trunk blood was collected by rapid decapitation at the designated time point after the winning experience. Blood was immediately centrifuged to obtain plasma, and stored at −80°C until assayed at the Wisconsin Primate Research Center. Details of hormone assay techniques are published elsewhere [10–13]. Briefly, samples were extracted with ethyl ether, and steroids were separated using celite chromatography. T was measured using enzyme immunoassay (T antibody R156 diluted to 1 : 35 000, University of California at Davis). The intra- and inter-assay coefficients of variation were 3.3 and 10.6 per cent, respectively (n = 2 plates).
To determine if T levels changed after a fight, a one-way ANOVA was used to compare post-encounter T levels at 5, 30, 45, 60 and 1440 min. To determine whether T levels at any of these time points differed from baseline, multiple t-tests were used to compare the average T level at each post-encounter time point with the average baseline T level in handled (control) mice. Holm's procedure for multiple comparisons was used to control type I error [28]. T levels were natural log-transformed [ln (X + 1)], because Q–Q plots showed that this yielded a normal distribution [29].
(c). Experiment 2: the winner effect in white-footed mice
With a new group of mice, we then tested whether male white-footed mice were capable of forming a winner effect if they experienced a post-victory surge in plasma T similar to male California mice. A total of 37 male white-footed mice were randomly assigned to one of three groups in which winning experience and post-encounter androgens were manipulated prior to a test encounter that assessed the formation of the winner effect. They received either (i) three wins that were each followed by a T injection (W + T males; n = 13); (ii) three wins that were each followed by a saline injection (W + S males; n = 12); or (iii) three handling experiences that were each followed by a T injection (H + T males; n = 12). A fourth group (iv) consisted of California mice that received three winning experiences which were each followed by a saline injection (Cal: W + S males; n = 12) prior to a test encounter. Presumably, California mice in the ‘Cal: W + S’ group experience a natural T surge after the three separate winning experiences that mediated the formation of the winner effect [16]; thus, this group served as a control for the winner effect to which we could compare a possible winner effect formed in white-footed mice and ensure that our behavioural assay of the winner effect worked. Resident males of both species were treated identically throughout the experiment.
On day 1, each male was paired with a single female to provide sexual experience. Each pair was housed in its own standard cage with food and water. On day 11, residents were randomly assigned to treatment groups (see above). Each pair was then moved from its standard cage to an observation cage, which was lined with fresh aspen bedding and contained food, water, and a nest-box. On days 13, 15 and 17, resident males received winning or handling experiences. Winning experiences were administered using the same resident–intruder paradigm described in the prior study. These fights were similarly biased in favour of the resident male by ensuring that intruders were smaller, sexually inexperienced and unfamiliar with the resident's home cage. An experimenter watched each of these three fights in real time and verified that residents always won, using the definition of ‘winning’ described above. Handling experiences were conducted identically to staged encounters (i.e. removal of female, insertion of opaque divider, etc.), but an intruder was never placed on the vacant side of the observation cage; thus, handled mice never received fighting experience with a conspecific [10–12]. In other work, similar ‘no-fight’/handle treatments are used as controls to provide baseline measures of behaviour and physiology [30,31], since other types of social experience, such as losing, induce their own set of behavioural or physiological changes [6,14].
Intraperitoneal injections of saline or T (36 µg kg–1, T-cyclodextrin inclusion complex) were administered 30 min following each handling experience or win, respectively. Prior studies show that such a T injection at this time point after a fight induces a rapid, transient pulse of T, which persists for approximately 15–20 min and mirrors the endogenous T pulse that normally occurs in California mice after a social dispute [27]. We confirmed in white-footed mice that the dose and timing of our T injections increased plasma T levels in the same manner and that the T pulse we induced was within the species' physiological range. Thus, 10 min after a T injection (n = 8) or saline injection (n = 7), plasma T levels measured 1420.3 ± 327.2 pg ml–1 (mean ± s.e.m.) and 827.6 ± 258.5 pg ml–1 (mean ± s.e.m.), respectively. Hormone assay procedures were conducted in the same manner described above, with intra- and inter-assay coefficients of variation equalling 2.7 and 2.3 per cent, respectively. Note that the purpose of administering saline injections as controls was to allow mice to experience their normal physiological response to winning, while controlling for any effect of stress caused by the injection itself.
On day 19, resident males were subjected to the test encounter that was used to assess whether the winner effect formed. Test encounters were staged using the same resident–intruder paradigm described above, but the test intruders in this instance were sexually experienced, had won a single fight on the prior day, and were 11.2 per cent (s.e.m. = ±2.1%) larger in body mass than resident males. This intruder advantage decreases the chances that a resident wins the test encounters at random, and thus enhances the sensitivity with which experience-induced changes in winning ability can be detected [10–13]. Test intruders were always assigned to resident males at random, which controlled for an effect of variation in test intruder ‘aggressiveness’ on resident behaviour during the test encounter.
All test encounters were videotaped, and an observer blind to treatment group noted the winner and loser of each encounter. The observer recorded each contestant's activity rate (i.e. time in which contestant engaged in self-propelled physical movement), attack latency (i.e. time between the encounter's onset to the animal's first attack), total attacks (i.e. sum of bites, chases and wrestling bouts) and total losing behaviour (i.e. sum of retreats, jumps away and freezes) [10–13]. Using this information, the observer computed the ratio of intruder losing behaviour to resident total attacks. This ratio is referred to herein as attack efficiency because it reflects the relative amount of losing behaviour residents elicit from opponents per attack; thus, a higher ratio indicates that residents are able to elicit more losing behaviour with each attack they direct at their opponent.
Group differences in the proportion of resident victories during test encounters were analysed using a Fisher's exact test that was modified for data arranged in a 2 × 4 table [32]. Post hoc comparisons of winning were analysed using standard 2 × 2 Fisher's exact tests, controlling type I error using Holm's procedure for multiple contrasts [28]. All behavioural metrics (total activity, attack latency, total attacks, total losing behaviour, ratio of intruder losing behaviour to resident total attacks) were analysed using a series of one-way ANOVAs, and significant models were followed by Student–Newman–Keuls (SNK) post hoc tests. For the purpose of analysis, attack latency, total attacks and total losing behaviour were natural log-transformed [ln (X + 1)], whereas ratios of intruder losing behaviour to resident total attacks were cube root-transformed. These transformations were selected because Q–Q plots showed that they yielded normally distributed data [29].
3. Results
(a). Experiment 1: post-victory androgen responsiveness of white-footed mice
Endogenous plasma T levels in adult male white-footed mice did not differ from each other at any time point measured after a single winning experience (figure 1; ANOVA: F4,40 = 0.64, p > 0.05). Post-victory T levels were statistically indistinguishable from baseline T levels as well (t-tests: p > 0.05). Given that hypothalamic activation of gonadal steroid release normally takes only 30–45 min [33–35], these data indicate that it is unlikely that white-footed mice increase circulating T in response to winning an aggressive social encounter.
Figure 1.
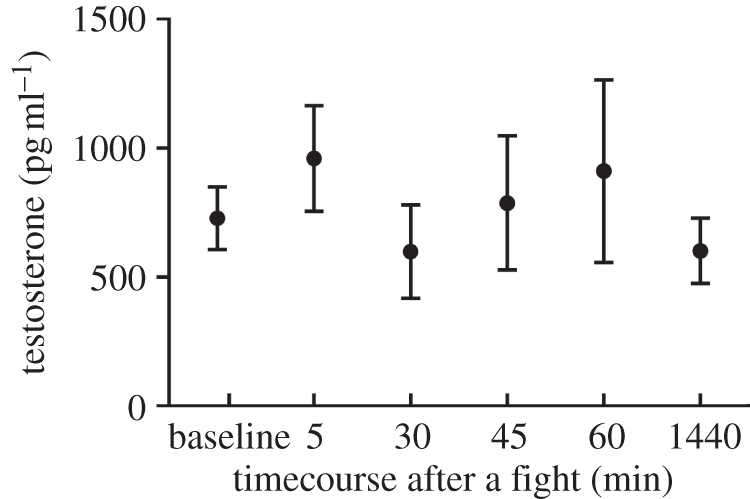
Post-victory T response of adult male white-footed mice at either 5, 30, 45, 60 or 1440 min after a single winning experience (n = 9 per group). T levels in response to a handling experience (i.e. no-fight control) are depicted as ‘baseline.’ Data represent mean ± s.e.m. For comparative purposes, baseline T in California mice typically ranges from 500–900 pg ml–1 [10,12,13,27,33].
Together with previous studies showing that the white-footed mice lack a winner effect and that California mice increase T after a win and form a subsequent winner effect [10–12,16], these data indicate that there is a positive association between a species' ability to increase T levels after winning a fight and its ability to form a strong winner effect. These results therefore form the basis for our second experiment.
(b). Experiment 2: the winner effect in white-footed mice
As predicted, the combination of winning experience and post-victory T has pronounced effects on plasticity in winning behaviour among white-footed mice (figure 2; Fisher's exact: p < 0.001). Male white-footed mice in the W + T group won a greater proportion of test encounters than males in either the W + S (Fisher's exact: p = 0.015) or H + T groups (Fisher's exact: p = 0.005). These results indicate that white-footed mice are capable of developing a full and robust winner effect in response to the engineered post-victory T response. Moreover, the proportions of male white-footed mice that won test encounters in the W + S and H + T groups were statistically indistinguishable (Fisher's exact: p = 1.0). On a comparative level, California mice (Cal: W + S) won a significantly higher proportion of test encounters than white-footed mice in the W + S group (Fisher's exact: p = 0.001) and the H + T group (Fisher's exact: p = 0.008); yet, California mice (Cal: W + S) won the same proportion of test encounters as white-footed mice in the W + T group (Fisher's exact: p = 1.0).
Figure 2.
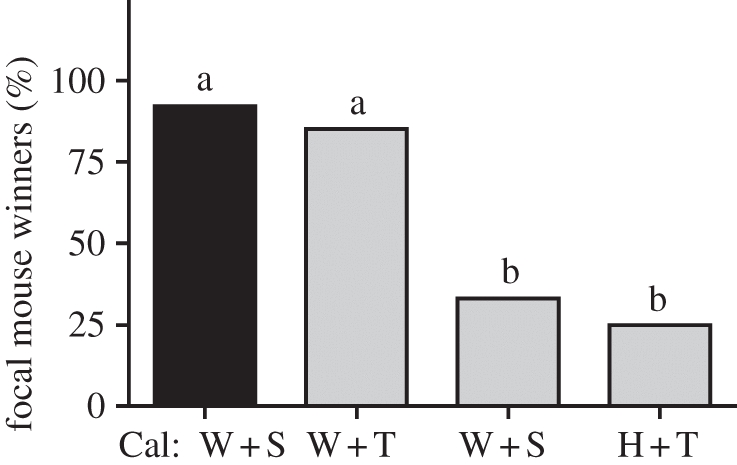
Proportion of focal males in each treatment group that won the test encounter. Labels on the horizontal axis denote treatment groups in which white-footed (grey bars) or California (black bars) mice received three prior wins that were each followed by a T injection (W + T; white-footed mice, n = 13; California mice, n = 12), three prior winning experiences that were each followed by a saline injection (W + S; n = 12), or three prior handling experiences that were each followed by a T injection (H + T; n = 12). Differences in the letters above the bars reflect significant between-group differences, with type I error being controlled by procedures outlined by Holm [28].
Different metrics of contest behaviour varied among the groups (table 1), particularly with respect to attack efficiency (ANOVA: F3,45 = 6.30, p < 0.05) and total losing behaviour (ANOVA: F3,45 = 4.37, p < 0.05). First, both white-footed mice in the W + T group and California mice in the Cal: W + S group elicited more losing behaviour from their opponents per attack than white-footed mice in either the W + S or H + T groups (SNK post hoc, p < 0.05). Second, both white-footed mice in the W + T group and California mice in the Cal: W + S group displayed significantly less losing behaviour than white-footed mice in the W + S group (SNK post hoc, p < 0.05), but not the H + T group (SNK post hoc, p > 0.05). Finally, there was a marginally significant difference among groups with respect to the average number of total attacks (ANOVA: F3,45 = 2.46, p < 0.08) and overall activity rates (ANOVA: F3,45 = 2.31, p < 0.09), whereas there was no difference among groups with respect to attack latency (ANOVA: F3,45 = 1.19, p > 0.05).
Table 1.
Contest behaviour of focal males (mean ± s.e.m.) during the test encounter. (Within each row, differences between the letters ‘a’ and ‘b’ denote significant difference between groups; SNK, p < 0.05.)
| behaviour | ANOVA results | treatment groups |
|||
|---|---|---|---|---|---|
| Cal: W + S (n = 12) | W + T (n = 13) | W + S (n = 12) | H + T (n = 12) | ||
| attack efficiency | F3,45 = 6.30; p < 0.05 | 1.27 ± 0.31a | 0.81 ± 0.21a | 0.33 ± 0.13b | 0.27 ± 0.17b |
| total losing behaviour | F3,45 = 4.37; p < 0.05 | 2.3 ± 1.5a | 6.8 ± 4.4a | 21.3 ± 7.3b | 11.0 ± 5.0a,b |
| total number of attacks | F3,45 = 2.46; p < 0.08 | 21.3 ± 4.2 | 51.5 ± 9.1 | 22.3 ± 5.1 | 74.9 ± 46.4 |
| attack latency (s) | F3,45 = 1.19; p < 0.32 | 114.7 ± 57.3 | 53.6 ± 18.7 | 17.8 ± 3.5 | 80.6 ± 31.4 |
| total activity (s) | F3,45 = 2.31; p < 0.90 | 379.5 ± 34.2 | 372.5 ± 27.6 | 272.3 ± 35.1 | 293.5 ± 44.1 |
4. Discussion
Our study suggests that male white-footed mice have the necessary neural and physiological machinery to enhance their fighting performance and develop a robust winner effect in response to accruing social victories. However, these males appear to lack the ability to naturally activate this machinery by releasing T into the bloodstream after a male–male challenge. We believe this explains why this species does not ordinarily form a robust winner effect.
From a comparative standpoint, our findings suggest that, when induced, the white-footed mouse's winner effect phenotype is similar to that of California mice. This interpretation comes from our results, which demonstrate that the combined effects of winning experience and post-victory T trigger similar changes to antagonistic behaviour in white-footed mice as they do to California mice [10,12,13].
Collectively, our data support the hypothesis that differences in the winner effect between these two species of Peromyscus have resulted from evolution of the internal mechanisms that govern post-victory T release.
(a). Post-victory T and plasticity in competitive behaviour
First and foremost, our study uncovers that white-footed mice maintain at least some of the latent machinery that helps individuals develop a strong winner effect. Past studies show that, under normal conditions, winning a fight does not cause males of this species to increase their circulating T levels or to enhance their capacity to win future fights [10,11]. Indeed, we confirm these results by showing that plasma T levels remain relatively steady within 1 h of winning a single aggressive encounter and that white-footed mice fail to form a robust winner effect after accumulating three separate wins that were each followed by the species-typical androgen response (i.e. no change in T). However, we demonstrate that when male white-footed mice are phenotypically engineered to experience a T pulse after each of their three separate victories, they develop a strong winner effect that lasts for multiple days. Thus, it appears as though the ‘induced’ winner effect in white-footed mice is somehow caused by an interaction between the effect of a social victory and the effect of a post-victory T surge. To this end, we can see from our data that T treatment by itself is probably not responsible for driving the full winner effect, because white-footed mice that were given T injections after handling experiences (i.e. no-win controls) failed to form a robust winner effect like white-footed mice that were given T injections after winning experiences.
The interactive effect between post-encounter T and winning experience appears to enhance elements of antagonistic performance in white-footed mice. Namely, individuals that received wins followed by T injections increased their attack efficiency and decreased their expression of losing behaviour in a competitive match. This, in effect, means that the mice become not only better at eliciting submissive behaviour from their opponents with each attack they initiate, but also better at suppressing their own expression of losing behaviour during a contest. It is therefore possible that these two types of contest behaviours heavily contribute to experience-induced plasticity in winning ability. Past research in white-footed mice supports this idea, showing that total losing behaviour is an important predictor of context-dependent flexibility in winning ability and possibly territoriality [36].
At the same time, our data suggest that the separate effects of T injections and winning experience have their own influence on select types of antagonistic behaviour. For example, results show that white-footed mice which receive T injections without winning experience exhibit a modest (albeit non-significant) reduction in total losing behaviour. This supports the intriguing idea that T is able to selectively modify neural circuits that influence an individual's display of losing or submissive behaviour in an aggressive context [31], and this effect might be intensified by cues associated with the experience of winning a fight. The notion that T and winning have selective effects on antagonistic behaviour is also supported by potential group differences in total attack behaviour, because the effect of T itself appears to increase total attacks compared with the effect of winning behaviour itself. These group differences in total attacks represent non-significant trends and therefore, should, be interpreted with caution. Future studies with larger sample sizes and increased statistical power are currently underway to tease apart the potentially selective effects that different behavioural and physiological components of winning have on future aggression.
Based on the behavioural data that we collected from both species of mouse, it seems as though administration of post-victory T in white-footed mice ‘turns on’ internal mechanisms, which produce a winner effect phenotype that is similar to the one documented in California mice [12]. Males from both species respond to victories and subsequent increases in T by experiencing a comparable increase in future winning ability. Additionally, the combination of winning and T triggers the same set of changes to attack efficiency and total losing behaviour (see above for description in white-footed mice). It is also interesting that the groups of white-footed and California mice which form a winner effect appear to increase levels of activity during aggressive disputes, unlike the other two groups. This latter result represents a non-significant trend and is therefore difficult to interpret; however, it may imply that in both species, post-victory T does not enhance winning or antagonistic behaviour by modulating rates of activity per se.
(b). Species differences in the winner effect
More broadly, our study helps reveal that evolution probably interacts with the mechanisms regulating post-victory T release in order to drive variation in the winner effect among Peromyscus rodents. If evolution had shaped these species differences by impacting a number of other neuroendocrine substrates, then T administration after a winning experience would not probably have been sufficient to induce a full winner effect in white-footed mice.
These results, however, should not be interpreted as evidence that white-footed mice are incapable of increasing T in response to social encounters. In male rodents, the release of gonadal T in response to a male–female encounter, regardless of whether mating occurs, is a hallmark physiological trait [37], and this suggests that male white-footed mice are also likely to emit a T pulse after they have a social experience with a female conspecific. Thus, we speculate that, in Peromyscus mice, evolutionary forces adjust the responsiveness of the T release system to different salient stimuli, as opposed to the overall ability to increase T itself. A recent study in birds supports this idea by showing that male black redstarts (Phoenicurus ochruros) do not normally mount a T response to male–male challenges, even though they have the physiological capacity to do so [38]. At this point, future studies need to explore whether evolutionary modification of the T release system occurs: (i) by adjusting endogenous pathways that govern gonadal T secretion (i.e. hypothalamic-pituitary-gonadal axis); (ii) by adjusting parts of the central nervous systems that integrate the perception of social experience and coordinate this information with the physiological pathways that regulate T release; or (iii) by adjusting both of these substrates.
If, in fact, evolution predominantly operates on the mechanisms that underlie T release to drive divergence in the winner effect, then this would provide compelling support for the so-called evolutionary potential hypothesis (EPH). Specifically, the EPH proposes that interspecific variation in behaviour results from selection independently acting on one component of a steroid signalling complex [2], and studies suggest that the EPH is relevant to the evolution of animal sociality [39,40]. However, to more conclusively address whether the EPH is relevant to the evolution of the winner effect, a greater understanding of the mechanisms through which androgens are detected in both white-footed and California mice is necessary. Recent work in California mice shows that males respond to social victories in the home territory by increasing androgen receptor (AR) expression in select areas of the mesolimbic system and that these changes in neural androgen sensitivity are positively associated with winning [17]. Although the persistence and functionality of this newly expressed AR are not yet clear, heightened androgen sensitivity throughout the mesolimbic system may help intensify the output and motivational elements of future aggression [41,42]. Given this, white-footed mice may naturally sustain levels of mesolimbic AR that are sufficient for post-victory T to enhance the reinforcing elements of aggression that compel mice to fight [43]. Future work must examine not only the winner effect, but also how androgen action mediates this phenomenon in white-footed mice, as well as other species of Peromyscus. Such experiments will help unravel how evolution interacts with endogenous steroid systems to guide species differences in the winner effect, and thus determine if such differences are the result of sexual or natural selection versus random effects, like drift.
5. Conclusions
In summary, our results show that by engineering white-footed mice to increase plasma T after a social victory, males that accrue winning experience form a strong winner effect. Such post-winning surges of T occur normally in California mice and contribute to the winner effect in this species, one of the white-footed mouse's congeners. Thus, white-footed mice seem to retain some of the physiological and neural architecture needed to modify antagonistic behaviour and winning ability, even though they do not normally activate these mechanisms on their own after male–male challenges. This suggests that evolutionary forces like selection potentially drive divergence in the winner effect between white-footed and California mice by interacting with the processes that control gonadal T release.
Acknowledgements
This research was approved by the University of Wisconsin–Madison Institutional Animal Use and Care Committee.
We thank K. Okpoguma and S. Mutnick for help with data collection and C. Snowdon, L. Mangiamele, K. Davidoff and J. Davidoff for comments on the manuscript. We thank D. Wittwer at the Wisconsin Primate Research Center for running hormone assays. NSF Graduate Research Fellowship (to M.J.F.), UW-Madison Zoology Department Research Grant (to M.J.F.), American Society of Mammalogists Grant-in-Aid of research (to M.J.F.), NSF grant IOS-0620042 (to C.A.M.) and the Wisconsin Alumni Research Foundation funded this research.
References
- 1.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Hau M. 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144 10.1002/bies.20524 (doi:10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- 3.Marler C. A., Moore M. C. 1988. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav. Ecol. Sociobiol. 23, 21–26 10.1007/BF00303053 (doi:10.1007/BF00303053) [DOI] [Google Scholar]
- 4.Ketterson E. D., Nolan V. 1999. Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25 10.1086/303280 (doi:10.1086/303280) [DOI] [PubMed] [Google Scholar]
- 5.Lynn S. E., Hayward L. S., Benowitz-Fredericks Z. M., Wingfield J. C. 2002. Behavioural insensitivity to supplementary testosterone during the parental phase in the chestnut-collared longspur, Calcarius ornatus. Anim. Behav. 63, 795–803 10.1006/anbe.2001.1980 (doi:10.1006/anbe.2001.1980) [DOI] [Google Scholar]
- 6.Hsu Y. Y., Earley R. L., Wolf L. L. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74 10.1017/S146479310500686X (doi:10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 7.Dugatkin L. A., Druen M. 2004. The social implications of winner and loser effects. Proc. R. Soc. Lond. B 271, S488–S489 10.1098/rsbl.2004.0235 (doi:10.1098/rsbl.2004.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guhl A. M., Collias N. E., Allee W. C. 1945. Mating behavior and the social hierarchy in small flocks of white leghorns. Physiol. Zool. 18, 365–390 [Google Scholar]
- 9.Fournier F., Fest-Bianchet M. 1995. Social dominance in adult female mountain goats. Anim. Behav. 49, 1449–1459 10.1016/0003-3472(95)90066-7 (doi:10.1016/0003-3472(95)90066-7) [DOI] [Google Scholar]
- 10.Fuxjager M. J., Marler C. A. 2010. How and why the winner effect forms: influences of contest environment and species differences. Behav. Ecol. 21, 37–45 10.1093/beheco/arp148 (doi:10.1093/beheco/arp148) [DOI] [Google Scholar]
- 11.Oyegbile T. O., Marler C. A. 2006. Weak winner effect in a less aggressive mammal: correlations with corticosterone but not testosterone. Physiol. Behav. 89, 171–179 10.1016/j.physbeh.2006.05.044 (doi:10.1016/j.physbeh.2006.05.044) [DOI] [PubMed] [Google Scholar]
- 12.Oyegbile T. O., Marler C. A. 2005. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267 10.1016/j.yhbeh.2005.04.007 (doi:10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- 13.Fuxjager M. J., Mast G., Becker E. A., Marler C. A. 2009. The ‘home advantage’ is necessary for a full winner effect and changes in post-encounter testosterone. Horm. Behav. 56, 214–219 10.1016/j.yhbeh.2009.04.009 (doi:10.1016/j.yhbeh.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 14.Oliveira R. F., Silva A., Canario A. V. M. 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects. Proc. R. Soc. B 276, 2249–2256 10.1098/rspb.2009.0132 (doi:10.1098/rspb.2009.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleason E. D., Fuxjager M. J., Oyegbile T. O., Marler C. A. 2009. Testosterone release and social context: when it occurs and why. Front. Neuroendocrin. 30, 460–469 10.1016/j.yfrne.2009.04.009 (doi:10.1016/j.yfrne.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 16.Marler C. A., Oyegbile T. O., Plavicki J., Trainor B. C. 2005. Response to Wingfield's commentary on ‘A continuing saga: the role of testosterone in aggression’. Horm. Behav. 48, 256–258 10.1016/j.yhbeh.2005.05.010 (doi:10.1016/j.yhbeh.2005.05.010) [DOI] [PubMed] [Google Scholar]
- 17.Fuxjager M. J., Forbes-Lorman R. M., Coss D. J., Auger C. J., Auger A. P., Marler C. A. 2010. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc. Natl Acad. Sci. USA 107, 12 393–12 398 10.1073/pnas.1001394107 (doi:10.1073/pnas.1001394107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlothlin J. W., Whittaker D. J., Schrock S. E., Gerlach N. M., Jawor J. M., Snajdr E. A., Ketterson E. D. 2010. Natural selection on testosterone production in a wild songbird population. Am. Nat. 175, 687–701 10.1086/652469 (doi:10.1086/652469) [DOI] [PubMed] [Google Scholar]
- 19.Voigt C., Goymann W. 2007. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii). Dev. Neurobiol. 67, 1560–1573 10.1002/dneu.20528 (doi:10.1002/dneu.20528) [DOI] [PubMed] [Google Scholar]
- 20.Hirschenhauser K., Oliveira R. F. 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277 10.1016/j.anbehav.2005.04.014 (doi:10.1016/j.anbehav.2005.04.014) [DOI] [Google Scholar]
- 21.Wingfield J. C., Hegner R. E., Dufty A. M., Ball G. F. 1990. The challenge hypothesis: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 10.1086/285134 (doi:10.1086/285134) [DOI] [Google Scholar]
- 22.Goymann W., Landys M. M., Wingfield J. C. 2007. Distinguishing seasonal androgen responses from male–male androgen responsiveness: revisiting the challenge hypothesis. Horm. Behav. 51, 463–476 10.1016/j.yhbeh.2007.01.007 (doi:10.1016/j.yhbeh.2007.01.007) [DOI] [PubMed] [Google Scholar]
- 23.Ribble D. O., Salvioni M. 1990. Social organization and nest-co-occupancy in Peromyscus californicus, a monogamous rodent. Behav. Ecol. Sociobiol. 26, 9–15 [Google Scholar]
- 24.Gubernick D. J., Teferi T. 2000. Adaptive significance of male parental care in a monogamous mammal. Proc. R. Soc. Lond. B 267, 147–150 10.1098/rspb.2000.0979 (doi:10.1098/rspb.2000.0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff J. O. 1986. Life history strategies of white-footed mice (Peromyscus leucopus). V. J. Sci. 37, 208–220 [Google Scholar]
- 26.Xia X., Millar J. S. 1991. Genetic evidence of promiscuity in Peromyscus leucopus. Behav. Ecol. Sociobiol. 28, 171–178 10.1007/2FBF00172168 (doi:10.1007/2FBF00172168) [DOI] [Google Scholar]
- 27.Trainor B. C., Bird I. M., Marler C. A. 2004. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 45, 115–121 10.1016/j.yhbeh.2003.09.006 (doi:10.1016/j.yhbeh.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 28.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 [Google Scholar]
- 29.Zar J. H. 1999. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- 30.Hasen N. S., Gammie S. C. 2005. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol. Behav. 84, 681–695 10.1016/j.physbeh.2005.02.010 (doi:10.1016/j.physbeh.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 31.Solomon M. B., Karom M. C., Norvelle A., Markham C. M., Erwin W. D., Huhman K. L. 2009. Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Horm. Behav. 56, 423–428 10.1016/j.yhbeh.2009.07.011 (doi:10.1016/j.yhbeh.2009.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman G. H., Halton J. H. 1951. Note on the exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 38, 141–149 10.2307/2F2332323 (doi:10.2307/2F2332323) [DOI] [PubMed] [Google Scholar]
- 33.Gleason E. D. 2010. Testosterone response to courtship in the male California mouse: does it predict future pair or paternal behavior? PhD dissertation, University of Wisconsin at Madison, USA [Google Scholar]
- 34.Jawor J. M., McGlothlin J. W., Casto J. M., Greives T. J., Snajdr E. A., Bentley G. E., Ketterson E. D. 2006. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen. Comp. Endocrinol. 149, 182–189 10.1016/2Fj.ygcen.2006.05.013 (doi:10.1016/2Fj.ygcen.2006.05.013) [DOI] [PubMed] [Google Scholar]
- 35.Millesi E., Hoffmann I. E., Steurer S., Metwaly M., Dittami J. P. 2002. Vernal changes in the behavioral and endocrine responses to GnRH application in male European ground squirrels. Horm. Behav. 41, 51–58 10.1006/hbeh.2001.1735 (doi:10.1006/hbeh.2001.1735) [DOI] [PubMed] [Google Scholar]
- 36.Fuxjager M. J., Montgomery J. L., Becker E. A., Marler C. A. 2010. Deciding to win: interactive effects of residency, resources and ‘boldness’ on contest outcome in white-footed mice. Anim. Behav. 80, 921–927 10.1016/j.anbehav.2010.08.018 (doi:10.1016/j.anbehav.2010.08.018) [DOI] [Google Scholar]
- 37.Nyby J. G. 2008. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Front. Neuroendocrin. 29, 199–210 10.1016/j.yfrne.2007.09.001 (doi:10.1016/j.yfrne.2007.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apfelbeck B., Goymann W. In press Ignoring the challenge? Male black redstarts (Phoenicurus ochruros) do not increase testosterone levels during territorial conflicts but they do so in response to gonadotropin-releasing hormone. Proc. R. Soc. B 278, 3233–3242 10.1098/rspb.2011.0098 (doi:10.1098/rspb.2011.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cushing B. S., Perry A., Musatov S., Ogawa S., Papademetriou E. 2008. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J. Neurosci. 28, 10 399–10 403 10.1523/JNEUROSCI.1928-08.2008 (doi:10.1523/JNEUROSCI.1928-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodson J. L., Schrock S. E., Klatt J. D., Kabelik D., Kingsbury M. A. 2009. Mesotocin and nonpeptide receptors promote songbird flocking behavior. Science 325, 862–866 10.1126/science.1174929 (doi:10.1126/science.1174929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sperry T. S., Wacker D. W., Wingfield J. C. 2010. The role of androgen receptors in regulating territorial aggression in male song sparrows. Horm. Behav. 57, 86–95 10.1016/j.yhbeh.2009.09.015 (doi:10.1016/j.yhbeh.2009.09.015) [DOI] [PubMed] [Google Scholar]
- 42.Juntti S. A., Tollkuhn J., Wu M. V., Fraser E. J., Soderborg T., Tan S., Honda S., Harada N., Shah N. M. 2010. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272 10.1016/j.neuron.2010.03.024 (doi:10.1016/j.neuron.2010.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Couppis M. H., Kennedy C. H. 2008. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology 197, 449–456 10.1007/s00213-007-1054-y (doi:10.1007/s00213-007-1054-y) [DOI] [PubMed] [Google Scholar]


