Abstract
Sexual selection was proposed by Darwin to explain the evolution of male sexual traits such as ornaments and elaborate courtship displays. Empirical and theoretical studies have traditionally focused on ornaments; the reasons for the evolution of elaborate, acrobatic courtship displays remain unclear. We addressed the hypothesis that females choose males on the basis of subtle differences in display performance, indicating motor skills that facilitate survival. Male golden-collared manakins (Manacus vitellinus) perform elaborate, acrobatic courtship displays. We used high-speed cameras to record the displays of wild males and analysed them in relation to male reproductive success. Females preferred males that performed specific display moves at greater speed, with differences of tens of milliseconds strongly impacting female preference. In additional males, we recorded telemetrically the heart rate during courtship using miniature transmitters and found that courtship is associated with profoundly elevated heart rates, revealing a large metabolic investment. Our study provides evidence that females choose their mates on the basis of subtle differences in motor performance during courtship. We propose that elaborate, acrobatic courtship dances evolve because they reflect motor skills and cardiovascular function of males.
Keywords: courtship, display, mate choice, motor skill, sexual selection, manakin
1. Introduction
Darwin proposed that elaborate ornaments and courtship displays evolve because they increase reproductive success of males, showing traits that are most attractive to females [1]. According to sexual selection theory, the large tail of the peacock and the courtship dances of male birds of paradise are the result of a preference by females to mate with males that have the largest tail or perform the best dance. In recent decades, theoretical and empirical work has focused on mechanisms that link female preference and male traits. The exaggerated ornaments of males in many vertebrate and invertebrate species were the object of most studies in this respect, because it is relatively simple to obtain quantitative measures of such traits. Thus, a number of characteristics—such as the size [2], coloration [3], symmetry [4] and other physical attributes of ornaments—have been shown to be objects of female choice. However, female choice appears to operate in many species in which males have no ornamentation. In these species, the attention of researchers has focused on what Darwin called ‘vigour’ (i.e. the performance intensity of activities that require considerable energetic investments) [1]. There is a long list of courtship displays that reflect vigour, from the display flights of many bird species to loud vocalization of frogs to leg-waving in spiders (reviewed by Byers et al. [5]). Nevertheless, a large proportion of variance in reproductive success among males cannot be explained by individual differences in ornament features or display activity even when females rely solely on these traits for selecting mates [6]. Furthermore, the relationship between details of elaborate courtship dances and performance vigour is not well understood. At present, there is scarce, if any, evidence for sexual selection being the driving force for the evolution of these elaborate courtship behaviours [5].
One obvious result of courtship displays is that of making ornaments more conspicuous (i.e. signal amplification; reviewed by Candolin [7]). Multi-component signals can be detected, discriminated and memorized better than single-component signals, particularly when multiple sensory modalities are involved (reviewed by Rowe [8]). Nevertheless, in many courtship displays, movements are very rapid and acrobatic, and it seems unlikely that their only function is that of catching the attention of the observers or amplifying the signal value. In a previous study, we proposed that display performance is an indicator of sensorimotor coordination and power, and thus of condition and overall quality [9]. This hypothesis was advanced also in a recent review on female choice based on male motor performance [5]. Females might use the skill with which individual males perform challenging actions as indicators of overall performance [5]. Challenging actions are those that require high precision in motor coordination, close to the limits allowed by anatomical and physiological characteristics (e.g. [10]). In an elaborate courtship display, males employ their neuromuscular and sensory systems, and need more than just good condition. Thus, females could evaluate from motor skills not only the good general health of males, but also their developmental history. To support this hypothesis, it is necessary to demonstrate that males differ significantly in the performance of courtship displays with respect to skills, and that female choice is associated with subtle differences in the performance of male displays (see also [5]).
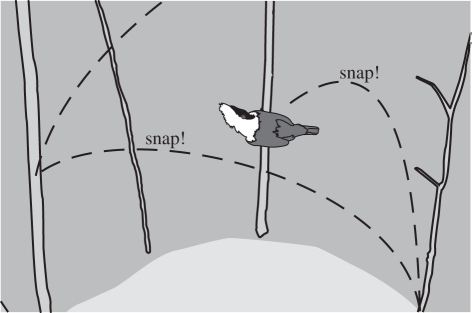
Manakins (Passeriformes; Pipridae) perform some of the most conspicuous and physically elaborate courtship dances in the animal world, including acrobatic routines and mechanically produced sounds [9,11,12]. The courtship dance of the male golden-collared manakin (Manacus vitellinus; hereafter GC manakin) is called the jump-snap display (figure 1; electronic supplementary material, movie S1). Males jump between small saplings that delimit an arena, which they clear on the forest floor. Mid-air in the jump, males powerfully flip their wings upwards to produce a loud ‘wingsnap’ and after touchdown they rapidly rotate their bodies to resume a statuary position in which they expose their erected bright yellow throat feathers (‘beard’) towards the centre of the arena (‘beard up’). After observing a male displaying, a female can engage with the male (courtship success) and then copulate (mating success). GC manakins have a lek mating system where females choose among several males courting concurrently. Mating success is highly skewed in Manacus spp. and a minority of males obtain most copulation [13–15]. Because males provide no direct benefits to their mates, courtship is the only cue that females can use to choose their partner. Male plumage and the position and background of the court are important factors for female choice, but they explain only a fraction of mating success [14–16]. The elaboration and high levels of specialization of the motor patterns strongly suggest that these components of the display are subject to sexual selection as well [12]. Phylogenetic analyses indicate that the complex motor patterns of manakin courtship evolved before the morphological traits [17], which may have arisen secondarily to increase the value of the visual stimulus and/or to highlight the motor skills [5].
Figure 1.
Illustration of the courtship dance of a male golden-collared manakin: the jump-snap display. The male jumps between saplings delimiting his court and produces loud wingsnaps in mid-air. Between jumps, the male freezes briefly on the saplings (on perch) with his golden throat feathers erected (‘beard up’); see also electronic supplementary material, movie S1.
In a previous study using high-speed videography, we showed that male display performance differs significantly between male GC manakins [9]. In particular, some males were faster than others in resuming the ‘beard up’ posture at the end of each jump and made shorter intervals between jumps [9]. We hypothesized that female GC manakins choose their mates based on their motor skills as shown in the courtship dances, which might reflect overall physiological qualities of males. In this work, we studied whether male differences in display performance are related to female preference. We found that females preferred males that performed specific dance moves at greater speed, with differences of tens of milliseconds strongly impacting female preference. A parallel study on additional males showed that heart rate (recorded telemetrically with miniature transmitters) increased during courtship compared with flight and other non-courtship activities, reaching extreme levels previously recorded only in very small hummingbirds. Thus, our study shows that female GC manakins prefer males that perform their very challenging courtship displays with greater skill.
2. Methods
(a). Behavioural observations and morphological measurements
The study was conducted between February and May, the most active period of the seven-month-long breeding season (January to July), in 2006 and 2009 in the secondary forests around Gamboa, a town along the Panama Canal in the Republic of Panama (09°07′ N, 79°42′ W). All males were marked with coloured leg bands. We measured tarsus, beard and wing length, and body weight. We used the focal sample method to record behaviours of male manakins at leks. During each 30 min observation session, we documented the following behavioural variables: number of jump-snap displays; number of wingsnaps produced during each display; number of cheepoo vocalizations; and number of rollsnaps. Rollsnaps are a rapid series of wingsnaps (up to 12 wingsnaps at 50 Hz). Cheepoos and rollsnaps are produced by males perched above or close to the arena and are involved in both female attraction and male–male interactions [13]. In addition, we recorded the courtship and mating success of each male. Courtship success is the number of times a female joins a male in his display arena, a measure that in Manacus is highly correlated to mating success, the number of times a male copulates [15]. We recorded a total of 895 observation units from 31 males of four different leks.
(b). Video recordings
High-speed video recordings (125 frames per second; see the electronic supplementary material, movie S2) were obtained using a MotionMeter camera (RedLake Inc., San Diego, CA, USA). The tripod-held camera was placed about 5 m from the court and controlled remotely. Video sequences were later analysed using The Observer Video Pro 4.0 (Noldus Information Technology, Wageningen, The Netherlands) [9]. We analysed a total of 87 high-speed video sequences from 17 of the 31 males [i.e. 5.12 ± 0.32 (s.e.m.) sequences of 9.45 ± 0.51 s per male] and measured the following variables: (i) jump duration; (ii) jump speed (distance between the two saplings/duration of the jump); (iii) wingsnap rate (number of wingsnaps per second during a jump-snap display); (iv) on perch (time spent on a sapling between two jumps); and (v) beard up (time required for the bird to resume his statuary posture with the erected beard at the end of the jump, from the moment of landing to the freezing of the posture).
(c). Heart rate recordings
Heart rate was recorded telemetrically using lightweight (approx. 1 g) transmitters (Sparrow systems, Fisher, IL, USA) that emit continuous-amplitude signals that are frequency-modulated by heart muscle potentials and that have been previously validated to record the heart rate of small birds as a measure of metabolic rate [18–20]. We were able to record the heart rate of four males during displays in parallel to wingsnap activity (see the electronic supplementary material, video S3). Files were analysed using Cool Edit 2000 (Syntrillium Software, Phoenix, AZ, USA). Band-pass filtering was applied to distinguish heart rates from the continuous transmitter carrying frequency, as well as from noise produced by skeletal muscle potentials. Heart rate was calculated only when a minimum of five sequential peaks was observed; skeletal muscle potentials demonstrated much less regularity. The relationship between heart rate and metabolic rate (oxygen consumption) for this species was established in a separate study [21].
(d). Statistics
SPSS Base 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. For behavioural analyses, we used individual mean values of each variable, and we used z-scores to normalize the data. We excluded the morphological variables of six males that had been ringed in previous years to reduce measurement error. Principal component analysis of courtship success and mating success was applied to extract a single factor (display success) with an eigenvalue of 1.889 that explained 94.5 per cent of the variance, and was positively associated with mating (0.972) and courtship success (0.972). Using display success as the dependent variable, step-wise multiple regression analyses were used to investigate which dance variables best predict male reproductive success.
3. Results
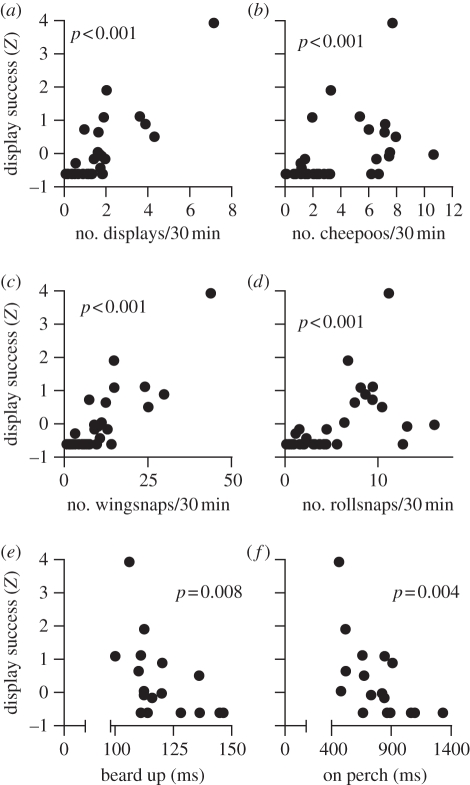
Female preference was strongly positively correlated with all measures of display activity (table 1 and figure 2). Males performing jump-snap displays, wingsnaps, rollsnaps and producing cheepoo vocalizations at higher frequency had a higher display success, an index that explains 94.5 per cent of courtship and mating success (figure 2a–d and table 1). Female preference was associated not only with display activity but also with the performance of specific moves of the jump-snap display. Males differed in these behaviours in the order of tens or hundreds of milliseconds (range of beard up: 0.100–0.146 s; range of on perch: 0.463–1.327 s). In particular, females preferred males who were fractions of a second faster in executing dance moves such as beard up and on perch (the time the beard up position was held), and produced more wingsnaps per second (figure 2e,f and table 1). The significance of the tests did not change substantially if the very successful male with a display success of 3.98 was removed from the analyses. Similarly, all tests with the exception of wingsnap rate remained significant after applying a serial Bonferroni's correction [22] (table 1). We found no significant correlation between morphological variables and display success (table 1).
Table 1.
Correlations matrix (Spearman, non-parametric) of display success with courtship and morphological variables. The rightmost column reports the significance of the test after application of serial Bonferroni's correction.
| variable | rS | n | p | corrected significance |
|---|---|---|---|---|
| display activity | ||||
| display frequency | 0.711 | 31 | <0.001 | S |
| wingsnap frequency | 0.756 | 31 | <0.001 | S |
| rollsnap frequency | 0.642 | 31 | <0.001 | S |
| cheepoo frequency | 0.562 | 31 | <0.001 | S |
| display choreography | ||||
| jump duration | 0.103 | 17 | 0.695 | n.s. |
| jump speed | −0.192 | 17 | 0.461 | n.s. |
| on perch | −0.663 | 17 | 0.004 | S |
| beard up | −0.620 | 17 | 0.008 | S |
| wingsnap rate | 0.526 | 17 | 0.030 | n.s. |
| morphology | ||||
| tarsus length | 0.101 | 27 | 0.615 | n.s. |
| beard length | 0.037 | 27 | 0.855 | n.s. |
| wing length | 0.275 | 27 | 0.164 | n.s. |
| body mass | 0.309 | 27 | 0.117 | n.s. |
Figure 2.
(a–d) Scatterplots of display activity variables versus display success, an index of reproductive success. (e,f) Scatterplots of two display choreography variables, beard up and on perch, versus display success. Among display activity variables, the wingsnap frequency was the best predictor of a male's display success in a regression analysis. On perch was the best predictor of a male's display success in a regression analysis including display choreography variables. The p-value refers to Spearman non-parametric correlation coefficients. See table 1 and text for details of the statistical analyses.
Multiple regression analyses were performed using display success as the dependent variable to determine which variables best predict male success. Using step-wise regression models for courtship activity (n = 31) with display frequency, wingsnap frequency, rollsnap frequency and cheepoo frequency as independent variables, the best model explaining (adjusted R2) 69.7 per cent of display success was obtained with a single predictor variable: the wingsnap frequency, which accounted for 84.1 per cent of the model predictive capacity (β; p < 0.001). Using courtship choreography variables jump speed, jump duration, beard up, on perch and wingsnap rate as independent variables, step-wise multiple regression analyses showed that the best model for predicting female preference included on perch alone as the predictor variable (adjusted R2, 31.9%; β = 60.1%; n = 17; p < 0.05). Differences in courtship between males did not depend on females: most displays (greater than 90%) were recorded when no female was in the court, and female presence during the display does not influence choreography variables [23].
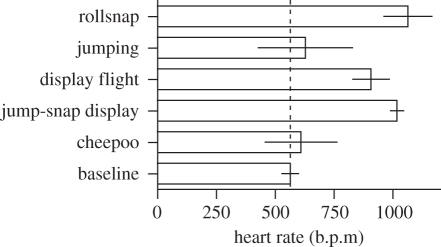
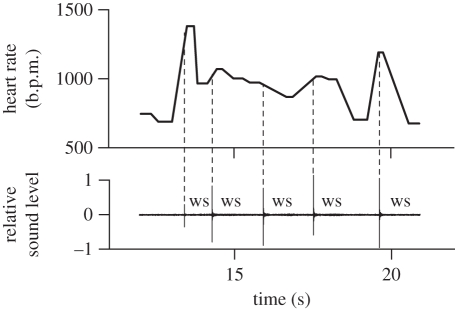
The velocity and the challenging moves of the courtship dance suggested that besides accurate neuromuscular coordination, it has high metabolic costs. We were able to record telemetrically the heart rate of four males during the courtship displays (see the electronic supplementary material, movie S3). An average daytime baseline heart rate of 563 ± 35 b.p.m. (mean ± s.e.m.) was recorded in males perched at the lek but not actively displaying. Heart rate nearly doubled during displaying, increasing significantly to 1017 ± 28 b.p.m. (figure 3). The production of wingsnaps in particular appears to be the most energetically demanding action during the jump-snap display, with a maximum recorded heart rate of 1374 b.p.m. (figure 4). An increase in heart rate from 563 to 1017 b.p.m. corresponds to an increase in metabolic rate from 60 to 120 kJ d−1 [21]. Thus, courting in manakins is energetically very expensive, and this is indicated also by a significant 18 per cent decrease in relative body mass (t = 8.79, d.f. = 6, p = 0.001) over the course of six to seven weeks of daily courting activity.
Figure 3.
The heart rate of male GC manakins doubles during courtship dances. Mean (±s.e.m.) heart rate of males recorded telemetrically during various daily activities. In baseline, the bird is perching or moving around the lek; in cheepoo, the male emits vocalizations; in jump-snap display, he jumps between the saplings delimiting his court, producing loud wingsnaps; in display flight, he performs a ‘noisy’ display flight near his court; in jumping, the bird jumps between the saplings of his court but he does not produce wingsnaps; in rollsnap, the bird produces a mechanical trill-like sound using the wings. The dashed line indicates the daytime baseline.
Figure 4.
The heart rate of male GC manakins increases to known vertebrate maxima during the acrobatic courtship display. The lower panel shows the sound recording of a jump-snap display in the field. Vertical spikes are single wingsnaps (ws): powerful sounds of the wings produced mid-air as males jump between saplings during their courtship display. The upper panel shows the heart rate of manakins recorded telemetrically during the same display. The dotted vertical lines indicate the occurrence of wingsnaps during the displays. At the beginning of the display, the heart rate rose from approximately 750 b.p.m. to approximately 1000 b.p.m., reaching peaks of 1374 b.p.m. during wingsnaps.
4. Discussion
Current theories of sexual selection state that, in the absence of direct benefits, females choose genetically superior males that will provide higher reproductive success and/or viability to the offspring [24–26]. In lekking species such as the GC manakin, males provide no parental investment; therefore, females can only rely on the courtship to assess potential mates. Typically, females assess male quality by examining indicators that signal costs borne by males, such as high levels of testosterone, parasite load and increased risk of predation [27,28]. Studies of other species have related display intensity to mating success; good indicators of success include the roar intensity of red deer [29], the calling rate of crickets [30], leg-raising rate of wolf spiders [31] and courtship display rate of greater prairie chickens [4]. Our work adds that acrobatic displays and motor skills are important indicators of male quality by providing novel evidence that female choice is associated with subtle differences in display performance in males.
Previous work on Manacus manacus found no correlation between displaying behaviour and mating success [14]. However, the investigators did not focus on courtship performance and observations were interrupted during females' visits [14]. The present study confidently illustrates very strong correlations between displaying activity and mating success in manakins. This is not a surprising result as the extreme specialization of manakin courtship appears to result from strong, ongoing sexual selection [12,17]. Thus, the intensity of displaying activity joins other courtship traits that have been shown to be associated with female choice in GC manakins, such as the colour of the plumage [15] and the contrast of the bird with the arena's background [16].
Courtship choreography may highlight morphological traits and thus enhance male conspicuousness [32]. Slow-motion analysis of M. vitellinus displays shows that males turn their brightly coloured collars towards the centre of the arena at the end of each jump—the beard up move [9]. This action might increase the apparent size of the beard (i.e. the beard is closer to the female) and its visibility. However, Prum [17] suggested that in manakins morphological specialization might be evolved to increase the visibility of motor patterns, and not vice versa. The duration of beard up shows a strong negative correlation with display success: the shorter the time required to restore the position after a jump, the higher the success. The examination of the jump-snap display at slow motion suggests indeed that the action of turning towards the court's centre at the end of each jump is one of the most demanding moves of the display. The whole action resembles the landing of a gymnast after a routine, technically defined as the reduction of total body momentum at touchdown [33]. In humans, such a task presents a significant challenge to the neuromuscular system [33]. Clearly, similar challenges are encountered by male manakins. The variable that regression analyses indicated as the best predictor of male success among choreography variables—on perch—reflects another obvious challenging limit to display performance, because males, after resuming the beard-up posture, need to reload the leg muscles to spring into the next jump. Altogether, our data support our hypothesis that the choreographic performance of the jump-snap display indicates to females males' sensorimotor coordination and power, and thus condition and overall quality [9]. They also provide novel evidence in support of the more general hypothesis that females might use the skill with which individual males perform challenging actions as indicators of overall performance [5].
Our results suggest that females visually discriminate differences in the male dance performance of tens to hundreds of milliseconds. The capacity of females to discriminate slight differences in male-choreographed motor patterns (dances) has previously been shown only in humans [34]. However, some data suggested that animals, and in particular manakins, have similar capacities. First, there is ample evidence that animals can discriminate slight differences in motor performance in contexts other than courtship (e.g. [5]). Second, flying species need to integrate information at a higher rate than other species [35], and in pigeons and domestic fowls, the critical fusion rate (the frequency at which a flickering light source is perceived as continuous) is two to three times higher than in humans (reviewed by Maddocks et al. [36]). Manakins are known for the quickness of their movements in the forest—presumably an adaptation for avoiding predation. Thus, it is not surprising that female manakins can discriminate male courtship moves differing by only fractions of seconds.
The other major finding of the present study was that courtship activity in manakins is accompanied by transient, exceptional increases in metabolic rate reflected in extraordinarily high heart rates, such as are recorded in tiny hummingbirds during hovering flight [37]. There is no consensus that energy expenditure is a cost evaluated by females, because high metabolic rate is found to be related to reproductive success in some species [38,39] but not in others [40]. However, conventional techniques to measure metabolic rate, such as body weight changes and doubly labelled water, lack fine temporal resolution [41,42]. Heart rate, on the contrary, provides a real-time measure of energy demand. This is one of the first studies in which heart rate and thus direct energy expenditure were measured during the performance of an elaborate, acrobatic courtship display, and we have shown that energy expenditure doubles during courtship compared with other activities performed immediately before and after the display. Therefore, manakin displays show off the energetic investment and the cardio-muscular capacity of the performer.
In conclusion, our study shows that female choice in GC manakins is associated with subtle differences in male motor performance during courtship displays—a challenging action for the nervous, neuromuscular and cardiac systems. We propose that elaborate, acrobatic courtship dances evolve because they reflect motor skills and cardiovascular function of males.
Acknowledgements
This research was supported by a National Geographic Society grant to L.F. and by NSF-IBN-021319 to B.A.S. We thank the Smithsonian Tropical Research Institute for support in Panama. Virginia Belloni, Francesca Coccon, Mileyka Santos and Giovanni Terranova helped with the fieldwork. We thank Tommaso Pizzari, Andrea Pilastro and Greg Grether for their comments on these studies.
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 2.Andersson S. 1992. Female preference for long tails in lekking jackson widowbirds—experimental evidence. Anim. Behav. 43, 379–388 10.1016/S0003-3472(05)80098-3 (doi:10.1016/S0003-3472(05)80098-3) [DOI] [Google Scholar]
- 3.Hamilton W. D., Zuk M. 1982. Heritable true fitness and bright birds—a role for parasites. Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 4.Nooker J. K., Sandercock B. K. 2008. Phenotypic correlates and survival consequences of male mating success in lek-mating greater prairie-chickens (Tympanuchus cupido). Behav. Ecol. Sociobiol. 62, 1377–1388 10.1007/s00265-008-0566-8 (doi:10.1007/s00265-008-0566-8) [DOI] [Google Scholar]
- 5.Byers J., Hebets E., Podos J. 2010. Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778 10.1016/j.anbehav.2010.01.009 (doi:10.1016/j.anbehav.2010.01.009) [DOI] [Google Scholar]
- 6.Hoglund J., Alatalo R. V. 1995. Leks. Monographs in Behavior and Ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Candolin U. 2003. The use of multiple cues in mate choice. Biol. Rev. 78, 575–595 10.1017/S1464793103006158 (doi:10.1017/S1464793103006158) [DOI] [PubMed] [Google Scholar]
- 8.Rowe C. 1999. Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921. 10.1006/anbe.1999.1242 (doi:10.1006/anbe.1999.1242) [DOI] [PubMed] [Google Scholar]
- 9.Fusani L., Giordano M., Day L., Schlinger B. 2007. High-speed video analysis reveals individual variability in the courtship displays of male golden-collared manakins. Ethology 113, 964–972 10.1111/j.1439-0310.2007.01395.x (doi:10.1111/j.1439-0310.2007.01395.x) [DOI] [Google Scholar]
- 10.Clark C. J. 2009. Courtship dives of Anna's hummingbird offer insights into flight performance limits. Proc. R. Soc. B 276, 3047–3052 10.1098/rspb.2009.0508 (doi:10.1098/rspb.2009.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman F. M. 1935. The courtship of Gould's manakin (Manacus vitellinus vitellinus) on Barro Colorado Island, Canal Zone. Am. Mus. Nat. Hist. Bull. 68, 472–521 [Google Scholar]
- 12.Prum R. O. 1998. Sexual selection and the evolution of mechanical sound production in manakins (Aves: Pipridae). Anim. Behav. 55, 977–994 10.1006/anbe.1997.0647 (doi:10.1006/anbe.1997.0647) [DOI] [PubMed] [Google Scholar]
- 13.Lill A. 1974. Sexual behaviour of the lek forming white-bearded manakin, M. manacus trinitatis. Z. Tierpsychol. 36, 1–36 10.1111/j.1439-0310.1974.tb02126.x (doi:10.1111/j.1439-0310.1974.tb02126.x) [DOI] [PubMed] [Google Scholar]
- 14.Shorey L. 2002. Mating success on white-bearded manakin (Manacus manacus) leks: male characteristics and relatedness. Behav. Ecol. Sociobiol. 52, 451–457 10.1007/s00265-002-0540-9 (doi:10.1007/s00265-002-0540-9) [DOI] [Google Scholar]
- 15.Stein A. C., Uy J. A. C. 2006. Plumage brightness predicts male mating success in the lekking golden-collared manakin, Manacus vitellinus. Behav. Ecol. 17, 41–47 10.1093/beheco/ari095 (doi:10.1093/beheco/ari095) [DOI] [Google Scholar]
- 16.Uy J. A. C., Endler J. A. 2004. Modification of the visual background increases the conspicuousness of golden-collared manakin display. Behav. Ecol. 15, 1003–1015 10.1093/beheco/arh106 (doi:10.1093/beheco/arh106) [DOI] [Google Scholar]
- 17.Prum R. O. 1990. Phylogenetic analysis of the evolution of display behaviour in the neotropical manakins (Aves: Pipridae). Ethology 84, 202–231 10.1111/j.1439-0310.1990.tb00798.x (doi:10.1111/j.1439-0310.1990.tb00798.x) [DOI] [Google Scholar]
- 18.Bisson I.-A., Butler L. K., Hayden T. J., Romero L. M., Wikelski M. C. 2009. No energetic cost of anthropogenic disturbance in a songbird. Proc. R. Soc. B 276, 961–969 10.1098/rspb.2008.1277 (doi:10.1098/rspb.2008.1277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochran W. W., Wikelski M. 2005. Individual migratory tactics of New World Catharus thrushes: current knowledge and future tracking options from space. In Birds of two worlds: the ecology and evolution of migration (eds Greenberg R., Marra P. P.), pp. 274–289 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 20.Steiger S. S., Kelley J. Â. P., Cochran W. W., Wikelski M. 2009. Low metabolism and inactive lifestyle of a tropical rain forest bird investigated via heart rate telemetry. Physiol. Biochem. Zool. 82, 580. 10.1086/605336 (doi:10.1086/605336) [DOI] [PubMed] [Google Scholar]
- 21.Barske J., Wikelski M., Fusani L., Schlinger B. A. In preparation. Metabolic rate of reproductively active male golden-collared manakins assessed by heart-rate telemetry. [Google Scholar]
- 22.Rice W. R. 1989. Analyzing tables of statistical tests. Evolution 43, 223–225 10.2307/2409177 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- 23.Barske J., Schlinger B. A, Fusani L. In preparation. Choreography of male courtship with and without females. [Google Scholar]
- 24.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 25.Fisher R. A. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press [Google Scholar]
- 26.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 27.Folstad I., Karter A. J. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622 10.1086/285346 (doi:10.1086/285346) [DOI] [Google Scholar]
- 28.Møller A. P., Petrie M. 2002. Condition dependence, multiple sexual signals, and immunocompetence in peacocks. Behav. Ecol. 13, 248–253 10.1093/beheco/13.2.248 (doi:10.1093/beheco/13.2.248) [DOI] [Google Scholar]
- 29.Charlton B. D., Reby D., McComb K. 2007. Female red deer prefer the roars of larger males. Biol. Lett. 3, 382–385 10.1098/rsbl.2007.0244 (doi:10.1098/rsbl.2007.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt J., Brooks R., Jennions M. D., Smith M. J., Bentsen C. L., Bussiere L. F. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027 10.1038/nature03084 (doi:10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 31.Hoefler C. D., Persons M. H., Rypstra A. L. 2008. Evolutionarily costly courtship displays in a wolf spider: a test of viability indicator theory. Behav. Ecol. 19, 974–979 10.1093/beheco/arn055 (doi:10.1093/beheco/arn055) [DOI] [Google Scholar]
- 32.Johnsgard P. A. 1994. Arena birds: sexual selection and behavior. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 33.McNitt-Gray J. L., Hester D. M., Mathiyakom W., Munkasy B. A. 2001. Mechanical demand and multijoint control during landing depend on orientation of the body segments relative to the reaction force. J. Biomech. 34, 1471–1482 10.1016/S0021-9290(01)00110-5 (doi:10.1016/S0021-9290(01)00110-5) [DOI] [PubMed] [Google Scholar]
- 34.Neave N., McCarty K., Freynik J., Caplan N., Hoenekopp J., Fink B. 2011. Male dance moves that catch a woman's eye. Biol. Lett. 7, 221–224 10.1098/rsbl.2010.0619 (doi:10.1098/rsbl.2010.0619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kare M. R. 1965. The special senses: the eye and vision. In Avian physiology (ed. Sturkie P. D.), pp. 407–418 Ithaca, NY: Cornell University Press [Google Scholar]
- 36.Maddocks S. A., Goldsmith A. R., Cuthill I. C. 2001. The influence of flicker rate on plasma corticosterone levels of European starlings, Sturnus vulgaris. Gen. Comp. Endocrinol. 124, 315–320 10.1006/gcen.2001.7718 (doi:10.1006/gcen.2001.7718) [DOI] [PubMed] [Google Scholar]
- 37.Bishop C. M. 1997. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Phil. Trans. R. Soc. Lond. B 352, 447–456 10.1098/rstb.1997.0032 (doi:10.1098/rstb.1997.0032) [DOI] [Google Scholar]
- 38.Hoglund J., Kalas J. A., Fiske P. 1992. The costs of secondary sexual characters in the lekking great snipe (Gallinago media). Behav. Ecol. Sociobiol. 30, 309–315 10.1007/BF00173942 (doi:10.1007/BF00173942) [DOI] [Google Scholar]
- 39.Vehrencamp S. L., Bradbury J. W., Gibson R. M. 1989. The energetic cost of display in male sage grouse. Anim. Behav. 38, 885–896 10.1016/S0003-3472(89)80120-4 (doi:10.1016/S0003-3472(89)80120-4) [DOI] [Google Scholar]
- 40.Hurd P. L. 2004. Conventional displays: evidence for socially mediated costs of threat displays in a lizard. Aggr. Behav. 30, 326–341 10.1002/ab.20020 (doi:10.1002/ab.20020) [DOI] [Google Scholar]
- 41.Bevan R. M., Speakman J. R., Butler P. J. 1995. Daily energy-expenditure of tufted ducks—a comparison between indirect calorimetry, doubly labeled water and heart-rate. Funct. Ecol. 9, 40–47 10.2307/2390088 (doi:10.2307/2390088) [DOI] [Google Scholar]
- 42.Butler P. J., Green J. A., Boyd I. L., Speakman J. R. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168–183 10.1111/j.0269-8463.2004.00821.x (doi:10.1111/j.0269-8463.2004.00821.x) [DOI] [Google Scholar]