Abstract
Background
Fatalistic beliefs about cancer have been implicated in low uptake of screening and delay in presentation particularly in low SES groups, but no studies have systematically evaluated inter-relationships between SES, fatalism, and early detection behaviours. We explored whether i) fatalism is associated with negative attitudes towards early detection, ii) lower SES groups are more fatalistic, and iii) SES differences in fatalism partly explain SES differences in attitudes towards early detection.
Methods
In a population-representative sample of adults in Britain using computer-based interviews in the home setting, respondents (N=2018) answered two questions to index fatalism (expectations of cancer survival and cure) and two items on early detection attitudes (the perceived value of early detection and fear of symptom reporting). SES was indexed with a social grade classification.
Results
Fatalism was associated with being less positive about early detection (β=−0.40, p<.001) and more fearful about seeking help for a suspicious symptom (β=0.24, p<.001). Lower SES groups were more fatalistic (β=−0.21, p<.001). Path analyses suggest that SES differences in fatalism might explain SES differences in attitudes about early detection.
Conclusions
In this population sample, SES differences in fatalism partly explained SES differences in the perceived value of early detection and fear of symptom presentation.
Impact
Fatalistic beliefs about cancer should be targeted in order to promote early presentation of cancer and this may be particularly important for lower SES groups.
Keywords: cancer fatalism, early detection, fear, social inequalities
INTRODUCTION
Fatalistic beliefs about cancer have been implicated in decision-making about cancer screening and symptomatic presentation (1-3). Fatalism is traditionally conceived as the perception that events and/or health issues are out of individual control (1-2). It differs from similar concepts such as low response efficacy and high external locus of control in that, in addition to believing they are powerless over the outcome of the event/health issue, a fatalistic individual assumes that the outcome will be negative ( e.g. that the disease will be fatal).Fatalistic views about the survivability of cervical cancer have been associated with a lower likelihood of being up-to-date with cervical cancer screening in a sample of Latino women (4), and fatalistic ideas about curability were associated with being less likely to have had a clinical skin exam (5). Fatalism has also been linked with avoidance of cancer-related information (6).
A number of sociological and psychological analyses have suggested that lower SES groups are more fatalistic (7-9). Qualitative studies consistently identify fatalistic attitudes about cancer in underserved groups (10, 11), and lower SES has been associated with being more fatalistic about surviving breast cancer (12).
Delay in symptomatic presentation is one of a number of ‘downstream’ factors attracting interest as a potential modifiable determinant of SES differences in cancer survival (13, 14). Von Wagner et al argued that the life experience of those living in more socioeconomically deprived environments could engender fatalistic beliefs about cancer, which in turn would reduce the likelihood of taking up opportunities for early detection (3). This idea gains support from one of the few population-based studies of cancer attitudes, which found that people from lower SES groups were more likely to cite fear of cancer as a deterrent to seeking medical advice (15).
However, the existing literature tends to focus either on fatalism or early detection. The aim of the present study was to assess both fatalism and attitudes towards early detection in a population-based sample. This was a preliminary exploration of the topic and focused on one aspect of cancer fatalism: ‘the inevitability of death’ (16), which was assessed by beliefs about cancer curability and survival. We explored whether: i) fatalism about cancer is associated with more negative attitudes about early detection (lower perceived value of early detection and higher fear of symptom reporting), ii) lower SES is associated with higher cancer fatalism and more negative early detection attitudes, and iii) SES differences in fatalism partly explain SES differences in attitudes towards early detection.
METHOD
Study Population
Data were collected from 2018 adults (937 male, 1081 female) as part of the British Market Research Bureau International’s (BMRB) face-to-face Omnibus survey of a nationally representative UK sample of 2,000 adults (aged >15 years) in September 2009. Interviews were carried out during household visits using a computer assisted system. BMRB uses a random location, quota-sampling technique ACORN strata are used to classify neighbourhoods according to census characteristics, which ensures that all area types are correctly represented, making SES quotas unnecessary. Within each sampling point, quota controls are set for sex, age and employment status, and additional controls are used to correct for variation in the likelihood of being at home at the time of the interview. The data were weighted using a rim weighting technique (17) that targets demographic variables so that the sample profiles match the population and allocates weights to each individual to balance the overall composition of the sample.
Measures
Single item measures were generated through a rapid review of the literature on cancer fatalism and early detection attitudes. A long list of items was collated and scrutinised by researchers with expertise in this field. We were able to include only two items relating to fatalism because of space restrictions. The first (cancer survival expectations) was selected to enable us to quantify the extent of fatalism through comparisons with actual survival rates: ‘Out of 100 people with a cancer diagnosis, how many do you think would be alive 5 years later. Responses were categorised into 10-percentage point intervals from 0-10 through to 91-100, and a ‘don’t know’ option. The second (perceived curability of cancer) used more natural language and reflected the way cancer fatalism in relation to survival had more typically been asked about in the literature (16): ‘Many people who get cancer can be completely cured’, with responses on a 5-point Likert scale labelled from ‘strongly disagree’ to ‘strongly agree’.
Two items related to early detection attitudes were selected on the basis of the Cancer Awareness Measure (15): i) the perceived value of early detection: ‘The earlier cancer is detected, the greater the chance of successful treatment’, and ii) fear of symptom reporting: ‘If I had a symptom that I thought might be cancer, I would be too frightened to seek medical advice’. Responses for both used a 5-point Likert scale labelled from ‘strongly disagree’ to ‘strongly agree’.
SES was determined using the social grade classification created by the National Readership Survey (18), which classifies people into AB (higher or intermediate managerial or professional occupations), C1 (supervisory or junior managerial occupations), C2 (skilled manual workers), D (semi-skilled and unskilled manual workers), and E (state pensioners or lowest grade workers). We combined D and E categories to equalise group sizes. Sex and age were recorded.
Statistical analyses
Data were analysed using the Statistical Package for the Social Sciences (SPSS) 17.0 and EQS Version 6.0 (19). Descriptive statistics were completed for SES, sex and age. SES differences in survival expectations were analysed using chi square. SES differences in perceived curability, perceived value of early detection, and fear of symptom reporting were explored using ANCOVA, controlling for age and sex, with polynomial contrasts to assess linear trends. We used path analyses to examine the indirect relationship (via fatalism) between SES and both fear of reporting symptoms and the perceived value of early detection.
The Mardia normalized estimate was 5.66, which shows significant multivariate kurtosis (p<.001). Therefore we used the robust maximum likelihood method, which takes non-normality into account and reports the Satorra Bentler scaled chi-square (SB χ2) (20). As the SB χ2 statistic is influenced by large sample sizes, we also calculated model fit according to accepted cut-offs (21); above 0.95 for the comparative fit index (CFI), above 0.90 for the normed fit index (NFI), and below 0.06 for the root mean square error of approximation (RMSEA) (22). For path analyses, curability and estimated survival were treated as a latent construct representing ‘fatalism’. Higher scores represented higher SES and higher fatalism. Cases with missing data, including ‘don’t know’ were excluded from analyses.
RESULTS
The average age of respondents was 47.4 ± 18.7 years, with slightly more women than men (54% vs. 46%). Respondents were distributed across social grade categories: AB (20%, N=408; C1 (27%, N=545); C2 (19%, N=390) and D/E (34%, N=675). AB and D/E participants were slightly older than the others, F(3,2014)=6.24, p<.001, but there were no gender differences between social grades.
There were no age differences in survival estimates but older people had higher expectations of cure (r=.20, p<.001). There was no age difference in fear of reporting symptoms, but older people rated early detection as more important (r=.08, p<.001). Age was controlled for in subsequent analyses. There were no significant sex differences in any responses.
Fatalism and attitudes to early detection
Fatalism was associated with lower perceived value of early detection (β=−.41, p<.001) and greater fear of reporting symptoms (β=.25, p<.001).
SES differences in cancer beliefs and attitudes towards early detection
Respondents were realistic about cancer survival; with the modal choice of ‘51-60%’; reflecting the current UK statistics of 51% for overall 5 year survival (23), although the spread of responses was wide. Because survival estimates were not normally distributed, we grouped them based on current survival figures (23) into ‘accurate’ (the 41-50% or 51-60% categories), ‘pessimistic’ (≤40%) and ‘optimistic’ (>60%). Respondents in the lowest SES category were less likely to be optimistic (lowest SES= 26%; highest SES= 41%;) and more likely to be pessimistic (29% vs 19%) [χ2(6)=25.80, p<.001]. There were significant linear trends for optimism (decreasing) and pessimism (increasing) from higher to lower SES groups [linear-by-linear association (1)=23.13, p<.001].
For the statement: ‘Many people who get [….] cancer can be completely cured’, the majority of respondents agreed or strongly agreed (56%). ANCOVA analyses controlling for age showed that lower SES groups were significantly less likely to believe that cancer could be cured [F(3, 2000)=4.58, p<.01], and there was a significant linear trend across SES groups (contrast estimate=−0.13, CI -.23, 04, p<.01). See Table 1.
Table 1.
Mean response by SES group
| SES | Perceived Curability (Mean, SD) |
Perceived Value of Early detection (Mean, SD) |
Fear of symptom reporting (Mean, SD) |
|---|---|---|---|
| AB | 3.48 (1.09) | 4.56 (0.84) | 1.53 (0.91) |
| C1 | 3.51 (1.00) | 4.48 (0.94) | 1.73 (1.09) |
| C2 | 3.37 (1.11) | 4.39 (0.93) | 1.76 (1.15) |
| D/E | 3.31 (1.04) | 4.30 (0.92) | 1.91 (1.19) |
|
| |||
| Fa (η2) | 4.58** (0.10) | 5.70*** (0.10) | 9.87*** (0.14) |
| Contrast estimate |
−0.13 ** | −0.19*** | 0.26*** |
F=ANCOVA analyses controlling for age and sex, η2= eta squared, contrast estimate=polynomial analyses
=p<.01
=p<.001
(AB=highest SES category, D/E=lowest SES category).
The majority of respondents (91%) agreed or strongly agreed with: ‘The earlier cancer is detected, the greater the chance of successful treatment’. Nonetheless, lower SES respondents were significantly less positive about the value of early detection than those in higher SES groups [F(3,2000)=5.70, p<.001], with a decreasing linear trend from higher to SES groups (contrast estimate=−0.19, p<.001). See Table 1.
Only 10% of respondents agreed or strongly agreed with ‘If I had a symptom that I thought might be cancer, I would be too frightened to seek medical advice’. However, as predicted, lower SES was associated with higher fear of reporting symptoms [F(3,2000)=9.87, p<.001], with a significant linear trend (contrast estimate=0.26, p<.001). See Table 1.
Path modelling
We examined the hypothesis that SES differences in early detection attitudes would be partly explained by SES differences in fatalism by assessing the indirect relationship between SES and these outcomes via the (latent) fatalism construct (see Figure 1).
Figure 1.
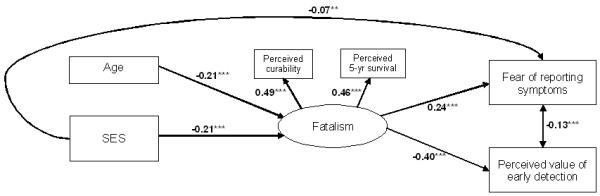
Path model for the relationship between SES, fatalism and fear of reporting symptoms/perceived value of early detection. Standardised coefficients reported. Residuals were estimated but are not included in the diagram for ease of reading, ***=p<.001
Consistent with the results described above, there was a direct relationship between SES and fear of reporting symptoms (β=−0.12, p<.001) and between SES and perceived value of early detection (β=0.12, p<.001). After including the latent fatalism vairable in the model, the relationship between SES and perceived value of early detection was fully mediated. The relationship between SES and fear of reporting symptoms was partially mediated (β=−0.07, p<.001) but the reduction was significant (Sobel test=−3.04, SE=p<.001). There were also significant indirect relationships: lower SES was associated with higher fatalism (β=−0.21, p<.001), which was associated with greater fear of reporting symptoms (β=0.24, p<.001) and lower perceived value of early detection (β=−0.40, p<.001). After using the Wald test to improve model fit by removing unnecessary pathways, the overall fit was acceptable. Although the SBχ2 was significant [(7)=32.67, p<.001], all other indices were indicative of a good-fitting model: NFI=0.92, CFI=0.93; RMSEA=0.04 (90% CI; 0.03, 0.06). The model explained 7% of the variance in fear of reporting symptoms and 17% of the variance in perceived value of early detection.
DISCUSSION
In this population-representative sample, fatalistic beliefs were comparatively rare and attitudes to cancer were broadly positive. The respondents’ estimated modal of 5-year survival rate was comparable to the ‘true’ survival figure and on average respondents were optimistic about cure. The majority also expressed a positive attitude towards early detection, and only a minority thought that fear would deter them from presenting with possible cancer symptoms. However, against this positive backdrop, lower SES respondents were, as predicted, more fatalistic, less positive about the value of early detection and more fearful of reporting symptoms. This is in line with previous research into demographic differences in cancer fatalism (24, 25). Individuals who were more fatalistic were also more fearful of reporting symptoms and had lower perceived value of early detection; supporting previous research indicating that cancer fatalism has a detrimental effect on early diagnosis (4, 24).
We used path analyses to test whether individuals from lower SES backgrounds would have more fatalistic beliefs about cancer and because of this, be less likely to value early detection (3). The results showed that there was an indirect association between SES and fear of reporting symptoms via the construct of fatalism, and there was no direct pathway between SES and perceived value of early detection when fatalism was included in the model. In other words, people from lower SES backgrounds are more fatalistic about cancer, and this is partly why they see it as less worthwhile to detect it early (16).
In contrast to previous findings (25), we found older respondents perceived cancer to be more curable than younger respondents. This may be because they have had more exposure to cancer screening programmes. However, because the findings relating age to fatalism have been mixed (2, 25), further clarification is required.
This study had several limitations. Fear was the only specific barrier to presentation that we examined, and although it has been identified as one of the most common barriers (14, 26), other factors play a role (15). Re-appraising symptoms as ‘not important’ has also been associated with greater delay (26), so it would be valuable to investigate SES differences in re-appraisal. An additional limitation was the use of single-item attitude measures which are less reliable than multiple-item scales and could have led to underestimates of SES differences. We also measured attitudes to early detection rather than behaviour and cannot assume that the results would be the same with objective measures. SES was defined in terms of occupational status only, and findings could be different with other indices of SES (e.g. years of education or income). The study was cross-sectional so we were unable to consider the temporal aspects of relationships between variables (3, 27). Despite the limitations, the results of this study highlight the potential importance of fatalism about cancer, and raise the possibility that SES differences in fatalism may ultimately translate into inequalities in cancer survival.
References
- (1).Straughan PT, Seow A. Fatalism reconceptualized: a concept to predict health screening behavior. J Gender Cult Health. 1998;3(2):85–100. [Google Scholar]
- (2).Niederdeppe J, Levy AG. Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev. 2007;16(5):998–1003. doi: 10.1158/1055-9965.EPI-06-0608. [DOI] [PubMed] [Google Scholar]
- (3).von Wagner C, Good A, Whitaker KL, Wardle J. Psychosocial predictors of socioeconomic inequalities in cancer screening participation: A conceptual framework. Epidemiol Rev. 2011 doi: 10.1093/epirev/mxq018. [DOI] [PubMed] [Google Scholar]
- (4).Chavez LR, Hubbell FA, Mishra SI, Valdez RB. The influence of fatalism on self-reported use of Papanicolaou smears. Am J Prev Med. 1997;13(6):418–24. [PubMed] [Google Scholar]
- (5).Michielutte R, Dignan MB, Sharp PC, Boxley J, Wells HB. Skin cancer prevention and early detection practices in a sample of rural women. Prev Med. 1996;25(6):673–83. doi: 10.1006/pmed.1996.0106. [DOI] [PubMed] [Google Scholar]
- (6).Miles A, Voorwinden S, Chapman S, Wardle J. Psychologic predictors of cancer information avoidance among older adults: The role of cancer fear and fatalism. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1872–9. doi: 10.1158/1055-9965.EPI-08-0074. [DOI] [PubMed] [Google Scholar]
- (7).Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Socioeconomic Status and Health in Industrial Nations. 1999;896:210–25. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- (8).Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. J Epidemiol Community Health. 2003;57(6):440–3. doi: 10.1136/jech.57.6.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pill R, Peters TJ, Robling MR. Social class and preventive health behavior - A British example. J Epidemiol Community Health. 1995;49(1):28–32. doi: 10.1136/jech.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Peek ME, Sayaa JV, Markwardt R. Fear, fatalism and breast cancer screening in low income African-American women: The role of clinicians and the health care system. J Gen Intern Med. 2008;23(11):1847–53. doi: 10.1007/s11606-008-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).James AS, Hall S, Greiner KA, Buckles D, Born WK, Ahluwalia JS. The impact of socioeconomic status on perceived barriers to colorectal cancer testing. Am J Health Promot. 2008;23(2):97–100. doi: 10.4278/ajhp.07041938. [DOI] [PubMed] [Google Scholar]
- (12).Grunfeld EA, Ramirez AJ, Hunter MS, Richards MA. Women’s knowledge and beliefs regarding breast cancer. Br J Cancer. 2002;86(9):1373–8. doi: 10.1038/sj.bjc.6600260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14(8):761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- (14).Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(Suppl 2):S92–S101. doi: 10.1038/sj.bjc.6605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Waller J, et al. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009;101(S2):S18–S23. doi: 10.1038/sj.bjc.6605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26(6):454–65. doi: 10.1097/00002820-200312000-00005. [DOI] [PubMed] [Google Scholar]
- (17). [Accessed 18th July 2011];BMRB Target Group Index. 2007 http://kantarmedia-tgigb.com/tgi-surveys/gb/
- (18).National Readership Survey (NRS) [Accessed 18th July 2011];2007 http://www.nrs.co.uk/lifestyle.html.
- (19).Bentler P. EQS 6 Structural Equations Program Manual. Multivariate Software, Inc; Encino, CA: 2006. [Google Scholar]
- (20).Tabachnick BG, Fidell LS. Using Multivariate Statistics. Fourth Edition Allyn & Bacon; Needham Heights, MA: 2001. [Google Scholar]
- (21).Ullman JB. Structural Equation Modeling. In: Tabachnick BG, Fidell LS, editors. Using Multivariate Statistics. Fourth Edition Allyn & Bacon; Needham Heights, MA: 2001. pp. 653–771. [Google Scholar]
- (22).Hu LT, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Coventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- (23).Cancer Research UK [Accessed 5th May. 2011];All cancers combined statistics - Key Facts. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@sta/documents/generalcontent/crukmig_1000ast-2750.pdf.
- (24).Perezstable EJ, Sabogal F, Oterosabogal R, Hiatt RA, Mcphee SJ. Misconceptions about cancer among latinos and anglos. JAMA. 1992;268(22):3219–23. doi: 10.1001/jama.1992.03490220063029. [DOI] [PubMed] [Google Scholar]
- (25).Mayo RM, Ureda JR, Parker VG. Importance of fatalism in understanding mammography screening in rural elderly women. J Women Aging. 2001;13(1):57–72. doi: 10.1300/J074v13n01_05. [DOI] [PubMed] [Google Scholar]
- (26).Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet. 2005;366(9488):825–31. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- (27).Whitaker KL, Good A, Miles A, Robb K, Wardle J, von Wagner C. Socioeconomic inequalities in colorectal cancer screening uptake: does time perspective play a role? Health Psychol. 2011 doi: 10.1037/a0023941. In Press. [DOI] [PubMed] [Google Scholar]


