Abstract
Background
Dyspnea is a common symptom experienced by many patients with chronic, life-threatening, and/or life-limiting illnesses. Although it can be defined and measured in several ways, dyspnea is best described directly by patients through regular assessment, as its burdens exert a strong influence on the patient's experience throughout the trajectory of serious illness. Its significance is amplified due to its impact on family and caregivers.
Discussion
Anatomic and physiologic changes associated with dyspnea, and cognitive perceptions related to patients and the underlying disease, provide insights into how to shape interventions targeting this oppressive symptom. Additionally, as described in the concept of “total dyspnea,” the complex etiology and manifestation of this symptom require multidisciplinary treatment plans that focus on psychological, social, and spiritual distress as well as physical components. Several validated assessment tools are available for clinical and research use, and choice of method should be tailored to the individual patient, disease, and care setting in the context of patient-centered care.
Conclusion
This article, the first in a two-part series, reviews the identification and assessment of dyspnea, the burden it entails, and the underlying respiratory and nonrespiratory etiologies that may cause or exacerbate it.
Introduction
The word “dyspnea” is derived from the Greek roots dys, meaning difficult, and pneuma meaning breath. Dyspnea, or breathlessness, is a common and oppressive symptom experienced by many patients throughout the trajectory of life-limiting illness. Dyspnea may be related to the illness, its comorbidities, therapy for either, or hypoxia. Often it is the result of a combination of all these. Dyspnea may occur at rest or with activity, may be continuous, intermittent or have a pattern of acute-on-chronic experiences and effects those with and without primary cardiopulmonary disorders alike. As breathing is a primal sensation of life, its disturbance creates a visceral sense of dread that, itself, incurs suffering. Dyspnea is a reason for consultation in over 10% of palliative care inpatient consultations1 and is the fourth most common reason for palliative care patients to visit the emergency department.2 In this first of a two-part series on dyspnea for the palliative care professional, we review the burden, pathophysiology, and measurement of dyspnea. The next review will appraise both pharmacologic and nonpharmacologic options for dyspnea management.
Definitions
The American Thoracic Society defines dyspnea as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary.”3 In more colloquial terms, dyspnea is an uncomfortable breathing sensation that is subjective and difficult to define by the outsider; patients “know it when they feel it.” Table 1 lists examples included in dyspnea assessment questionnaires to describe the symptom. Descriptors used by patients to express degrees of dyspnea or breathlessness fall into the general categories of difficulty with air movement (“I feel that my breathing is more rapid.” “My breath does not go out all the way.”), increased effort (“I feel that I am breathing more.” “I feel hunger for more air.”), and general distress (“I feel I am suffocating.” “I feel that I am smothering.”).4 This symptom may be constant (i.e., continuous dyspnea) or present in episodes (i.e., breakthrough dyspnea) as described in a cohort of cancer patients by Reddy et al.5 Breakthrough dyspnea was the predominant type, with over 80% of patients reporting short spells of breathlessness interrupting long symptom-free periods throughout the day. Continuous dyspnea was reported by only 39% of patients. On average, breakthrough episodes occurred 5–6 times per day and lasted less than 5 minutes, indicating that tailored treatment paradigms with quick onset of action are needed to counteract this more common type.
Table 1.
Patient Descriptors of Dyspnea
| I feel… | My… | I cannot…. |
|---|---|---|
| That I am smothering | Breathing requires effort | Take a full breath |
| That my breath stops | Chest feels tight | Get enough air |
| That I am suffocating | Breathing is fast | Stop thinking about my breathing |
Although dyspnea has traditionally been described in the medical literature by physiologic measures, there is a growing acknowledgment that it is ultimately a patient-centered symptom that does not necessarily correlate with findings of hypoxia, hypercarbia, or tachypnea. Correspondingly, patient-reported experience of the symptom has become the standard for assessment, and an increasingly accepted method for determining its severity and impact. Because of its many correlates with the patient's underlying disease, demographic background, concomitant symptoms, and emotional state, each patient's experience of dyspnea becomes as unique to the individual as is their journey with life-limiting illness.
Burden and Consequences in Advanced Disease
Dyspnea is either an element of the disease course or a component of the final stages of most etiologies that cause significant morbidity and mortality in the United States. Dyspnea is experienced at some point by most patients with advanced cancer,6 heart failure,7 and chronic lung disease8 and in the last 3 days of life in the imminently dying.9 In fact, Currow et al.10 recently reported that only 11.4% of patients receiving end-of-life care report “no breathlessness.” Given that cancer, heart failure, and chronic lung disease represent, respectively, the first, second, and fourth most common causes of death in the United States and account for over 50% of all deaths,11 dyspnea therefore afflicts a large population of patients. Dyspnea is also a significant part of the symptom milieu of patients with dementia,12 advanced age,13 and HIV.14 Correspondingly, dyspnea remains a major target for intervention in palliative care. Among patients receiving end-of-life care, shortness of breath was the seventh most observed symptom, affected almost 50% of all patients, was reported as “severe” in half of those experiencing it,15 and increased significantly between 3 months and 1 month before death.9,16
The presence of dyspnea in patients with life-altering disease can exert a profound effect on patient and caregiver quality of life. Often patients notice and caregivers observe a gradual loss of independence, progressing from initial difficulty with pleasurable activities to ultimate loss of ability to perform even the most routine activities of daily living. Patient reports of increasing levels of dyspnea have correlated with lower quality-of-life scores overall17 and with physical, emotional, and cognitive changes including anorexia, fatigue, poor concentration, memory loss, and decreased mastery.18
A recent review of over 1600 patients with severe obstructive lung disease from the National Emphysema Treatment Trial showed that dyspnea was strongly and inversely associated with health-related quality of life (HRQOL) independent of forced expiratory volume in 1 second (FEV1) measure.19 Interventions associated with increased HRQOL included pulmonary rehabilitation and supplemental oxygen. Another recent study conducted in a population of patients with chronic obstructive pulmonary disease (COPD), 94% of whom reported dyspnea, found a positive association between increasing symptom burden and decreased quality of life.20 In a multivariate analysis, Reddy et al.5 recently reported an association between dyspnea and depression, and documented the effect of increasing dyspnea intensity on interference with general activity, mood, and enjoyment of life. The impact of uncontrolled dyspnea extends beyond the patient to affect family members and other caregivers, health care staff, and the health care delivery system. In the setting of worsening dyspnea, patients and families are often faced with difficult decisions regarding care advancement (e.g., mechanical ventilation versus noninvasive positive pressure ventilation versus other symptom treatment) or change in locations of care (e.g., hospital versus home). Dyspnea is a risk factor for hospitalization in patients with lung cancer21 and for in-hospital death, independent of underlying illness, for anyone with advanced disease who is enrolled in home-based health care.22 Significant associations have been reported between dyspnea and decreased patient and family well-being and increased staff anxiety, highlighting the impressive effect of this one symptom on the patient, his/her support network, and the health care delivery system.
Not surprisingly, dyspnea is frequently included as a component of prognostic models of death from pulmonary and nonpulmonary disease. Two decades ago, Carpenter et al.23 reported that symptoms of breathlessness are a predictor of death from all causes over a period of 27 years—even when the symptom was episodic. The Bode Scale, a tool used to determine prognosis of COPD patients over 1 to 3 years, has as its only patient-experienced component the measurement of dyspnea on a scale from 0–4.24 The Mortality Risk Index Score incorporates dyspnea as an important part of the 6-month assessment of prognosis in elderly, nursing home residents.25 The Palliative Prognostic Score, a tool to stratify patients into groups based on predicted 30-day survival, also uses the presence of dyspnea as an integral part of its survival prediction.26
Etiologies of Dyspnea
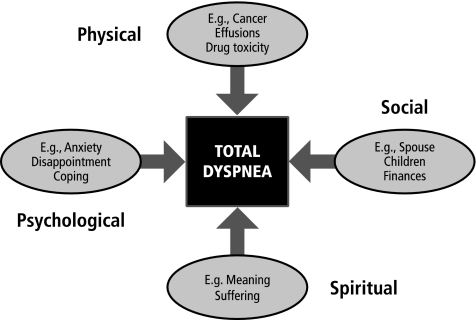
Patterned after the concept of total pain first described by Dame Cicely Saunders in the 1960s, the concept of “total dyspnea” creates a framework for understanding dyspnea etiology in a way that captures its full impact on the patient and caregiver.27 Encompassing the four domains of physical, psychological, interpersonal, and existential distress, total dyspnea describes the patient's experience of the symptom from multiple perspectives that, synergistically, combine to shape the symptom and to determine its impact (Fig. 1). A mnemonic encompassing these various biopsychosocial components is presented in Figure 2. This approach is wholly consistent with the multidisciplinary nature central to palliative care.
FIG. 1.
Elements of the biopsychosocial model of “total dyspnea.”
FIG. 2.
Mnemonic of the biopsychosocial elements of dyspnea.
Physiologically, dyspnea results from three main abnormalities: increased load requiring greater respiratory effort (e.g., obstruction), an increase in the proportion of respiratory muscle required to maintain a normal workload (e.g., weakness), and an increase in ventilator requirements (e.g. fever, anemia). The anatomic condition and underlying disease giving rise to dyspnea may be pulmonary obstruction (COPD, reactive airways, cough/secretions, mass lesions), pulmonary restriction (fibrosis or other interstitial disease, effusions, fibrosis, infections, kyphosis, obesity), perfusion/oxygenation mismatch (anemia, pulmonary hypertension, heart failure, pulmonary embolism), and fatigue/weakness (multiple sclerosis, amyotrophic lateral sclerosis, cancer fatigue). Although abnormalities can often be measured, imaged, or assumed based on underlying disease, etiologies of dyspnea are often related to systemic effects of illness. For example, data from the National Hospice Study reported that 24% of patients with no known cardiopulmonary process (e.g., local cancer involvement, pleural effusions, pulmonary infections) experience dyspnea.6 Additionally, in hospice patients with no known cardiopulmonary disorder, both prevalence and severity of dyspnea increase significantly as death approaches.10 In such cases, systemic changes such as asthenia and cachexia, both of which are present in greater than 80% of people with advanced cancer, are postulated as etiologies.28 Other nonanatomic correlates of dyspnea include affect and spiritual and existential distress. Patient anxiety and panic both play an important role in the development of dyspnea and, in turn, are exacerbated by its presence—setting up a pernicious spiral of cause and symptom (Fig. 3). The association between symptoms of breathlessness and anxiety, particularly panic attacks, is well-documented. This association bears notice given the high prevalence of anxiety in palliative care populations. One recent study reported that 22% of patients in an inpatient palliative care unit met Hospital Anxiety and Depression Scale (HADS) criteria for anxiety.29 It has also been observed that, compared to control patients, patients with underlying anxiety or panic disorders have an exaggerated experience of dyspnea.30 Additionally the effect of spiritual distress on dyspnea has been recently described.22
FIG. 3.
Overview of a rational approach to dyspnea.
The central nervous system (CNS) also plays a critical role in the perception of dyspnea. In the accepted neurophysiologic model, it is thought that dyspnea arises when sensory receptors involved with respiration are activated, sending an afferent impulse to the CNS; the CNS then directs an efferent impulse toward the muscles of respiration. Mismatch between these signals may contribute to dyspnea through modulation of any of these systems.31 It has been postulated since 1985 that endogenous opioids, known as endorphins, may attenuate the dyspnea sensation at the CNS level.32 An important recent investigation antagonizing endogenous endorphins in opioid-naïve volunteers has added to the understanding of the efficacy of exogenous opioids as the mainstay of global therapy for dyspnea. Mahler et al.33 conducted an investigation measuring beta-endorphin levels at rest and post-exercise in 17 patients with COPD undergoing a 10-minute treadmill exercise test. Patients were given either intravenous saline or naloxone, measured for beta-endorphin serum levels, and queried for numerical rating score of dyspnea. A threefold increase in serum beta-endorphin levels was observed from rest to postexercise. Mean ratings of dyspnea throughout exercise were significantly higher in patients when they received naloxone.33 This recent proof of concept suggests that further studies are warranted to identify ways in which the endogenous opioid effect on dyspnea can be accentuated.
Additionally, increasing investigations with positron emission tomography (PET)34–36 and functional magnetic resonance imaging (fMRI) have added to the knowledge of how dyspnea activates cortical and cerebellar systems.37
Measurement of Dyspnea
Various validated measurement tools are available to assist the palliative care professional in quantitatively and qualitatively assessing the patient's dyspnea. These instruments range from standard single-item ordinal scales such as the visual analogue scale (VAS), numerical rating scale (NRS, e.g., 0 [no breathlessness] to 10 [worst possible breathlessness]), and modified Borg scale38 to measure the intensity of breathlessness to functional assessment scales such as the Medical Research Council Dyspnea Scale and Baseline Dyspnea Index (BDI). Research studies typically employ more complex measurements of physiologic parameters such as the 6-minute walk test, alone or in combination with surveys describing the patient's symptom experience39 (e.g., “I feel out of breath”, “My chest feels tight”). Certain categorical tools such as the Memorial Symptom Assessment Scale and Edmonton Symptom Assessment Scale (ESAS) incorporate numerical ratings of dyspnea into an overall symptom inventory. Dorman et al.40 recently presented a comprehensive, systematic review of measurement scales for dyspnea in palliative care that includes many other scales not covered here.
Recent attention has been drawn to the need for a practical assessment tool that describes the patient's experience of dyspnea and is useful in the clinical setting as well as for research purposes. While several validated scales are used in the research setting to measure the impact of dyspnea on HRQOL, most of these are too long and arduous to be practical in routine clinical care. To better reflect the patient's experience rather than focusing on physiologic parameters, Tanaka et al.41 developed the Cancer Dyspnea Scale, a 12-item multidimensional dyspnea scale for patients with cancer to assess effort, anxiety, and discomfort. Originally validated in a Japanese population, it has subsequently been validated in Swedish-speaking42 and English-speaking43 cohorts. This effort represents a step forward in incorporating the patient's symptom experience into dyspnea assessment, but falls short of assessing the full impact of dyspnea. To date, no single assessment tool considers all of the various components of this multifaceted symptom and its impact on people.
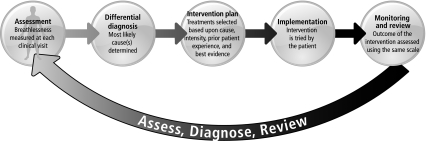
Ultimately, when selecting assessment measures in palliative care, the clinician must take into account the goals of care, purpose of the assessment, ease of administration, and patient burden, with the aim of gathering useful information while not detracting from quality of life by being tedious, distressing, intensive, or complicated. In clinical practice, the most fruitful way of measuring experience is simply to ask, intervene, follow up, and ask again – using the same scale each time (Figure 3). Clinically, a straightforward standardized scale (e.g., 0–10 NRS) alone or as a part of a longer symptom list (e.g., ESAS) is likely best. More complex tools (e.g., Cancer Dyspnea Scale) have not yet demonstrated their role in clinical practice, but in the research setting they can help identify etiologies and document change in complex outcomes in response to therapies. Additionally, the evolving understanding that most dyspnea is intermittent, as opposed to continuous, means that a thorough assessment involves questioning both the patient's current symptom burden and experiences over the last 24 hours.
Conclusion
Dyspnea, a complex and commonly experienced symptom, worsens in prevalence and intensity for many palliative care patients. Its dramatic effects on independence and quality of life make prompt recognition and characterization of underlying causes and comorbidities paramount. Various tools to measure dyspnea are available to the clinician and researcher, but often the most useful and well-received are those that are short, to the point, and used regularly to monitor symptom status. Most importantly, the actual words of the patient reflect the true meaning of dyspnea, its burden, and the impact of therapeutic interventions. The field of palliative care is expanding and the patient population evolving. An increasing proportion of inpatient palliative care consultations are being performed on patients without cancer, such as individuals receiving advanced therapies for COPD and heart failure. As the population of those with life-limiting illnesses referred to palliative care grows, further research and clinician education are critical; we must continue to refine and improve our understanding of, ability to recognize, and capacity to effectively treat this disabling symptom.
In a subsequent article, we will explore pharmacologic and nonpharmacologic interventions for dyspnea.
Author Disclosure Statement
Amy Abernethy receives research funding from the Agency for Healthcare Quality and Research, National Cancer Institute, Nation Institute of Nursing Research (National Institutes of Health [NIH]), National Institute of Aging (NIH), and The Robert Wood Johnson Foundation. She receives industry funding for clinical research from Pfizer, Lilly, Bristol Myers Squibb, Helsinn, Amgen, Kanglaite, and Abbott Laboratories. She is a consultant (less than $10,000/year) for Helsinn, Proventys, and GlaxoSmithKline. Jane Wheeler, Arif Kamal, Jennifer Maguire, and David Currow have no funding to disclose.
References
- 1.Kamal A SK. Liu H. Ruegg S. Carey EC. Whitford K. Bock FA. Creagan ET. Moynihan TJ. Kaur JS. Survival trends in palliative care patients with cancer: A Mayo Clinic 5 year review. J Clin Oncol. 2009;27(Suppl) doi: 10.1200/JOP.2010.000067. abstract 9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbera L. Taylor C. Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 182:563–568. doi: 10.1503/cmaj.091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyspnea. Mechanisms, assessment, and management: A consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 4.Caroci Ade S. Lareau SC. Descriptors of dyspnea by patients with chronic obstructive pulmonary disease versus congestive heart failure. Heart Lung. 2004;33:102–110. doi: 10.1016/j.hrtlng.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Reddy SK. Parsons HA. Elsayem A. Palmer JL. Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12:29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 6.Reuben DB. Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89:234–236. doi: 10.1378/chest.89.2.234. [DOI] [PubMed] [Google Scholar]
- 7.Nordgren L. Sorensen S. Symptoms experienced in the last six months of life in patients with end-stage heart failure. Eur J Cardiovasc Nurs. 2003;2:213–217. doi: 10.1016/S1474-5151(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds P. Karlsen S. Khan S. Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med. 2001;15:287–295. doi: 10.1191/026921601678320278. [DOI] [PubMed] [Google Scholar]
- 9.Lynn J. Teno JM. Phillips RS. Wu AW. Desbiens N. Harrold J. Claessens MT. Wenger N. Kreling B. Connors AF., Jr Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;126:97–106. doi: 10.7326/0003-4819-126-2-199701150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Currow DC. Smith J. Davidson PM. Newton PJ. Agar MR. Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39:680–690. doi: 10.1016/j.jpainsymman.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Faststats. Leading causes of death. 2011. http://www.cdc.gov/nchs/fastats/lcod.htm. [Mar 01;2011 ]. http://www.cdc.gov/nchs/fastats/lcod.htm
- 12.Mitchell SL. Teno JM. Kiely DK. Shaffer ML. Jones RN. Prigerson HG. Volicer L. Givens JL. Hamel MB. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho SF. O'Mahony MS. Steward JA. Breay P. Buchalter M. Burr ML. Dyspnoea and quality of life in older people at home. Age Ageing. 2001;30:155–159. doi: 10.1093/ageing/30.2.155. [DOI] [PubMed] [Google Scholar]
- 14.Fantoni M. Ricci F. Del Borgo C. Bevilacqua N. Izzi I. Damiano F. Marasca G. Symptom profile in terminally ill AIDS patients. AIDS Patient Care STDs. 1996;10:171–173. doi: 10.1089/apc.1996.10.171. [DOI] [PubMed] [Google Scholar]
- 15.Kutner JS. Kassner CT. Nowels DE. Symptom burden at the end of life: Hospice providers' perceptions. J Pain Symptom Manage. 2001;21:473–480. doi: 10.1016/s0885-3924(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 16.Elmqvist MA. Jordhoy MS. Bjordal K. Kaasa S. Jannert M. Health-related quality of life during the last three months of life in patients with advanced cancer. Support Care Cancer. 2009;17:191–198. doi: 10.1007/s00520-008-0477-2. [DOI] [PubMed] [Google Scholar]
- 17.Smith EL. Hann DM. Ahles TA. Furstenberg CT. Mitchell TA. Meyer L. Maurer LH. Rigas J. Hammond S. Dyspnea, anxiety, body consciousness, and quality of life in patients with lung cancer. J Pain Symptom Manage. 2001;21:323–329. doi: 10.1016/s0885-3924(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 18.Bernhard J. Ganz PA. Psychosocial issues in lung cancer patients (Part 2) Chest. 1991;99:480–485. doi: 10.1378/chest.99.2.480. [DOI] [PubMed] [Google Scholar]
- 19.Moy ML. Reilly JJ. Ries AL. Mosenifar Z. Kaplan RM. Lew R. Garshick E. National Emphysema Treatment Trial Research Group: Multivariate models of determinants of health-related quality of life in severe chronic obstructive pulmonary disease. J Rehabil Res Dev. 2009;46:643–654. doi: 10.1682/JRRD.2008.09.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blinderman CD. Homel P. Billings JA. Tennstedt S. Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38:115–123. doi: 10.1016/j.jpainsymman.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Skaug K. Eide GE. Gulsvik A. Hospitalisation days in patients with lung cancer in a general population. Respir Med. 2009;103:1941–1948. doi: 10.1016/j.rmed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Edmonds P. Higginson I. Altmann D. Sen-Gupta G. McDonnell M. Is the presence of dyspnea a risk factor for morbidity in cancer patients? J Pain Symptom Manage. 2000;19:15–22. doi: 10.1016/s0885-3924(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter L. Beral V. Strachan D. Ebi-Kryston KL. Inskip H. Respiratory symptoms as predictors of 27 year mortality in a representative sample of British adults. BMJ. 1989;299:357–361. doi: 10.1136/bmj.299.6695.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celli BR. Cote CG. Marin JM. Casanova C. Montes de Oca M. Mendez RA. Pinto Plata V. Cabral HJ. The body-mass index, airflow olbstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell SL. Kiely DK. Hamel MB. Park PS. Morris JN. Fries BE. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291:2734–2740. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 26.Glare P. Eychmueller S. Virik K. The use of the palliative prognostic score in patients with diagnoses other than cancer. J Pain Symptom Manage. 2003;26:883–885. doi: 10.1016/s0885-3924(03)00335-x. [DOI] [PubMed] [Google Scholar]
- 27.Abernethy AP. Wheeler JL. Total dyspnoea. Curr Opin Support Palliat Care. 2008;2:110–113. doi: 10.1097/SPC.0b013e328300cad0. [DOI] [PubMed] [Google Scholar]
- 28.Bruera EF. RL: Clinical management of cachexia and anorexia. In: Doyle D, editor. Oxford Textbook of Palliative Medicine. Oxford: Oxford Medical; 1993. pp. 330–337. [Google Scholar]
- 29.Smith EM. Gomm SA. Dickens CM. Assessing the independent contribution to quality of life from anxiety and depression in patients with advanced cancer. Palliat Med. 2003;17:509–513. doi: 10.1191/0269216303pm781oa. [DOI] [PubMed] [Google Scholar]
- 30.Nardi AE. Freire RC. Zin WA. Panic disorder and control of breathing. Respir Physiol Neurobiol. 2009;167:133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell DE. Banzett RB. Carrieri-Kohlman V. Casaburi R. Davenport PW. Gandevia SC. Gelb AF. Mahler DA. Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: A roundtable. Proc Am Thorac Soc. 2007;4:145–168. doi: 10.1513/pats.200611-159CC. [DOI] [PubMed] [Google Scholar]
- 32.Santiago TV. Edelman NH. Opioids and breathing. J Appl Physiol. 1985;59:1675–1685. doi: 10.1152/jappl.1985.59.6.1675. [DOI] [PubMed] [Google Scholar]
- 33.Mahler DA. Murray JA. Waterman LA. Ward J. Kraemer WJ. Zhang X. Baird JC. Endogenous opioids modify dyspnoea during treadmill exercise in patients with COPD. Eur Respir J. 2009;33:771. doi: 10.1183/09031936.00145208. [DOI] [PubMed] [Google Scholar]
- 34.Banzett RB. Mulnier HE. Murphy K. Rosen SD. Wise RJ. Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–2020. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- 35.Parsons LM. Egan G. Liotti M. Brannan S. Denton D. Shade R. Robillard R. Madden L. Abplanalp B. Fox PT. Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proc Natl Acad Sci USA. 2001;98:2041–2046. doi: 10.1073/pnas.98.4.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liotti M. Brannan S. Egan G. Shade R. Madden L. Abplanalp B. Robillard R. Lancaster J. Zamarripa FE. Fox PT. Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proc Natl Acad Sci USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans KC. Banzett RB. Adams L. McKay L. Frackowiak RS. Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88:1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 38.Kendrick KR. Baxi SC. Smith RM. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26:216–222. doi: 10.1016/s0099-1767(00)90093-x. [DOI] [PubMed] [Google Scholar]
- 39.Harver A. Mahler DA. Schwartzstein RM. Baird JC. Descriptors of breathlessness in healthy individuals: Distinct and separable constructs. Chest. 2000;118:679–690. doi: 10.1378/chest.118.3.679. [DOI] [PubMed] [Google Scholar]
- 40.Dorman S. Byrne A. Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21:177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K. Akechi T. Okuyama T. Nishiwaki Y. Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: A multidimensional, brief, self-rating scale. Br J Cancer. 2000;82:800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henoch I. Bergman B. Gaston-Johansson F. Validation of a Swedish version of the Cancer Dyspnea Scale. J Pain Symptom Manage. 2006;31:353–361. doi: 10.1016/j.jpainsymman.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Uronis HE BS. Bosworth H. Ahmedzai S. Rogers E. Currow D. Fazekas B. Abernethy AP. An examination of the psychometric properties of an English version of the Cancer Dyspnea Scale (CDS) for evaluating dyspnea in patients with advanced lung cancer. St. Gallen, Switzerland: International MASCC/ISOO Symposium; 2007. [Google Scholar]