Abstract
Unlike most animals studied so far in which the activity with no circadian rhythms is pathological or linked to deteriorating performance, worker bees and ants naturally care for their sibling brood around the clock with no apparent ill effects. Here, we tested whether bumble-bee queens that care alone for their first batch of offspring are also capable of a similar chronobiological plasticity. We monitored locomotor activity of Bombus terrestris queens at various life cycle stages, and queens for which we manipulated the presence of brood or removed the ovaries. We found that gynes typically emerged from the pupae with no circadian rhythms, but after several days showed robust rhythms that were not affected by mating or diapauses. Colony-founding queens with brood showed attenuated circadian rhythms, irrespective of the presence of ovaries. By contrast, queens that lost their brood switched again to activity with strong circadian rhythms. The discovery that circadian rhythms in bumble-bee queens are regulated by the life cycle and the presence of brood suggests that plasticity in the circadian clock of bees is ancient and related to maternal behaviour or physiology, and is not a derived trait that evolved with the evolution of the worker caste.
Keywords: circadian rhythms, maternal behaviour, plasticity, Bombus terrestris, social evolution
1. Introduction
Worker bumble-bees (Bombus terrestris), honeybees (Apis mellifera) and harvester ants (Pogonomyrmex occidentalis) show remarkable chronobiological plasticity that is associated with the division of labour that organizes their society. Workers that care for the brood (‘nurses’) are active around the clock with no circadian rhythms in behaviour or clock gene expression, whereas foragers have strong behavioural and molecular rhythms and a consolidated nightly rest [1–7]. Honeybee nurses are active around the clock even when exposed to a synchronizing light signal, and foragers continue to exhibit strong circadian rhythms when placed in constant conditions, suggesting that the variation in the environment experienced by foragers (alternating light : dark regime) and nurses (mostly constant conditions) cannot account for task-related plasticity in circadian rhythms (reviewed in [8]). The evidence for chronobiological plasticity in taxonomically distinct species (bees and ants) and in species differing in the organization of the division of labour (age-related in honeybees versus size-related in bumble-bees) is consistent with the hypothesis that this form of plasticity is functionally significant because it improves task specialization and colony efficiency. Around-the-clock activity is assumed to improve brood care, whereas a functioning circadian clock enables foragers to time their visits to flowers and rely on the constantly moving sun as a compass for orientation [8]. The lack of circadian rhythms in nurse bees and ants and in a few other species (e.g. [9–11]) contrasts with evidence from most animals studied so far, including humans, in which around-the-clock activity is accompanied by increased pathologies and deterioration in performance [12].
Traits that appear to be specific to the worker caste, such as those associated with the division of labour, are commonly thought to represent a derived stage in the evolution of sociality. However, given the strong association between around-the-clock activity and brood care [5,7], we wondered whether mother bees that need to nurse their offspring brood exhibit a similar plasticity in circadian rhythms, as workers do. Egg-laying queens of ants [13–15] and honeybees [16–18] are indeed active around the clock. However, the queens of these species are highly derived; they specialize in reproduction, and engage in little, if any, brood care. Their around-the-clock activity can be therefore better explained by selection acting to improve fecundity rather than care for the brood. On the other hand, bumble-bees such as B. terrestris provide an excellent model with which to test the hypothesis that plasticity in circadian rhythms is anciently linked to brood care behaviour because their queens care alone for the first batch of brood. Newly emerged virgin gynes mate in the late summer, and spend the winter in diapause. In the spring, each queen finds a nest site, collects food, lays eggs and cares for the first batches of brood. When the first workers emerge, they gradually take over most brood care duties allowing the queen to devote her time to other activities such as reproduction and policing [19].
We found that bumble-bee queens indeed exhibit brood care-related plasticity in circadian rhythms. Since the ovaries and the steroid hormones they release have profound influences on circadian rhythms in both mammals and insects [20–23], we next tested whether plasticity in circadian rhythms in bumble-bee queens is modulated by the queen's highly active ovaries. We found that in the presence of brood, queens are active around the clock even in the absence of ovaries. These findings unveiled profound brood-related plasticity in circadian rhythms of bumble-bee queens that is similar to plasticity in circadian rhythms of nurse workers.
2. Material and methods
(a). General procedures
For the locomotor activity monitoring, we placed cages with B. terrestris queens (obtained from Polyam Pollination Services, Yad-Mordechai, Israel) in an environmental chamber (29 ± 1°C, 40–50% relative humidity (RH)). The chamber was constantly illuminated with two dim light sources (60 V each) covered by a combination of filters (106 primary red and 126 mauve; ‘Lee filters’) blocking the transmission of most light wavelengths below 650 nm. Similar dim red light illumination is commonly used for monitoring locomotor activity in circadian studies (e.g. [4,5,7,24–26]). We monitored locomotor activity and analysed circadian rhythms with the ClockLab data acquisition and analysis system (Actimetrics Co., Wilmette, IL, USA). The distance covered by a bee during a fixed time interval was used as an index for locomotor activity. Empty cages served as a control for spontaneous background noise. To determine the day of onset of rhythmicity, we performed a circadian analysis on a sliding window of overlapping 3 day intervals. The onset of rhythmicity was defined as the first day in which a periodogram analysis over two consecutive intervals produced a statistically significant rhythm with a period of 19–29 h. Additional details can be found in Yerushalmi et al. [7], and in Shemesh et al. [4,5].
(b). Experiment 1: the effect of life stage on circadian rhythms in queens
We monitored locomotor activity of queens at four different life stages: (i) virgin gynes (0–1 days of age), (ii) mated queens before diapause, (iii) mated queens after diapause, and (iv) colony-founding queens with their first batches of brood (‘queens + brood’). We performed an additional trial for testing the ontogeny of circadian rhythms in virgin gynes (‘trial 2’). In these two trials, we placed each focal queen in an individual monitoring cage made of a modified Petri dish (diameter = 90 mm, height = 30 mm), which was provisioned with commercial sugar syrup (64% w/w; obtained from Polyam Pollination Services), and a pea size ball of pollen (collected by honeybees) mixed with commercial sugar syrup. We performed all treatments under dim red light. The sample sizes were as follows: ‘virgins’: trial 1, n = 21; trial 2, n = 8; ‘before diapause’: n = 20; ‘after diapause’: n = 17 and ‘queens + brood’: n = 10. We only included in the analyses queens that had lived for 5 days or more. The per cent survival after 4 and 10 days, respectively, was: ‘virgins’: trial 1 = 100 and 64 per cent, trial 2 = 100 and 100 per cent, ‘before diapause’: 100 and 76 per cent; ‘after diapause’: 95 and 65 per cent and ‘queens + brood’: 93.5 and 29 per cent. Technical difficulties prevented us from recording activity between 02.00 and 09.39 h on the fourth day of the first trial of this experiment. We therefore did not calculate the power of rhythmicity for bees in this trial, but the data were good enough to allow us to unequivocally determine the presence of rhythmicity (electronic supplementary material, figure S1).
(c). Experiment 2: the influence of brood presence on circadian rhythms in queens
Because survival in the modified Petri dishes used in experiment 1, specifically for colony-founding queens, was relatively low (see above), we used wooden cages (12 × 7 × 4 cm) with glass side walls in experiments 2 and 3. In experiment 2, we monitored locomotor activity for three groups of queens: (i) virgin gynes, (ii) colony-founding queens with their first batches of brood (‘queens + brood’), and (iii) colony-founding queens for which we removed the first batches of brood prior to locomotor activity monitoring (‘queens−brood’). We provisioned all cages with two tubes, each containing 10 ml of commercial sugar syrup, and a cube (approx. 2 × 1 × 1 cm) of pollen (see §2b above). The sample sizes were as follows: ‘virgins’: n = 12, ‘queens + brood’: n = 15 and ‘queens−brood’: n = 17. The overall survival rate was 88–100%. The improved survival of these queens and the emergence of worker bees inside the cages of colony-founding queens indicate that this experimental set-up provided an appropriate environment for the queens. Importantly, this experimental set-up enabled high-quality data acquisition for queens, which probably showed the normal behaviour associated with nest funding and brood rearing. We performed all treatments under dim red light. We subtracted the average activity values of the empty cages from the activity data of all bees because the background noise was relatively high in this particular experiment.
(d). Experiment 3: the influence of ovariectomy and the presence of brood on circadian rhythms in queens
We used the same experimental set-up described for experiment 2 to compare the following four groups: (i) virgin gynes, (ii) ovariectomized colony-founding queens placed with their first batches of brood (‘O− B+’), (iii) sham-operated colony-founding queens placed with their first batches of brood (‘O+ B+’), and (iv) sham-operated colony-founding queens for which we removed the first batches of brood prior to the locomotor activity monitoring (‘O+ B−’). We performed three trials of this experiment and pooled the data for the analyses presented in figure 3 and table 1 (sample size for trials 1, 2 and 3, respectively: ‘virgins’: n = 0, 0 and 12; ‘O− B+’: n = 3, 3 and 3; ‘O+ B+’: n = 4, 4 and 6 and ‘O+ B−’: n = 5, 7 and 2). The survival rate was 100 per cent for virgin gynes and ‘O+ B−’ queens, 93 per cent for ‘O+ B−’ queens and 56 per cent for ‘O− B+’ queens. Ten out of the 14 queens for which we removed the brood (‘O+ B−’) had laid again during the course of the experiment. These queens were not included in the analysis presented in figure 3, but their data were analysed separately and summarized in table 1. To determine the presence of brood, we visually inspected each cage on 2–3 different days (days 6 and 10 in trial 1, days 2, 4 and 10 in trial 2 and days 4 and 10 in trial 3).
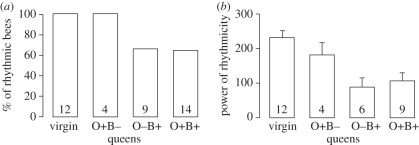
Figure 3.
The influence of the ovaries and the presence of brood on circadian rhythms in locomotor activity in queens. (a) The percentage of queens with circadian rhythms in locomotor activity during days 1–5 (Pearson χ2, p = 0.02). (b) The power of circadian rhythms in locomotor activity for rhythmic bees (mean ± s.e.) during days 1–5 (Kruskal–Wallis test, p = 0.006). Numbers inside bars depict sample size. The data for 10 additional queens that laid again after their brood was removed are summarized in table 1. Other details as in figure 1.
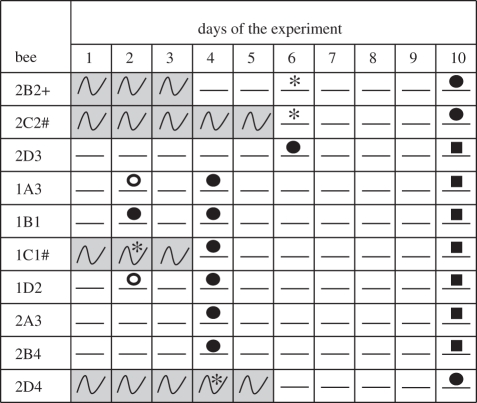
Table 1.
Circadian rhythms and brood presence for colony-founding queens with ovaries that had laid again following brood removal (experiment 3). (White boxes with a straight line—days with no circadian rhythms in locomotor activity; grey boxes with a sinus sign—days with circadian rhythms in locomotor activity. The presence of brood was monitored during the course of the experiment. An asterisk indicates an inspection in which no brood was present; an empty circle indicates an inspection in which the queen was observed constructing egg cells; a filled circle indicates an inspection in which sealed egg cups were present; a filled square indicates an inspection in which sealed larvae cells were present. A plus symbol indicates a queen for which an actogram is presented in figure 2 and a hash indicates a queen for which an actogram is presented in the electronic supplementary material, figure S2.)
 |
For ovariectomy and sham operation, we used the following procedures. Colony-founding queens were anaesthetized on ice for approximately 20 min and harnessed on an ice-cold metal mould. We made two small incisions in the abdominal membrane between the fourth and the fifth sternites from which we pulled out the ovaries with fine forceps. Following the operation, the queens were reintroduced to their cages and placed in an incubator (31 ± 1°C, approx. 70% RH) for 1 day for recovery, before being transferred to the locomotor activity monitoring chambers. Sham-operated (control) queens were treated similarly but their ovaries were only touched with the forceps, and not removed. During handling and operation, the queens were exposed to the normal illumination of the laboratory for approximately 30 min.
At the end of the experiment, we dissected the ovariectomized queens and found that the entire ovaries were successfully removed for all of the queens. Importantly, both ovariectomized and sham-operated colony-founding queens successfully reared brood that pupated. The adult bees emerging from these pupae look perfectly normal, suggesting that despite the experimental procedure, the queens showed normal behaviours associated with brood rearing.
3. Results
(a). Experiment 1: the effect of life stage on circadian rhythms in queens
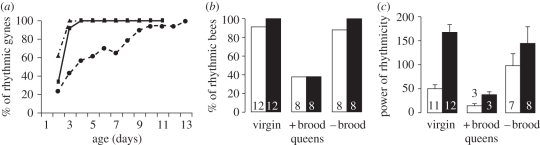
Virgin gynes typically showed an ontogeny of circadian rhythms in locomotor activity (figures 1 and 2a). The proportion of gynes with circadian rhythms (figure 1a) and their overall level of activity (see the electronic supplementary material, table S1) increased with age; all the gynes exhibited significant circadian rhythms by the end of the experiment (figure 1a; similar results were also obtained for gynes in experiment 3, see below). Mated queens before or after diapause were more similar to virgin gynes than to colony-founding queens in that most exhibited robust circadian rhythms in locomotor activity (see the electronic supplementary material, figure S1). This finding suggests that mating or diapause does not affect circadian rhythms in B. terrestris queens. Colony-founding queens that had brood in their cages (‘queens + brood’) had no, or only weak, circadian rhythms in locomotor activity (electronic supplementary material, figure S1). This experiment shows that the expression of circadian rhythms in B. terrestris queens changes during their normal life cycle.
Figure 1.
The effect of age and brood on circadian rhythms in locomotor activity in B. terrestris queens. (a) Ontogeny of circadian rhythms in locomotor activity for virgin gynes (experiment 1: trial 1, n = 21; trial 2, n = 8 and experiment 2, n = 12). A bee that showed significant circadian rhythms during two consecutive 3 day intervals was considered rhythmic thereafter. (b) The percentage of queens with circadian rhythms in locomotor activity, during days 1–4 (open bars) and 5–8 (filled bars) (Pearson χ2, p = 0.01 for days 1–4; p < 0.001 for days 5–8). (c) The power of circadian rhythms in locomotor activity for rhythmic bees (mean ± s.e.), during days 1–4 (open bars) and days 5–8 (filled bars; Kruskal–Wallis tests, p = 0.04 for days 1–4; p = 0.03 for days 5–8). Numbers inside bars depict sample size. The figure shows only the data for queens for which the presence of brood did not change during the course of the experiment. Data for additional queens are summarized in the electronic supplementary material, table S2. (a) Circles with dashed lines, trial 1; triangles with dashed lines, trial 2; squares with solid line, experiment 2.
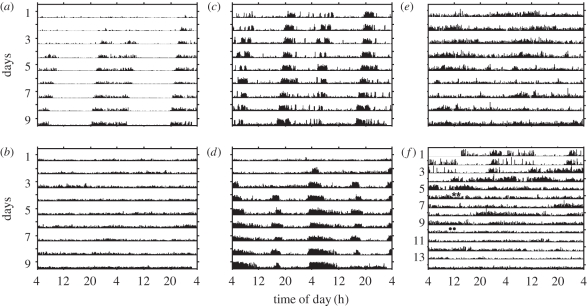
Figure 2.
Representative double-plot actograms for the locomotor activity of queens in various reproductive states. The numbers on the y-axes in each plot depict days; the height of the bars within each day corresponds to the level of locomotor activity in 10 min bins. (a) A virgin gyne. This bee developed significant circadian rhythms on day 3. (b) A colony-founding queen left with her first batches of brood. This bee is active around the clock with no circadian rhythms. (c) A colony-founding queen whose brood was removed. This bee exhibited circadian rhythms from day 1. (d) A colony-founding queen whose brood was left intact, but the brood died during the experiment. This bee exhibited significant circadian rhythms from day 3. (e) An ovariectomized colony-founding queen left with her first batches of brood. (f) A colony-founding queen that had laid again after her brood was removed. This bee exhibited significant circadian rhythms during the first 3 days, but later was active around the clock with no circadian rhythms. Asterisks indicate brood inspections in which there were no eggs found inside the cage; filled circles indicate an inspection in which eggs were observed inside the cage. (a) Experiment 2-queen 2A4; (b) experiment 2-queen 2D2; (c) experiment 2-queen 2B3; (d) experiment 2-queen 1E3; (e) experiment 3-queen 2C3 and (f) experiment 3-queen 2B2.
(b). Experiment 2: the influence of brood presence on circadian rhythms in queens
To investigate whether activity with no circadian rhythms in colony-founding queens relates to their life cycle stage or to the presence of brood in their cages, we manipulated the presence of brood in incipient colonies. As in experiment 1, colony-founding queens with brood were active around the clock with no circadian rhythms (figures 1b,c and 2b). However, nearly all the queens whose brood was removed and that did not lay eggs again, exhibited robust circadian rhythms in locomotor activity (figures 1b,c and 2c). The differences between colony-founding queens with and without brood were not owing to differences in their overall level of activity (electronic supplementary material, table S1). The free-running period of circadian rhythms was similar for virgin gynes, colony-founding queens with brood and without brood (see the electronic supplementary material, table S1).
To further test the relationship between the presence of brood and circadian rhythms, we analysed circadian rhythms of queens for which the presence of brood changed during the course of the experiment. These queens, whose data are summarized in the electronic supplementary material, table S2, were not included in the analysis presented in figure 1. Eight of the 17 queens for which we removed the brood had brood in their cages at the end of the experiment (see the electronic supplementary material, table S2), indicating that they had laid again and reared the brood after their first batches of brood were removed. In the cage of an additional queen, we found only dead larvae at the end of the experiment, indicating that she had laid again, but the developing larvae did not survive (queen 1E5 in the electronic supplementary material, table S2 and figure S2b). This queen did not show significant circadian rhythms during the first 8 days (during which she probably had brood developing in her cage), whereas on day 9 she started to exhibit strong circadian rhythms again (probably after her brood had died).
The queens that had laid again after their brood was removed were active around the clock with no circadian rhythms, and were as likely to exhibit circadian rhythms as colony-founding queens for which we did not remove the brood (only queen 1D5 had significant circadian rhythms during days 1–4, with a very weak power of 15.4; none of the queens had significant rhythms during days 5–8; Mann–Whitney test based on days 1–4: rhythm, p = 0.4; power, p = 0.3; electronic supplementary material, table S2 and figure S2a). Although we did not monitor the presence of brood during the course of this experiment, these results indicate that some queens were active around the clock in the presence of eggs, or perhaps even prior to egg-laying.
Seven of the 15 colony-founding queens for which we left the brood lost it before the end of the experiment (see the electronic supplementary material, table S2). Six of these queens switched from activity with no circadian rhythms to activity with significant circadian rhythms over the course of the experiment (see the electronic supplementary material, table S2). In the cage of the seventh queen (queen 2E3; electronic supplementary material, table S2) who did not show significant circadian rhythms, we found dead larvae at the end of the experiment, suggesting that the brood had died shortly before the inspection. This experiment establishes a strong association between the presence of brood and attenuated circadian rhythms in locomotor activity in colony-founding queens.
(c). Experiment 3: the influence of ovariectomy and the presence of brood on circadian rhythms in queens
In this experiment, we visually inspected each cage two to three times during the course of the experiment and therefore unequivocally determined the presence or absence of brood. Our observations confirmed and extended the findings of experiment 2, suggesting that the expression of circadian rhythms in locomotor activity by the queen is linked to the presence of brood or her reproductive state (table 1). Both ovariectomized and sham-operated queens that were left with their brood had no circadian rhythms in locomotor activity or their rhythms were very weak (figure 3). All of the queens for which we removed the brood but had laid again (n = 10), were active around the clock with no circadian rhythms. Importantly, four of these queens (2B2, 2C2, 1A3, and 1D2; table 1; figure 2f and electronic supplementary material, figure S2c) were active with no circadian rhythms prior to egg-laying, suggesting that the around-the-clock activity of these queens is related to their reproductive state.
We next examined whether the highly active ovaries of the queens influence circadian rhythmicity. Virgin gynes had no apparent rhythm in locomotor activity during their first day following emergence (data not shown), as in experiments 1 and 2 (figure 1), but latter developed strong circadian rhythms. Sham-operated queens with no brood (‘O+ B− queens’) also showed strong circadian rhythms in locomotor activity (figure 3 and electronic supplementary material, figure S2e). By contrast, both ovariectomized and sham-operated queens that were left with their brood (‘O− B+’ and ‘O+ B+’ queens, respectively) had no circadian rhythms in locomotor activity or their rhythms were very weak (figure 2e and electronic supplementary material, figure S2c). In addition, the queens with and without ovaries did not differ in their free-running period and overall level of activity (see the electronic supplementary material, table S3). These findings show that the ovaries are not necessary for the expression of around-the-clock activity by B. terrestris queens.
4. Discussion
Our study reveals remarkable plasticity in circadian rhythms of bumble-bee queens. Gynes typically emerge from the pupae with no circadian rhythms in locomotor activity. They develop robust circadian rhythms during the first days following emergence that persist after mating and diapause. However, queens with their first batch of brood are again active around the clock with no circadian rhythms. We further show that the queens can switch between activity with and without circadian rhythms in accordance with the presence of brood in their nest, and that this plasticity is not influenced by the ovaries.
To the best of our knowledge, this is the first study showing an ontogeny of circadian rhythms in queens of any social insect. The ontogeny of circadian rhythms is endogenous because it occurs under constant conditions and rhythms free-run with a period of about, but not exactly, 24 h. Bumble-bee and honeybee workers show a similar development of rhythm [7,27]. Solitary insects, by contrast, typically rely on their circadian clock to time their emergence from the pupa, and shortly after eclosion show circadian rhythms in locomotor activity [28–30]. A possible explanation for the apparent association between the ontogeny of circadian rhythms and sociality is that social insects emerge into a protected environment and therefore do not need to instantly adjust their behaviour and physiology to the day/night fluctuation in ambient conditions [8]. Later in life however, virgin queens probably need to rely on the circadian clock for timing their mating activity. Species-specific mating rhythms have been reported for many species of vertebrates and invertebrates and are thought to be adaptive because they coordinate the activities of males and females, reduce risky exposures to predators and save time and energy (e.g. [31–35]).
In contrast to virgin gynes and mated queens before and after diapause, colony-founding queens with brood are active around the clock, with weak or no circadian rhythms. Colony-founding queens can nonetheless show robust circadian rhythms again if their brood is removed or lost. This close association between brood care and around-the-clock activity in queens is similar to that of bumble-bee workers that care for the brood around the clock, but forage with strong circadian rhythms [7]. In honeybees, it was recently shown that direct contact with the brood is necessary for nurses to be active around the clock with no circadian rhythms [5].
What is the mechanism linking the presence of brood and around-the-clock activity in queens? One possibility is that interactions with the brood, such as brood care, modulate the circadian system of the mother queen. According to this hypothesis, the brood requires continuous care and the mother bee is highly sensitive and responsive to this need. Signals from the brood repeatedly reset the circadian clock of the queen or alter it in other ways.
Another, not mutually exclusive hypothesis is that circadian rhythmicity is modulated by the reproductive physiology of the queen. This hypothesis can account for the observations that queens with eggs but no larvae, and even queens just before egg-laying are active around the clock with no circadian rhythms (figure 2f and electronic supplementary material, figure S2c; table 1 and electronic supplementary material, table S2). Thus, begging signals from the larvae or the act of food provision are not compulsory for inducing around-the-clock activity in mother queens.
The ovaries are obvious candidates for this influence because in both mammals and insects, ovariectomy has diverse and significant effects on circadian rhythms. Moreover, in many studies, the modifications caused by ovariectomy were at least partially reversed by treatment with ovarian steroid hormones [20–23]. In cockroaches, there is evidence suggesting that active ovaries may mask circadian rhythms; mated females show low levels of activity with no circadian rhythms but start to exhibit strong circadian rhythms following ovary removal [21]. In addition, bumble-bee nurses that are active around the clock typically have more developed ovaries compared with foragers [36–38]. Even in honeybee workers, in which the ovaries are typically at a basal state, there is evidence that the division of labour is influenced by a reproductive regulatory network that includes the ovaries [39,40]. Our overiectomy experiments, however, indicate that in bumble-bee queens the ovaries, or the endocrine signals they release (e.g. ecdysteroids, [41]), are not necessary for the queen to be active around the clock. Ovariectomized queens were indistinguishable from sham-operated and intact queens; queens from all these groups had no, or only weak, circadian rhythms in the presence of brood (figure 3). Additional studies are needed for identifying the physiological factors regulating plasticity in circadian rhythms in queens. Nevertheless, our study suggests that multiple factors including the presence of brood and the behaviour or physiology associated with egg cup construction, can induce queens to be active around the clock; both queens with no ovaries but with brood (‘O− B+’ queens in experiment 3) and queens before egg-laying (table 1, figure 2f and electronic supplementary material, figure S2c) were active around the clock with no circadian rhythms.
Our findings that mother bees are capable of activity with no circadian rhythms indicate that brood-related plasticity in circadian rhythms is not a derived trait limited to the worker caste. Rather, around-the-clock activity in nurse bees caring for sibling brood can stem from the same ancient pathways linking maternal physiology and the circadian system and allowing mother bees to care for their young around the clock. This explanation is consistent with the hypothesis that nursing behaviour in workers evolved from maternal traits of solitary insects [42–44]. Interestingly, an association between maternal care and around-the-clock activity was also reported for killer-whales and bottlenose-dolphins in which neonates and their mothers are active around the clock for the first postpartum month [9]. Thus, the interplay between maternal behaviour or physiology and the circadian system may be more common than previously thought. The adaptive value of this association is perhaps that around-the-clock activity enables better care during crucial stages of offspring development. In social insects, around-the-clock activity of queens and workers may be further selected to allow for fast population growth despite the fact that only a single or a few females produce all the workers, which are necessary for colony growth and reproduction.
Acknowledgements
We thank three anonymous reviewers for their excellent comments on previous versions of this manuscript. This research project was funded by the US-Israel Binational Science Foundation (BSF, no. 2007465), and the Israel Science Foundation (ISF, no. 452/07, and no. 606/02) to G.B.
References
- 1.Bloch G., Toma D. P., Robinson G. E. 2001. Behavioural rhythmicity, age, division of labour and period expression in the honey bee brain. J. Biol. Rhythms 16, 444–456 10.1177/074873001129002123 (doi:10.1177/074873001129002123) [DOI] [PubMed] [Google Scholar]
- 2.Bloch G., Rubinstein C. D., Robinson G. E. 2004. Period expression in the honey bee brain is developmentally regulated and not affected by light, flight experience, or colony type. Insect Biochem. Mol. Biol. 34, 879–891 10.1016/j.ibmb.2004.05.004 (doi:10.1016/j.ibmb.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 3.Ingram K. K., Krummey S., LeRoux M. 2009. Expression patterns of a circadian clock gene are associated with age-related polyethism in harvester ants, Pogonomyrmex occidentalis. BMC Ecol. 9, 7. 10.1186/1472-6785-9-7 (doi:10.1186/1472-6785-9-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shemesh Y., Cohen M., Bloch G. 2007. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 21, 2304–2311 10.1096/fj.06-8032com (doi:10.1096/fj.06-8032com) [DOI] [PubMed] [Google Scholar]
- 5.Shemesh Y., Eban-Rothschild A., Cohen M., Bloch G. 2010. Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J. Neurosci. 30, 12 517–12 525 10.1523/JNEUROSCI.1490-10.2010 (doi:10.1523/JNEUROSCI.1490-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toma D. P., Bloch G., Moore D., Robinson G. E. 2000. Changes in period mRNA levels in the brain and division of labour in honey bee colonies. Proc. Natl Acad. Sci. USA 97, 6914–6919 10.1073/pnas.97.12.6914 (doi:10.1073/pnas.97.12.6914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yerushalmi S., Bodenhaimer S., Bloch G. 2006. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 209, 1044–1051 10.1242/jeb.02125 (doi:10.1242/jeb.02125) [DOI] [PubMed] [Google Scholar]
- 8.Bloch G. 2010. The social clock of the honeybee. J. Biol. Rhythms 25, 307–317 10.1177/0748730410380149 (doi:10.1177/0748730410380149) [DOI] [PubMed] [Google Scholar]
- 9.Lyamin O., Pryaslova J., Lance V., Siegel J. 2005. Continuous activity in cetaceans after birth. Nature 435, 1177. 10.1038/4351177a (doi:10.1038/4351177a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Veen D. R., Le Minh N., Gos P., Arneric M., Gerkema M. P., Schibler U. 2006. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc. Natl Acad. Sci. USA 103, 3393–3398 10.1073/pnas.0507825103 (doi:10.1073/pnas.0507825103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Oort B. E. H., Tyler N. J. C., Gerkema M. P., Folkow L., Schytte Blix A., Stokkan A. 2005. Circadian organization in reindeer. Nature 438, 1095–1096 10.1038/4381095a (doi:10.1038/4381095a) [DOI] [PubMed] [Google Scholar]
- 12.Dunlap J. C., Loros J. J., DeCoursey P. J. 2004. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 13.McCluskey E. S. 1967. Circadain rhythms in female ants and loss after mating flight. Comp. Biochem. Physiol. 23, 665–677 10.1016/0010-406X(67)90418-5 (doi:10.1016/0010-406X(67)90418-5) [DOI] [PubMed] [Google Scholar]
- 14.Sharma V. K., Lone S. R., Goel A. 2004. Clocks for sex: loss of circadian rhythms in ants after mating? Naturwissenschaften 91, 334–337 [DOI] [PubMed] [Google Scholar]
- 15.Sharma V. K., Lone S. R., Goel A., Chandrashekaran M. K. 2004. Circadian consequences of social organization in the ant species Camponotus compressus. Naturwissenschaften 91, 386–390 [DOI] [PubMed] [Google Scholar]
- 16.Free J. B., Ferguson A. W., Simpkins J. R. 1992. The behaviour of queen honeybees and their attendants. Physiol. Entomol. 17, 43–55 10.1111/j.1365-3032.1992.tb00988.x (doi:10.1111/j.1365-3032.1992.tb00988.x) [DOI] [Google Scholar]
- 17.Harano K., Sasaki M., Sasaki K. 2007. Effects of reproductive state on rhythmicity, locomotor activity and body weight in the European honeybee, Apis mellifera queens (Hymenoptera, Apini). Sociobiology 50, 189–200 [Google Scholar]
- 18.Johnson J. N., Hardgrave E., Gill C., Moore D. 2010. Absence of consistent diel rhythmicity in mated honey bee queen behaviour. J. Insect Physiol. 56, 761–773 10.1016/j.jinsphys.2010.01.004 (doi:10.1016/j.jinsphys.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 19.Michener C. D. 1974. The social behaviour of the bees. Cambridge, MA: The Belknap Press of Harvard University Press [Google Scholar]
- 20.Colvin G. B., Whitmoye D. I., Sawyer C. H. 1969. Circadain sleep-wakefulness patterns after ovariectomy and treatment with estrogen. Exp. Neurol. 25, 616–625 10.1016/0014-4886(69)90104-6 (doi:10.1016/0014-4886(69)90104-6) [DOI] [PubMed] [Google Scholar]
- 21.Lin T. M., Lee H. J. 1998. Parallel control mechanisms underlying locomotor activity and sexual receptivity of the female German cockroach, Blattella germanica (L.). J. Insect Physiol. 44, 1039–1051 10.1016/S0022-1910(98)00069-9 (doi:10.1016/S0022-1910(98)00069-9) [DOI] [PubMed] [Google Scholar]
- 22.Ruiz de Elvira M. C., Persaud R., Coen C. W. 1992. Use of running wheels regulates the effects of the ovaries on circadian rhythms. Physiol. Behav. 52, 277–284 10.1016/0031-9384(92)90271-3 (doi:10.1016/0031-9384(92)90271-3) [DOI] [PubMed] [Google Scholar]
- 23.Thomas E. M., Armstrong S. M. 1989. Effect of ovariectomy and estradiol on unity of female rat circadian rhythms. Am. J. Physiol. 257, R1241–R1250 [DOI] [PubMed] [Google Scholar]
- 24.Shen S., Spratt C., Sheward W. J., Kallo I., West K., Morrison C. F., Coen C. W., Marston H. M., Harmar A. J. 2000. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc. Natl Acad. Sci. USA 97, 11 575–11 580 10.1073/pnas.97.21.11575 (doi:10.1073/pnas.97.21.11575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheward W. J., Maywood E. S., French K. L., Horn J. M., Hastings M. H., Seckl J. R., Holmes M. C., Harmar A. J. 2007. Entrainment to feeding but not to light: circadian phenotype of VPAC2 receptor-null mice. J. Neurosci. 27, 4351–4358 10.1523/JNEUROSCI.4843-06.2007 (doi:10.1523/JNEUROSCI.4843-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prosser H. M., Bradley A., Chesham J. E., Ebling F. J., Hastings M. H., Maywood E. S. 2007. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc. Natl Acad. Sci. USA 104, 648–653 10.1073/pnas.0606884104 (doi:10.1073/pnas.0606884104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore D. 2001. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 47, 843–857 10.1016/S0022-1910(01)00057-9 (doi:10.1016/S0022-1910(01)00057-9) [DOI] [Google Scholar]
- 28.Fantinou A. A., Alexandri M. P., Tsitsipis J. A. 1998. Adult emergence rhythm of the egg-parasitoid Telenomus busseolae. Biocontrol 43, 141–151 10.1023/A:1009984318289 (doi:10.1023/A:1009984318289) [DOI] [Google Scholar]
- 29.Fleury F., Allemand R., Vavre F., Fouillet P., Bouletreau M. 2000. Adaptive significance of a circadian clock: temporal segregation of activities reduces intrinsic competitive inferiority in Drosophila parasitoids. Proc. R. Soc. Lond. B 267, 1005–1010 10.1098/rspb.2000.1103 (doi:10.1098/rspb.2000.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders D. S. 2002. Insect clocks, 3rd edn. Amsterdam, The Netherlands: Elsevier Press [Google Scholar]
- 31.Eskes G. A. 1984. Neural control of the daily rhythm of sexual-behaviour in the male golden-hamster. Brain Res. 293, 127–141 10.1016/0006-8993(84)91460-4 (doi:10.1016/0006-8993(84)91460-4) [DOI] [PubMed] [Google Scholar]
- 32.Koeniger N., Koeniger G. 2000. Reproductive isolation among species of the genus Apis. Apidologie 31, 313–339 10.1051/apido:2000125 (doi:10.1051/apido:2000125) [DOI] [Google Scholar]
- 33.McCluskey E. S. 1992. Periodicity and diversity in ant mating flights. Comp. Biochem. Physiol. 103, 241–243 10.1016/0300-9629(92)90574-A (doi:10.1016/0300-9629(92)90574-A) [DOI] [PubMed] [Google Scholar]
- 34.Sakai T., Ishida N. 2001. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc. Natl Acad. Sci. USA 98, 9221–9225 10.1073/pnas.151443298 (doi:10.1073/pnas.151443298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvegren G., Lofstedt C., Rosen W. Q. 2005. Circadian mating activity and effect of pheromone pre-exposure on pheromone response rhythms in the moth Spodoptera littoralis. J. Insect Physiol. 51, 277–286 10.1016/j.jinsphys.2004.11.013 (doi:10.1016/j.jinsphys.2004.11.013) [DOI] [PubMed] [Google Scholar]
- 36.Duchateau M. J., Velthuis H. H. W. 1989. Ovarian development and egg laying in workers of Bombus terrestris. Entomol. Exp. Appl. 51, 199–213 [Google Scholar]
- 37.Foster R. L., Brunskill A., Verdirame D., O'Donnell S. 2004. Reproductive physiology, dominance interactions, and division of labour among bumble bee workers. Physiol. Entomol. 29, 327–334 10.1111/j.0307-6962.2004.00388.x (doi:10.1111/j.0307-6962.2004.00388.x) [DOI] [Google Scholar]
- 38.Van Doorn A. 1987. Investigations into the regulation of dominance behaviour and of the division of labour in bumblebee colonies (Bombus terrestris). Netherlands J. Zool. 37, 255–276 10.1163/002829687X00080 (doi:10.1163/002829687X00080) [DOI] [Google Scholar]
- 39.Amdam G. V., Csondes A., Fondrk M. K., Page R. E. 2006. Complex social behaviour derived from maternal reproductive traits. Nature 439, 76–78 10.1038/nature04340 (doi:10.1038/nature04340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Kaftanoglu O., Siegel A. J., Page R. E., Amdam G. V. 2010. Surgically increased ovarian mass in the honey bee confirms link between reproductive physiology and worker behaviour. J. Insect Physiol. 56, 1816–1824 10.1016/j.jinsphys.2010.07.013 (doi:10.1016/j.jinsphys.2010.07.013) [DOI] [PubMed] [Google Scholar]
- 41.Geva S., Hartfelder K., Bloch G. 2005. Reproductive division of labour, dominance, and ecdysteroid levels in hemolymph and ovary of the bumble bee Bombus terrestris. J. Insect Physiol. 51, 811–823 10.1016/j.jinsphys.2005.03.009 (doi:10.1016/j.jinsphys.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 42.Linksvayer T. A., Wade M. J. 2005. The evolutionary origin and elabouration of sociality in the aculeate hymenoptera: maternal effects, sib-social effects, and heterochrony. Q. Rev. Biol. 80, 317–336 10.1086/432266 (doi:10.1086/432266) [DOI] [PubMed] [Google Scholar]
- 43.West-Eberhard M. J. 1987. Flexible strategy and social evolution. In Animal societies: theories and facts (eds Itö Y., Brown J. L., Kikkawa J.), pp. 35–51 Tokyo, Japan: Japan Scientific Societies Press [Google Scholar]
- 44.West-Eberhard M. J. 1996. Wasp societies as microcosms for the study of development and evolution. In Natural history and evolution of paper-wasps (eds Tiurilazzi S., West-Eberhard M. J.), pp. 290–317 New York, NY: Oxford University Press, Inc [Google Scholar]