Abstract
OBJECTIVE
To investigate the effect of sensory neuron-specific receptors (SNSRs) activation on the micturition reflex in rats.
MATERIALS AND METHODS
Continuous cystometrograms (CMG, 0.04ml/min) were performed in female Sprague-Dawley rats under urethane anesthesia. After stable micturition cycles were established, a selective rat SNSR1 agonist, bovine adrenal medulla 8–22 (BAM8-22), was administered intravenously or intrathecally in normal rats or rats pretreated with capsaicin 4 days before the experiments. Micturition parameters were recorded and compared before and after drug administration.
RESULTS
Intravenous administration of BAM8-22 (3 to 100 μg/kg) significantly increased intercontraction intervals in dose dependent fashion, but did not affect residual urine or baseline pressure at any doses tested. Intrathecal administration of BAM8-22 (0.01 to 0.3 μg) also increased intercontraction intervals in dose dependent fashion, but did not affect residual urine or baseline pressure at any doses tested. These inhibitory effects of intravenous (30 μg/kg) or intrathecal (0.3 μg) administration of BAM8-22 were still observed after capsaicin pretreatment.
CONCLUSION
These results indicate that in urethane-anesthetized rats activation of SNSRs can inhibit the micturition reflex via the pathways independent of capsaicin sensitive C-fibers. Thus SNSRs could be a potential target for the treatment of bladder dysfunction such as overactive bladder.
Keywords: bladder, sensory-neuron-specific receptors, capsaicin, spinal cord, rats
INTRODUCTION
Previous studies have suggested that hyperexcitability of C-fiber bladder afferents, which are silent under normal conditions, is involved in the emergence of overactive bladder and bladder pain in various pathological conditions, such as spinal cord injury, bladder outlet obstruction or interstitial cystitis [1]. Thus, it has been postulated that targeting afferent hyperexcitability could be effective for treating detrusor overactivity and pain symptoms [2].
A family of G-protein-coupled receptors has been identified in rat dorsal root ganglia (DRG) and named as sensory neuron-specific receptors (SNSRs) [3]. These receptors are expressed exclusively in a subset of small-diameter sensory neurons in DRG and trigeminal ganglia [3]. Based on several analyses, this family of receptors is comprised of four to six members in human (MrgX1-4 or SNSR1-6) and 32 receptors in mouse classified into three major subfamilies Mrg A, B, and C [3–6]. Initially, only one SNSR gene was identified in the rat [3]. Recently, it has been demonstrated that more than one rat SNSR/Mrg subtype exists [7]. These receptors have been subclassified in a similar scheme as described for human and mouse, rMrg A, B, C, and D. For the sake of simplicity, we refer to rSNSR/rMrgC as rSNSR1 in this study, which corresponds to the first gene described in small DRG neurons [3]. It appears that rSNSR1 is pronociceptive as intrathecal (i.t.) and intradermal administration of its agonists, bovine adrenal medulla 8–22 (BAM8-22) and (Tyr6)-γ2-MSH-6-12, enhance acute nociception [8]. In contrast, it was reported that activation of rSNSR1 produces analgesia in the persistent pain model [9]. However, to our knowledge it is not known whether SNSRs are involved in the regulation of neural mechanisms controlling the micturition reflex. Therefore, we examined the effects of activation of rSNSR1 on the micturition reflex in urethane anesthetized rats.
MATERIALS AND METHODS
Adult female Sprague-Dawley rats weighing 248 to 267 g were used. All experiments were performed in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
DRUG
BAM8-22 (Tocris Bioscience, Ellisville, Missouri) was used. For intravenous and intrathecal administration BAM8-22 was dissolved in saline (0.9% NaCl).
INTRAVENOUS ADMINISTRATION OF BAM8-22
Rats were anesthetized with isoflurane followed by urethane (1.2 g/kg subcutaneously) (Sigma Chemical Co., St. Louis, Missouri). Thereafter the abdomen was opened through a midline incision and a polyethylene catheter (Clay-Adams, Parsippany, New Jersey, PE-60) connected to a pressure transducer and an amplifier was implanted into the bladder through the bladder dome. This catheter was used to record intravesical pressure during cystometry. The PowerLab (ADInstruments Pty, Ltd., Castle Hill, New South Wales, Australia) was used for data acquisition and manipulation. The catheter was also used to fill the bladder by continuous infusion of saline. Intravenous (i.v.) injections were made through a cannula (PE-10) inserted into the right jugular vein. After intravesical catheter insertion saline was continuously infused for about 2 hours at a rate of 0.04 ml per minute to record cystometrograms during a control period. BAM8-22 (3, 10, 30 and 100 μg/kg, n = 8 per dose) was then administered intravenously and changes in bladder activity were monitored. In another group of animals BAM8-22 (30 μg/kg, n = 6) was administered intravenously after pretreatment with subcutaneous injection of capsaicin (Sigma Chemical Co., St. Louis, Missouri) (125 mg/kg) 4 days before the experiments to determine whether the effect of BAM8-22 was mediated by capsaicin sensitive C-fiber afferent pathways. I.v. BAM8-22 was administered in a volume of 0.1ml/100 g body wt. The intercontraction interval (ICI), maximum voiding pressure (MVP), pressure threshold (PT), baseline pressure (BP) were measured before and after drug administration. Post-void residual volume (PVR) also was measured in a separate group of 8 animals before BAM8-22 administration and at the end of 2 micturition cycles after drug administration. After constant voided volumes were collected bladder infusion was stopped and PVR was measured by withdrawing intravesical fluid through the catheter by gravity.
INTRATHECAL ADMINISTRATION OF BAM8-22
A PE-10 intrathecal (i.t.) catheter was implanted using isoflurane anesthesia via an incision in the dura at the Th11 vertebra 3 days before the experiments. The catheter was directed caudally into the spinal subarachnoid space and positioned at the level of the L6-S1 spinal cord. The volume of fluid in the catheter was kept constant at 6 μl. Single doses of drugs were then administered in a volume of 2 μl, followed by a 7 μl flush with saline. At the end of experiments, a laminectomy was performed to verify the location of the catheter tip and the distribution of an injected dye (2 μl methylene blue flushed with 7 μl saline). Intravesical catheter (PE-60) was placed as described, and cystometrograms were recorded for about 2 hours under urethane anesthesia (1.2 g/kg subcutaneously). Then BAM8-22 was administered intrathecally (0.01, 0.03, 0.1 and 0.3 μg, n = 6 per dose). In another group of animals BAM8-22 (0.3 μg, n = 6) was administered intrathecally after capsaicin pretreatment 4 days before the experiments. ICI, MVP, PT and BP were measured before and after drug administration. PVR before and after i.t. administration of BAM8-22 was also measured in a separate group of 6 animals as described above.
STATISTICS
All data values are expressed as the mean ± SEM. In experiments with i.v. and i.t. administration of BAM8-22 ICI, MVP, PT, BP values during 30 minutes before and after drug administration were averaged in each rat and then the averages in a group of animals were combined. A one-way ANOVA followed by Dunnett’s multiple comparison test was used for the statistical analysis between the vehicle and drug-treated groups. Student’s paired t-test was used to compare cystometric variables before and after treatment, with p <0.05 considered to indicate statistical significance.
RESULTS
INTRAVENOUS ADMINISTRATION OF BAM8-22
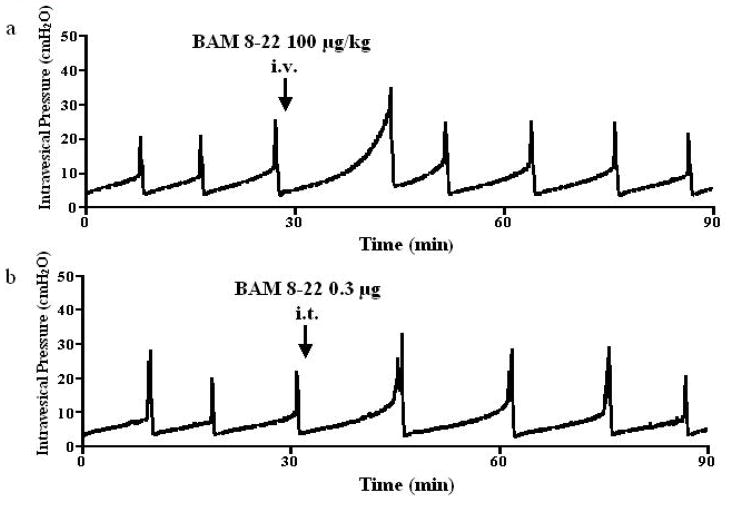
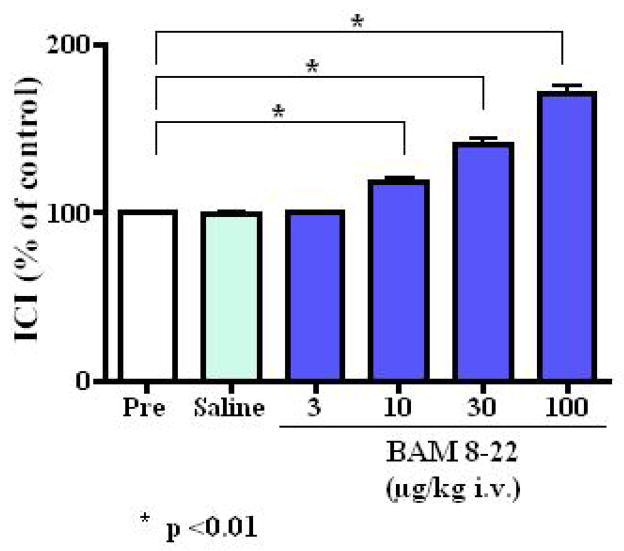
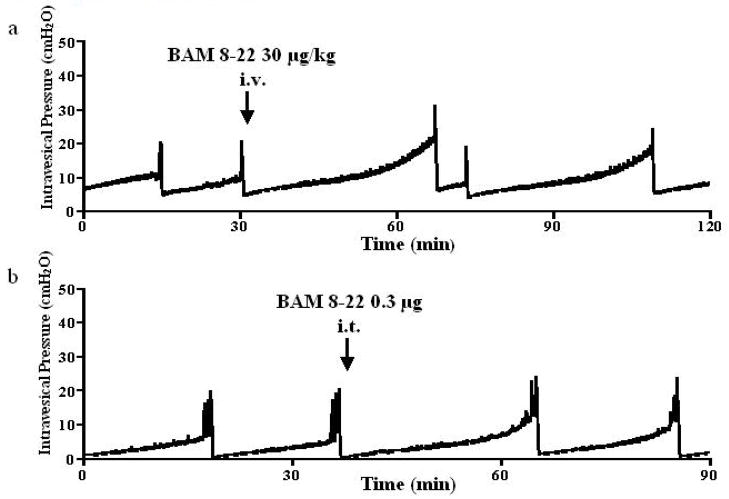
Intravenously applied BAM8-22 inhibited the micturition reflex (Fig. 1a). BAM 8–22 at 3, 10, 30 and 100 μg/kg increased ICI at doses of 10 μg/kg or higher in a dose dependent fashion to 100.2 ± 2.0%, 118.1 ± 7.8%, 141.1 ± 7.7% and 170.4 ± 12.4% of the control value, respectively (at 10, 30 and 100 μg/kg, p <0.01) (Fig. 2). This inhibitory effect was seen immediately after administration and returned to the pre-control level within 50 minutes (Fig. 1a). BAM8-22 also increased PT and MVP in a dose dependent fashion, but did not affect BP or PVR at any doses tested (Table 1). Injection of vehicle (saline) had no effect on ICI, PT, MVP, BP or PVR (Table 1). In rats with capsaicin pretreatment, the inhibitory effect of BAM 8–22 (30 μg/kg, n = 6) was observed (Fig. 3a). Intravenous administration of BAM8-22 in capsaicin pretreated rats increased the ICI with a similar efficacy observed in normal rats, and increased the PT and MVP significantly (Fig. 3a and Table 2). There was no significant change in BP or PVR (Table 2).
FIG. 1.
Representative cystometrograms showing the effects of intravenous (i.v.) (a) or intrathecal (i.t.) (b) of BAM 8–22 (100 μg/kg and 0.3 μg, respectively) on bladder activity in normal rats. The timing of the drug application is indicated by arrows.
FIG. 2.
Changes in intercontraction interval (ICI) (% of control [Pre]) after intravenous (i.v.) administration of vehicle (saline) or BAM 8–22 (3–100 μg/kg) in normal rats. *<0.01 vs vehicle administration (Dunnett’s multiple comparison test)
TABLE 1.
Changes in cystometric parameters after intravenous (i.v.) BAM 8–22 administration in normal rats.
| Variable | Vehicle | BAM 8–22, μg/kg
|
|||
|---|---|---|---|---|---|
| 3 | 10 | 30 | 100 | ||
| Number of rats | 4 | 8 | 8 | 8 | 8 |
| Mean ± SE | |||||
| ICI, mins | |||||
| before treatment | 9.69 ± 2.26 | 9.96 ± 1.77 | 10.14 ± 1.36 | 9.99 ± 1.29 | 10.12 ± 1.44 |
| after treatment | 9.65 ± 2.11 | 9.99 ± 1.83 | 11.90 ± 1.04 | 14.16 ± 2.44*† | 16.91 ± 3.08*† |
| BP, cmH2O | |||||
| before treatment | 5.80 ± 0.77 | 5.44 ± 2.13 | 5.75 ± 1.68 | 5.40 ± 1.32 | 5.62 ± 1.51 |
| after treatment | 5.58 ± 1.06 | 5.50 ± 2.18 | 5.70 ± 1.59 | 5.42 ± 1.28 | 5.49 ± 1.40 |
| PT, cmH2O | |||||
| before treatment | 7.12 ± 0.45 | 7.99 ± 1.03 | 8.53 ± 1.64 | 8.12 ± 1.76 | 7.98 ± 1.75 |
| after treatment | 7.28 ± 0.52 | 8.04 ± 1.05 | 9.68 ± 1.55 | 12.44 ± 3.83*† | 20.28 ± 4.26*† |
| MVP, cmH2O | |||||
| before treatment | 27.5 ± 4.86 | 26.9 ± 3.65 | 26.1 ± 2.39 | 25.7 ± 2.08 | 26.1 ± 2.70 |
| after treatment | 27.7 ± 4.06 | 26.0 ± 3.22 | 26.1 ± 2.50 | 35.5 ± 1.62*† | 40.4 ± 2.71*† |
| PVR, ml | |||||
| before treatment | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.02 |
| after treatment | 0.08 ± 0.04 | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.03 |
P <0.01 (paired t-test);
P <0.01 vs vehicle injection (Dunnett’s multiple comparison test)
FIG. 3.
Representative cystometrograms showing the effects of intravenous (i.v.) (a) or intrathecal (i.t.) (b) of BAM 8–22 (30 μg/kg and 0.3 μg, respectively) on bladder activity in capsaicin-pretreated rats. The timing of the drug application is indicated by arrows.
TABLE 2.
Changes in cystometric parameters after intrathecal (i.t.) BAM 8–22 administration in normal rats.
| Variable | Vehicle | BAM 8–22, μg
|
|||
|---|---|---|---|---|---|
| 0.01 | 0.03 | 0.1 | 0.3 | ||
| Number of rats | 4 | 6 | 6 | 6 | 6 |
| Mean ± SE | |||||
| ICI, mins | |||||
| before treatment | 10.91 ± 1.94 | 10.82 ± 2.21 | 11.34 ± 1.69 | 10.04 ± 1.92 | 10.76 ± 1.64 |
| after treatment | 10.88 ± 1.75 | 10.60 ± 2.12 | 14.08 ± 2.26**†† | 14.52 ± 2.98*†† | 17.01 ± 3.35*† |
| BP, cmH2O | |||||
| before treatment | 5.71 ± 1.24 | 5.65 ± 1.91 | 5.71 ± 1.59 | 6.12 ± 1.64 | 6.51 ± 2.23 |
| after treatment | 5.89 ± 1.55 | 5.64 ± 1.89 | 5.67 ± 1.54 | 6.09 ± 1.58 | 6.51 ± 2.17 |
| PT, cmH2O | |||||
| before treatment | 7.58 ± 1.09 | 7.68 ± 1.11 | 7.99 ± 0.65 | 7.51 ± 1.22 | 8.11 ± 1.33 |
| after treatment | 7.55 ± 1.06 | 7.71 ± 1.04 | 8.34 ± 0.65 | 12.43 ± 1.03*† | 16.94 ± 2.41*† |
| MVP, cmH2O | |||||
| before treatment | 24.6 ± 2.10 | 27.0 ± 3.00 | 25.1 ± 2.24 | 26.8 ± 1.38 | 26.6 ± 2.45 |
| after treatment | 24.4 ± 1.03 | 26.8 ± 2.61 | 26.4 ± 2.38 | 30.7 ± 1.58*† | 36.3 ± 2.20*† |
| PVR, ml | |||||
| before treatment | 0.11 ± 0.02 | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.09 ± 0.03 |
| after treatment | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.03 | 0.11 ± 0.02 | 0.10 ± 0.04 |
P <0.01,
P <0.05 (paired t-test);
P <0.01,
P<0.05 vs vehicle injection (Dunnett’s multiple comparison test)
INTRATHECAL ADMINISTRATION OF BAM8-22
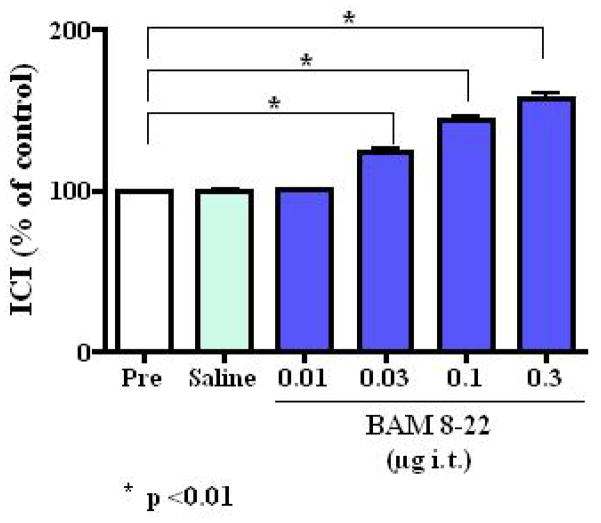
Intrathecal administration of BAM 8–22 also inhibited the micturition reflex (Fig. 1b). BAM 8–22 at 0.01, 0.03, 0.1 and 0.3 μg increased the ICI at dose of 0.03 μg or higher in a dose dependent fashion to 100.6 ± 1.6%, 124.0 ± 5.4%, 144.3 ± 5.8% and 157.4 ± 8.6% of the control value, respectively (at 0.03, 0.1 and 0.3 μg, p <0.01) (Fig. 4). The inhibitory effect of BAM 8–22 decreased with time and returned to the pre-control level within 50 minutes (Fig. 1b). BAM 8–22 also dose-dependently increased PT and MVP (Table 3). However, there were no significant changes in BP, PVR (Table 3). Intrathecally injected vehicle (saline) had no effect on ICI, PT, MVP, BP or PVR (Table 3). The inhibitory effect of intrathecally applied BAM 8–22 (0.3 μg, n = 6) was still observed after capsaicin pretreatment (Fig. 3b). Intrathecal administration of BAM8-22 in capsaicin pretreated rats increased the ICI with a similar efficacy observed in normal rats, and increased the PT and MVP significantly (Fig. 3b and Table 4). There was no significant change in BP or PVR (Table 4).
FIG. 4.
Changes in intercontraction interval (ICI) (% of control [Pre]) after intrathecal (i.t.) administration of vehicle (saline) or BAM 8–22 (0.01–0.3 μg/kg) in normal rats. *<0.01 vs vehicle administration (Dunnett’s multiple comparison test)
TABLE 3.
Changes in cystometric parameters after i.v.BAM 8–22 administration in capsaicin pretreated rats.
| Variable | Vehicle | BAM 8–22, μg/kg
|
|---|---|---|
| 30 | ||
| Number of rats | 4 | 6 |
| Mean ± SE | ||
| ICI, mins | ||
| before treatment | 16.6 ± 1.95 | 16.7 ± 1.93 |
| after treatment | 16.8 ± 1.63 | 23.0 ± 5.26*†† |
| BP, cmH2O | ||
| before treatment | 4.03 ± 1.13 | 4.32 ± 0.91 |
| after treatment | 4.03 ± 1.10 | 4.47 ± 0.95 |
| PT, cmH2O | ||
| before treatment | 11.3 ± 1.71 | 11.5 ± 1.47 |
| after treatment | 11.0 ± 1.62 | 22.9 ± 2.06*† |
| MVP, cmH2O | ||
| before treatment | 24.6 ± 4.78 | 25.2 ± 4.27 |
| after treatment | 24.9 ± 4.48 | 32.3 ± 4.47**†† |
| PVR, ml | ||
| before treatment | 0.10 ± 0.02 | 0.10 ± 0.01 |
| after treatment | 0.10 ± 0.03 | 0.11 ± 0.02 |
P <0.01,
P <0.05 (paired t-test);
P <0.01,
P<0.05 vs vehicle injection (Dunnett’s multiple comparison test)
TABLE 4.
Changes in cystometric parameters after i.t. BAM 8–22 administration in capsaicin pretreated rats.
| Variable | Vehicle | BAM 8–22, μg
|
|---|---|---|
| 0.3 | ||
| Number of rats | 4 | 6 |
| Mean ± SE | ||
| ICI, mins | ||
| before treatment | 15.3 ± 2.25 | 15.7 ± 1.89 |
| after treatment | 15.3 ± 3.08 | 23.5 ± 3.45*† |
| BP, cmH2O | ||
| before treatment | 2.83 ± 1.42 | 3.17 ± 1.29 |
| after treatment | 2.84 ± 1.36 | 3.26 ± 1.18 |
| PT, cmH2O | ||
| before treatment | 7.99 ± 2.28 | 7.95 ± 1.72 |
| after treatment | 7.97 ± 2.21 | 15.95 ± 1.75*† |
| MVP, cmH2O | ||
| before treatment | 21.8 ± 2.18 | 22.3 ± 1.36 |
| after treatment | 22.1 ± 2.64 | 26.2 ± 1.73*† |
| PVR, ml | ||
| before treatment | 0.10 ± 0.02 | 0.10 ± 0.01 |
| after treatment | 0.09 ± 0.01 | 0.10 ± 0.02 |
P <0.01 (paired t-test);
P <0.01 vs vehicle injection (Dunnett’s multiple comparison test)
DISCUSSION
The goal of this study was to assess the effects of activation of rSNSR1 on the micturition reflex in urethane anesthetized rats. Our findings indicate that activation of rSNSR1 by BAM8-22 has an inhibitory effect on the micturition reflex. These were demonstrated by the data showing that intravenous or intrathecal administration of BAM8-22 dose-dependently increased ICI and PT in normal rats.
BAM8-22 is a synthesized peptide with 15 amino acids. It differs from bovine adrenal medulla 22 (BAM22), an opioid peptide with 22 amino acids and one of the natural cleavage products of proenkephalin A [10], in that BAM8-22 does not contain the N-terminal YGGFM motif of BAM22 [3]. It has also been demonstrated that in vitro BAM8-22 does not interact directly with the opioid receptor [3]. Furthermore, it has been demonstrated that the effect of BAM8-22 on nociception was not mediated by opioid receptors since this peptide displayed identical efficacy in inhibiting the nocifensive behaviors in the absence or presence of naloxone, a non-selective opioid receptor antagonist [9]. In addition, endogenous BAM8-22 has not been found to exist up to date although BAM22, an opioid peptide, is widely distributed in the central nervous system [11, 12]. Therefore, it seems reasonable to assume that the inhibitory effects of BAM8-22 on normal bladder activity were mediated by activation of rSNSR1.
An in vitro study with electrophysiological technique has shown that activation of SNSR4 produces inhibition as application of BAM8-22 induces marked inhibition of synaptic responses (presynaptic inhibition) in hippocampal neurons expressing human SNSR4 [9]. BAM8-22 also induces inhibition of high voltage-activated Ca2+ current in rat DRG and superior cervical ganglion neurons expressing human SNSR4 [13], which may lead to inhibition of excitatory neurotransmitter release at synapses formed between small-diameter DRG neurons and the spinal dorsal horn neurons [14]. These events may underline the results obtained in the present study. If this is true, SNSRs could be categorized as the type of receptors that are expressed in the small-sized neurons in the DRG and function to negatively modulate excitability of the central terminals of primary afferents, similar to opioid [15], acetylcholine [16] and GABA receptors [17].
The current study also indicated that the activation of rSNSR1 can inhibit the micturition reflex via the pathways independent of capsaicin sensitive C-fiber afferents. In the present study, ICI and PT in capsaicin pretreated rats were significantly increased compared to normal rats, as reported in previous studies using anesthetized rats with afferent desensitization induced by pretreatment with capsaicin, a C-fiber neurotoxin [18]. The inhibitory effects of intravenous or intrathecal administration of BAM8-22 were still observed after capsaicin pretreatment. An study using in situ hybridization (ISH) analysis, rat SNSR mRNA was detected in a subset of small neurons within trigeminal ganglia and DRG, but not in the sympathetic superior cervical ganglion or in the nodose ganglion, indicating SNSR is uniquely associated with sensory afferents in the rat [19]. Moreover, in RT-PCR analyses of 25 different human tissues using oligonucleotides designed to conserved regions of SNSR 1–6, SNSR mRNA expression was detected exclusively in human DRG [19]. Lembo et al. [19] used double-labeling studies combining ISH with immunohistochemical detection of neuronal markers such as substance P, calcitonin gene-related peptide (CGRP), vanilloid receptor (TRPV1), isolectin B4 (IB4) on rat DRG tissue sections to identify the neuronal phenotype of SNSR-expressing cells. Most SNSR-positive neurons were found in the non-peptidergic, IB4-positive population. Also, a half of them were not co-localized with TRPV1 capsaicin receptor. Therefore, it is possible that the effects of BAM8-22 are mediated by capsaicin-resistant C-fibers. The site of action may be afferent fibers in the bladder and/or the spinal cord although it is not known whether intravenously applied BAM8-22 can pass through the blood-brain barrier.
In the present study, intrathecal or intravenous administration of BAM8-22 increased MVP in urethane anesthetized rats. There may be three possible mechanisms involved in the elevation of MVP. First of all, BAM8-22 may increase urethral pressure during voiding by affecting the urethral reflex. Secondly, BAM8-22 may increase MVP because voiding has to be done for larger volume after the increase in ICI. Lastly, BAM8-22 may increase smooth muscle activity via efferent pathway stimulation. However, the first or second mechanism may be more reasonable because SNSRs are not expressed in smooth muscle or autonomic neurons although further studies using isovolumetric cystometry or urethral perfusion pressure measurements are needed to clarify the mechanism inducing the BAM8-22-mediated increase in MVP.
Many receptors that can modulate micturition are also distributed in the neurons pathways that regulate other functions, such as respiration and brain activity, etc. Targeting this type of receptors concurrently results in modulating the micturition reflex and producing unwanted effects. For example, opioids have been used clinically as effective analgesics for many pain conditions, but their use is limited by their considerable central nervous system-mediated side effects. To date, it has been found that SNSRs are exclusively expressed in a subset of sensory neurons in the DRG and the trigeminal ganglion [3, 8, 19]. Thus, it is assumed that the sensory neuron-specific receptor could be an effective target for the pharmacological treatment of bladder dysfunction with less side effects.
In conclusion, the results of our study have shown that activation of SNSRs can inhibit the micturition reflex via suppression of afferent pathways independent of capsaicin sensitive C-fibers. These findings raise the possibility that modulating SNSRs could be effective for treating bladder overactivity in various pathological conditions.
Acknowledgments
This study was supported by grants from NIH (DK057267 and DK068557).
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002;168:1897–913. doi: 10.1016/S0022-5347(05)64261-9. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59:61–7. doi: 10.1016/s0090-4295(01)01639-9. [DOI] [PubMed] [Google Scholar]
- 3.Lembo P, Grazzini E, Groblewski T, et al. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–9. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 4.Dong X, Han SK, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–32. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 5.Bender E, Buist A, Jurzak M, et al. Characterization of an orphan G protein-coupled receptor localized in the dorsal root ganglia reveals adenine as a signaling molecule. Proc Natl Acad Sci USA. 2002;99:8573–8. doi: 10.1073/pnas.122016499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han SK, Dong X, Hwang JI, et al. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci USA. 2002;99:14740–5. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA. 2003;100:10043–8. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grazzini E, Puma C, Roy MO, et al. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci USA. 2004;101:7175–80. doi: 10.1073/pnas.0307185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Cai Q, Hong Y. Intrathecal sensory neuron-specific receptor agonists bovine adrenal medulla 8–22 and (Tyr6)-gamma2-MSH-6-12 inhibit formalin-evoked nociception and neuronal Fos-like immunoreactivity in the spinal cord of the rat. Neuroscience. 2006;141:965–75. doi: 10.1016/j.neuroscience.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Dores RM, Mcdonald LK, Steveson TC, Sei CA. The molecular evolution of neuropeptides: prospects for the ’90s. Brain Behav Evol. 1990;36:80–99. doi: 10.1159/000115300. [DOI] [PubMed] [Google Scholar]
- 11.Khachaturian H, Lewis ME, Watson SJ. Colocalization of proenkephalin peptides in rat brain neurons. Brain Res. 1983;279:369–73. doi: 10.1016/0006-8993(83)90212-3. [DOI] [PubMed] [Google Scholar]
- 12.Pittius CW, Seizinger BR, Pasi A, Mehraein P, Herz A. Distribution and characterization of opioid peptides derived from proenkephalin A in human and rat central nervous system. Brain Res. 1984;304:127–36. doi: 10.1016/0006-8993(84)90868-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Ikeda SR. Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci. 2004;24:5044–53. doi: 10.1523/JNEUROSCI.0990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao J, Li JJ, Perl ER. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci. 1998;18:8740–50. doi: 10.1523/JNEUROSCI.18-21-08740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beland B, Fitzgerald M. Mu- and delta-opioid receptors are downregulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain. 2001;90:143–50. doi: 10.1016/s0304-3959(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 16.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–82. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 17.Stoyanova I, Dandov A, Lazarov N, Chouchkov C. GABA- and glutamate-immunoreactivity in sensory ganglia of cat: a quantitative analysis. Arch Physiol Biochem. 1998;106:362–9. doi: 10.1076/apab.106.5.362.4360. [DOI] [PubMed] [Google Scholar]
- 18.Maggi CA, Santicioli P, Giuliani S, et al. The capsaicin-sensitive innervation of the rat urinary bladder: further studies on mechanisms regulating micturition threshold. J Urol. 1986;136:696–700. doi: 10.1016/s0022-5347(17)45030-0. [DOI] [PubMed] [Google Scholar]
- 19.Lembo PM, Grazzini E, Groblewski T, et al. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–9. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]