Abstract
Tendon stem cells (TSCs) have been proposed to play a major role in the development of tendinopathy, which refers to pathological changes, such as calcification, in affected tendons. Using a human TSC (hTSC) culture model, this study investigated the effects of PGE2, an inflammatory mediator present in injured tendons, on hTSC proliferation and differentiation as well as the molecular mediator for such PGE2-induced effects. We found that PGE2 treatment of hTSCs decreased cell proliferation and caused osteogenic differentiation of hTSCs in a dose- dependent manner. Also, PGE2 treatment of hTSCs induced dose-dependent BMP-2 production in culture, and moreover, addition of BMP-2 to hTSC culture decreased cell proliferation and induced hTSC differentiation into osteoblasts. Finally, addition of BMP-2 antibodies to hTSC culture treated with PGE2 nearly abolished PGE2 effects on both cell proliferation and osteogenic differentiation. Taken together, the findings of this study showed that BMP-2 mediates PGE2-induced reduction of proliferation and osteogenic differentiation of hTSCs. We suggest that such a mechanism may be partially responsible for the formation of calcified tissues in tendinopathic tendons seen in clinical settings.
Keywords: Tendinopathy, tendon stem cells, proliferation, differentiation, osteogenesis
INTRODUCTION
Tendons are fibrous bands of connective tissue that connect muscles to bones. Traditionally, tendons are thought to contain mainly tenocytes, or fibroblast-like cells responsible for the maintenance and repair of tendons. However, in recent years, a new type of tendon cells termed tendon stem cells (TSCs) have been identified in humans, mice 1; 2, rabbits 3, and rats 4. TSCs differ from tenocytes in that they possess multi-differentiation potential 3; for example, under appropriate induction conditions, these tendon-specific stem cells can differentiate into osteoblasts, or bone cells, among other non-tenocyte lineages of cells.
The function of tendons is to transmit muscular forces to bone, thus enabling joint movements. During such force transmission, large mechanical loads act on the tendons; consequently, tendons are frequently injured. Tendon injuries are divided into two categories, namely acute tendon injury (or traumatic injury) and chronic tendon injury. The latter is also referred to tendinopathy 5. A prevalent tendon disorder in both occupational and athletic settings, tendinopathy is associated with pain, localized tenderness, swelling, and impaired performance. Histopathologically, tendinopathy at later stages (or the degenerative stages 6) is characterized by variable zones of reduced number of cells 7; 8 and calcific tissues, among other symptoms, in tendon lesions 9. While the precise mechanisms for the development of tendinopathy remain elusive, previous studies 10–12 suggest that prostaglandin E2 (PGE2), a major inflammatory mediator of pain and acute inflammation in injured tendons 13, may be involved due to its catabolic effects on tendon cells. PGE2 treatment of tenocytes, for instance, decreases cell proliferation and cellular production of collagen 14. In addition, when rabbit TSCs were treated with PGE2 in vitro, the treatment suppressed cell proliferation and induced TSC differentiation into osteoblasts 13. Despite these observations of PGE2 effects, the molecular mediators responsible for such effects on TSCs were not known. Considering that bone morphogenetic protein-2 (BMP-2) is a bone-inducing differentiation factor 15 that causes mesenchymal stem cells (MSCs) to differentiate into osteoblasts 16, we hypothesized that PGE2 induced reduction of TSC proliferation and osteogenic differentiation is mediated through BMP-2. To test this hypothesis, we performed cell culture experiments using human tendon stem cells (hTSCs). We found that BMP-2 mediated the reduction of proliferation and osteogenic differentiation of hTSCs due to PGE2 treatment. The findings of this study suggest a TSC-based mechanism for the development of calcific tendinopathy.
MATERIALS AND METHODS
hTSC culture
hTSCs were derived from the patellar tendons of six human donors, ages 26 to 49 years old. Briefly, using our published protocol 3, a single-cell suspension was obtained from core portions of patellar tendons and cultured in flasks at 37°C with 5% CO2. After 8–10 days in culture, hTSCs formed colonies on the culture surface of the flask. These colonies were isolated by local treatment with 0.25% trypsin and cultured in growth medium (DMEM plus 20% FBS). hTSCs were expanded once (or passage 1), and cells at passage 1 were used for all cell culture experiments described as follows.
The PGE2 effect experiment on cell proliferation
hTSCs were seeded in 6-well plates at a density of 6 × 104/well and cultured in growth medium with three concentrations (1, 10, and 100 ng/mL) of PGE2. Cells without PGE2 treatment were used as controls. The medium was changed every three days, with addition of fresh PGE2 each time. The cell proliferation was measured at 3 and 7 days by counting cells using a hemocytometer.
The measurement of BMP-2 levels in culture
In the cell proliferation experiment above, cell-conditioned medium at 7 days was collected, and BMP-2 levels in culture were measured using an ELISA kit (R&D Systems).
The PGE2 effect experiment on osteogenic differentiation of hTSCs
In a separate experiment, hTSCs were seeded in 6-well plates at a density of 24 × 104/well and cultured in growth medium with various concentrations of PGE2 for 14 days. Alizarin S Red assay 3 was used to assess osteogenic differentiation of hTSCs. In addition, immuno-staining for osteocalcin expression was also performed to verify osteogenesis of hTSCs by PGE2. Osteocalcin is a non-collagenous protein produced solely by osteoblasts 17; hence, it was used as a marker of osteogenesis of hTSCs in this study.
The BMP-2 effect experiment on cell proliferation and differentiation
hTSCs were seeded in 6-well plates at a density of 9 × 104/well and cultured in growth medium for two days. Then the cells were divided into five groups as follows. Group 1: the control cells without any treatment; group 2: the cells treated with PGE2 (100 ng/ml); group 3: the cells treated with the same amount of PGE2 plus a saturated concentration of BMP-2 antibodies (100 ng/ml); group 4: the cells treated with BMP-2 (10 ng/ml), and group 5: the cells treated with the same amount of BMP-2 plus a saturated concentration of BMP-2 antibodies (100 ng/ml).
The cells were grown in culture for up to 15 days. During culture time, media were replaced with new growth media containing fresh PGE2 and anti-BMP-2 antibodies every 3 days. The proliferation of hTSCs in each group was assessed by measuring cell population doubling time (PDT) 3, and the differentiation of hTSCs in each group was assessed using Alizarin Red S assay for staining calcium deposits and immunostaining for osteocalcin.
Alizarin Red S Assay
Osteogenesis of hTSCs induced by PGE2 or BMP-2 was evaluated by Alizarin Red S assay. After removing culture medium, cells were fixed in ice-cold 70% ethanol for 1 hr. Then the cells were rinsed with distilled water 2 times, each 5 min, and stained with Alizarin Red S (Millipore, Cat. # 2003999) at room temperature for 30 min. The stained cells were examined on the microscope, with images taken by a CCD camera and analyzed by SPOT imaging software. The osteoblasts containing mineral deposits would be stained brown by Alizarin Red S assay, which is an established method to assess osteogenesis of stem cells 1; 3.
Immunostaining of hTSCs and differentiated cells
Immunocytochemistry was performed on the stem cell markers, including Oct-4, SSEA-4, and nucleostemin, using the protocol previously described 3. In addition, immunostaining was performed on differentiated cells to detect the expression of osteocalcin. Briefly, cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and blocked in 2% mouse or goat or rabbit serum for 1 hr. Then the cells were incubated at room temperature with mouse anti-human Oct-4 (1:400; Millipore, Cat. # P20263) for 5 hrs, mouse anti-human SSEA-4 (1:350; Invitrogen, Cat. # 414000) for 5 hrs, goat anti-human nucleostemin (1:500; Neuromics, Cat. # GT1505) for 4 hrs, and rabbit anti-osteocalcin (1:350; Millipore, Cat. # AB10911) for 3 hrs. After washing the cells with PBS for three times, Cy3-conjugated goat anti-rabbit IgG (1:500; Invitrogen, Cat. # A10521) for Oct-4 and SSEA-4, Cy3-conjugated Donkey anti-goat IgG (1:500; Millipore, Cat. # AP180C) for nucleostemin, and Cy3-conjugated goat anti-rabbit IgG (1:500; Millipore, Cat. # AP132C) for osteocalcin were applied. Negative controls were used during immunostaining by omitting primary antibodies, and at least two independent stainings were performed. The stained cells were examined and color images of cells were obtained on a fluorescence microscope.
Statistical analysis
Unless otherwise indicated, data were expressed in mean ± SD. For statistical data analysis, one-way ANOVA was used, followed by Fisher’s PLSD test for multiple comparisons. A P-value less than 0.05 was considered to be significantly different.
RESULTS
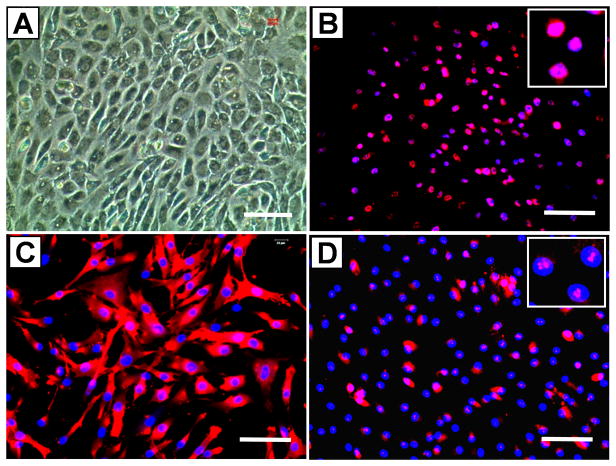
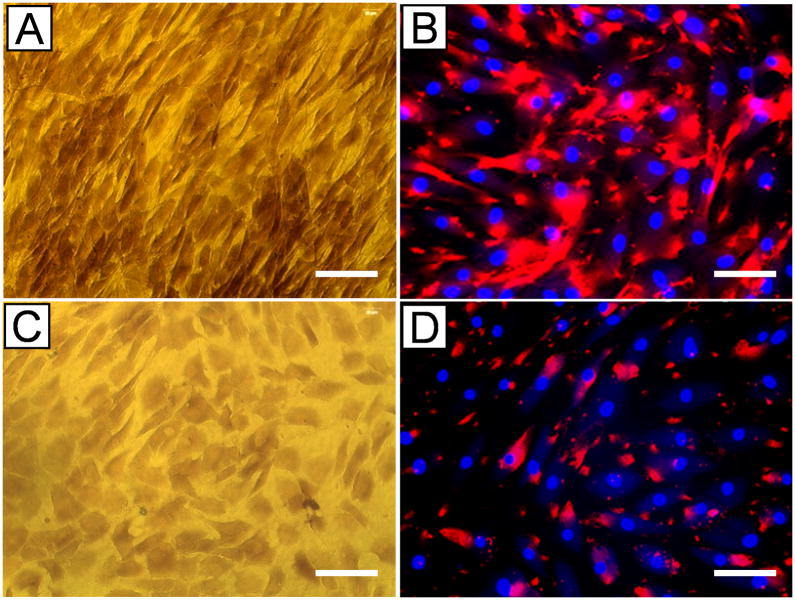
Human TSCs (hTSCs) exhibited a cobblestone-like shape in confluent conditions (Fig. 1A), in contrast to highly elongated fibroblast-phenotype of tenocytes (not shown). Immunostaining also showed that hTSCs expressed stem cell markers Oct-4, SSEA-4, and nucleostemin (Fig. 1B, C, D), thus verifying their stem cell identity.
Fig. 1.
The identity of human tendon stem cells (hTSCs) was verified. Specifically, hTSCs exhibited a cobblestone-like shape when grown to confluence (A). However, human tenocytes were highly elongated in shape (not shown). Moreover, hTSCs in culture expressed stem cell markers Oct-4 (B, pink dots in the inset), SSEA-4 (C, red), and nucleostemin (D, pink dots in the inset). Bar: 100 μm.
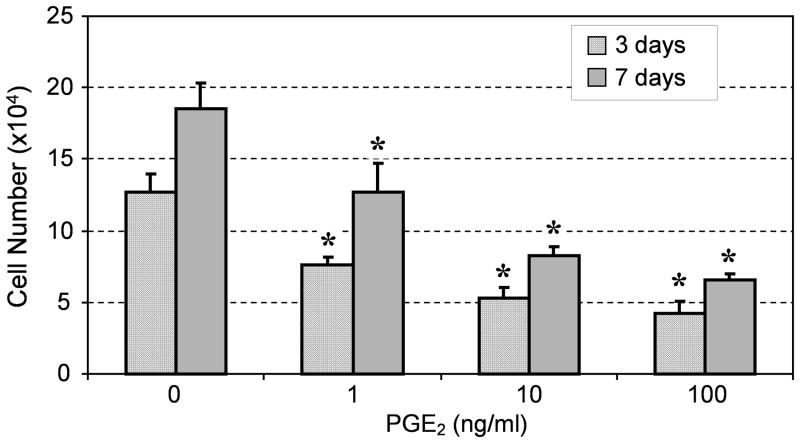
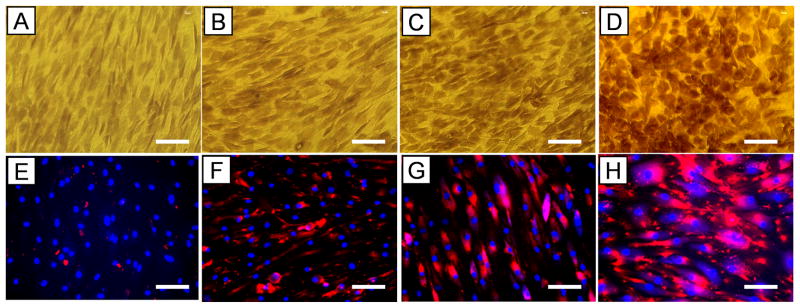
After hTSCs were cultured in PGE2-containing growth medium for 3 and 7 days, cell proliferation decreased at both time points in a dose-dependent manner (Fig. 2). Moreover, PGE2 treatment induced osteogenic differentiation of hTSCs, as evidenced by the accumulation of calcium deposits (Fig. 3A-D) and the expression of osteocalcin (Fig. 3E-H), a marker for osteoblasts. The PGE2 effects on osteogenic differentiation of hTSCs also appeared to be dose dependent.
Fig. 2.
PGE2 treatment of hTSCs decreased cell proliferation at both time points (3 and 7 days) in a dose-dependent manner (*P < 0.05, with respect to control and to each other).
Fig. 3.
PGE2 treatment induced osteogenic differentiation of hTSCs. A, E. Controls (i.e. cells without PGE2 treatment); B, F. PGE2 treatment at a low dose (1 ng/ml); C, G. PGE2 treatment at a medium dose (10 ng/ml); and D, H. PGE2 treatment at a high dose (100 ng/ml). With increased PGE2 dosage, more calcium deposits (A-D) were stained, and more extensive expression of osteocalcin were detected (E-H, red). Bar: 100 μm.
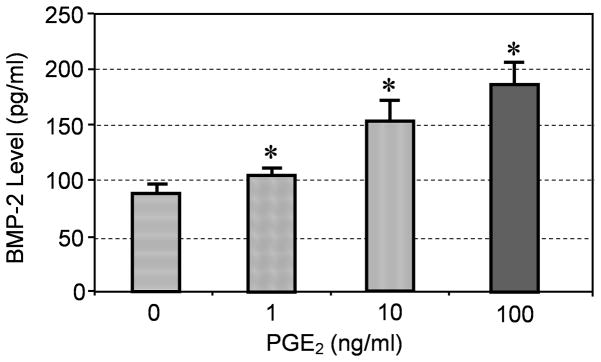
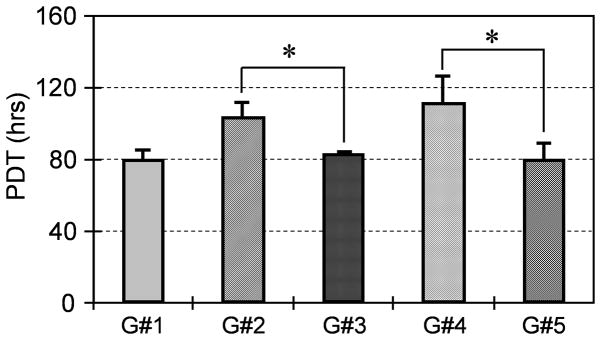
In addition, PGE2 treatment of hTSCs induced production of BMP-2 in the medium, with increasing levels dependent on PGE2 dose (Fig. 4). Addition of PGE2 (100 ng/ml) or BMP-2 (10 ng/ml) to hTSC culture decreased cell proliferation, but each inhibitory effect was abolished by co-addition of BMP-2 antibodies, as cell proliferation returned to control levels (Fig. 5). Furthermore, hTSCs treated with BMP-2 (10 ng/ml) underwent osteogenic differentiation (Fig. 6A, B), as shown by staining of calcium deposits and osteocalcin; however addition of BMP-2 antibodies to hTSC culture treated with PGE2 (100 ng/ml) largely blocked hTSC osteogenesis as well (Fig. 6C, D).
Fig. 4.
PGE2 treatment of hTSCs increased BMP-2 production in culture in an apparent dose- dependent manner (*P < 0.05, with respect to control and to each other.)
Fig. 5.
BMP-2 treatment mediates reduction in proliferation of PGE2-treated hTSCs. Cell proliferation was assessed by measuring the population doubling time (PDT) of cells in culture. Note that G#1 is the control cells without any treatment, G#2 is the cells treated with PGE2 (100 ng/ml); G#3 is the cells treated with the same amount of PGE2 plus a saturated concentration of BMP-2 antibodies (100 ng/ml); G#4 is the cells treated with BMP-2 (10 ng/ml), and G#5 is the cells treated with the same amount of BMP-2 plus a saturated concentration of BMP-2 antibodies (100 ng/ml). As indicated, G#3 was significantly different from G#2, so was G#5 with respect to G#4 (*P < 0.05). Also, note that G#2 and G#4 were significantly different from G#1 (the control group), respectively. However, there was no significant difference between G#2 and G#4.
Fig. 6.
BMP-2 mediated osteogenic differentiation of PGE2-treated hTSCs. When treated with BMP-2, hTSCs underwent osteogenic differentiation, as evidenced by the accumulation of abundant calcium deposits (A, compared to Fig. 3D) and extensive osteocalcin expression (B, red). Addition of BMP-2 antibodies to hTSC culture treated with PGE2 (100 ng/ml) markedly decreased the extent of osteogenic differentiation, as indicated by light staining of calcium (C, compared to Fig. 3A) and osteocalcin (D, compared to Fig. 3E ). Bar: 100 μm.
DISCUSSION
To determine how PGE2 treatment of hTSCs affects cell proliferation and differentiation and whether BMP-2 mediates such PGE2-induced effects, we first derived hTSCs and verified their stemness from their cobblestone shape in confluent conditions and their expression of Oct-4, SSEA-4, and nucleostemin, three tendon stem cell markers 3. We then performed an hTSC culture experiment and showed that PGE2 treatment of hTSCs decreased cell proliferation and induced osteogenic differentiation, which was consistent with our previous findings on rabbit TSCs 13. We further showed that BMP-2 was produced in PGE2 treated hTSC cultures and that BMP-2 treatment of hTSCs also resulted in reduced cell proliferation and osteogenic differentiation, similar to the results of PGE2 treatment. When BMP-2 antibodies were added to BMP-2 or PGE2-containing hTSC cultures, however, cell proliferation went back to control levels, and osteogenic differentiation was largely abolished. Taken together, the results indicate that PGE2 treatment of hTSCs suppressed cell proliferation and induced osteogenic differentiation and that the two cellular events were mediated by BMP-2 produced in culture due to PGE2 treatment.
PGE2 is a prostaglandin belonging to the eicosanoid family that plays a prominent role in inflammatory and immune responses as well as various other tissue responses 18; 19. It is also known that PGE2 is produced in human, rabbit, and mouse tendons 13; 20; 21. Taken together with the findings of this study, we believe that PGE2, acting as a local hormone in tendons, may contribute to the development of tendinopathy through two parallel, cellular mechanisms: a) decreasing the number of TSCs, and b) inducing osteogenic differentiation of TSCs. Mediated by BMP-2, both cellular events may lead to reduction in TSCs available for tendon repair and calcification often seen in tendons at later stages of tendinopathy 9.
BMP-2 is a known osteogenic growth factor and has been demonstrated to induce bone formation in vivo; for example, implantation of BMP-2 in a collagen sponge induces new bone formation, and this approach is widely used for the treatment of bony defects, delayed union, and non-union 22. In vitro, BMP-2 causes a dose-dependent decrease in proliferation of human fetal osteoblastic cells (hFOB 1.19) 23 and induces osteogenic differentiation of BMSCs 16. The cellular events caused BMP-2 are likely two separated but coordinated processes 24. In this study, when hTSCs were treated with BMP-2, they also differentiated to osteoblasts, as indicated by the presence of abundant calcium deposits and the expression of osteocalcin. Therefore, our finding that BMP-2 treatment of hTSCs leads to reduced cell proliferation and osteogenic differentiation is consistent with the known osteogenic functions of BMP-2 as well as its effect on osteoblastic cells.
BMP-2 has been found in injured tendons, and it has been suggested to be responsible for the calcification of tendinous tissues in vivo 25. In addition, mechanical loading of so called tendon-derived stem cells increases BMP-2 expression 26. However, because PGE2 treatment of hTSCs leads to BMP-2 production (Fig. 4), and mechanical loading of tendons and tenocytes increases PGE2 production 10; 12; 27, we suspect that PGE2 acts as an upstream molecule of BMP-2 that suppresses hTSC proliferation while inducing osteogenic differentiation. This argument is also supported by the previous finding that treatment of human MSCs (hMSCs) with NS-398, which blocks cellular production of PGE2, among others, by inhibiting COX-2 28, down-regulates BMP-2 expression 29.
In this study, PGE2 at all three doses (1, 10, 100 ng/ml, or 2.84, 28.4, 284 nM) was shown to decrease hTSC proliferation. A previous study 30 showed that while PGE2 at 25 and 250 nM decreased proliferation of hMSCs, as measured by enhanced 3H-thymidine incorporation, at low concentrations (< 25 nM), PGE2 slightly increased hMSC proliferation. Therefore, Kleiveland’s study suggests that PGE2 at lower concentrations than those used in his study could increase instead of decrease hTSC proliferation. While this possibility is definitely worthy of further investigation, there are two important differences between this study and our study. These include: a) hMSCs vs. hTSCs. Another previous study showed that the two types of stem cells differ from each other in terms of gene profiles 1; and b) hMSCs in serum starvation conditions vs. hTSCs in cultures with 20% FBS. Stem cells in culture are metabolically active 1; 3. Therefore, there is a possibility that such serum starvation may change the stemness of stem cells. Finally, MSCs may respond differently to PGE2 treatment than hTSCs, as a previous study showed that addition of PGE2 to rat MSC cultures increased cell number in a concentration-dependent manner 31. One cautionary note is, however, that the stemness of MSCs in this study and Kleiveland’s study were not characterized.
It is now known that tendon tissues produce PGE2 in response to mechanical loading placed on tendons 13; 32. So the question is: are there any mechanisms in tendons that can minimize the catabolic effects on TSCs? While future studies are obviously required to look into this important question, we speculate that like tendon fibroblasts, leukotriene B4 may be able to counter-balance the negative effects induced by PGE2 on TSCs 33.
Now a few comments on the limitations of this study are in place. First, this was a cell culture study that did not take into account the effects of mechanical loading on hTSCs. However, since tendons are subject to large mechanical loading in vivo, future studies should investigate the possible synergistic effects of PGE2 and mechanical loading on TSCs. Second, in addition to BMP-2, there are other BMPs 15, such as BMP-4 and BMP-7, that could also play a role in mediating PGE2 effects on TSC proliferation and osteogenic differentiation. A previous study showed that BMP-4 and BMP-7 were expressed by chondrocyte-like cells in a rat injury model 34, suggesting that during tendon injury, these BMPs could also mediate non-tenocyte differentiation of TSCs (e.g. chondrogenesis). Third, the influence of tenocytes on TSCs in terms of their response to PGE2 treatment was not examined. Future investigation should be done to determine the interactions between the two types of tendon cells. Fourth, in our TSC culture model, exogenous instead of endogenous PGE2 was used to assess its effects on TSCs. There might be differences between the two sources of PGE2 in their effects on TSCs. Finally, the molecular signaling pathways of BMP-2 that lead to osteogenic differentiation of TSCs in this study are not known and remain to be determined in future studies. It is likely, however, that p38 kinase and Smad1/5 could be involved in BMP-2 signaling 35.
In summary, this study showed that PGE2 treatment of hTSCs decreases cell proliferation and induces osteogenic differentiation and that these effects are mediated through BMP-2 produced in response to PGE2 treatment. We suggest that such BMP-2 mediated effects on hTSCs may contribute to the formation of calcified tissues in tendinopathic tendons.
Acknowledgments
We gratefully acknowledge the funding support from NIH grants AR049921 and AR049921S2 (JHW).
References
- 1.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Pan T, Liu Y, Wang JH. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28:1178–1183. doi: 10.1002/jor.21123. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010 doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 6.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 7.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford) 2006;45:508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- 8.Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- 10.Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Medicine & Science in Sports & Exercise. 1993;25:603–607. [PubMed] [Google Scholar]
- 11.Wang JH, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- 12.Flick J, Devkota A, Tsuzaki M, et al. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin Biomech (Bristol, Avon) 2006;21:99–106. doi: 10.1016/j.clinbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28:198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 14.Cilli F, Khan M, Fu F, Wang JH. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14:232–236. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Carlsen B, Wulur I, et al. BMP-2 exerts differential effects on differentiation of rabbit bone marrow stromal cells grown in two-dimensional and three-dimensional systems and is required for in vitro bone formation in a PLGA scaffold. Exp Cell Res. 2004;299:325–334. doi: 10.1016/j.yexcr.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 17.Puchacz E, Lian JB, Stein GS, et al. Chromosomal localization of the human osteocalcin gene. Endocrinology. 1989;124:2648–2650. doi: 10.1210/endo-124-5-2648. [DOI] [PubMed] [Google Scholar]
- 18.Gross S, Tilly P, Hentsch D, et al. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J Exp Med. 2007;204:311–320. doi: 10.1084/jem.20061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Langberg H, Boushel R, Skovgaard D, et al. Cyclo-oxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J Physiol. 2003;551:683–689. doi: 10.1113/jphysiol.2003.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshima H, Kondo S, Mishima S, et al. Expression of interleukin-1beta, cyclooxygenase-2, and prostaglandin E2 in a rotator cuff tear in rabbits. J Orthop Res. 2007;25:92–97. doi: 10.1002/jor.20241. [DOI] [PubMed] [Google Scholar]
- 22.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Gautschi OP, Cadosch D, Zellweger R, et al. Apoptosis induction and reduced proliferation in human osteoblasts by rhBMP-2, -4 and -7. J Musculoskelet Neuronal Interact. 2009;9:53–60. [PubMed] [Google Scholar]
- 24.Alvarez-Rodriguez R, Barzi M, Berenguer J, Pons S. Bone morphogenetic protein 2 opposes Shh-mediated proliferation in cerebellar granule cells through a TIEG-1-based regulation of Nmyc. J Biol Chem. 2007;282:37170–37180. doi: 10.1074/jbc.M705414200. [DOI] [PubMed] [Google Scholar]
- 25.Lui PP, Chan LS, Cheuk YC, et al. Expression of bone morphogenetic protein-2 in the chondrogenic and ossifying sites of calcific tendinopathy and traumatic tendon injury rat models. J Orthop Surg Res. 2009;4:27. doi: 10.1186/1749-799X-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rui YF, Lui PP, Ni M, et al. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011;29:390–396. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- 27.Wang JH-C, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: A novel in vitro model study. Conn Tiss Research. 2002 doi: 10.1080/03008200390223909. in press. [DOI] [PubMed] [Google Scholar]
- 28.Meyer-Siegler K. COX-2 specific inhibitor, NS-398, increases macrophage migration inhibitory factor expression and induces neuroendocrine differentiation in C4-2b prostate cancer cells. Mol Med. 2001;7:850–860. [PMC free article] [PubMed] [Google Scholar]
- 29.Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 2004;200:400–406. doi: 10.1002/jcp.20031. [DOI] [PubMed] [Google Scholar]
- 30.Kleiveland CR, Kassem M, Lea T. Human mesenchymal stem cell proliferation is regulated by PGE2 through differential activation of cAMP-dependent protein kinase isoforms. Exp Cell Res. 2008;314:1831–1838. doi: 10.1016/j.yexcr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Scutt A, Bertram P. Bone marrow cells are targets for the anabolic actions of prostaglandin E2 on bone: induction of a transition from nonadherent to adherent osteoblast precursors. J Bone Miner Res. 1995;10:474–487. doi: 10.1002/jbmr.5650100320. [DOI] [PubMed] [Google Scholar]
- 32.Langberg H, Skovgaard D, Karamouzis M, et al. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol. 1999;515 ( Pt 3):919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thampatty BP, Im HJ, Wang JH. Leukotriene B4 at low dosage negates the catabolic effect of prostaglandin E2 in human patellar tendon fibroblasts. Gene. 2006;372:103–109. doi: 10.1016/j.gene.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee Lui PP, Wong YM, Rui YF, et al. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. J Orthop Res. 2010 doi: 10.1002/jor.21313. [DOI] [PubMed] [Google Scholar]
- 35.Noth U, Tuli R, Seghatoleslami R, et al. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291:201–211. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]