Abstract
Chronic constipation affects almost one in six adults and is even more frequent in the elderly. In the vast majority of patients, there is no obstructive mucosal or structural cause for constipation and, after excluding relatively rare systemic diseases (commonest of which is hypothyroidism), the differential diagnosis is quickly narrowed down to three processes: evacuation disorder of the spastic (pelvic floor dyssynergia, anismus) or flaccid (descending perineum syndrome) varieties, and normal or slow transit constipation. Treatment of chronic constipation based on identifying the underlying pathophysiology is generally successful with targeted therapy. The aims of this review are to discuss targeted therapy for chronic constipation: behavioural treatment for outlet dysfunction and pharmacological treatment for constipation not associated with outlet dysfunction. In particular, we shall review the evidence that behavioural treatment works for evacuation disorders, describe the new treatment options for constipation not associated with evacuation disorder, and demonstrate how `targeting therapy' to the underlying diagnosis results in a balanced approach to patients with these common disorders.
INTRODUCTION
Chronic constipation affects almost one in six adults and is even more frequent in the elderly. In the vast majority of patients, there is no obstructive mucosal or structural cause for constipation and, after excluding relatively rare systemic diseases (commonest of which is hypothyroidism), the differential diagnosis is quickly narrowed down to three processes: evacuation disorder of the spastic (pelvic floor dyssynergia, anismus) or flaccid (descending perineum syndrome) varieties, and normal or slow transit constipation.1
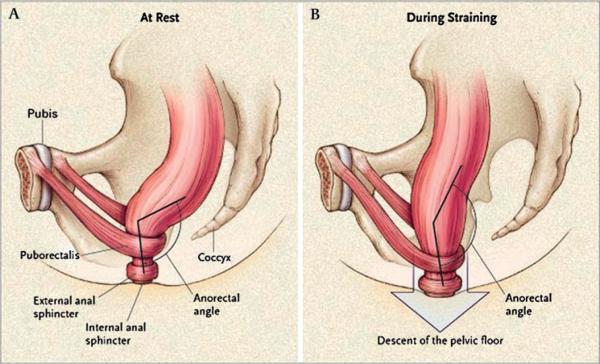
Figure 11 illustrates the function of the pelvic floor and anal sphincters during the process of defecation. The coordinated relaxation of the pelvic floor and anal sphincters, together with propulsion of content in the distal colon and raised intra-abdominal pressure during straining, allow the straightening of the rectoanal angle and comfortable, unimpeded evacuation of stool.
Figure 1.
Function of the pelvic floor and anal sphincters during defaecation. The coordinated relaxation of the pelvic floor and anal sphincters, together with propulsion of content in the distal colon and raised intra-abdominal pressure during straining allow the straightening of the recto-anal angle and comfortable, unimpeded evacuation of stool. Reproduced from Lembo T, Camilleri M.1
Treatment of chronic constipation based on identifying the underlying pathophysiology is generally successful with targeted therapy. The aims of this review are to discuss targeted therapy for chronic constipation: behavioural treatment for outlet dysfunction and pharmacological treatment for constipation not associated with outlet dysfunction. In particular, we shall review the evidence that behavioural treatment works for evacuation disorders, describe the new treatment options for constipation not associated with evacuation disorder, and demonstrate how `targeting therapy' to the underlying diagnosis results in a balanced approach to patients with these common disorders.
ALGORITHM FOR THE MANAGEMENT OF CHRONIC CONSTIPATION
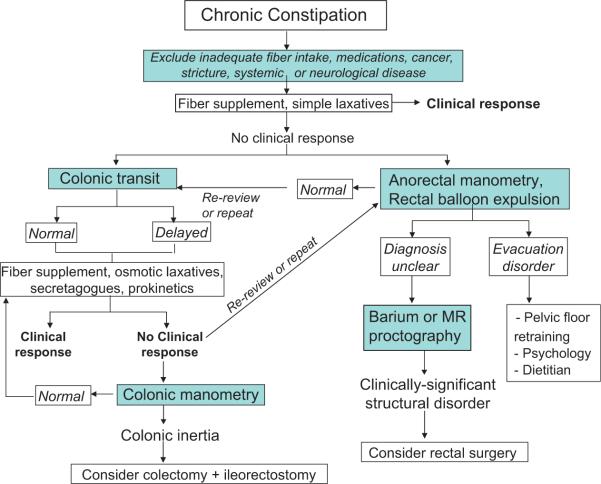
Figure 2 illustrates the algorithm used in our practice for the management of patients with chronic constipation. After excluding underlying diseases such as cancer, strictures, hypothyroidism and the adverse effects of medications and ensuring the patient has received an adequate trial of fibre supplementation (at least 12 g per day), there are assessments that are essential to guiding management: a test of evacuation function, typically ano-rectal manometry with balloon expulsion test,2 and a test of colonic transit, typically a radio-opaque marker transit test (figure 3).3,4 Alternatively, transit can be measured by radioscintigraphy5,6 or a wireless motility capsule.7 While the performance characteristics of the latter two transit methods have been extensively documented,6,8 they are not generally available or approved for use in some countries, and the most widely used transit method is based on radio-opaque markers. In our practice, almost half the patients referred with constipation not responding to first-line therapies have a disorder of rectal evacuation.9 It is important to note that delayed colonic transit may be the result of an evacuation disorder. Hence, colonic transit measurements have to be interpreted within the context of the evacuation dynamics. While it may not be essential to assess colonic transit initially in patients with defaecatory disorders, this test has been positioned at an early stage in the algorithm because many practitioners are more likely to have access to colonic transit than ano-rectal testing in their practice.
Figure 2.
Algorithm for managing patients with chronic constipation.
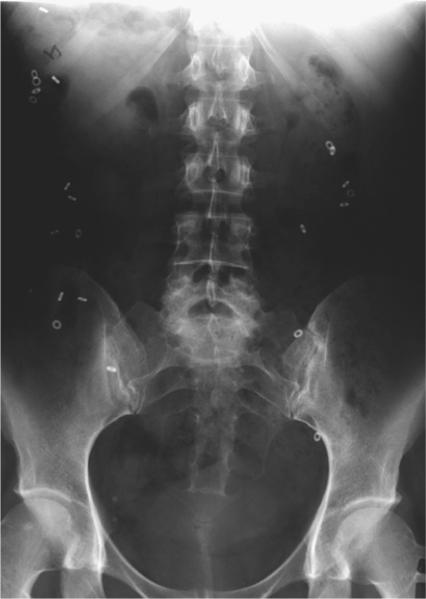
Figure 3.
Example of radio-opaque marker colonic transit measurement. Plain x-ray obtained 4 days after ingestion of 72 radio-opaque markers on days 1–3 (ie, Metcalf technique) shows 22 markers scattered throughout the colon, suggestive of normal colonic transit.
In selected patients, other tests may be required, as second-line approaches, such as magnetic resonance defaecography to evaluate defecation dynamics.10 Barium or magnetic resonance defaecation proctography may reveal anatomical disorders (eg, internal prolapse, intussusception, persistent rectocele that does not empty) that are amenable to surgical intervention.10,11 Similarly, colonic manometry and/or barostat testing may be needed to assess colonic motor activity in patients with severe slow transit constipation that is unresponsive to medical therapy, if the patient is being considered for colectomy.12,13
WHAT IS THE EVIDENCE THAT BEHAVIOURAL TREATMENT WORKS FOR EVACUATION DISORDERS?
The predominant behavioural treatment is biofeedback. Through biofeedback therapy, patients are taught to appropriately use their abdominal and pelvic floor muscles during defaecation; patients receive feedback of anal and pelvic floor muscle activity recorded by surface electromyographic (EMG), anal pressure sensors, or digital examination by a therapist. Generally, patients are taught how to use their abdominal muscles to increase intra-abdominal pressure and keep the pelvic floor muscles relaxed during evacuation, and then employ these techniques to evacuate an air-filled rectal balloon while a therapist assists by providing external traction. Sensory retraining, in which patients learn to recognise weaker rectal filling sensation, can also be provided.
After several uncontrolled trials, there have been controlled trials assessing the role of behavioural therapy in the form of retraining with biofeedback. These studies started in the paediatric population, but recent data also included adults and the elderly. While childhood constipation is different from constipation in adults, we have included information from paediatric practice to provide a more comprehensive assessment, and because there were lessons learned from the paediatric experience. The trials are summarised in table 1.14–27 Data from eight biofeedback therapy trials in the literature have been subjected to meta-analyses using fixed effect models and computing OR and 95% CI of treatment effects.28 In four trials, electromyographic (EMG) biofeedback was compared to non-biofeedback treatments (laxatives, placebo, sham training and botulinum toxin injection). In the other four studies, EMG biofeedback was compared to other forms of biofeedback (balloon pressure, verbal feedback). Three randomised controlled trials, summarised in a meta-analysis (table 128), show that biofeedback therapy is better (OR 3.657, 95% CI 2.127 to 6.290) than placebo (ie, laxatives, a muscle relaxant (ie, diazepam) and sham biofeedback) for improving symptoms and ano-rectal functions in adults with defaecatory disorders.13–27 This improvement is sustained for up to 2 years. Moreover, in contrast to earlier studies from the St. Mark's group, more recent data demonstrate that biofeedback therapy benefits patients with defaecatory disorders but not isolated slow transit constipation.22 Thus, biofeedback therapy is the treatment of choice for functional defaecation disorders. The evidence in children and the elderly is somewhat weaker. In contrast, differences between EMG versus other forms of biofeedback therapy were not significantly different (OR 1.436, CI 0.692 to 3.089). Enck et al28 recommended caution in the interpretation of the meta-analysis, since the included trials showed a substantial lack of quality and harmonisation; for example, use of variable endpoints and missing psychological assessment across studies. Further studies are required to compare different types of instrumented therapy and also to compare instrumented versus non-instrumented feedback (ie, teaching pelvic floor exercises by digital examination with verbal feedback) are necessary.
Table 1.
Controlled trials of behavioural treatment for rectal evacuation disorders
| Reference | Patients | Behavioural treatment | Design and comparator | Main results |
|---|---|---|---|---|
| Children | ||||
| Wald 198714 | 50 encopretic children; 18 FFR | BF | Single blind, versus mineral oil | At 12 months, FFR remission or markedly improved: 6/9 (BF) vs 3/9 on mineral oil |
| Loening-Baucke 199015 | 43 children: impaction, encopresis | BF+laxatives | DB, RCT, versus laxatives | At 12 months, 50% (BF) vs 16% (laxatives) symptom resolution; 55% (BF) vs 5% defecation dynamic response |
| Van der Plas 199616 | 192 children constipation, not all FFR | EMG BF+laxatives | DB, RCT, versus laxatives | No symptomatic benefit from BF but improved defecation dynamics |
| Nolan 199817 | 29 children with anismus | EMG BF + CMT | RCT versus CMT | No symptomatic benefit from BF but improved defecation dynamics |
| ADULTS | ||||
| Bleijen-berg 199418 | 20 adults with constipation+PD | Intra-anal EMG BF | RCT versus balloon training | 73% (EMG BF) vs 22% (balloon BF) symptom response rate |
| Koutso-manis 199519 | 60 adults constipation; 47/60 PD | EMG and rectal balloon BF | RCT versus muscular coordination training +balloon | Relative to baseline, both arms (EMG and pressure) of BF effective, but no difference between the 2 Rx arms |
| Heymen 199920 | 36 adults with constipation | 4 anal EMG BF arms | RCT | Relative to baseline, EMG BF alone as effective as EMG + balloon training, home training, or both. |
| Glia 199721 | 20 adults with constipation + PD | Peri-anal EMG BF | RCT versus pressure BF+balloon training | Relative to baseline, both arms (EMG and pressure) of BF effective, but no difference between the 2 Rx arms |
| Chiarioni 22 | 52 adults with STC: 32 PD, 6 mixed, and 12 STC alone | Anal EMG and balloon BF to teach relaxation | Open trial:+abdo muscle training to teach straining | At 6 months, symptom response (≥3 BM/ week) in 71% PD group, vs 8% STC group; improvements were maintained at 24 months of follow-up. Improved defaecation dynamics |
| Chiarioni 200623 | 99 adults with PD | BF | RCT versus PEG (14.6–29.2 g/d) + counselling | At 6 months, major clinical improvement 80% (BF) group versus 20% PEG group; results sustained 2 years |
| Rao 200724 | 77 adults with constipation + PD | BF | RCT versus Sham (relaxation Rx) | 88% (BF) satisfactory response vs 48% on control; improved defecation dynamics |
| Heymen 200725 | 84 adults with constipation and PD | EMG BF+pelvic floor exercises balloon pressure BF | 3-arm RCT versus diazepam or placebo 1–2 h before attempt to defaecate | Adequate relief of constipation: 70% (BF) vs 23% (diazepam) vs 38% (placebo); more unassisted BMs and reduced strain |
| Farid 200926 | 48 adults with anismus | balloon pressure BF | RCT; Botulinum toxin (BTX) -A to EAS | 1 month improvement: BF versus BTX-A 71% (p=0.008); 1 year improvement 25 vs 33% |
| Simon 200927 | 30 elderly constipated with PD | EMG BF | Counselling on behavioural mechanisms in defaecation | Improved symptoms and EMG results in biofeedback group at 4 weeks and 2 months |
2 BF, biofeedback; BM, bowel movements; CMT, conventional medical therapy; DB, double-blind; EAS, external anal sphincter; EMG, electromyography; FFR, functional faecal retention; PD, puborectalis dyssynergia; RCT, randomised controlled trial.
Three issues unique to biofeedback training deserve emphasis. First, it requires concentration and cognitive processing that may be beyond the abilities of younger children. Second, it requires skilled and experienced therapists and an optimal therapist–patient relationship; the required skill level and experience is not widely available. Third, the efficacy of biofeedback retraining in flaccid disorders of evacuation (such as descending perineum syndrome) has not been evaluated in controlled studies, and the data from observational studies suggest it may be efficacious in only ~50% of patients.29 In addition, while the St. Mark's group had suggested it is equally effective for patients with slow transit as for those with evacuation disorder,30 this was not confirmed by Chiarioni et al,22 and most centres reserve this treatment for patients with evacuation disorders. Approximately 50% of patients with a defaecatory disorder have delayed colonic transit. Some patients with evacuation disorders continue to experience constipation after retraining; they usually have a combination of evacuation disorder and slow transit constipation and, typically, the constipation resolves with standard treatment with fibre and osmotic or stimulant laxatives, as long as the pelvic floor dysfunction has been rehabilitated.
What are the new treatment options for constipation not associated with evacuation disorder?
The efficacy of dietary fibre supplementation, osmotic laxatives, particularly polyethylene glycol, and stimulant laxatives (eg, bisacodyl) for chronic constipation is supported by rigorously conducted controlled trials.31–33 In addition to improving symptoms, these agents also accelerate colonic transit. For example, bisacodyl and sorbitol accelerate ascending colon emptying and colonic transit respectively in healthy subjects.34,35 A placebo-controlled study observed that bisacodyl, 10 mg/day for three consecutive days, was an effective rescue agent for chronic constipation.33 In another study, bisacodyl also improved stool frequency and consistency and straining at 14 and 28 days.36 These inexpensive approaches should be tried initially, particularly for patients who do not have an underlying evacuation disorder and in primary care.
Patients who do not respond to or tolerate these therapies may have a more complicated disorder such as an evacuation disorder, slow transit constipation or iatrogenic (usually drug-induced) constipation, as shown previously.37
The next section briefly reviews drugs in the pipeline for treatment of chronic constipation based on either recent regulatory approved in some countries or published data including at least phase II trials, based on a PubMed Search. There are two general categories of medications that are being developed for the treatment of chronic constipation: colonic prokinetics in the serotonin receptor subtype 4 (5-HT4) agonist class and intestinal secretagogues.
5-HT4 agonists
Of the 5-HTreceptor subtypes in the gut, 5-HT3 and 5-HT4 receptors have been most extensively studied as potential targets of prokinetic drugs in humans. They have the potential to enhance laxation through the induction of fast excitatory postsynaptic potentials in intrinsic neurons, release neurotransmitters such as the excitatory acetylcholine, and induce mucosal secretion by activating submucosal neurons. With the withdrawal of cisapride and tegaserod because of cardiac or potential vascular adverse events and the appreciation that serotonin receptors modify vascular function (eg, 5-HT1B receptors induce contraction of arterioles and venules, and 5-HT1D, 5-HT2B, 5-HT4 and 5-HT7 receptors induce relaxation of venules), all new drugs in this class have to be devoid of cardiac effects (eg, arrhythmogenic effects and prolongation of QTc interval) and selective for 5-HT4 receptors over other receptors (eg, 5-HT2B, 5-HT7) and channels (eg, delayed rectifier potassium channel) and safety through studies of arrhythmogenic potential and effects on QTc interval. For example, it has been demonstrated that tegaserod has significant effects on receptors other than 5-HT4 that could conceivably influence vascular function.38 Table 2 is a summary of the three main candidate 5-HT4 agonists in development: prucalopride, velusetrag and ATI-7505. The properties of these newer agents, and in particular, their specificity and cardiovascular safety, differ from those of older 5-HT4 agonists.39,40
Table 2.
Comparison of novel 5-HT4 agonists
| Prucalopride | Velusetrag | ATI-7505 | |
|---|---|---|---|
| Chemistry | Benzofuran carboxamide | Quinolinone carboxamide | Benzamide |
| Selectivity and affinity for 5-HT4 receptor | Highly selective, high-affinity; weak affinity for human D4 and Σ1, and mouse 5-HT3 receptors at concentrations exceeding the Ki for 5-HT4 receptors by 290-fold | High affinity and selectivity for h5-HT4c over other biogenic amine receptors; >500-fold selective over other 5-HT receptors (including h5-HT2B, h5-HT3A) | Specific 5-HT4 full agonist activity in the GI tract, but a partial agonist activity in the heart |
| Metabolism | Limited hepatic, not CYP 3A4 | CYP 3A4 | Hydrolytic esterase, not CYP 3A4 |
| Pharmacodynamic efficacy in humans | Accelerated colonic transit in health and chronic constipation | Accelerated colonic transit in health in dose-related fashion | Accelerated colonic transit in health |
| Clinical trial efficacy | Phase II and III portfolio in chronic constipation | Phase IIBPhaseIB | Phase IB |
| Open label effectiveness | Open label experience of ~ 1000 cumulative patient-years | – | – |
| Arrhythmogenicity | No arrhythmic activity in human atrial cells; inhibited hERG channel only at μmol/l concentration (IC50~4.9 10−6 mol/l); no clinically relevant cardiac AEs in clinical trials of > 4000 humans | At 3 μmol/l, no effect on hERG channel current; safety ratio versus cisapride > 1000-fold; no effect on QT in health or 400 patients with constipation | At 100 μmol/l, no effect on hERG channel; affinity ratio between IKr and 5-HT4 receptors of > 1000-fold. |
| Cardiovascular safety including elderly | Healthy subjects 'thorough' QTc study; safety in elderly cohort 80% on CV drugs | Healthy subjects 'thorough' QTc study; transient increase in heart rate not different from placebo | Healthy subjects 'thorough' QTc study; |
| Commonest AEs | Diarrhoea, nausea,headache | Diarrhoea, nausea, headacheDiarrhoea,headache | Diarrhoea, nausea,headache |
| Approval status | EMEA | – | – |
EMEA, European Medicines Agency; hERG, human ether-à-go-go-related gene.
The largest body of evidence 41–47 on pharmacodynamic and clinical efficacy in disease (chronic constipation) is available for prucalopride, with several thousand patients exposed for assessing safety (at least 2000 in phase III clinical trials and 1000 patient-years cumulative follow-up). The European Agency for Evaluation of Medicinal Products (EMEA) approved the medication for chronic constipation at a dose of 2 mg per day in adults and 1 mg per day in the elderly.
Velusetrag, which shows specificity and safety in vitro and in vivo,48,49 has also been tested in pharmacodynamic studies in humans50 and in a large (400 patient) phase IIB study.51 While a single dose of velusetrag also accelerated colonic transit in a dose-dependent manner, there was tachyphylaxis with repeat dosing, particularly at the highest doses tested (eg, 50 mg daily).50 However, there was no evidence of tachyphylaxis during the 4-week clinical trial. Velusetrag has one metabolite which is almost as potent as the parent drug.
ATI-7505 has only recently entered into clinical trials, but the pharmacodynamic efficacy appears promising.52,53 The lack of CYP3A4 metabolism of prucalopride and ATI-7505 is also potentially advantageous to avoid drug interactions.
In conclusion, the new generation of 5-HT4 agonists appears effective and safe. Prucalopride has been approved for marketing at a standard dose of 2 mg per day for adults and a starting dose of 1 mg per day for elderly patients. The velusetrag development programme includes one completed phase IIB study51 that confirms efficacy. There is reason for optimism in medical treatment of chronic constipation that is unresponsive to current therapy, as shown for prucalopride in the phase III programme44–46 in which patients had an average of less than one spontaneous bowel movement per week and ~80% reported insufficient response to current treatment with laxatives.
Intestinal secretagogues
In addition to being troublesome per se, hard stools are also more difficult to evacuate, providing the rationale for intestinal secretagogues to relieve constipation. Both secretagogues for chronic constipation increase intestinal chloride secretion which is followed by secretion of water into the lumen. There are several different classes of chloride channels (ClC) including ClC-2 and ClC-3 which are expressed in most cells. Epithelial chloride transport induces fluid secretion: chloride enters into the enterocyte or colonocyte through the basolateral Na+-K+-2Cl− co-transporter (with the cations being exported through the Na+ pump (Na+, K+, ATPase) and KCNQ1/KCNE3 heteromeric K+ channels which are needed for K+ recycling) (figure 4). Secretory pathways in the apical membrane of the enterocyte include cystic fibrosis transmembrane regulator (CFTR) and ClC-2 chloride channels, which allow chloride secretion.54‒56
Figure 4.
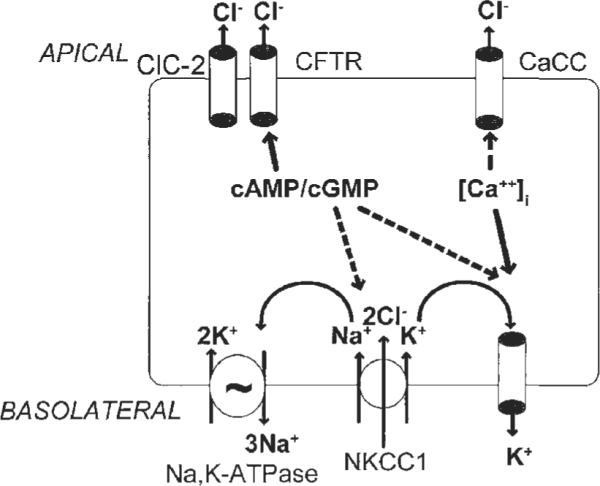
Chloride secretory mechanisms in intestinal epithelial cells can be stimulated by increases in cyclic nucleotides (cAMP/cGMP) or cytosolic calcium ([Ca2+]i). Major targets for regulation of secretion include channels in the apical membrane: CFTR, cystic fibrosis transmembrane conductance regulator; CaCC, calcium-activated chloride channel and ClC-2 (chloride channel type 2). Ion channels in the basolateral membrane deliver chloride into the enterocytes (NKCC1 (sodium/potassium/2 chloride co-transporter type 1) and ensure that obligatorily co-transported potassium and sodium ions are extruded by energy-dependent (eg, ATP) mechanisms, such as the sodium pump and different potassium transporters (eg, IK [intermediate conductance potassium channel]; K-cAMP channel, and KCNQ1/KCNE3 heteromeric K+ channels). Adapted from Barrett KE, Keely SJ,55 and reproduced from Camilleri M.56
Lubiprostone
Lubiprostone is a bicyclic fatty acid that is derived from prostaglandin E1. It selectively activates apical membrane CIC-2 channels to increase intestinal and colonic secretion of chloride-rich fluid into the intestinal lumen. Lubiprostone increased electrogenic chloride transport with a 50% effective concentration (EC50) of ~18 nmol/l in vitro54 and dose dependently increased water and chloride secretion in rats in vivo.57 Though initial studies suggested it does not activate CFTR channels, more recent data suggest that CFTR is necessary58 and prostaglandin EP receptors may be activated, too.59
Lubiprostone accelerated intestinal and colonic transit in healthy subjects,60 but had no significant effect on colonic motility or sensation61 in humans or smooth muscle in vitro.62 Lubiprostone may enhance mucosal barrier function.63 Clinical trials demonstrate its efficacy and safety in chronic constipation, and it is FDA approved at a dose of 24 mg twice daily for this indication.64,65 Lubiprostone is reported to cause nausea in about 20% of patients.
Guanylcyclase C
Guanylcyclase C (GC-C) is the principal receptor for heat-stable enterotoxins (STa), a major causative factor in Escherichia coli-induced secretory diarrhoea. GC-C is enriched in intestinal epithelium, though it is detected in other epithelia.66 It consists of an extracellular receptor domain, a single transmembrane domain, a kinase homology domain, and a catalytic domain. It is modified by N-linked glycosylation and, at least in the small intestine, by proteolysis, resulting in an STa receptor that is coupled non-covalently to the intracellular domain. The enteric bacterial peptides in the heat-stable enterotoxin family (ST peptides) (19 AAs) induce secretion by activating this surface receptor. There are two endogenous ligands of GC-C: the small cysteine-rich peptides, guanylin (15AA) and uroguanylin (16AA), which are released in an autocrine or paracrine fashion into the intestinal lumen, but may also function as endocrine hormones in gut–kidney communication and as regulators of ion transport in extra-intestinal epithelia.
Activation of GC-C occurs by inducing a conformational change in the extracellular portion of the homotrimeric GC-C complex, which allows two of the three intracellular catalytic domains to dimerise and form two active catalytic clefts. In the intestine, activation of GC-C results in stimulation of chloride and bicarbonate secretion through the opening of apical CFTR chloride channels and inhibition of sodium absorption through blockade of an apical Na/H exchanger. The principal effector of the GC-C effect on ion transport is cGMP-dependent protein kinase type II which, together with GC-C and the ion transporters, may form a supra-molecular complex at the apical border of epithelial cells.
Linaclotide
Linaclotide is a 14 amino acid peptide that contains three disulfide bonds required for GC-C activation. The active metabolite, MM-419447, is produced after loss of the C-terminal tyrosine through the action of carboxypeptidase A. By increasing cyclic guanosine monophosphate (cGMP), linaclotide induces signalling pathways which stimulate chloride and bicarbonate secretion through CFTR channel-dependent and, to a lesser extent, channel-independent mechanisms.67 Linaclotide also inhibits sodium absorption from the lumen by a sodium proton exchanger.68 Phase IIA placebo-controlled studies of 2 weeks and 5 days in duration showed that linaclotide improved symptoms and accelerated colonic transit.69–71 A phase IIB study of 310 patients with chronic constipation who were treated with placebo or one of four doses of linaclotide (75, 150, 300 or 600 μg once daily) for 4 weeks confirmed that all four doses improved constipation symptoms.72 Table 3 summarises the properties of these two chloride secretagogues.
Table 3.
Comparison of secretagogues, lubiprostone and linaclotide
| Lubiprostone | Linaclotide | |
|---|---|---|
| Chemistry | Bicyclic fatty acid called a prostone | 14 amino acid peptide, analogue of guanylin |
| Target receptor | Chloride channel (ClC2); ? CFTR involved | Guanylate cyclase C receptor activation with CFTR-mediated secretion |
| Pharmacodynamics in humans | Accelerated small bowel and colonic transit in health | Accelerated colonic transit in IBS-C in dose-related fashion |
| Clinical trial efficacy | Phase II and III portfolio in chronic constipation and C-IBS | Phase IIB in chronic constipation and IBS-C |
| Open label effectiveness | Clinical practice experience | – |
| Arrhythmogenicity | No arrhythmic activity | Low bioavailability, no arrhythmic activity |
| Cardiovascular safety | Healthy subjects 'thorough' QTc study | Healthy subjects 'thorough' QTc study |
| Commonest AEs | Diarrhoea, nausea | Diarrhoea |
| Potential other actions | Mucosal protection | Anti-neoplastic |
| Approval status | FDA | – |
ACHIEVING A BALANCE IN THE CLINICAL MANAGEMENT OF CHRONIC CONSTIPATION
While the stepwise approach shown in figure 2 has not been formally evaluated, it is widely employed and, in our experience, provides a logical, balanced and effective approach to managing constipation in clinical practice. This algorithm is underpinned by the concepts that: (1) dietary fibre supplementation and osmotic agents should be initially tried for patients with chronic constipation, particularly in primary care; and (2) thereafter, management should be guided by the results of colonic transit and ano-rectal function tests in patients who do not respond to the first line of treatment. These tests should be considered earlier if there is a strong clinical suspicion for defaecatory disorders.
For patients with normal or slow transit constipation, it is customary to start treatment with fibre and an osmotic laxative such as a magnesium salt or polyethylene glycol, adding a stimulant laxative such as bisacodyl on an as-needed basis. These agents are relatively safe, inexpensive, widely used, and in many cases their efficacy has been proven in controlled trials. Newer medications that seem to be efficacious and safe should be considered in patients who do not respond to these older agents or do not tolerate them. These agents include 5-HT4 agonist prokinetics, of which prucalopride is approved in Europe, and secretagogues like lubiprostone, which is approved in the United States. Colonic motor assessments with intraluminal techniques are useful for identifying colonic motor dysfunction and identifying patients who may benefit from subtotal colectomy. A subtotal colectomy should be considered in patients with medically refractory chronic constipation who do not have a defaecatory disorder.
Defaecatory disorders can be diagnosed by careful clinical assessments and ano-rectal testing and are managed by biofeedback therapy. However, the expertise necessary to provide pelvic floor retraining is not widely available. Many patients with defaecatory disorders have structural abnormalities (ie, rectocoeles, rectal mucosal intusussception, enterocoele, and descending perineum syndrome), which may be transient (ie, related to straining) or persistent, and may occur in isolation or in association with functional disturbances.
Managing structural abnormalities is guided by several considerations. Not all abnormalities (eg, small rectocoeles) cause symptoms and some may be secondary to a functional disturbance (eg, excessive straining, non-relaxing pelvic floor). Thus, pelvic floor retraining should be considered even in some patients with structural abnormalities. However, the response to pelvic floor retraining in patients with structural abnormalities has not been evaluated in controlled studies. Surgery should be considered for anatomical abnormalities (eg, large enterocoeles) that obstruct defaecation.
In controlled trials, up to 75% of patients with a defaecatory disorder have satisfactory bowel habits after pelvic floor retraining at specialised centres. Non-behavioural options (eg, sacral nerve stimulation, pelvic floor botulinum toxin) for patients with pelvic floor dysfunction persistent despite retraining are of unproven efficacy. Persistent constipation after resolution of pelvic floor dysfunctions may be due to colonic motor dysfunction which may need specific treatment with laxatives, prokinetics and rarely colectomy, as described above.
Box 1
-
▶
Diagnostic tests are useful for identifying defaecatory disorders and characterising colonic transit in chronic constipation; this classification facilitates management.
-
▶
Controlled studies suggest that behavioural and pharmacological treatments improve symptoms in patients with and without defaecatory disorders respectively.
-
▶
Rigorous trials support the efficacy of simple measures (fibre supplementation, osmotic and stimulant laxatives) for chronic constipation.
-
▶
Newer agents should be considered for patients who do not respond to older therapies.
Acknowledgments
Funding AEB is supported by NIH RO1 DK 78924, and MC by NIH R01-DK079866 and NIH 1RC1-DK086182 for studies in lower gastrointestinal motility disorders.
Footnotes
Competing interests MC has received research grants from Aryx (for ATI-7505), Johnson and Johnson (for prucalopride), Microbia (for linaclotide), Takeda/Sucampo (for lubiprostone), and Theravance (for velusetrag); honoraria below the US federal threshold for significant COI from Theravance, Takeda, and Ironwood; and CDA with no personal remuneration from Movetis. AEB has received research grants from Pfizer, and honoraria below the US federal threshold for significant COI from American Medical Systems, and Helsinn HealthCare.
Provenance and peer review Commissioned; externally peer reviewed.
REFERENCES
- 1.Lembo T, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–8. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 2.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Locke GR, III, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement guidelines on constipation. Gastroenterology. 2000;119:1761–6. doi: 10.1053/gast.2000.20390. [DOI] [PubMed] [Google Scholar]
- 4.Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–7. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 5.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2009 December 21; doi: 10.1111/j.1365-2982.2009.01442.x. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2009 December 18; doi: 10.1111/j.1365-2982.2009.01441.x. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–44. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Thorne N, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Gastroenterology. 2010;138:S224–5. doi: 10.1111/j.1365-2982.2010.01517.x. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surrenti E, Rath DM, Pemberton JH, et al. Audit of constipation in a tertiary-referral gastroenterology practice. Am J Gastroenterol. 1995;90:1471–5. [PubMed] [Google Scholar]
- 10.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Bharucha AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol. 2006;40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Bharucha AE, Di Lorenzo C, et al. Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–82. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 13.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2010;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald A, Chandra R, Gabel S, et al. Evaluation of biofeedback in childhood encopresis. J Pediatr Gastroenterol Nutr. 1987;6:554–8. doi: 10.1097/00005176-198707000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Loening-Baucke V. Modulation of abnormal defecation dynamics by biofeedback treatment in chronically constipated children with encopresis. J Pediatr. 1990;116:214–22. doi: 10.1016/s0022-3476(05)82877-x. [DOI] [PubMed] [Google Scholar]
- 16.van der Plas RN, Benninga MA, Buller HA, et al. Biofeedback training in treatment of childhood constipation: a randomized controlled study. Lancet. 1996;348:776–80. doi: 10.1016/s0140-6736(96)03206-0. [DOI] [PubMed] [Google Scholar]
- 17.Nolan T, Catto-Smith T, Coffey C, et al. Randomised controlled trial of biofeedback training in persistent encopresis with anismus. Arch Dis Child. 1998;79:131–5. doi: 10.1136/adc.79.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleijenberg G, Kuijpers HC. Biofeedback treatment of constipation: a comparison of two methods. Am J Gastroenterol. 1994;89:1021–6. [PubMed] [Google Scholar]
- 19.Koutsomanis D, Lennard-Jones JE, Roy AJ, et al. Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut. 1995;37:95–9. doi: 10.1136/gut.37.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heymen S, Wexner SD, Vickers D, et al. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Dis Colon Rectum. 1999;42:1388–93. doi: 10.1007/BF02235034. [DOI] [PubMed] [Google Scholar]
- 21.Glia A, Gylin M, Gullberg K, et al. Biofeedback retraining in patients with functional constipation and paradoxical puborectalis contraction: comparison of anal manometry and sphincter electromyography for feedback. Dis Colon Rectum. 1997;40:889–95. doi: 10.1007/BF02051194. [DOI] [PubMed] [Google Scholar]
- 22.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–64. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–8. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum. 2007;50:428–41. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 26.Farid M, El Monem HA, Omar W, et al. Comparative study between biofeedback retraining and botulinum neurotoxin in the treatment of anismus patients. Int J Colorectal Dis. 2009;24:115–20. doi: 10.1007/s00384-008-0567-0. [DOI] [PubMed] [Google Scholar]
- 27.Simón MA, Bueno AM. Behavioural treatment of the dyssynergic defecation in chronically constipated elderly patients: a randomized controlled trial. Appl Psychophysiol Biofeedback. 2009;34:273–7. doi: 10.1007/s10484-009-9100-7. [DOI] [PubMed] [Google Scholar]
- 28.Enck P, Van der Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterol Motil. 2009;21:1133–41. doi: 10.1111/j.1365-2982.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 29.Harewood GC, Coulie B, Camilleri M, et al. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94:126–30. doi: 10.1111/j.1572-0241.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 30.Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut. 1998;42:517–21. doi: 10.1136/gut.42.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bijkerk CJ, de Wit NJ, Muris JWM, et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ. 2009;339:b3154. doi: 10.1136/bmj.b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dipalma JA, Cleveland MV, McGowan J, et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102:1436–41. doi: 10.1111/j.1572-0241.2007.01199.x. [DOI] [PubMed] [Google Scholar]
- 33.Kienzle-Horn S, Vix JM, Schuijt C, et al. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:1479–88. doi: 10.1111/j.1365-2036.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 34.Manabe N, Cremonini F, Camilleri M, et al. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment Pharmacol Ther. 2009;30:930–6. doi: 10.1111/j.1365-2036.2009.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoog SM, Bharucha AE, Camilleri M, et al. Effects of an osmotically active agent on colonic transit. Neurogastroenterol Motil. 2006;18:300–6. doi: 10.1111/j.1365-2982.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 36.Kienzle-Horn S, Vix JM, Schuijt C, et al. Comparison of bisacodyl and sodium picosulphate in the treatment of chronic constipation. Curr Med Res Opin. 2007;23:691–9. doi: 10.1185/030079907x178865. [DOI] [PubMed] [Google Scholar]
- 37.Voderholzer WA, Schatke W, Mühldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol. 1997;92:95–8. [PubMed] [Google Scholar]
- 38.Beattie DT, Smith JA, Marquess D, et al. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol. 2004;143:549–60. doi: 10.1038/sj.bjp.0705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi: 10.1111/j.1365-2982.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 40.De Maeyer JH, Prins NH, Schuurkes JA, et al. Differential effects of 5-hydroxytryptamine4 receptor agonists at gastric versus cardiac receptors: an operational framework to explain and quantify organ-specific behavior. J Pharmacol Exp Ther. 2006;317:955–64. doi: 10.1124/jpet.106.101329. [DOI] [PubMed] [Google Scholar]
- 41.Camilleri M, Deiteren A. Prucalopride for constipation. Exp Opin Pharmacother. 2010;11:451–61. doi: 10.1517/14656560903567057. [DOI] [PubMed] [Google Scholar]
- 42.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–60. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 43.Sloots CE, Poen AC, Kerstens R, et al. Effects of prucalopride on colonic transit, anorectal function and bowel habits in patients with chronic constipation. Aliment Pharmacol Ther. 2002;16:759–67. doi: 10.1046/j.1365-2036.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 44.Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–54. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 45.Quigley EM, Vandeplassche L, Kerstens R, et al. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipatione–a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315–28. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]
- 46.Tack J, van Outryve M, Beyens G, et al. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58:357–65. doi: 10.1136/gut.2008.162404. [DOI] [PubMed] [Google Scholar]
- 47.Camilleri M, Beyens G, Kerstens R, et al. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009;21:1256–63. doi: 10.1111/j.1365-2982.2009.01398.x. [DOI] [PubMed] [Google Scholar]
- 48.Smith JA, Beattie DT, Marquess D, et al. The in vitro pharmacological profile of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:125–37. doi: 10.1007/s00210-008-0282-y. [DOI] [PubMed] [Google Scholar]
- 49.Beattie DT, Armstrong SR, Shaw JP, et al. The in vivo gastrointestinal activity of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:139–47. doi: 10.1007/s00210-008-0281-z. [DOI] [PubMed] [Google Scholar]
- 50.Manini ML, Camilleri M, Goldberg M, et al. Effects of velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil. 2009 August 18; doi: 10.1111/j.1365-2982.2009.01378.x. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg MR, Li Y-P, Pitzer K, et al. TD-5108, a selective 5-HT4 agonist, is consistently better than placebo regardless of response definition in patients with chronic constipation. Gastroenterology. 2008;133:A547. [Google Scholar]
- 52.Dennis D, Palme M, Irwin I, et al. ATI-7505 is a novel, selective 5HT(4) receptor agonist that causes gastrointestinal prokinetic activity in dogs. Gastroenterology. 2004;126:A641. [Google Scholar]
- 53.Camilleri M, Vazquez-Roque MI, Burton D, et al. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30–8. doi: 10.1111/j.1365-2982.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 54.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol. 2004;287:C1173–83. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 55.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–72. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 56.Camilleri M. Review article: new receptor targets for medical therapy in irritable bowel syndrome. Aliment Pharmacol Ther. 2010;31:35–46. doi: 10.1111/j.1365-2036.2009.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKeage K, Plosker GL, Siddiqui MAA. Lubiprostone. Drugs. 2006;66:873–9. doi: 10.2165/00003495-200666060-00015. [DOI] [PubMed] [Google Scholar]
- 58.Bijvelds MJ, Bot AG, Escher JC, et al. Activation of intestinal Cl-secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–85. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 59.Bassil AK, Borman RA, Jarvie EM, et al. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126–35. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol. 2006;290:G942–7. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 61.Sweetser S, Busciglio IA, Camilleri M, et al. Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol. 2009;296:G295–301. doi: 10.1152/ajpgi.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuppoletti J, Malinowska DH, Chakrabarti J, et al. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat. 2008;86:56–60. doi: 10.1016/j.prostaglandins.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Moeser AJ, Nighot PK, Engelke KJ, et al. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol. 2007;292:G647–56. doi: 10.1152/ajpgi.00183.2006. [DOI] [PubMed] [Google Scholar]
- 64.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther. 2007;25:1351–61. doi: 10.1111/j.1365-2036.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 65.Johanson JF, Morton D, Geenen J, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103:170–7. doi: 10.1111/j.1572-0241.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 66.Vaandrager AB. Structure and function of the heat-stable enterotoxin receptor guanylyl cyclase C. Mol Cell Biochem. 2002;230:73–83. [PubMed] [Google Scholar]
- 67.Joo NS, London RM, Kim HD, et al. Regulation of intestinal Cl- and HCO3-secretion by uroguanylin. Am J Physiol. 1998;274:G633–44. doi: 10.1152/ajpgi.1998.274.4.G633. [DOI] [PubMed] [Google Scholar]
- 68.Donowitz M, Cha B, Zachos NC, et al. NHERF family and NHE3 regulation. J Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–8. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 70.Johnston JM, Kurtz CB, Drossman DA, et al. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol. 2009;104:125–32. doi: 10.1038/ajg.2008.59. [DOI] [PubMed] [Google Scholar]
- 71.Johnston J, MacDougall J, Lavins B, et al. Linaclotide significantly improved abdominal pain, constipation and global assessments in adults with irritable bowel syndrome with constipation: results from a large twelve-week, randomized, double blind, placebo-controlled study. Am J Gastroenterol. 2008;103:S460–1. [Google Scholar]
- 72.Lembo AJ, Kurtz CB, MacDougall JE, et al. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology. 2010;138:886–95. doi: 10.1053/j.gastro.2009.12.050. [DOI] [PubMed] [Google Scholar]