Abstract
BACKGROUND & AIMS
Sodium chenodeoxycholate (CDC) accelerates colonic transit in health. Our aim was to examine pharmacodynamics (colonic transit, bowel function) and pharmacogenetics of CDC in constipation-predominant irritable bowel syndrome (IBS-C).
METHODS
In a double-blind placebo-controlled study, 36 female patients with IBS-C were randomized to treatment with delayed-release oral formulations of placebo, 500 mg CDC, or 1000 mg CDC for 4 days. We assessed gastrointestinal and colonic transit, stool characteristics, and associations of transit with fasting serum 7αC4 (surrogate of bile acid synthesis) and FGF19 (negative regulator of bile acid synthesis) levels. Candidate genetic polymorphisms involved in regulation of bile acid synthesis were analyzed in the 36 patients with IBS-C and 57 healthy volunteers to assess genetic influence on effects of CDC on transit.
RESULTS
Overall colonic transit and ascending colon emptying (AC t½) were significantly accelerated in the CDC group compared with placebo (P = .005 and P = .028, respectively). Looser stool consistency (P = .003), increased stool frequency (P = .018), and greater ease of passage (P = .024) were noted with CDC compared with placebo. The most common side effect was lower abdominal cramping/pain (P = .01). Fasting serum 7αC4 (but not FGF19) was positively associated with colonic transit (rs = 0.749, P = .003, placebo group). Genetic variation in FGFR4 was associated with AC t½ in response to CDC (uncorrected P = .015); αKlothoβ variant showed a gene-by-treatment interaction based on patient subgroup (uncorrected P = .0088).
CONCLUSIONS
CDC accelerates colonic transit and improves bowel function in female patients with IBS-C. The rate of bile acid synthesis influences colonic transit. Genetic variation in negative feedback inhibition of bile acid synthesis may affect CDC-mediated acceleration of colonic transit.
Keywords: Bile Acid, 7α, C4, FGF19, FGFR4
Bile acids have been used in the treatment of patients with gallstones and cholestatic liver diseases. Long-term treatment is generally well tolerated other than the consistent side effect of diarrhea,1 which mimics the chronic loose stools observed in patients with a disrupted enterohepatic circulation from ileal disease (eg, Crohn’s disease, surgical resection, or radiation ileitis), resulting in spillage of bile acid into the colon.2 This appears to be in part a secretory phenomenon because studies have shown that colonic infusion of di-α-hydroxy bile acids induces fluid and electrolyte secretion in mammalian and human colon.3,4 The mechanisms involved in promoting secretion include intracellular activation of adenylate cyclase,5 increased mucosal permeability,6,7 and inhibition of apical Cl−/OH− exchange.8 Bile acids also induce propulsive contractions in the mammalian and human colon.9,10
Chenodeoxycholic acid (CDCA), a primary bile acid previously used for dissolution of gallstones, elicited diarrhea at dosages of 750 to 1000 mg/day but not at 250 to 500 mg/day.11 CDC (with hydroxyl groups in the 3α, 7α positions) promoted colonic secretion in comparison to its 3α, 7β epimer, ursodeoxycholic acid.6,12 Over time, ursodeoxycholic acid has replaced CDCA in the treatment of biliary diseases due to its superior patient tolerability.
In a head-to-head comparison with ursodeoxycholic acid, CDC was shown to improve constipation in patients with gallstones receiving oral bile acids.13 Bazzoli et al also reported that CDCA administered to patients with gallstones who had chronic constipation resulted in a significant increase in the frequency of bowel movements and loosening of stools compared with placebo.14 In our previous study in healthy volunteers who received 500 to 1000 mg CDC in a delayed-release capsule, we observed accelerated colonic transit, increased stool frequency, and looser stool consistency compared with the group that received placebo.15
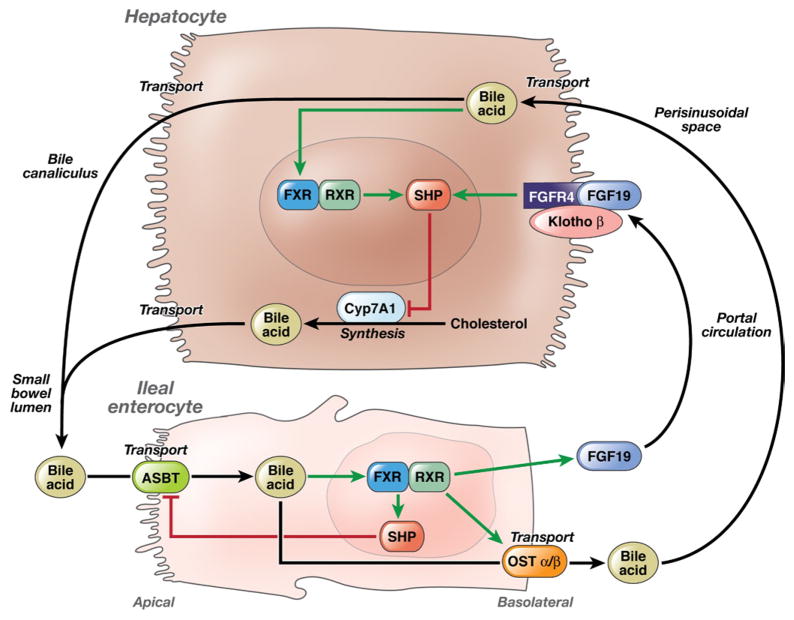
The intestinal uptake, transport, hepatic extraction, and synthesis of bile acids are controlled by a complex series of carrier proteins, enzymes, and receptors in ileal enterocytes and hepatocytes that are under genetic control (see Figure 1). Bile acid synthesis is regulated homeostatically by fibroblast growth factor 19 (FGF19)-mediated feedback inhibition of hepatic bile acid synthesis. This pathway is initiated by detection of high bile acid concentrations at the ileal enterocyte, which results in secretion of FGF19 into the mesenteric and portal circulation. FGF19 then binds to fibroblast growth factor receptor 4 (FGFR4) and Klothoβ (KLB) on the hepatocyte cell surface to initiate intracellular signaling cascades that suppress CYP7A1 expression and decrease bile acid synthesis. Bile acids also bind to the steroid receptor farnesoid X receptor to induce expression of small heterodimer partner (SHP), which ultimately suppresses transcription of CYP7A1 (Figure 1). It is unclear whether the endogenous level of bile acid synthesis and the genetic control of bile acid homeostasis influence colonic transit or its response to CDC.
Figure 1.
Illustration of bile acid enterohepatic circulation and homeostasis and relevant proteins with genetic variation tested in this study.
Walters et al recently suggested that plasma FGF19 levels reflect the strength of the negative feedback suppression of hepatic bile acid synthesis.16 The rate of synthesis is reflected by the serum level of 7α-hydroxy-4-cholesten-3-one (7αC4), which is closely related to the fecal excretion of bile acids as measured by the 75SeHCAT retention test.17 Higher levels of 7αC4 are therefore associated with lower levels of FGF19.
Our study had 3 primary aims: (1) to evaluate the effect of ileocolonic delivery of CDC on gastrointestinal (GI) and colonic transit and bowel function in patients with constipation-predominant irritable bowel syndrome (IBS-C), (2) to assess whether fasting serum 7αC4 or plasma FGF19 levels influence colonic transit response to CDC, and (3) to examine whether single nucleotide polymorphisms (SNPs) of candidate genes involved in bile acid homeostasis affect CDC-induced changes in colonic transit.
Patients and Methods
Study Design, Randomization, and Medication
This was a double-blind, placebo-controlled, randomized study evaluating the effects of orally administered CDC or placebo in patients with IBS-C selected on the basis of Rome III criteria. Participants were enrolled by one study coordinator between April 17 and November 20, 2009, when active participation in the trial ended as all required studies were completed. The study was approved by the Mayo Clinic Institutional Review Board and was registered on the ClinicalTrials.gov web site (NCT00912301). All data were collected in the Clinical Research Unit (National Institutes of Health Clinical and Translational Science Award grant RR0024150).
Patients with IBS-C were recruited from a preexisting database of 800 patients with different functional GI diseases residing within 150 miles of Rochester, Minnesota, or through local advertisements. We did not place a restriction on sex when recruiting patients for this study. All medical records were screened for major exclusion criteria that could affect GI function, including the use of medications known to alter GI transit. At the time of the initial screen, all participants signed informed consent. Fasting blood levels of 7αC4 and FGF19 were measured to assess for underlying disturbances in bile acid synthesis. The Hospital Anxiety and Depression Scale,18 Symptom Checklist 90 (somatization),19 and validated daily bowel diaries, including the Bristol Stool Form Scale,20 were also completed. GI and colonic transit were evaluated by a scintigraphic method during the last 48 hours of drug ingestion, which were days 3 and 4 of treatment.
CDC was purchased from Calbiochem, EMD Biosciences Inc (San Diego, CA), and the Mayo research pharmacy prepared capsules with identical appearance containing either placebo or CDC. All capsules were coated with the pH-sensitive polymer methacrylate (Eudragit S-100; Rohm Pharmaceuticals, Darmstadt, Germany), which is acid resistant but dissolves at the neutral pH of the distal ileum and thus prevents proximal small intestinal absorption of the drug.
Patients were randomized to receive one oral capsule daily containing placebo, 500 mg CDC, or 1000 mg CDC for 4 days. The study doses were selected based on the observations of diarrhea in previous gallstone dissolution trials1; in addition, daily synthesis to replenish fecal bile acid loss in humans is ~600 mg.17 An independent Mayo Clinic statistician who was otherwise not involved in the study generated the 3-group randomization code by computer, and allocation was concealed from the study investigators. The pharmacy implemented the randomization schedule, which was blinded from the study investigators including the study biostatistician, and only assigned research pharmacists had access to the schedule in case of emergency. Participants were assigned by the research pharmacist in accordance with the randomization schedule and allocation sequence. All clinical and laboratory study personnel were therefore blinded throughout the study until data were locked and analyzed. Safety was monitored for the duration of the study.
GI Transit Measurements
An adaptation of our established scintigraphic method was used to measure GI and colonic transit. The method has been reported in hundreds of patients and volunteers,21 and its performance characteristics are well established.22,23 111In was adsorbed onto activated charcoal particles and delivered to the colon by an oral methacrylate-coated capsule. The capsule was ingested following an overnight fast. After the capsule emptied from the stomach, a 99mTc-sulfur colloid radiolabeled meal (of standardized composition and calorie content) was ingested to measure gastric and small bowel transit. Subjects also ingested standardized meals for lunch and dinner at 4 and 8 hours after the radiolabeled meal, respectively. Abdominal scintiscans were obtained every hour for the first 6 hours and at 8, 24, and 48 hours after ingestion of the capsule containing 111In-charcoal.
Transit Data Analysis
Transit was measured by quantification of the radioactive counts in the stomach and 4 colonic regions (ascending [AC], transverse, descending, and combined sigmoid and rectum) with a variable region of interest program. Counts were corrected for isotope decay, tissue attenuation, and downscatter of 111In counts in the 99mTc window.22
Gastric emptying half-life (GE t½) was defined as the time for 50% of the radiolabeled tracer to empty from the stomach. Colonic filling at 6 hours (CF6) was the percentage of the radiolabeled meal that reached the colon at 6 hours, indirectly reflecting small bowel transit time. Overall colonic transit was computed as the colonic geometric center (GC), which is the weighted average of counts in the different colonic regions (AC, transverse, descending, rectosigmoid) and stool, respectively, numbered 1 to 5. At any time point, the GC equals the proportion of counts in each colonic region multiplied by its weighting factor: (percent AC × 1 + percent transverse × 2 + percent descending × 3 + percent rectosigmoid × 4 + percent stool × 5)/100. Thus, a higher GC reflects a faster colonic transit. AC t½ was calculated by linear interpolation of values on the AC emptying curve.
The primary end points were the colonic GC at 24 hours (GC 24) and AC t½. Secondary transit end points were colonic GC at 48 hours (GC 48), GE t½, and CF6. As an end point, GC 24 has been shown in previous pharmacodynamic studies to be responsive to a variety of treatments in patients with IBS-C or functional constipation.24
Daily Stool Diaries
During the 7 days before initiation of the study and the 4 days of treatment, each patient maintained a diary, recording the time of each bowel movement, the description of stool consistency according to the Bristol Stool Form Scale,20 ease of passage of stool (range: 1 = manual disimpaction to 7 = incontinence), and completeness of evacuation (1 = yes and 0 = no).
7αC4 and FGF19 Measurements
Serum 7αC4 levels were quantified by high-performance liquid chromatography with tandem mass spectrometry.25 In our laboratory, a value of <61 ng/mL is established as the 95th percentile for the fasting serum 7αC4 level in healthy volunteers.25 Plasma FGF19 levels were measured using the FGF19 Quantikine Enzyme-Linked Immunosorbent Assay Kit (R&D Systems, Minneapolis, MN).
Genotyping
We assessed candidate genes involved in bile acid homeostasis, that is, genes controlling proteins involved in the regulation of ileal absorption, hepatic uptake, and synthesis of bile acid (see Table 1). We selected SNPs in these genes that are nonsynonymous and have a minor allele frequency (MAF) >9% according to the HapMap-CEU population (see Table 1). These coding SNPs result in missense mutations with potential functional changes in the amino acid sequence of the synthesized protein. Two genes, OSTβ and CYP7A1, do not have coding SNPs with an MAF >9%; therefore, tag SNPs in these genes with r2 > 0.8 and MAF >9% were used. In total, 16 SNPs or tag SNPs in 7 candidate genes were genotyped.
Table 1.
List of Genotypes Analyzed
| Gene | SNP rs ID | Major allele | Minor allele | Hardy–Weinberg equilibrium P value | Predicted MAF | Observed MAF | Function | Amino acid change | Amino acid position |
|---|---|---|---|---|---|---|---|---|---|
| NR0B2 (SHP) | rs6659176 | C | G | 1 | 0.092 | 0.035 | Missense | Ala → Gly | 171 |
| SC10A2 (ASBT) | rs188096 | G | T | 1 | 0.192 | 0.123 | Missense | Ala → Ser | 171 |
| KLB | rs17618244 | G | A | .565 | 0.155 | 0.202 | Missense | Arg → Gln | 728 |
| KLB | rs4975017 | C | A | .888 | 0.325 | 0.395 | Missense | Gln → Lys | 1020 |
| FGFR4 | rs1966265 | G | A | 1 | 0.224 | 0.211 | Missense | Val → Ile | 10 |
| FGFR4 | rs376618 | T | C | .264 | 0.225 | 0.175 | Missense | Leu → Pro | 136 |
| FGFR4 | rs351855 | G | A | 1 | 0.283 | 0.386 | Missense | Gly → Arg | 388 |
| OST3 | rs939885 | G | A | .126 | 0.458 | 0.491 | Missense | Val → Ile | 202 |
| OST3 | rs2946676 | A | G | .792 | 0.14 | 0.125 | Near-gene-3 (dbSNP) | ||
| OST3 | rs4238399 | C | T | 1 | 0.37 | 0.43 | Near-gene-3 (dbSNP) | ||
| CYP7A1 | rs10957057 | C | T | 1 | 0.14 | 0.149 | Intergenic (GVS) | ||
| CYP7A1 | rs8192879 | C | T | .107 | 0.37 | 0.412 | Utr-3 (dbSNP) | ||
| CYP7A1 | rs8192877 | A | G | 1 | 0.18 | 0.167 | Intron (dbSNP) | ||
| CYP7A1 | rs11786580 | C | T | .558 | 0.24 | 0.246 | Intron (dbSNP) | ||
| CYP7A1 | rs3808607 | T | G | .785 | 0.37 | 0.377 | Near-gene-5 (dbSNP) | ||
| CYP7A1 | rs7833904 | T | A | .359 | 0.41 | 0.404 | Intergenic (GVS) |
NOTE. Predicted MAF is based on the HapMap-CEU population. Observed MAF is derived from genotypes of the 93 subjects in this study. Hardy–Weinberg equilibrium P value >.05 denotes that the study sample is in Hardy–Weinberg equilibrium for the corresponding SNP.
Genomic DNA was isolated from blood using standard methods. Genotyping was performed using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions, with 10 to 20 ng of DNA. Following polymerase chain reaction amplification, end reactions were analyzed on the ABI 7300 Real-Time PCR System using Sequence Detection Software (Applied Biosystems).
Statistical Analysis
An analysis of covariance (ANCOVA) compared the responses overall among the treatment groups and was used to assess the treatment effects of CDC on the primary and secondary end points, with body mass index (BMI) as a covariate. For each of the bowel pattern responses, the corresponding baseline value obtained from the 7 days before initiation of the study was also used as a covariate. Two pairwise comparisons (eg, each dose of CDC against placebo) were also examined using Dunnett’s test. Another set of ANCOVA models incorporated baseline 7αC4 and FGF19 levels separately as covariates to assess whether treatment effects with placebo were affected by baseline levels of these enzymes.
Associations of fasting serum 7αC4 levels with colonic transit in the placebo group and the association between fasting 7αC4 and FGF19 levels in all groups were assessed using the Spearman correlation.
Sample Size Considerations for the Pharmacodynamic Effects of CDC on Colonic Transit
Sample size assessment was based on the results of primary end points in a previous study of patients with IBS-C in our laboratory.26 For the primary end point of GC 24, the mean value was 1.9 and coefficient of variation was 15%. The estimated effect size demonstrable in GC 24 was 16%; this effect size is the difference in group means as a percentage of the overall mean based on a 2-sample t test with n = 12 per group. For the other primary end point of AC t½, the mean value was 17 hours and coefficient of variation was 41%. The estimated effect size demonstrable in AC t½ was 43% based on a 2-sample t test with n = 12 per group.
It was anticipated that the ANCOVA would provide similar power for somewhat smaller effect sizes by pooling residual variation across all 3 treatment groups and by incorporating relevant covariates.
Analysis of Relationship of Genotype and Colonic Transit Response to CDC
An ANCOVA (with adjustments for BMI) was used to assess the association of genotypes with potential differential CDC effects on colonic transit variables (GC 24 and AC t½). To increase the power for the analysis, data from the 36 patients with IBS-C in this study were pooled with that of 57 of 60 healthy volunteers who had genetic data available and who participated in a similar study evaluating the effect of the same doses of CDC and placebo on transit.15 We used a dominant genetic model comparing the major allele homozygotes with the combined group of heterozygotes and minor allele homozygotes, adjusting for BMI and phenotype (ie, health and IBS-C). We did not adjust α for the multiple genes tested. Given the differences in the MAFs of the different candidate genotypes, we did not assess the statistical power based on the sample size for each of the candidate genes. However, we assessed post hoc the power for the 2 genes that were associated with AC t½ with P values less than the nominal .05 (see Discussion).
Results
Participants, Study Conduct, and Completion
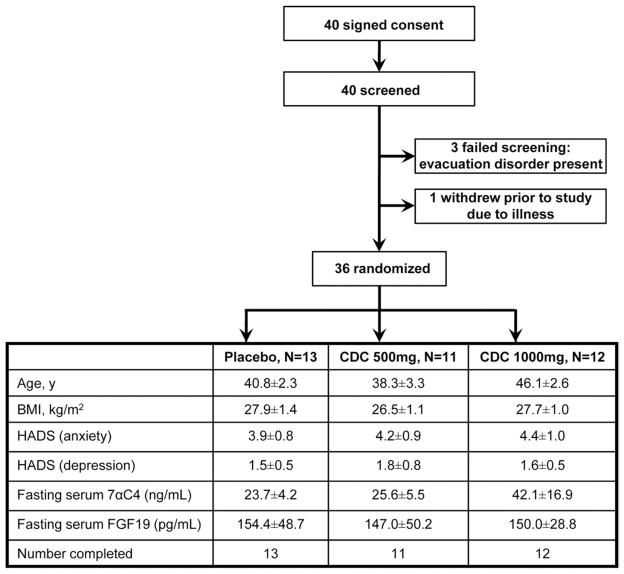
Forty participants with IBS-C (Figure 2) were recruited. Three participants were deemed ineligible based on the initial screen, and one dropped out before randomization secondary to major illness. Demographic data of the 36 female participants randomized to placebo, 500 mg CDC, or 1000 mg CDC are shown in Figure 2; all treatment groups had similar age and BMI. All patients completed the studies, and there was 100% medication compliance (based on coordinator interview and pill count).
Figure 2.
Study flow chart and patient demographics.
Effect of CDC on Colonic Transit in Female Patients With IBS-C
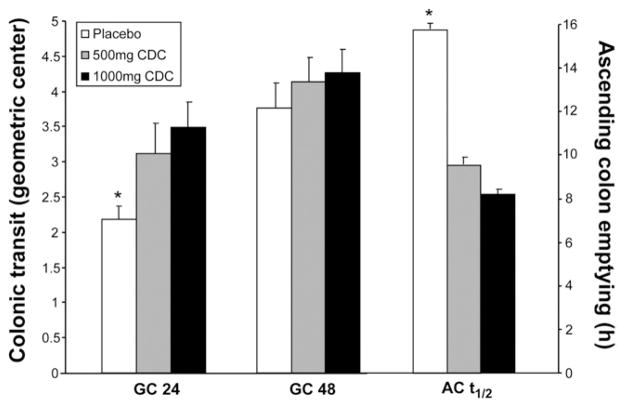
Data are summarized in Table 2 and illustrated in Figure 3. CDC accelerated overall colonic transit at 24 hours (ANCOVA, P = .005, overall CDC vs placebo) with a greater effect observed for the 1000-mg dose than the 500-mg dose compared with placebo (Dunnett’s test, P = .012 and P = .066, respectively). There was also acceleration of AC t½ with CDC treatment (ANCOVA, P = .028, overall CDC vs placebo). The 1000-mg dose of CDC accelerated AC emptying relative to placebo (P = .058, Dunnett’s test), but the effects of 500 mg CDC and placebo were not different (P = .18).
Table 2.
Effects of CDC on GI Transit and Bowel Function
| Placebo (n = 13) | CDC 500 mg (n = 11) | CDC 1000 mg (n = 12) | P value (contrast of drug vs placebo) | |
|---|---|---|---|---|
| GE t½ (min) | 116.5 ± 4.3 | 138.3 ± 11.7 | 134.1 ± 10.3 | .079 |
| CF6 (%) | 50.6 ± 6.7 | 52.6 ± 6.7 | 54.8 ± 7.1 | .71 |
| GC 24 | 2.2 ± 0.2 | 3.1 ± 0.4 | 3.5 ± 0.4 | .005 |
| GC 48 | 3.8 ± 0.3 | 4.1 ± 0.3 | 4.3 ± 0.3 | .130 |
| AC t½ (h) | 15.8 ± 2.5 | 9.5 ± 2.9 | 8.2 ± 1.8 | .028 |
| Stool frequency (daily) | 1.2 ± 0.2 | 1.6 ± 0.2 | 2.1 ± 0.3a | .018 |
| Stool consistency (Bristol Stool Form Scale) | 2.9 ± 0.3 | 4.4 ± 0.5b | 4.4 ± 0.4a | .003 |
| Ease of stool passage (scale 1–7) | 3.7 ± 0.1 | 4.2 ± 0.2 | 4.4 ± 0.2 | .024 |
| Sense of complete evacuation (1 = yes, 0 = no) | 0.30 ± 0.09 | 0.55 ± 0.11 | 0.44 ± 0.09 | .20 |
NOTE. Values are expressed as mean ± SEM unless otherwise noted.
P = .010 vs placebo.
P = .031 vs placebo.
Figure 3.
Effects of placebo or CDC on overall colonic transit and time for AC emptying. *Overall significant difference among placebo and CDC groups (ANCOVA P = .005 for GC 24 and P = .028 for AC t½).
Effect of CDC on Bowel Function in Female Patients With IBS-C
There were significant overall treatment effects of CDC compared with placebo on bowel function, including looser stool consistency (P = .003), increased stool frequency (P = .018), and greater ease of passage (P = .024) (see Table 2 and Supplementary Figure 1).
Effect of CDC on Gastric and Small Bowel Transit in Female Patients With IBS-C
CDC treatment was associated with prolongation of mean GE t½ by an average of 22 and 18 minutes in the 500-mg and 1000-mg CDC groups respectively, relative to the mean GE t½ in the placebo group (ANCOVA, P = .079 overall CDC vs placebo, see Table 2). Significant treatment effects on CF6 were not observed (Table 2).
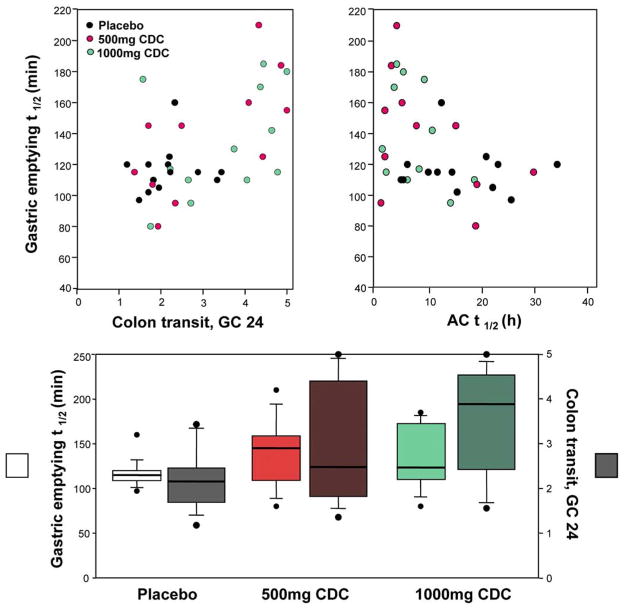
We also assessed the relationship between gastric emptying and colonic transit in response to treatment. There was a significant inverse correlation between GE t½ and colonic transit in the entire cohort (treated with placebo or CDC [see Figure 4, upper panel]); thus, the faster the colonic transit, the slower the stomach emptied (GC 24: rs = 0.464, P = .0043; AC t½: rs = −0.431, P =.009). This relationship was also present in participants who received placebo. Thus, an analysis removing the treatment effects of CDC indicates a correlation between GE t½ and colon transit at 24 hours (r = 0.52 [P = .002]), colon transit at 48 hours (r = 0.42 [P = .014]), and AC t½ (r = −0.39 [P = .026]). However, there was an additional effect of CDC treatment on the relationship between colonic transit and gastric emptying such that, as the colonic transit was accelerated by CDC, there was a greater retardation of gastric emptying (Figure 4, bottom panel).
Figure 4.
Correlation between gastric emptying and colonic transit for each treatment group.
Blood Levels of 7αC4 and FGF19 in Female Patients With IBS-C
No clinically important differences were found among the treatment groups (Figure 2) in fasting serum 7αC4 or plasma FGF19 levels. An inverse correlation was, however, found between the 7αC4 and FGF19 measurements (rs = −0.371, P = .028).
Relationship of Baseline Fasting 7αC4 and FGF19 Levels and Colonic Transit With Placebo or CDC
There was a positive correlation between fasting serum 7αC4 level and colonic transit (GC 24) for the placebo group. Thus, the higher the 7αC4 level, the faster the overall colonic transit observed (rs = 0.749, P = .0032). Supplementary Figure 2 shows the relationship between (the rank transformation of) baseline serum 7αC4 level and observed GC 24 values for the placebo group. There was no significant correlation between fasting serum. 7αC4 level and AC t½.
ANCOVA models assessing treatment effects of CDC on colonic transit indicated an influence of 7αC4 on GC 24 (P = .055) and on GC 48 (P = .019).
There was no correlation between plasma FGF19 level and GC 24 or GC 48 for the placebo or the CDC groups.
Associations of Variations in Candidate Genes Controlling Bile Acids and Colonic Transit
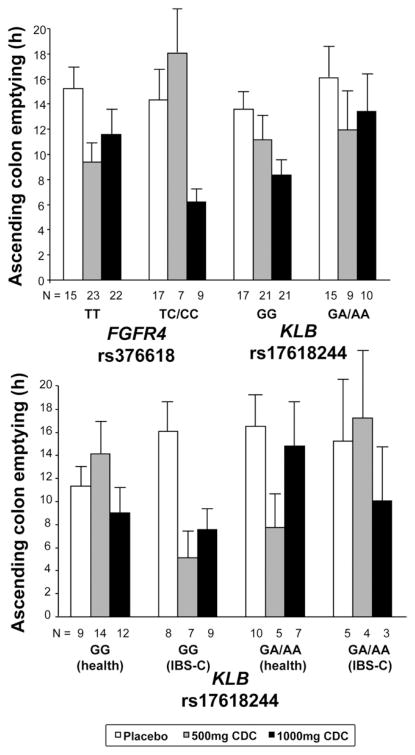
The association between SNP rs376618 from the FGFR4 gene and GC 24 in the entire cohort of IBS-C and healthy participants was not significant (P = .126). SNP rs376618 was, however, associated with AC t½ (uncorrected P = .015) in the entire cohort. The TT genotype was associated with acceleration of transit with both doses of CDC; in contrast, participants with the TC/CC genotypes did not show accelerated transit with the 500-mg dose of CDC, only with the 1000-mg dose of CDC (Figure 5).
Figure 5.
Effects of treatment on AC colon emptying in relation to genotype. The upper panel shows results by genotype in the entire cohort (health and IBS-C). The lower panel shows results for KLB for the different phenotype subgroups and illustrates the genotype-by-treatment-by-phenotype interaction.
SNP rs17618244 from the KLB gene was not associated with colonic transit in the entire cohort (Figure 5). However, a genotype-by-treatment interaction depending on participant subtype was shown (uncorrected P = .0088). Thus, in patients with IBS-C, the GG genotype was associated with accelerated AC t½ with 500-mg and 1000-mg doses of CDC, whereas GA/AA genotypes showed accelerated transit with 1000 mg not with 500 mg of CDC. In contrast, in healthy volunteers with the GG genotype, AC t½ was accelerated by 1000 mg but not by 500 mg of CDC, whereas AC t½ was accelerated in GA/AA carriers by 500 mg but not 1000 mg of CDC.
There were nonsignificant potential associations of CF6 with SNP rs7833904 from the CYP7A1 gene (P = .0891) and of CF6 (P = .0854) and GC24 (P = .0858) with rs6659176 from the SHP gene. The other 12 SNPs analyzed did not correlate with any of the measured transit parameters (data not shown).
Adverse Events With CDC
The most common adverse events with CDC were lower abdominal cramping/pain (0% with placebo, 45% with 500 mg CDC, and 42% with 1000 mg CDC; P = .01 by Fisher exact test), diarrhea (0% with placebo, 18% with 500 mg CDC, and 17% with 1000 mg CDC; P = .36), and nausea (0% with placebo, 9% with 500 mg CDC, and 25% with 1000 mg CDC; P = .14). Headache was observed at similar rates (15%–18%) between the placebo and 2 treatment groups. Gas, heartburn, and light-headedness were also experienced by >10% of participants in the CDC arms (P = not significant). No participants withdrew from the study due to an adverse event.
Discussion
Our study provides insight on the potential use of bile acids for the treatment of bowel dysfunction in patients with IBS-C and helps elucidate the influence of endogenous bile acid synthesis (via indirect serum markers) and genes involved in control of bile acid homeostasis on the response to exogenous CDC. Overall, our data support the role of CDC as a physiologic laxative, accelerating overall and AC colonic transit and improving bowel function, and as a potential treatment in patients with constipation and IBS-C. Genetic variations in the proteins involved in the FGF19-mediated feedback inhibitory pathway of bile acid synthesis may influence the effects of CDC on AC colon emptying.
Pharmacodynamics of CDC in IBS-C
In female patients with IBS-C, doses of CDC previously used for dissolution of gallstones accelerated whole colonic transit, and the higher dose of 1000 mg appeared more efficacious. The acceleration in colonic transit was accompanied by looser stool form, increased stool frequency, and greater ease of passage. A post hoc analysis showed that the coefficient of variation in primary end points observed was similar to that on which the study sample was calculated, and therefore the sample size in each group was appropriate and the results are reliable.
The fasting serum 7αC4 level was associated with colonic transit, suggesting that the endogenous rate of bile acid synthesis influences overall colonic transit in the absence of treatment. This is consistent with the concept that an idiopathic decrease in bile acid levels in the colon is associated with constipation in children.27 Alterations in bile acid synthesis have also been reported in adults with slow transit constipation28 and, conversely, chronic diarrhea in “idiopathic bile acid malabsorption” appears to result from reduced negative feedback by the hormone FGF19.16
Given the postulated role of FGF19 in the feedback control of bile acid synthesis,16 we assessed whether FGF19 was a significant covariate in response to CDC. Our data in female patients with IBS-C replicate the inverse relationship previously observed in health15 and chronic diarrhea16 between fasting serum 7αC4 and FGF19 levels. However, we did not find a significant correlation between fasting FGF19 and CDC-induced colonic transit, in contrast with the significant association of serum 7αC4 level with colonic transit.
Bile salts accelerate colonic transit by inducing fluid and electrolyte secretion or by stimulation of motility. Concentrations of >5 mmol/L bile acids infused directly into the human sigmoid and rectum stimulated colonic phasic contractions10; however, such concentrations are seldom achieved in the colon in the absence of ileal resection.2 In fact, bile acid concentrations in the proximal colon in constipation states are likely to be lower. It is estimated that the human bile acid pool is 3 to 5 mmol, with 6 to 10 enterohepatic circulations each day. The daily bile acid synthesis is about 600 mg29; thus, the daily dose administered in our study may have simply doubled or tripled the load available in the bile acid pool. The efficacy of exogenous bile acids in stimulating colonic secretory or motor function may depend on the baseline endogenous concentrations to which the colon is exposed. This is illustrated by the observation that ANCOVA models assessing treatment effects of CDC on colonic transit indicated an influence of 7αC4 on transit at 24 and 48 hours. We found that in subjects with lower bile acid synthesis rates, as suggested by relatively lower 7αC4 levels, CDC was more effective in accelerating transit. The clinical potential of CDC in patients with IBS-C will require longer administration or repeated administration of short courses. Identifying the subset of patients with IBS-C with a diminished rate of endogenous bile acid synthesis may help target those individuals most likely to respond to CDC treatment. CDC appears to be safe because no major adverse events occurred throughout this study. In addition, there have been no reports of colon cancer or liver damage observed in longer-term follow-up of patients with gallstones who were treated with CDCA and patients who underwent partial ileal bypass for hyperlipidemia.30
In a prior study, fecal parameters did not respond in a subgroup of patients with chronic constipation treated with up to 1 g oral CDCA.14 Our results suggest that the delivery of CDC to the ileocolonic region may be more efficacious, but a direct comparison between oral and delayed release CDC is required to prove this.
A post hoc analysis showed a significant inverse correlation between gastric and colonic transit, consistent with the hypothesis that stimulation of the ileal brake by delivery of bile acids to the ileocolonic region for 4 days provided negative feedback to the stomach. Bile acids have been shown to promote glucagon-like peptide 1 secretion in cell culture.31 The mechanism of in vivo inhibition of gastric emptying by bile acids may involve glucagon-like peptide 1, oxyntomodulin, peptide YY, and neurotensin; future studies are warranted to elucidate this further. Our recent study15 showed a similar delay in gastric emptying in patients with diarrhea-predominant IBS who received the bile acid sequestrant colesevelam, which stimulates glucagon-like peptide 1 and has been shown to enhance glycemic control in diabetes.32 Therefore, it is possible that both bile acids and colesevelam may increase release of incretins or other hormones (eg, peptide YY, oxyntomodulin) from enteroendocrine cells, which then inhibit gastric emptying.
Pharmacogenetics of CDC in IBS-C and Health
Our study also explored potential genetic factors that might impact the therapeutic efficacy of CDC. To assess this hypothesis, we developed assays for common SNPs or tag SNPs in genes associated with bile acid homeostasis, including enterocyte uptake and efflux, hepatic synthesis, and feedback inhibition of bile acid production. To increase the power of our study, we combined data from our previous report of healthy subjects15 with the data from the patients with IBS-C in this study; the 2 studies had identical study design with the same doses of CDC used. Our data suggest that the genes of 2 proteins critical in FGF19-mediated feedback inhibition of hepatic bile acid synthesis impact the acceleration in colonic transit (specifically AC colonic emptying) by CDC. The SNP rs376618 in FGFR4 was associated with differences in the effects of CDC on colonic transit, and patients with the rs376618 TT genotype responded to a lower concentration of CDC delivered to the colon. These data also raise the hypothesis that carriers of the TT genotype are more sensitive to effects of endogenous bile acids and are thus more susceptible to the syndrome of bile acid malabsorption and chronic diarrhea. The magnitude of the effect of this genetic polymorphism is consistent with the influence of fasting serum 7αC4 on treatment response to CDC.
A weaker association was identified with SNP rs17618244 in KLB. In a separate study of the genetic association of this SNP with intermediate phenotypes of diverse colonic functions, we found a significant interaction between rs17618244 and colonic transit.33 In the current study, there was an effect of rs17618244 genotype on dose response in patients with IBS-C, similar to what was observed with rs376618 in FGFR4. The additional finding of an interaction of genotype-by-treatment effects with participant subgroup, with a different effect observed in healthy subjects compared with patients with IBS-C, may be spurious given the relatively small sample size and MAF of 15.5% of rs17618244. Nevertheless, if these SNPs are functional, genetic variations in these 2 proteins involved in ileal feedback suppression of hepatic bile acid synthesis by CYP7A1 (the rate-limiting enzyme in the synthesis of bile acid from cholesterol) may ultimately be critical for controlling the size of the total body bile acid pool, the dynamics of the enterohepatic circulation, and hence the amount of bile acids reaching the colon to promote laxation. FGFR4 and KLB are both transmembrane proteins expressed on the surface of hepatocytes, where they serve together in a receptor complex for binding their ligand FGF19 and activating downstream intracellular signaling pathways to suppress bile acid synthesis. Our results therefore raise the tantalizing hypothesis that variations in bile acid homeostatic genes are associated with colonic transit in response to exogenously administered bile acids.
The genetic associations observed are clearly hypothesis generating given the relatively small sample size (n = 93) and multiple SNPs (n = 16) tested. However, we do perceive that there was adequate power to detect clinically meaningful associations in this study, because we estimated the differences in physiologic responses that could be identified between subgroups defined by genotype. This study examined several SNPs; therefore, assuming a dominant genetic model, there were differing numbers of subjects in the subgroups for each SNP. For 2 of the SNPs studied, FGFR4 (rs371688) and KLB (rs17618244), the breakdown of minor allele homozygotes plus heterozygotes (CC/TT for FGFR4 and GA/AA for KLB) versus major allele homozygotes (TT for FGFR4 and GG for KLB) was almost identical: 33 versus 60 and 34 versus 59, respectively. Thus, the difference in AC t½ that could be detected with ≥80% power (using a 2-sided α level of .05) is calculated to be 5.3 hours, based on univariate associations, for groups of 33 and 60. Figure 5 shows that in response to treatment with 1000 mg CDC, there is a >5-hour difference in AC t½ in one genotype grouping versus the other for the 2 genes of interest. In summary, this post hoc assessment shows that the study had sufficient power to detect meaningful differences in AC t½ for these 2 different genotypes.
In summary, CDC accelerates colonic transit and improves bowel function in female patients with IBS-C. The rate of bile acid synthesis influences colonic transit. Genetic variations in bile acid synthesis may affect CDC-mediated acceleration of colonic transit and deserves further study.
Supplementary Material
Acknowledgments
The authors thank Cindy Stanislav for excellent secretarial support.
Funding
Supported by National Institutes of Health grants DK-54681, DK-079866, and DK-086182 (to M.C.) and National Institutes of Health Clinical and Translational Science Award grant RR0024150.
Abbreviations used in this paper
- AC
ascending
- ANCOVA
analysis of covariance
- BMI
body mass index
- CDC
(sodium) chenodeoxycholate
- CF6
colonic filling at 6 hours
- FGF19
fibroblast growth factor 19
- FGFR4
fibroblast growth factor receptor 4
- GC
geometric center
- GC 24
geometric center at 24 hours
- GC 48
geometric center at 48 hours
- GE
gastric emptying half-life
- IBS-C
constipation-predominant irritable bowel syndrome
- KLB
Klothoβ
- MAF
minor allele frequency
- 7αC4
7α-hydroxy-4-cholesten-3-one
- SNP
single nucleotide polymorphism
- t½
half-life
Footnotes
Conflicts of interest
The authors disclose no conflicts.
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2010.07.052.
References
- 1.Iser JH, Sali A. Chenodeoxycholic acid: a review of its pharmacological properties and therapeutic use. Drugs. 1981;21:90–119. doi: 10.2165/00003495-198121020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell WD, Findlay JM, Prescott RJ, et al. Bile acids in the diarrhoea of ileal resection. Gut. 1973;14:348–353. doi: 10.1136/gut.14.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M, Murphy R, Chadwick VS. Dose-related effects of chenodeoxycholic acid in the rabbit colon. Dig Dis Sci. 1980;25:433–438. doi: 10.1007/BF01395507. [DOI] [PubMed] [Google Scholar]
- 4.Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conley DR, Coyne MJ, Bonorris GG, et al. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am J Dig Dis. 1976;21:453–458. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 7.Raimondi F, Santoro P, Barone MV, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 8.Alrefai WA, Saksena S, Tyagi S, et al. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 9.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 10.Kirwan WO, Smith AN, Mitchell WD, et al. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok HY, Bell GD, Dowling RH. Effect of different doses of chenodeoxycholic acid on bile-lipid composition and on frequency of side-effects in patients with gallstones. Lancet. 1974;2:253–257. doi: 10.1016/s0140-6736(74)91415-9. [DOI] [PubMed] [Google Scholar]
- 12.Caspary WF, Meyne K. Effects of chenodeoxy- and ursodeoxycholic acid on absorption, secretion and permeability in rat colon and small intestine. Digestion. 1980;20:168–174. doi: 10.1159/000198436. [DOI] [PubMed] [Google Scholar]
- 13.Fromm H, Roat JW, Gonzalez V, et al. Comparative efficacy and side effects of ursodeoxycholic and chenodeoxycholic acids in dissolving gallstones. A double-blind controlled study. Gastroenterology. 1983;85:1257–1264. [PubMed] [Google Scholar]
- 14.Bazzoli F, Malavolti M, Petronelli A, et al. Treatment of constipation with chenodeoxycholic acid. J Int Med Res. 1983;11:120–123. doi: 10.1177/030006058301100211. [DOI] [PubMed] [Google Scholar]
- 15.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters JR, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Sauter GH, Munzing W, von Ritter C, et al. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 20.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 21.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293–300. e282. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 23.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. e495. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M, Talley NJ. Pathophysiology as a basis for understanding symptom complexes and therapeutic targets. Neurogastroenterol Motil. 2004;16:135–142. doi: 10.1111/j.1365-2982.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:e734–e743. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann AF, Loening-Baucke V, Lavine JE, et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008;47:598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 28.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, et al. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol. 2008;43:1483–1488. doi: 10.1080/00365520802321212. [DOI] [PubMed] [Google Scholar]
- 29.Pattni S, Walters JR. Recent advances in the understanding of bile acid malabsorption. Br Med Bull. 2009;92:79–93. doi: 10.1093/bmb/ldp032. [DOI] [PubMed] [Google Scholar]
- 30.Buchwald H, Varco RL, Boen JR, et al. Effective lipid modification by partial ileal bypass reduced long-term coronary heart disease mortality and morbidity: five-year posttrial follow-up report from the POSCH. Program on the Surgical Control of the Hyperlipidemias. Arch Intern Med. 1998;158:1253–1261. doi: 10.1001/archinte.158.11.1253. [DOI] [PubMed] [Google Scholar]
- 31.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RB, Holman R, Drucker DJ. Clinical decisions. Management of type 2 diabetes. N Engl J Med. 2008;358:293–297. doi: 10.1056/NEJMclde0708469. [DOI] [PubMed] [Google Scholar]
- 33.Wong BS, Camilleri M, Carlson P, et al. Klotho-beta gene polymorphism is associated with colonic transit in health and lower functional gastrointestinal disorders. Gastroenterology. 2010;138:S624. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.