Abstract
Inhibiting unwanted thoughts, actions and emotions figures centrally in daily life, and the prefrontal cortex is widely viewed as a source of this inhibitory control. We argue that the function of the prefrontal cortex is best understood in terms of representing and actively maintaining abstract information such as goals, which produces two types of inhibitory effects on other brain regions. Inhibition of some subcortical regions takes a directed, global form, with prefrontal regions providing contextual information relevant to when to inhibit all processing in a region. Inhibition within neocortical (and some subcortical) regions takes an indirect, competitive form, with prefrontal regions providing excitation of goal-relevant options. These distinctions are critical for understanding the mechanisms of inhibition and how they can be impaired or improved.
Executive control over inhibition
We constantly need to inhibit – whether our critical thoughts about a partner, our reaching for seconds at dinner, or our fear while boarding a plane. Failures to inhibit are observed in a variety of clinical disorders [1], in children [2] and in the elderly [3], and are associated with learning difficulties [4] and behavioral problems [5]. Understanding how we inhibit and how variations in underlying mechanisms contribute to inhibitory deficits is essential for advancing training, remediation, and clinical intervention. Although the concept of inhibition has had a long and controversial history in cognitive science [6,7], we believe that emerging research across a variety of disciplines support a unifying framework for understanding the cognitive and neural mechanisms that support this fundamental process.
It is widely believed that the prefrontal cortex (PFC) serves as a source of inhibitory control over other brain areas. In this article, we focus on specifying more precisely its role in inhibition. A prevalent view is that certain PFC regions are specialized for inhibitory control per se, suggesting, for example, that the right inferior frontal gyrus (rIFG) is a specialized response inhibition area [8]. We argue instead for a more unified framework for understanding inhibitory control in the broader context of PFC function. We take as a point of departure the characterization of PFC areas as primarily specialized for actively representing and maintaining abstract information (such as goals, contexts, and task sets) relevant for cognitive control over behavior [9,10], with inhibitory control as one of many down-stream effects of this specialization [11-14]. Supporting such a perspective, a recent factor analysis of executive function (EF) [15] reveals that there is no inhibition-specific factor. Instead, tasks thought to tap inhibitory function, such as the anti-saccade task, load on a common EF factor, observed across all EF tasks, which is separable from two more specific EF factors: shifting and working memory updating. A parsimonious interpretation is that this common EF factor reflects active goal maintenance in PFC [15], which supports inhibition among other processes.
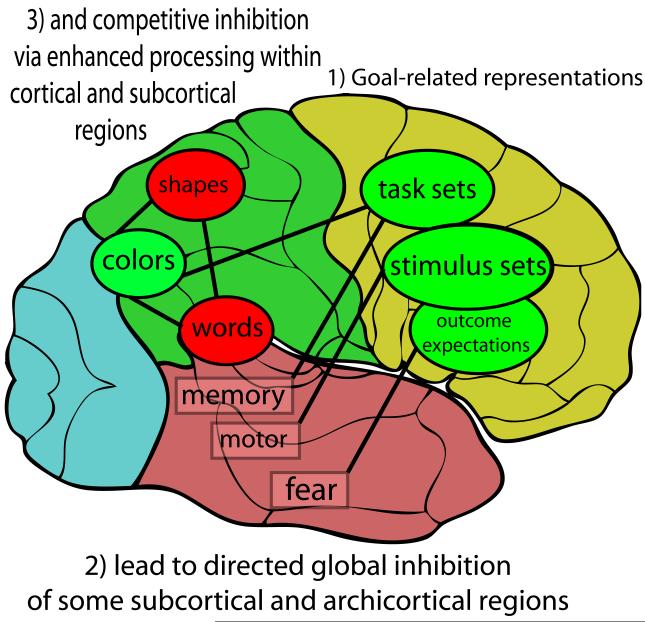
We argue that this specialization for actively representing and maintaining abstract information in the PFC produces two distinct types of inhibitory effects on other brain regions (Figure 1). First, some instances of PFC projections to subcortical and archicortical areas support directed, global inhibition, where PFC projections activate GABAergic interneurons in the target area, and inhibit its overall function. In this case, the role of PFC areas in inhibition is to maintain the abstract information that is relevant to when to inhibit the function of the target region. In contrast, we argue that, within the neocortex and some subcortical regions, a more indirect, competitive inhibition dynamic provides a better way of capturing the nature of PFC inhibitory control. In this case, PFC neurons directly excite goal-relevant processing areas. This allows them to compete better with other possible processing pathways, yielding a collateral effect of inhibiting competitors. This competitive dynamic helps a winner to emerge, rather than leading to a global shutdown.
Figure 1. Prefrontal control over directed global and competitive inhibition.
We argue that two distinct forms of inhibition – directed global vs. indirect competitive – can be understood within a unified framework that builds upon the well-accepted characterization of prefrontal areas as specialized for actively representing and maintaining abstract goal-related information, rather than for inhibition per se. While the specific type of content represented across each of the various prefrontal regions is still debated, we argue that inhibitory control arises as one downstream effect of these goal representations. Prefrontal cortex provides targeted global inhibition of some subcortical and archicortical regions (e.g., those related to fear, motor, and memory processing), and supports indirect competitive inhibition in neocortical and subcortical regions by enhancing relevant regions or representations (e.g., for processing colors), whose increased activity then causes reduced activation of competing areas or representations (e.g., for processing words or shapes). Targeted global inhibition thus leads to a global shutdown of associated regions, whereas competitive inhibition helps a winner to emerge from among a variety of competitors. To support basic survival processes (e.g., avoiding harm), subcortical regions are relatively “dumb” and inflexible, in the sense that they just turn on responses that can be adaptive (e.g. a fear response) but are physiologically and psychologically costly. Prefrontal regions support decisions about such responses, and globally inhibit subcortical circuitry and associated responses when the context indicates it is appropriate to do so (e.g., when stressors are controllable). Compared to subcortical processing systems, cortical processing systems are more flexible (changing more over the course of development and learning), and are less clearly linked to physiological and psychological benefits from being globally inhibited. These characteristics may explain why the prefrontal cortex excites the relevant cortical regions for a given goal rather than globally inhibiting irrelevant cortical regions. Regions whose activity is increased or enhanced are shown in green, while those whose activity is inhibited (globally or competitively) are shown in red; neocortical regions are labeled in ovals, while non-neocortical regions are labeled in rectangles.
Distinguishing between these two types of inhibition has important implications for understanding differences in how we inhibit across a range of situations. By considering the well-established role of the PFC in representing and maintaining abstract information, our approach allows for a unifying framework for understanding these two distinct types of inhibition in the broader context of PFC function.
Directed global inhibition of subcortical and archicortical regions by PFC
We consider three examples of directed global inhibition of subcortical and archicortical systems: coping with stressors, inhibiting responses, and suppressing memory retrieval. These examples span several regions of the PFC (medial and lateral, dorsal and ventral) and highlight two neural mechanisms that support directed global inhibition. First, PFC excitatory projections can synapse directly onto GABAergic interneurons in the target area, as occurs in coping with stressors. Second, PFC excitatory projections can synapse onto excitatory neurons in a region, which in turn synapse preferentially onto GABAergic interneurons in the target area, as occurs in response inhibition.
Ventromedial prefrontal cortex, dorsal raphe nucleus, and coping
Compelling evidence from research with animals demonstrates that the ventromedial prefrontal cortex (vmPFC) supports coping with controllable stressors, by directly inhibiting the fear-related dorsal raphe nucleus (DRN) – for a review, see [16]. Specifically, controllable stress (e.g., an escapable shock) activates neurons in the vmPFC that project to the DRN [17]. These excitatory projections from the vmPFC synapse preferentially onto inhibitory interneurons, which then inhibit the serotonergic cells of the DRN [18]. Activation of these DRN serotonergic cells increases fear behavior and decreases coping responses (e.g., fight or flight), via projections to other brain regions. Thus, behavioral control over stressors is viewed as activating the vmPFC’s inhibitory control over the fear-related DRN. Stimulating the vmPFC inhibits the DRN, and leads animals to treat uncontrollable stressors (such as inescapable shock, which does not tend to activate the vmPFC) as if they were controllable [19]. Conversely, inactivating the vmPFC leads animals to treat controllable stressors as if they were uncontrollable [20]. A similar, well-established circuit operates with prefrontal connectivity to the amygdala, and provides inhibitory control over fear conditioning [21] and emotion regulation [22-24].
This inhibitory control of the vmPFC over the DRN is global in nature; it inhibits the whole area, not specific neurons/representations within it. (This is true of all of the examples in this section; Box 1 considers mixed evidence for the idea that directed inhibition can be more selective.) Thus, this vmPFC-DRN account of coping serves as a clear example of directed global inhibition. However, rather than being specialized for inhibition per se, the vmPFC represents abstract information (e.g., outcome expectations, [25] – in this case, regarding behavioral control) which triggers inhibition specifically of fear responses.
BOX 1: Selective inhibition via the basal ganglia?
We have argued that directed inhibition of subcortical regions takes a global form, but some researchers have suggested that more selective directed inhibition is possible via the basal ganglia (BG; [66,67]). The BG is a major subcortical system with extensive connectivity to the frontal cortex, composed almost entirely of inhibitory GABAergic neurons, with competing Go and NoGo pathways that control the initiation of motor and cognitive actions at a relatively selective, fine-grained level [68,69]. Although NoGo signals might appear to represent a highly specific form of direct inhibition, evidence for their importance to common behavioral measures of inhibition is lacking or inconclusive.
The output of the BG exclusively causes disinhibition of excitatory loops through the cortex. Thus, the NoGo signal can only block the disinhibition (i.e., net excitation) that would have otherwise come from a Go signal – it does not directly inhibit the cortex, and thus has a relatively weak effect on controlling cognitive states. This characteristic may explain why individuals with altered function of BG do not show impairments on classical inhibition tasks such as Stroop and Stop-Signal. For example, Parkinson’s patients on medication that impairs NoGo pathway function perform the same as age-matched controls on these tasks [70]. Thus, the selectivity of the NoGo pathway does not appear relevant to standard inhibitory control tasks, perhaps because the blocking of BG disinhibition (which translates into blocking a boost from a Go Signal to word reading in the Stroop task, for example) is too weak to overcome the strong prepotent associations that are typically tested in such tasks.
Recently it has been proposed that rIFG may support selective motor inhibition via the slower and more selective NoGo pathway (as opposed to faster global inhibition via STN). This proposal is based on evidence that response inhibition for one hand is slower and exerts less interference on responses of the other hand when subjects have foreknowledge about what response may need to be stopped [66]. This foreknowledge is thought to support the use of a slower but more selective response inhibition circuit. (Additional evidence is considered in Box 2). However, other evidence suggests that the two effects taken as diagnostic of selective inhibitory control may arise from more domain-general features of motor function, rather than reflecting inhibitory control per se. First, bimanual interference is also observed in tasks lacking inhibitory demands, as long as different actions must be performed by each hand. This interference is decreased when the assignment of response rules to effectors is simplified [71]. Simplification of such effector rules is clearly afforded by foreknowledge, so that the reduction in interference with foreknowledge on response inhibition tasks may reflect something much more general than an act of inhibitory control. Second, response inhibition can be prolonged by manipulations that are not thought to involve the use of the NoGo pathway [72].
Therefore, the evidence on selective direct inhibition through BG influences is inconclusive or ambiguous, even for motor actions. One possibility is that the NoGo pathway serves to delay, not suppress, motor actions.
The subthalamic nucleus and response inhibition
The subthalamic nucleus (STN) provides global inhibition over the output of the basal ganglia, and is thought to be important for pausing motor output until a satisfactory motor plan has been settled upon by frontal motor control areas [6,26]. Thus, PFC excitatory projections to excitatory neurons of the STN support directed global inhibition of all motor output. Many prefrontal areas project to the STN, with anterior cingulate cortex (ACC) and pre-SMA providing strong innervation [27,28]. A compelling directed inhibition account is that signals of conflict, difficulty, and uncertainty, which have been widely associated with the ACC [29,30,Cf 31] and pre-SMA [30], drive activation of the STN, which then globally inhibits all motor output until these signals are resolved.
Another prefrontal area – the rIFG – also projects to the STN (though less strongly than other prefrontal regions [27,28]), and is also reliably engaged by tasks in which motor responses are interrupted in the middle of preparation (e.g., the Stop Signal task). This evidence has been used to argue that response inhibition is the defining function of the rIFG [8]. However, recent data suggest that the role of the rIFG in directed inhibition may actually be more associated with monitoring the environment for task-relevant stimuli, such as those that indicate a stop signal trial (Box 2). For example, the rIFG is also recruited when the environment must be monitored for stimuli that indicate a response must be initiated [32-36]. In fact, the information content of neural activity within the rIFG is so highly similar across response initiation and response inhibition that the identity of individual subjects can be decoded using a multivariate analysis of neural activity patterns in rIFG (but not in other brain regions) across these conditions [34]. Thus, rather than being specialized for inhibition per se, rIFG (like the rest of PFC) may be defined more by its ability to maintain task-relevant information (e.g., the environmental context required for responses). Multiple such PFC regions (including ACC and pre-SMA), each representing different types of task-relevant information (e.g. sets of appropriate motoric actions), may lead to the pausing of motor output (Box 3) as well as supporting other functions. This interpretation is consistent with findings showing that, in other contexts, the rIFG provides top-down excitation to task-relevant posterior cortical regions [37-39].
BOX 2: Challenges in studying inhibition: The case of TMS and response inhibition.
Even with advances in cognitive neuroscience methods, differentiating the roles of brain regions in inhibition is challenging. For example, evidence from transcranial magnetic stimulation (TMS) has been interpreted as supporting a specifically inhibitory role of right inferior frontal gyrus. Although disrupting function of a region (e.g., rIFG) via TMS can indicate whether that region is involved in a task (e.g., Stop Signal), it does not necessarily provide evidence on how a region supports normal task performance (e.g., monitoring for task-relevant signals vs. inhibition per se).
However, some patterns of TMS results can allow for solid inferences. For example, if disruption of a region leads to similar deficits across multiple tasks, it can be inferred that the region’s function is nonspecific to the individual tasks. Similarly, if disruption of multiple regions leads to similar loss of function, those functions may be general across those regions. These informative data patterns have been obtained, as outlined below, and challenge the view of rIFG as a single area specialized for inhibition:
Classical “virtual lesion” repetitive TMS (rTMS) protocols are used to inhibit the function of an area. They have demonstrated rIFG is involved not only in tasks with stimuli indicating a response must be stopped [73], but also in tasks with stimuli indicating a response must be committed [74].
Single-pulse TMS (spTMS) protocols are used to probe motor excitability, in terms of the motor potential evoked by a single excitatory pulse to motor cortex. They have demonstrated a lateralized decrease in motor excitability following cues indicating that a response may soon need to be stopped [67], which has been interpreted in terms of selective inhibition (Box 1). However, a lateralized decrease in motor excitability is also observed following cues indicating that a response may soon need to be committed [75].
Paired-pulse TMS procedures (ppTMS) provide an additional excitatory TMS pulse to a different neural locus, to probe regional interactions. These studies demonstrate reduced motor excitability following rIFG stimulation [76], but also following DLPFC stimulation [77].
Together, these studies suggest that rIFG’s role is a more general attentional one related to monitoring for task-relevant signals. The reduced motor excitability that is observed after TMS to this region occurs while motor plans are being developed and as a relatively general effect of frontal lobe stimulation. Such an interpretation is consistent with PFC regions being specialized for maintaining task-relevant information, with multiple regions contributing to pausing motor output until a plan is resolved.
Box 3. Questions for future research.
Our framework emphasizes commonalities across prefrontal regions in representing and maintaining abstract information, with regions varying in the content of this information and in connectivity with other brain regions that are biased or inhibited by prefrontal regions. Are there differences in neural mechanisms that distinguish prefrontal regions to create gradations or diversity in this overall framework?
What is the best way to characterize the content of prefrontal representations in individual regions? This question has been debated extensively [78-81], but how do the answers about functional role change when viewed with the emphasis on information content we use here?
What is the relationship between prefrontal areas that lead to directed global inhibition versus those that support competitive inhibition? (Identical, overlapping, mutually exclusive?)
Could the prefrontal cortex also support directed global inhibition of cortical regions, but researchers just haven’t discovered it yet? We believe this possibility is unlikely (e.g., given the adaptive arguments in Fig 1), but it would be convincingly demonstrated by: a) the existence of prefrontal regions synapsing preferentially onto inhibitory neurons in a cortical region, with activation of the prefrontal neurons leading to inhibition of the excitatory neurons in the other cortical region, or b) a similar functional role for the tiny minority of long-range projections that are inhibitory – currently these projections are thought to primarily serve to synchronize excitatory firing [54].
What is the relationship between delaying a response and preventing a response altogether? Do they tap the same neural mechanisms? In the case of working memory, the basal ganglia is thought to signal when information should be maintained in PFC (via activation of the NoGo pathway) and when the PFC should update to encode new information (via activation of the Go pathway [82]). Applying this idea to motor control, the NoGo pathway could delay the initiation of a motor action, rather than outright inhibit it. What kinds of targeted experiments could test this account?
Can indirect competitive and directed global inhibitory dynamics themselves be inhibited, or might they fall beyond the scope of cognitive control?
Right middle frontal gyrus, hippocampus, and memory suppression
The right middle frontal gyrus (rMFG) appears to exert inhibitory control over memory-related areas such as the hippocampus, to support suppression of memory retrieval in the Think No-Think (TNT) paradigm [40-42]. After subjects learn pairs of stimuli (cue-target), they are presented with only the cues, and are instructed repeatedly for certain items to “think” (T) about the associated target, and for other items to “not think” (NT) of the associated target. Recall of NT items is worse than both T items and baseline items [43-45]. Moreover, mid-lateral PFC areas (particularly rMFG), which are anatomically connected to the hippocampal memory system [46,47] (although preferential synapsing onto inhibitory interneurons has not been established), get more active as participants get practice in inhibiting no-think items, while hippocampal activation gets correspondingly decreased [41].
These patterns are compatible with the idea that the rMFG provides directed global inhibition of the hippocampus and associated memory retrieval processes [41,42]. Repeated inhibition of the entire hippocampal system in response to NT cues may lead these cues (and corresponding targets) to become associated with the rMFG representations that trigger inhibition of the hippocampus. As a result, the global inhibition process is triggered in response to NT cues (or other cues associated with the targets). This account is compatible with the idea that rather than being specialized for inhibition per se, rMFG (like other areas of PFC) maintains relevant abstract information, in this case about the relevance of the cue for a specific goal, which ultimately leads to reduced activity in the hippocampus and inhibition of memory retrieval.
Computational models have also demonstrated how transient activation of specific items in memory can lead to learning effects that mimic inhibitory processes [48,49]. For example, if a NT target is activated by a cue, then de-activated through directed inhibition, this results in a long-term depression for the synaptic connections for the NT target item, making it more difficult to activate. This learning account opens an important new dimension in thinking about inhibition as occurring via long-term changes to the strength of the representation itself.
Indirect competitive inhibition within cortical and subcortical regions
In contrast to the direct inhibition of subcortical and archicortical areas, data from neuroscience and computational models suggest that inhibition within neocortical regions takes an indirect, competitive form. In this type of inhibition, rather than representing “Don’t do X”, prefrontal regions provide top-down support for the representations related to “Do Y” [37]. As a result, alternative representations are inhibited, via diffuse lateral inhibitory connectivity, which serves to amplify activity in the most active representations (e.g., those receiving the most top-down, lateral, and bottom-up support) and suppress competitors. This type of indirect competitive mechanism is commonly discussed in theories of selection and attention [50-53].
Evidence that inhibition within neocortical regions takes a competitive form comes from several sources. First, the vast majority of long-range interregional connectivity is mediated through excitatory pyramidal cells (99.9% by some estimates;[54]). Although these excitatory projections can synapse on local inhibitory interneurons, there is no evidence of preferential connectivity onto inhibitory neurons as would be required to create the kind of directed inhibition observed in non-neocortical regions (e.g., in the vmPFC->DRN pathway). Moreover, relative to excitatory neurons, inhibitory interneurons have broader, more diffuse patterns of connectivity, and considerably more coarse tuning functions, such that they represent and transmit information with less specificity than excitatory neurons [55-57]. These data are consistent with inhibitory interneurons serving to regulate overall levels of activity, so that only the most competitive (active) neurons rise above the inhibition, rather than directly targeting specific cortical representations or areas. Such local competitive inhibition occurs throughout the brain, from sensory visual areas [50] to multimodal parietal areas [51], and within prefrontal cortex itself [52], as well as within some subcortical regions (e.g., the thalamic reticular nucleus, [53]), which can thus be subject to both competitive and global inhibition.
At a broader level, cortical areas also compete with one another (e.g., [58]) potentially via competitive inhibition within associated subcortical regions and via excitatory top-down and lateral biases [12]. For example, activation of a given cortical region can act to reduce support for processing in other cortical regions. One potential mechanism underlying this effect is competition within the thalamic reticular nucleus, a region that allows for competition among thalamic regions connecting to nearly all areas of cortex [53]. This type of competition among regions may suggest alternatives to some directed global inhibition accounts. For example, although this account is not favored by researchers working in this domain, one might ask whether increased rMFG activation in the Think No-Think paradigm may reflect people strategically directing their thoughts to something other than the target, with hippocampal activations decreasing as a result of competitive inhibitory dynamics among regions, in the context of a focus on cognitive processes other than retrieval.
Finally, computational models have demonstrated how competitive inhibition can explain behaviors thought to require direct inhibitory processes [11,59]. On inhibitory tasks, actively-maintained PFC representations (e.g., simulating a maintained “color” representation in dorsolateral PFC during the Stroop task) provide an excitatory top-down bias to enhance associated representations (e.g., of color information in posterior cortical regions); those enhanced representations then competitively inhibit alternative representations (e.g., of word meaning) [12]. When the models cannot sufficiently maintain goal information, they mimic the apparent inhibitory deficits (such as perseverative behavior) that occur after prefrontal damage or during childhood when the frontal lobes are not fully developed. However, the deficits occur not because of problems with directed global inhibition, but because prepotent responses win over task-relevant ones in the competitive inhibitory dynamic.
Concluding remarks
Inhibitory phenomena clearly do not share one common neural substrate. As such, the important psychological construct of inhibition becomes more nuanced when viewed through the lens of neuroscience (Box 3). We have argued that what is commonly called “inhibition” at the functional level is actually subserved by at least two distinct types of neural mechanisms, targeted global inhibition and indirect competitive inhibition. There are many facets to prefrontal function and many contributions to executive function from distributed networks extending beyond the PFC [60,61]; we have focused on the PFC given its specialization for the most robust maintenance of goal-relevant information [62,63] and given the centrality of this function in inhibitory processes[15]. What distinguishes prefrontal regions and their roles in distinct types of inhibition is the nature of their connectivity with other brain regions and the content of the abstract information represented.
We have emphasized the distinction between directed global inhibition and competitive inhibition, but we note that they are not mutually exclusive and can work in concert. For instance, should competitive inhibition fail to focus processing on the ink color in the Stroop task, global inhibition of the response (through conflict signals activating the STN) could prevent an erroneous response ([64,65]). For both types of inhibition, the contribution from prefrontal cortical regions can be understood in terms of actively representing and maintaining abstract goal-related information (e.g., to identify the ink color, and conflict associated with this task), rather than representing what should be inhibited (e.g., “Don’t attend to or read the word”). This unifying framework suggests that prefrontally-mediated inhibitory deficits can reflect problems in knowing or maintaining the context that signals when to inhibit or in activating alternative behaviors to reach a goal, rather than problems in inhibition per se, and that treatments should be tailored accordingly (Box 4).
Box 4: Clinical implications.
The unifying model proposed here has implications for psychopathology. A variety of externalizing disorders, all of which begin to manifest during childhood and adolescence, including attention-deficit hyperactivity disorder (ADHD), substance use disorders, oppositional defiant disorder and conduct disorder, are thought to be characterized by behavioral disinhibition (e.g., [1]) -- the inability to inhibit impulses and to forego attractive potential rewards when they are socially inappropriate and/or may result in negative consequences. Deficits in response inhibition are often observed in individuals with externalizing disorders, and more so than deficits in working memory updating or set shifting [83]. With regard to the neural substrates of such difficulties, abnormalities in prefrontal brain activation, most notably in mid- and inferior prefrontal areas, are observed in individuals with these disorders when performing tasks related to response inhibition (e.g., ADHD: [42,84]; Substance Use Disorders: [85]; Conduct Disorder: [84]).
The current framework suggests that these disruptions in response inhibition reflect problems in using environmental cues to activate and maintain information in PFC about which actions are most appropriate in a given context, rather than problems in downstream inhibitory effects (e.g., in the STN global inhibition of the outputs of the basal ganglia). As such, it is unlikely that the problem in a child with conduct disorder is solely one of inhibiting the motor movement of hitting another child, or that the child with ADHD has a specific problem in inhibiting their vocalizations. Rather, these children may have difficulties maintaining a task set such as gaining social acceptance. Consistent with this perspective, the deficits in response inhibition observed in externalizing disorders are attributable to deficits in a common executive function factor, which is tapped across executive function tasks and is interpreted in terms of goal maintenance (Friedman et al., unpublished data; see also [86]).
These considerations lead to the prediction that effective therapeutic interventions for these disorders will have somewhat similar characteristics (e.g., as observed for ADHD and conduct disorder; [87]), and should be geared toward supporting PFC maintenance of the appropriate contextual information. Effective pharmacological interventions are likely to be ones that affect prefrontal and basal ganglia activity in neurologically-normal individuals (e.g. [88]) and normalize the activity of prefrontal cortex in individuals with clinical disorders (e.g., [89]). Effective behavioral approaches are likely to be those that emphasize what behaviors are appropriate in which contexts, including positive reinforcement, token economies, and time outs.
Acknowledgements
We thank Jessica Andrews-Hannah, Tim Curran, Naomi Friedman, Tom Hazy, Akira Miyake, Hannah Snyder, Melanie Stollstorff, and other members of the P50 center on Executive Function and Dysfunction for useful discussions and comments on the manuscript, and Eden Davis and Andrei Semenov for assistance with manuscript preparation. The writing of this paper was supported by grants from the National Institutes of Health (P50-MH079485 and RO1HD37163).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iacono WG, et al. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annu. Rev. Clin. Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 2.Diamond A. A model system for studying the role of dopamine in prefrontal cortex during early development in humans. In: Johnson MH, Munakata Y, Gilmore RO, editors. Brain Development and Cognition: a Reader. Blackwell Press; 2002. pp. 441–493. [Google Scholar]
- 3.Hasher L, et al. Inhibitory mechanisms and the control of attention. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in Working Memory. Oxford University Press; 2007. pp. 227–249. [Google Scholar]
- 4.Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 5.Friedman NP, et al. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychol. Sci. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 6.Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod CM, et al. In opposition to inhibition. In: Ross BH, editor. Psychology of Learning and Motivation. Vol. 43. Academic Press; 2003. [Google Scholar]
- 8.Tabibnia G, et al. Different forms of self-control share a neurocognitive substrate. J. Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle V, editors. Handbook of Physiology: The Nervous System. 1987. pp. 373–417. Am. Physiol. Soc. [Google Scholar]
- 10.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychol. Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Herd S, et al. Neural mechanisms of cognitive control: An integrative model of Stroop task performance and fMRI data. J. Cogn. Neurosci. 2006;18:22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- 13.Stedron JM, et al. Common mechanisms for working memory and attention: The case of perseveration with visible solutions. J. Cogn. Neurosci. 2005;17:623–631. doi: 10.1162/0898929053467622. [DOI] [PubMed] [Google Scholar]
- 14.Velanova K, et al. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman NP, et al. Individual differences in executive functions are almost entirely genetic in origin. J. Exp. Psychol. Gen. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baratta M, et al. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur. J. Neurosci. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajos M, et al. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- 19.Amat J, et al. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 21.Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 22.Beauregard M, et al. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan KL, et al. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenbaum G, et al. A new perspective on the role of the orbitofrontal cortex in adaptive behavior. Nat. Rev. Neurosci. 2010;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank MJ, et al. Hold your horses: Impulsivity, deep brain stimulation, and medication in Parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 27.Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70:1991–1995. doi: 10.1212/01.wnl.0000313022.39329.65. [DOI] [PubMed] [Google Scholar]
- 28.Nambu A, et al. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci. Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 29.Botvinick M, et al. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 30.Garavan H, et al. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 31.Grinband J, et al. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011:1–9. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampshire A, et al. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodds CM, et al. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb. Cortex. 2011;21:1155–1165. doi: 10.1093/cercor/bhq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatham CH, et al. Cognitive control reflects context monitoring, not stopping, in response inhibition. PloS ONE. doi: 10.1371/journal.pone.0031546. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharp DJ, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. P. Natl. Acad. Sci. USA. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chikazoe J, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb. Cortex. 2008;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- 37.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 38.Jackson MC, et al. Strategic resource allocation in the human brain supports cognitive coordination of object and spatial working memory. Hum. Brain Mapp. 2010;32:1330–1348. doi: 10.1002/hbm.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fink GR, et al. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: An fMRI study with clinical implications. Neuroimage. 2003;20:1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Anderson MC. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 41.Depue BE, et al. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- 42.Depue BE, et al. Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: Evidence from deficits in individuals with ADHD. Neuropsychologia. 2010;48:3909–3917. doi: 10.1016/j.neuropsychologia.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:131–134. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- 44.Wessel I, et al. Dissociation and memory suppression: A comparison of high and low dissociative individuals’ performance on the Think-No Think task. Pers. Indiv. Differ. 2005;39:1461–1470. [Google Scholar]
- 45.Depue BE, et al. Suppression of emotional and nonemotional content in memory. Psychol. Sci. 2006;17:441–447. doi: 10.1111/j.1467-9280.2006.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman-Rakic PS, et al. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 47.Morris R, et al. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J. Comp. Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 48.Norman KA, et al. How inhibitory oscillations can train neural networks and punish competitors. Neural Comput. 2006;18:1577–1610. doi: 10.1162/neco.2006.18.7.1577. [DOI] [PubMed] [Google Scholar]
- 49.Norman KA, et al. A neural network model of retrieval-induced forgetting. Psychol. Rev. 2007;114:887–953. doi: 10.1037/0033-295X.114.4.887. [DOI] [PubMed] [Google Scholar]
- 50.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 51.Cohen JD, et al. Mechanisms of spatial attention: The relation of macrostructure to microstructure in parietal neglect. J. Cogn. Neurosci. 1994;6:377–387. doi: 10.1162/jocn.1994.6.4.377. [DOI] [PubMed] [Google Scholar]
- 52.Snyder HR, et al. Neural inhibition enables selection during language processing. P. Natl. Acad. Sci. USA. 2010;107:16483–16486. doi: 10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAlonan K, et al. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamamaki N, Tomioka R. Long-range GABAergic connections distributed throughout the neocortex and their possible function. Front. Neurosci. 2010;4:202. doi: 10.3389/fnins.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 56.Roerig B, Chen B. Relationships of local inhibitory and excitatory circuits to orientation preference maps in ferret visual cortex. Cereb. Cortex. 2002;12:187–198. doi: 10.1093/cercor/12.2.187. [DOI] [PubMed] [Google Scholar]
- 57.Swadlow HA, Gusev AG. Receptive-field construction in cortical inhibitory interneurons. Nat. Neurosci. 2002;5:403–404. doi: 10.1038/nn847. [DOI] [PubMed] [Google Scholar]
- 58.Kaster S, Ungerleider L. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 59.Morton JB, Munakata Y. Active versus latent representations: A neural network model of perseveration, dissociation, and decalage in childhood. Dev. Psychobiol. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- 60.Dosenbach NUF, et al. Distinct brain networks for adaptive and stable task control in humans. P. Natl. Acad. Sci. USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luna B, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 62.Miller EK, et al. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi AF, et al. The prefrontal cortex and the executive control of attention. Exp. Brain Res. 2008;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egner T, et al. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 65.Silton RL, et al. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aron AR, Verbruggen F. Stop the presses: Dissociating a selective from a global mechanism for stopping. Psychol. Sci. 2008;19:1146–1153. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 67.Cai W, et al. A proactive mechanism for selective suppression of response tendencies. J. Neurosci. 2011;31:5965–5969. doi: 10.1523/JNEUROSCI.6292-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mink J. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog. Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 69.Frank MJ, et al. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cogn. Affect Behav. Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 70.Cools R, et al. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albert NB, Ivry RB. The persistence of spatial interference after extended training in a bimanual drawing task. Cortex. 2009;45:377–385. doi: 10.1016/j.cortex.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padmala S, Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2010;48:558–565. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chambers CD, et al. Executive “brake failure” following deactivation of human frontal lobe. J. Cogn. Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 74.Verbruggen F, et al. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. P. Natl. Acad. Sci. USA. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb. Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neubert F, et al. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. P. Natl. Acad. Sci. USA. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reis J, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 2007;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banich MT. Executive function: The search for an integrated account. Curr. Dir. Psychol. Sci. 2009;18:89–94. [Google Scholar]
- 79.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 80.O’Reilly RC. The what and how of prefrontal cortical organization. Trends Neurosci. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Phil Trans. Royal Soc. London B Biol. Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314:91–94. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- 83.Young SE, et al. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J. Abnorm. Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubia K, et al. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum. Brain Mapp. 2009;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garavan H, et al. Acute effects of cocaine on the neurobiology of cognitive control. Phil. Trans. Royal Soc. London B Biol. Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman NP, et al. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Dev. Psychol. 2011 doi: 10.1037/a0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Connor DF, et al. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J. Dev. Behav. Pediatr. 2010;31:427–440. doi: 10.1097/DBP.0b013e3181e121bd. [DOI] [PubMed] [Google Scholar]
- 88.Chamberlain SR, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol. Psychiat. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 89.Epstein JN, et al. ADHD and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J. Child Psychol. Psyc. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]