SUMMARY
In the adult, both neurologic recovery and anatomical growth after a CNS injury are limited. Two classes of growth inhibitors, myelin associated inhibitors (MAIs) and extracellular matrix associated inhibitors, limit both functional recovery and anatomical rearrangements in animal models of spinal cord injury. Here we focus on how MAIs limit a wide spectrum of growth that includes regeneration, sprouting, and plasticity in both the intact and lesioned CNS. Three classic myelin associated inhibitors, Nogo-A, MAG, and OMgp, signal through their common receptors, Nogo-66 Receptor-1 (NgR1) and Paired-Immunoglobulin-like-Receptor-1 (PirB), to regulate cytoskeletal dynamics and inhibit growth. Initially described as inhibitors of axonal regeneration, subsequent work has demonstrated that MAIs also limit activity and experience-dependent plasticity in the intact, adult CNS. MAIs therefore represent a point of convergence for plasticity that limits anatomical rearrangements regardless of the inciting stimulus, blurring the distinction between injury studies and more “basic” plasticity studies.
Spinal cord injuries (SCIs) have classically been regarded as devastating injuries from which there is no recovery due to restricted axonal growth in the adult central nervous system (CNS). This notion has been modified in recent years by reports of limited recovery in patients suffering from a variety of SCIs [1, 2]. Understanding the basis of recovery, as well as the systems that limit growth in the CNS, has been the goal of a century of research. While our understanding remains incomplete, myriad studies have described how injured neurons can be stimulated to grow, overcome physical and chemical barriers, and make functional connections. Here we focus on studies demonstrating the role of myelin-associated inhibitors (MAIs) in restricting axonal growth in the CNS with particular emphasis on the Nogo-Nogo Receptor axis.
Pioneering work demonstrated that MAIs inhibit axonal regeneration; however, their physiological role remained unclear. Importantly, when MAIs have been antagonized in animal models of SCI, functional recovery exceeds frank axonal regeneration per se. The search for an anatomical correlate to increased functional recovery led to the insight that MAIs limit a wide spectrum of injury-induced growth that includes axonal regeneration, sprouting, and plasticity. Genetic dissection of the roles played by MAIs in SCI led to two important insights. First, SCI recovery is dependent on both regenerative and non-regenerative anatomical growth, significantly complicating our analysis of neurological recovery from CNS damage. Second, the existence of an inhibitory system regulating a wide spectrum of growth emphasizes the physiological role of MAIs, and led to the appreciation of a common regulatory system for both experience and activity-dependent plasticity. The existence of a shared system for limiting anatomical reorganization in the naïve and injured CNS blurs the distinction between translational injury repair studies and more “basic” plasticity studies.
Axonal Regeneration
Regeneration is a remarkable feat that requires extensive coordinated biology: the lesioned axon must survive, find permissive cues for growth, receive appropriate trophic support, extend towards relevant synaptic targets, and ultimately make functional connections. Cajal first described the ability of PNS (peripheral nervous system), but not CNS, neurons to regenerate in 1928[3]. Despite this early observation, nearly 60 years passed before a clear picture emerged as to why regeneration rarely succeeds in the CNS. Aguayo and colleagues elegantly demonstrated that adult CNS neurons are capable of growth but are limited by their environment. By transplanting peripheral nerve grafts into transected spinal cords, they demonstrated that CNS axons could grow into the grafted tissue[4, 5], suggesting that a CNS-specific component, absent from the PNS, constitutively inhibits the growth of damaged axons in the CNS. Subsequent studies demonstrated that neurite outgrowth is limited by CNS white matter homogenates[6], and that growth cone collapse can be induced by oligodendrocytes and CNS myelin[7]. Together, these data demonstrate that CNS myelin is inhibitory to axon growth, and that inhibitors present on oligodendrocyte membranes and within the myelin sheath limit axon regeneration in vivo.
While myelin is an important source of inhibition in the CNS, axon growth can also be modulated by a variety of other molecules including increased neurotrophic support[8] and digestion of extracellular matrix protein side-chains[9], both of which have been shown to support axon regeneration in vitro and in vivo. Broadly speaking, we currently understand the inhibitory environment of the CNS to be composed of two major molecular classes: MAIs and inhibitors associated with the extracellular matrix, typified by chondroitin sulfate proteoglycans (CSPGs). MAIs are a wide-ranging category including Myelin Associated Glycoprotein (MAG), Oligodendrocyte Myelin glycoprotein (OMgp), Nogo-A, Ephrins, Semaphorins, and their relevant receptors, Nogo-Receptor-1 and 2 and Paired Immunoglobulin Like ReceptorB, Ephs, and Plexins, respectively (Figure 1). Here, we focus on MAIs, and in particular, how the Nogo-Nogo Receptor-1 axis limits regeneration, sprouting, and plasticity in both the injured and naïve CNS.
Figure 1. Growth Inhibition in the Central Nervous System.
There are two classes of known growth inhibitors in the CNS: inhibitors associated with the ECM and those associated with myelin. CSPGs (in brown) are associated with the ECM and astrocytes (in green), and signal at least in part through PTPσ to inhibit growth. Nogo-A, MAG, and OMgp are inhibitors present on the membranes of oligodendrocytes (in red). Nogo-A has two distinct inhibitory domains, a hydrophilic 66 amino acid domain (Nogo-66) and a larger N-terminal domain (amino-Nogo), and immunolocalization studies have demonstrated multiple topologies for Nogo-A that include the one depicted here as well as other topologies, such as extracellular amino-Nogo and intracellular Nogo-66. Despite their lack of sequence homology, all three ligands can bind to two different neuronal (in yellow) receptors, NgR1 and PirB. NgR1 is a GPI linked protein that requires coreceptors to signal intracellularly. It can form one of at least two complexes after binding Nogo-66; NgR1-LINGO-p75 or NgR1-LINGO-TROY. p75 and TROY mediate Nogo-NgR1 dependent activation of the Rho, ROCK signaling cascade that ultimately leads to cytoskeletal changes and growth inhibition. The intracellular signaling cascade between PirB or PTPσ and axonal growth inhibition are not well defined, although both ultimately lead to cytoskeletal changes that mediate growth inhibition. Importantly, both Nogo-A and NgR1 are localized pre and post-synaptically, suggesting an additional role in regulating synaptic plasticity (lower right corner). (CSPGs, chondroitin sulfate proteoglycans; ECM, extracellular matrix; GPI, glycosylphosphatidylinositol; MAG, myelin associated glycoprotein; NgR1, Nogo-Receptor-1; OMgp, oligodendrocyte myelin glycoprotein; PirB, Paired-Immunoglobulin-like Receptor; PTPσ, Protein-Tyrosine-Phosphatase-σ; TROY, TNF-alpha orphan receptor; see text for details.)
Nogo-A
In a screen for inhibitory components of CNS myelin, Schwab and colleagues initially isolated 35 and 250kD fractions from CNS myelin that were found to be necessary for myelin inhibition of fibroblast spreading in vitro[10]. They later raised an antibody (IN-1) against these two antigens that significantly reduced the inhibitory effect of CNS myelin in vitro and enhanced corticospinal tract (CST) axon regeneration after a spinal cord injury in young rats[11]. Using peptide sequence information from the IN-1 antibodies, three independent groups indentified the mammalian gene, Nogo, or Reticulon 4 (Rtn4) [12–14].
Nogo is differentially spliced into two isoforms, Nogo A and B; Nogo C is the product of an alternate promoter[12, 13]. All isoforms share a common C-terminal domain with reticulon homology[12, 13] and hence belong to the reticulon family. Nogo-A is the principle isoform of CNS Nogo, and its expression in the CNS, but not PNS[15, 16], suggests that Nogo-A may account for limited CNS regeneration. Nogo-A is predominantly expressed by oligodendrocytes[15, 16], although Nogo-A can also be detected in neurons[15–18]. Using immunoelectron microscopy in oligodendrocytes, Nogo-A has been localized to the innermost and outermost myelin membranes as well as the endoplasmic reticulum[15, 16]. Neuronal expression of Nogo-A has been detected in various subsets of mature CNS neurons[15–18], and interestingly, at both the pre and post-synaptic side of hippocampal synapses[15, 19]. The localization of Nogo-A at both the periaxonal sheath and synapses suggests roles for Nogo-A in both axonal growth and synaptic plasticity.
The carboxyl segment of Nogo-A shares homology with reticulon family proteins and contains the hydrophilic 66-amino acid segment (Nogo-66) between two hydrophobic domains[13] (Figure 1). The amino terminus of Nogo-A (amino-Nogo) does not share sequence homology with the reticulon family or with other proteins. Interestingly, both the hydrophilic amino-Nogo and the Nogo-66 domains potently inhibit neurite outgrowth in vitro through biochemically distinct mechanisms[12–14, 20, 21]. Amino-Nogo disrupts integrin function[22] whereas Nogo-66 inhibits growth through its neuronal receptors, NgR1 and PirB[20, 23]. Subsequent studies have demonstrated that both amino-Nogo and Nogo-66 are localized both intracellularly and extracellularly, suggesting multiple topologies for Nogo-A[13, 21] that provide an explanation for how both domains might inhibit growth.
Nogo-A has been repeatedly shown to initiate growth cone collapse and inhibit neurite outgrowth in vitro by a wide number of laboratories[12–14], and to limit regeneration of the CST after spinal cord injury in mammalian animal models using genetic deletion, neutralizing antibodies, and pharmacologic antagonists in vivo[11, 24–29]. Moreover, ectopic expression of either Nogo-C[30] (which expresses the Nogo-66 domain but not amino-Nogo) or Nogo-A[31] (which expresses both Nogo-66 and amino-Nogo) in PNS Schwann cells limits otherwise routine peripheral nerve regeneration after sciatic nerve injury. The fact that Nogo-A is expressed in the CNS, where regeneration is limited, but not in the PNS, where regeneration occurs, correlates with in vitro and in vivo reports of Nogo-A-dependent growth inhibition, suggesting a causal role for Nogo-A in restricting growth in the CNS.
Even though in vitro and in vivo models have pointed to the importance of Nogo-A in limiting regeneration and recovery after SCI, data from Nogo-A deletion mutants has been less simple to interpret. Three independent laboratories created Nogo-A, Nogo-A/B, and Nogo-A/B/C mutants, with varying regeneration phenotypes[26, 27, 32, 33]. Subsequent studies have demonstrated that age[34], strain[35], and type of lesion[36] also modulate the Nogo null phenotype, complicating the interpretation of the data from the three different deletion mutants. Ongoing studies are characterizing a compensatory genetic difference (our unpublished observations) between two Nogo-A deletions: one with a preserved N-terminal fragment and a marked post-SCI regenerative and recovery phenotype, and the complete Nogo-A deletion that is not associated with enhanced regeneration or recovery in mouse models of SCI. Despite the inherent complexity of genetic compensation in genetic deletion studies, careful interpretation of both positive and negative data should clarify the role of Nogo-A in vivo and explain the recovery phenotypes observed in mouse, rat, and primate models of SCI[11, 26, 27, 29, 37–40].
MAG
A member of the immunoglobulin superfamily, myelin associated glycoprotein (MAG), is a cell surface protein with five extracellular immunoglubulin-like domains, a transmembrane domain, and two alternatively spliced C-terminal tails[41] (Figure 1). In the CNS, MAG is expressed by oligodendrocytes in the periaxonal layer of myelin during the first phases of axonal myelin ensheathment and persists in a strictly periaxonal distribution, suggesting a stabilizing glial-axonal interaction[42, 43]. Two independent groups later identified MAG as the first myelin associated protein recognized to limit neurite outgrowth in vitro. Recombinant MAG potently inhibits neuronal cultures in vitro, and this inhibition is rescued by immunodepletion of MAG[44, 45]. Although MAG is expressed by myelinating glial cells in both the CNS and PNS[42], rapid myelin clearance in the PNS, but not CNS, after injury may leave residual MAG only in the CNS to limit regeneration in vivo[46]. Nevertheless, there is no enhancement of neurite outgrowth on CNS myelin from mice lacking MAG[47–50], and MAG null mice do not have enhanced CST regeneration or functional recovery in vivo in mouse models of SCI[47, 48].
However, the relief of inhibition from MAG deletion may be masked by the presence of other, more potent inhibitors. The first evidence for this hypothesis came from experiments showing that neurite outgrowth is differentially affected by the inhibitory fractions of CNS myelin from which MAG was originally isolated. In vitro, total CNS myelin derived from MAG knock-out mice inhibits neurite outgrowth to a similar degree as total CNS myelin from wild-type mice, whereas distinct myelin subfractions from MAG null mice are significantly less inhibitory to neurite outgrowth than the equivalent fractions from their wild-type litter mates[49]. This result suggests that other more potent MAIs in the other myelin fractions obscured the role of MAG in axon growth inhibition by total myelin. Recent evidence that Nogo-A masks the effect of MAG and OMgp inhibition (see below) in vitro and in vivo[48] supports the hypothesis that MAG is an important, but not principal, inhibitor of regeneration in vivo (Figure 2a).
Figure 2. Myelin Associated Inhibitors Limit Injury-Induced Growth.
(A–E). Anatomical Rearrangements of Lesioned Fibers in Response to Injury are Limited by MAIs. 2–4 month year old female wild-type or mutant mice were subjected to a dorsal hemisection at T6. 4 weeks later, biotin dextran amine (BDA) was unilaterally injected into primary motor cortex to trace the CST, and mice perfused with 4% paraformaldehyde, and 30 µm parasagittal sections were cut on a vibratome. (A) A sagittal section of thoracic spinal cord shows a tight bundle of CST fibers rostral (left) descending until the lesion, and the absence of any fibers caudal to the lesion. Scale bar represents 1 mm. (B) Inset of lesion depicted in (A), demonstrating higher magnification view of abrupt CST interruption at the lesion. Scale bar represents 100 µm. (C) A sagittal section of thoracic spinal cord shows 6 weeks after a dorsal hemisection in mice null for Nogo-A, OMgp, and MAG. Rostral to the lesion, the CST is no longer a tight bundle, reflecting extensive collateral growth of the lesioned tract in response to the injury (arrowhead). Caudal to the lesion, CST fibers can be detected for several millimeters, demonstrating axonal regeneration in an adult mammalian CNS. Scale bar represents 1 mm. (D,E) Insets of spinal cord caudal to the lesion demonstrating regenerating axons fibers (arrowhead). Scale bar represents 100 µm.
(F–H). Anatomical Rearrangements of Unlesioned Fibers in Response to Injury are Limited by MAIs. A unilateral lesion to the descending CST at the level of the medullary pyramids (pyramidotomy) was made in either wild-type or NgR1-null adult mice. The intact CST was traced 4 weeks later using unilateral, cortical injections of BDA. Mice were perfused 2 weeks later with 4% paraformaldehyde and 30 µm axial sections were prepared from the cervical spinal cord, caudal to the lesion. Scale bar represents 500 µm. (F,G) In wild-type mouse subjected to a sham surgery (F) or pyramidotomy (G), CST terminals are noted in the ipsilateral grey matter, but very few are seen contralaterally. (H) In NgR1-null mice subject to a pyramidotomy, the CST makes both ipsilateral and contralateral terminals in the grey matter, demonstrating the potential for collateral growth from intact fiber tracts in response to CNS injury.
(I–L). Anatomical Rearrangements in Synaptic Structures. Apical tufts of L5 pyramidal cells in S1 of P180 thy-EGFP-M mice were imaged every four days for 28 days through a cranial window. Days 0, 4, 8 and 28 are shown above. Scale bar represents 2 µm. Although most spines persist, a fraction of spines turnover (green asterisk). Of newly formed spines (yellow and red asterisk), only a small percentage persist (yellow asterisk) and make new synapses. Based on their role in limiting a wide spectrum of injury-induced growth, MAIs can potentially regulate dendritic spine elimination, formation, and or persistence.
OMgp
A screen for glycosylphosphatidylinositol (GPI) -anchored myelin inhibitors led to the description of a third MAI, oligodendrocyte myelin glycoprotein (OMgp)[51] (Figure 1). OMgp was originally described as a GPI anchored peanut agglutinin-binding glycoprotein whose expression was exclusively found in the CNS, enriched in white matter tracts, and paralleled myelination[52]. Originally isolated from CNS myelin, OMgp is detectable on membranes of several neuronal types, oligodendrocytes, and periaxonal myelin[53, 54]. Recombinant OMgp potently inhibits neurite outgrowth of cerebellar granule neuron cultures and induces growth cone collapse in adult DRG cultures[51, 55]. OMgp is present on periaxonal processes of oligodendrocyte-like glia in the CNS, and interestingly, immunohistochemistry and immunoelectronmicrography demonstrate a perinodal distribution of OMgp[54]. Consistent with this localization, nodal architecture is disrupted in mice lacking OMgp, and there is a significant increase in the number of axons that sprouted from these disrupted nodes in vivo[54]. Recent work, however, has challenged this model, suggesting that OMgp antisera used in this previous study may have additionally bound to a CSPG component, thus questioning the perinodal distribution of OMgp [56]. While further work will be needed to clarify these findings, it is clear that OMgp inhibits neurite outgrowth in vitro[51].
Nonetheless, rodent SCI models show a minimal, if any, recovery phenotype in the absence of OMgp[48, 57]. Similar to MAG, it is possible that the presence of other more potent inhibitors mask the effect of OMgp deletion in vitro and in vivo. When MAG and OMgp are deleted individually or together, neurite outgrowth is unaffected in vitro and in vivo[44, 45, 47–49], whereas Nogo-A deletion decreases growth inhibition and increases functional recovery and CST axonal regeneration in mouse models of SCI[12–14, 21, 26, 27, 48]. When MAG and OMgp are deleted in a Nogo-A-null background, there is a synergistic decrease of growth inhibition in vitro, and greatly enhanced CST regeneration and functional recovery after a dorsal hemisection in a mouse model of SCI[48] (Figure 2a). It should be noted that a small N-terminal fragment of Nogo-A is retained in these genetic mutants, and that in mice completely null for Nogo-A, there is no relief of inhibition nor any additional benefit with the deletion of MAG and OMgp[58]. Further studies are required to determine the role of this N-terminal domain and the genetic differences underlying these phenotypes. Nonetheless, these results demonstrate a model of genetic redundancy in growth inhibition, with Nogo-A masking the inhibitory effect of MAG and OMgp in vitro and in vivo, suggesting that therapeutics targeting all three classic MAIs may provide the greatest effect on regeneration and SCI recovery.
NgR1
The identification of Nogo-A and description of the Nogo-66 inhibitory fragment led to the discovery of Nogo-Receptor-1 (NgR1), a CNS specific receptor that binds Nogo-66 with high affinity and mediates growth cone collapse in vitro[20]. Surprisingly, further studies have revealed that NgR1 is also a high affinity receptor for MAG[59] and OMgp[60], serving as a point of convergence for all three classic MAIs despite their lack of sequence homology. NgR1 protein expression is postnatal and is detectable by P15 in mice[15], and can be localized to axonal membranes[15] and post-synaptic compartments[15, 19]. This localization suggests that NgR1 is expressed where it can interact with glial Nogo-A, MAG, and OMgp in the myelin sheath as well as neuronal Nogo-A at the synapse, suggesting a role in both axonal growth inhibition and synaptic plasticity.
NgR1 is a leucine-rich-repeat (LRR) protein with LRR N and C termini flanking a core of 8 LRR segments[20, 61, 62], and deletion of either LRR terminus, or any pair of LRRs in the core of the protein, abolishes the ability of NgR1 to bind Nogo-66[62]. Homologous proteins Nogo-Receptor-2 and -3 (NgR2 and NgR3, respectively) do not show binding to Nogo-A, OMgp, or MAG under similar conditions[61], although NgR2 can tightly bind MAG in a sialic acid dependent manner[63].
NgR1 is a GPI anchored protein that is concentrated in lipid rafts and capable of oligomerization[61, 62], likely contributing to macromolecular receptor complexes that may magnify receptor-ligand interactions in vivo. As a GPI anchored protein with no transmembrane domain[61, 62], activation of NgR1 is likely transduced intracellularly by membrane-associated co-receptors (Figure 1). Subsequent studies have identified a large and varying receptor complex that includes the low-affinity neurotrophin receptor p75 that interacts with LINGO-1 to transduce NgR1 activation and induce RhoA activation in a subset of neurons[64]. Nonetheless, the distribution of p75 does not fully overlap with NgR1, leading to the discovery that a closely related TNF-alpha orphan receptor, TROY, could replace p75 in the NgR1-p75-LINGO-1 complex to mediate intracellular NgR1 signaling and activation of RhoA[65, 66].
While there may be yet more co-receptors that transduce the effects of NgR1 in distinct neuronal populations, several studies have demonstrated that activation of NgR1 and signaling through its co-receptors leads to increased activation of the small GTPase, RhoA[67–70], and its effectors ROCK and LIMK1[67–69], while negatively regulating Rac1 activity[70]. Together, these studies suggest that activated NgR1 and its co-receptors signal via a Rho and Rac kinase signaling system to tip the balance against actin polymerization and neurite outgrowth, providing a molecular mechanism for growth phenotypes seen in vitro and in vivo.
As a bridge between extracellular inhibitory signals and intracellular actin regulation, NgR1 is required for myelin-induced growth cone collapse[20, 71] and neurite outgrowth inhibition[72] in vitro. Using either a dorsal hemisection or complete transection model of SCI, rubrospinal and raphespinal, but not CST, axons regenerate past the lesion in the absence of NgR1[73]. Importantly, these anatomical phenotypes correlate with a significant increase in locomotor recovery as well as transcranial magnetic stimulation induced electromyographic responses in the gastrocnemius muscles of lesioned animals lacking NgR1[73], demonstrating a reestablished cortical-spinal motor neuron connection. Multiple strategies have been employed to antagonize NgR1 action including a competitive antagonist for the Nogo-66 binding site (NEP1–40)[25] and a decoy receptor, NgR(310)ecto-Fc, made from the ectodomain of NgR1 fused to the Fc portion of an immunoglobulin to deprive endogenous NgR1 from ligand activation [62, 74]. Antagonizing NgR1 signaling through either of these interventions decreases myelin induced growth cone collapse and inhibition of neurite outgrowth in vitro, and enhances CST regeneration and functional recovery in animal models of SCI in vivo[25, 62, 75, 76].
Interestingly, the most potent myelin associated intervention tested thus far has been antagonizing all three classic MAIs, Nogo-A, MAG, and OMgp. In murine models of SCI, triple ligand deletion permits significantly more regeneration and functional recovery than the deletion of their common receptor NgR1[73] (presumably due to the presence of additional receptors), or anti-Nogo-A therapy[76, 77] (due to the presence of other inhibitors of regeneration). Currently, NgR(310)ecto-Fc is the only therapeutic method that targets all three ligands, thus blocking activation of all endogenous receptors of these three ligands[62, 75, 76]. When using a contusion model of SCI in rodents, intracerebroventricular delivery of NgR(310)ecto-Fc leads to axon regeneration and significantly enhanced neurologic recovery [78], while there have been no reports for similar benefits using anti-Nogo-A therapy after contusion injury. NgR1 is therefore not just a point of convergence for signaling of myelin inhibition, but a therapeutic target to enhance axonal regeneration and SCI recovery in humans.
PirB
While NgR1 mediates a substantial portion of Nogo-66 mediated inhibition[20], the absence of NgR1 does not completely desensitize neurites to the inhibitory effects of Nogo-66 or myelin[20], suggesting the presence of other receptors. In a screen for novel receptors for Nogo-66, Atwal and colleagues identified Paired Immunoglobulin-like Receptor B (PirB) as a high affinity receptor for Nogo-66, MAG, and OMgp[23](Figure 1). Genetic deletion of PirB dampens the inhibitory effect of myelin and recombinant Nogo-66 on CGN neurite outgrowth[23], while anti-PirB treatment decreases growth cone collapse in vitro[23]. PirB was originally described as a modulator of immune cell regulation with an immunoglobulin-like ectodomain and a long cytoplasmic tail with 4 tandem immunoreceptor tyrosine based inhibitory motifs[79–81]. Interestingly, PirB expression in the intact adult CNS is limited[82, 83]. PirB expression is almost undetectable in mature cortical neurons in vitro[72]. In vivo, in situ hybridization[84] and immunoblots [58] detect trace amounts of PirB in the CNS relative to other MAIs, and in particular, no PirB is detected in the CST using immunohistochemistry[82]. In the only published study on the role of PirB in SCI, there was no PirB-dependent effect on functional recovery or CST regeneration in a dorsal hemisection model of SCI[85]. Nevertheless, its potent inhibitory role in vitro[23] and effect on cortical plasticity in vivo[83] (see below) suggest that further studies will be necessary to fully evaluate the role of PirB in SCI repair.
Regeneration, Sprouting, and Plasticity: A Continuum of Repair
A wide spectrum of inhibitors has been observed to limit neurite outgrowth in vitro, and when antagonized in animal models of SCI, cause significant regeneration of severed axons in vivo. Nonetheless, the extent of the observed regeneration alone does not explain the levels of functional recovery observed in rodent and primate models of SCI. In classic spinal hemisection models targeting the corticospinal tract in rodents and primates, significant neurological recovery is observed despite scant CST axonal regeneration. Thus, while axon regeneration is the principal goal of SCI research, it is not the only substrate for recovery.
Since SCI is fundamentally a problem of interrupted communication between the brain and the spinal cord, motor recovery from an SCI ultimately depends on reestablishing a connection between cortical projection neurons and spinal motor neurons. Conceptually, this can be divided into two classes of reconnection: direct and indirect. Axonal regeneration provides a direct or monosynaptic connection between two cells that need to communicate with one another. An indirect or multi-synaptic connection, on the other hand, occurs when communication between two cells is mediated by synaptic connections with one or more intermediate neurons. In humans, where CNS regeneration is extremely limited, some tetraplegic patients can recover ambulation depending on the amount of tissue spared from the SCI[1], suggesting that indirect connections between cortical and spinal motor neurons can also restore function. The search for a substrate of recovery beyond regeneration has led to the discovery of a wide spectrum of compensatory anatomical growth patterns in SCI repair that is limited by a common regulatory system. Ranging from frank regeneration to subtle axonal sprouting, these anatomical changes represent the fundamental malleability of the CNS and provide a model to study the mechanisms that limit SCI repair.
Beyond Regeneration
At one end of an injury-induced spectrum of axonal growth, regeneration describes regrowth from the severed end of an damaged axon over long distances (e.g., cm) and time intervals (Figure 2a), while, at the other end of the spectrum, plasticity represents rapid anatomical changes over microns (Figure 2c). Sprouting refers to medium length (on the order of mm) collateral growth from either intact or lesioned axons (Figure 2b). Unfortunately, these distinctions imply boundaries where none are well defined, slowing an appreciation for how these phenotypes represent a dynamic continuum of anatomic growth (reviewed elsewhere[86]). Appreciating that regeneration is just one point along a continuum of growth has led to the discovery that a common molecular system participates in multiple phenotypes.
The vast majority of human SCIs are contusions, sparing a significant amount of white and gray matter circuitry that can provide a substrate for recovery[87]. The importance of spared tissue in recovery was first demonstrated when either the intact or lesioned CST was traced in rats subjected to a unilateral lesion of the CST (pyramidotomy). Anterograde labeling of the lesioned CST reveals a significant amount of sprouting rostral to the lesion, suggesting compensatory anatomical growth to develop indirect connections between the brain and spinal cord[88, 89]. In the intact CST, fibers sprout from the unlesioned side across the midline into the denervated side both rostral and caudal to the lesion, suggesting that the intact CST also creates novel connections that somehow mediate recovery[88]. Using a dorsal hemisection model of SCI, Schwab and colleagues elegantly demonstrated a role for injury-induced collateral projections in functional recovery. After a dorsal hemisection, lesioned CST fibers rostral to the injury sprout collaterals into the ipsilateral grey matter, creating novel CST-propriospinal neuron (PSN) connections that can serve as a bypass to route cortical stimuli around the injury to spinal motor neurons below the lesion[90]. Re-injuring the severed CST at the level of the medulla (more rostrally) reverses the recovery of hindlimb function, suggesting that injury-induced CST-PSN connections in the cervical cord lead to a novel CST-dependent pathway to control the hindlimbs in a hemisected spinal cord[90]. These results suggest that rearrangements in the spared circuitry from both intact and lesioned projections provide a mechanism for indirect routing of cortical signals to spinal motor neurons, and thus, represent a novel substrate for plasticity and recovery.
Myelin Limits Injury-Induced Growth and Sprouting
The first evidence that myelin limits collateral sprouting from axons came from Schwab and colleagues when they examined whether myelin regulated the proper formation of the tight bundle that forms the CST in rats. Depleting myelin by irradiating oligodendrocytes, or antagonizing MAIs via IN-1 (anti-Nogo) antibody delivery, leads to significant sprouting of aberrant CST fibers in the developing spinal cord[91]. Treatment of the mature rat spinal cord with IN-1 causes a significant increase in collaterals that cross the midline in both the intact CST (above and below the injury) and lesioned CST (above the injury)[88]. Evidence that oligodendrocyte associated factors, and Nogo-A in particular, restrict collateral sprouting in the developing CNS suggest that MAIs may also limit a spectrum of non-regenerative growth in the injured CNS.
In murine models of SCI, deletion of Nogo-A leads to a significant increase in collateral sprouting that crosses the midline and correlates with a significant improvement in functional recovery [26, 27]. When anti-Nogo-A antibodies are used in primate models of SCI, there is a significant improvement in functional recovery that correlates with the increase in sprouting of CST collaterals, but not CST regeneration, in vivo [29, 37]. Deletion of NgR1 leads to increased levels of recovery and collateral sprouting, but not CST regeneration, after a thoracic hemisection[73]. Antagonizing NgR1 or its ligands via NEP1–40 or NgR(310)ecto-Fc, respectively, leads to increased regeneration, sprouting, and recovery [25, 75, 92]. If recovery is separated into regeneration-dependent and -independent components, then it is clear that the Nogo-NgR1 axis limits the entire spectrum of anatomical growth and thus, functional recovery. Interestingly, if rats are allowed to enter the chronic phase of recovery (over 2 months post-injury) and are then treated with NgR(310)ecto-Fc, there is a significant increase in collateral sprouting and recovery of hindlimb function (Wang, Cafferty, and Strittmatter, unpublished). Thus, the Nogo-NgR1-axis limits non-regenerative growth and functional recovery in both the acute and chronic phases of SCI recovery, suggesting that this axis has a homeostatic role in limiting growth in the adult CNS.
Murine models of stroke provide an additional model system to look for a substrate of recovery in a setting where axon regeneration does not confound the interpretation. Genetic or pharmacologic antagonism of Nogo-A or NgR1 lead to a significant increase in functional recovery of the lesioned forepaw as well as anatomical rearrangements in several murine models of stroke. For instance, in mice with either Nogo-A or NgR1 deletions, increased neurological recovery correlates with significant sprouting of the intact (contra-lesional) CST into the denervated side at the level of the midbrain and cervical spinal cord[93]. Even if treatment is delayed for a week after the infarction, antagonism of the Nogo-NgR pathway with either IN-1[94] or NgR(310)ecto-Fc[93] in rats leads to a significant increase in CST sprouting and functional recovery. These results demonstrate that non-regenerative anatomical plasticity is a substrate for functional recovery, and that the Nogo-NgR1 axis is a potent inhibitor of this growth in vivo.
There is less evidence for the role of other MAIs in limiting the growth of collaterals in SCI repair. While double mutants of MAG and OMgp do not have any increase in functional recovery or sprouting rostral to spinal cord lesion, double mutants in the background of Nogo-A deletion have extensive growth of CST axons including regeneration and collateral sprouting after a dorsal hemisection in mice[48]. Similar to regeneration, the inhibitory effect of MAG and OMgp on the sprouting of spared and injured CST axons is masked by the more potent inhibitory effect of Nogo-A.
Limited literature on the role of PirB in injury-induced plasticity has not shown a neuroanatomical or neurological phenotype with PirB deletion after injury[82, 85]. Using mouse models of traumatic brain injury (TBI)[82] or SCI[85], PirB does not limit the sprouting of collateral axons from the intact CST, regeneration of the lesioned CST, or the functional recovery from either lesion[82, 85]. Further work is required since these are the only published studies on the role of PirB in CNS injury.
Myelin and Plasticity In the Naïve Brain
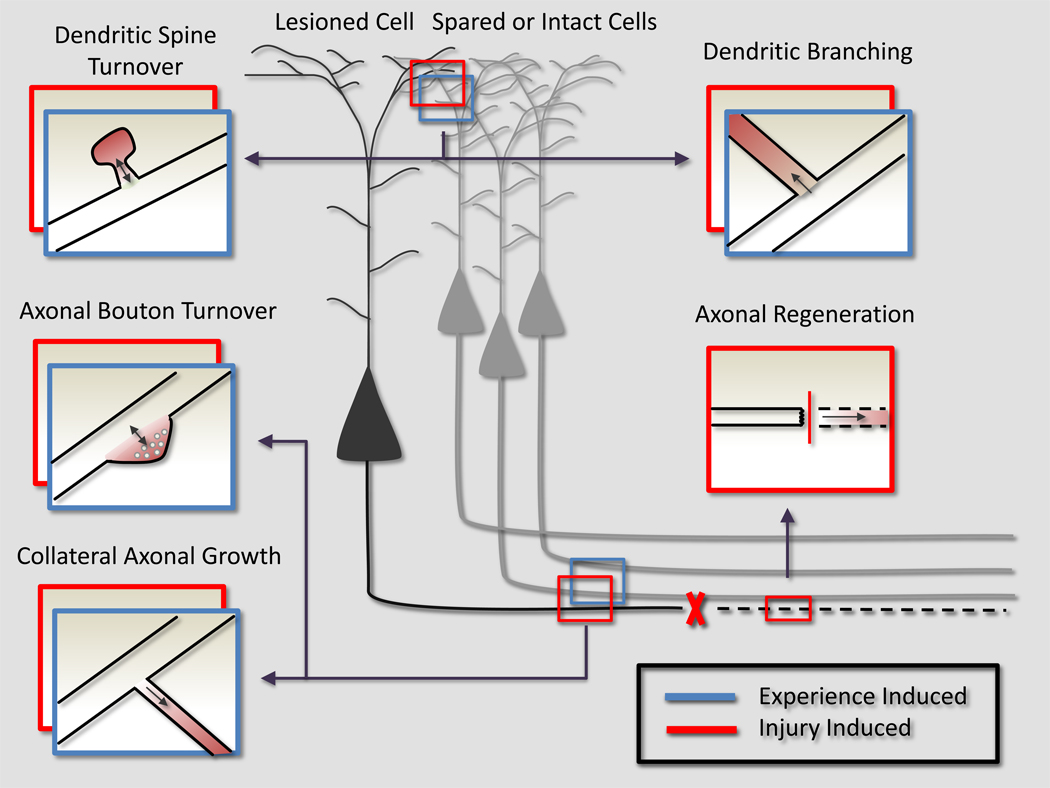
The search for inhibitors of CNS regeneration led to the discovery of MAIs that limit a wide spectrum of injury-induced anatomical growth. Injury, however, is not the only stimulus for plasticity. The understanding that MAIs limit a wide spectrum of injury-induced anatomical plasticity led investigators to test if this system also limits plasticity in the uninjured CNS. It has now been demonstrated that MAIs limit both experience and activity dependent plasticity in the adult mammalian brain, suggesting a physiological role for this system in the intact CNS. Ultimately, cytoskeletal rearrangements link the entire of spectrum of anatomical plasticity, regardless of the initial stimulus (injury, experience, etc). Thus, both injury and experience can induce a wide range of anatomical growth that includes synaptic turnover and collateral neurite branching (Figure 3). The discovery that MAIs limit plasticity both in the intact and lesioned CNS demonstrates that a continuum of plasticity is fundamentally regulated by a common system inducing cytoskeletal rearrangements that limit the entire spectrum of plasticity, regardless of the initial stimuli.
Figure 3. Myelin Inhibits a Spectrum of Growth in both the Intact and Injured CNS.
An injury (red boxes) that severs an axon of a projection neuron leads to a wide spectrum of compensatory growth (represented as a red, emerging process off the original neurite). Anatomical rearrangements in either injured (black) or spared (grey) neurons contribute to functional recovery. This entire spectrum, in both injured and uninjured neurons, is limited by MAIs. In the absence of MAIs, the severed axon could regenerate through the lesion. More rostrally, the injured neuron could sprout collateral dendritic or axonal processes to create multisynaptic connections to bypass the lesion. More subtle changes in the injured neurons synaptic connections, such as differential dendritic spine and axonal bouton turnover and formation, could also redirect signals from the injured neuron around the lesion. In the setting of an injury, spared neurons (grey) can also sprout collateral dendrites or axons, or change their pre or post-synaptic connectivity in an attempt to reroute signaling into the denervated tissue.
Experience (blue boxes) stimulates anatomical rearrangements that encode the plastic response to experience. MAIs are known to limit experience-dependent plasticity, and we propose that this is because they also limit anatomical rearrangements in the intact CNS. Experience can be encoded through anatomy by promoting dendritic or axonal collateral growth, or more subtly, the differential stabilization of pre or post-synaptic structures. Ultimately, a common molecular system that regulates cytoskeletal rearrangements links the entire of spectrum of growth, regardless of the initial stimulus (injury or experience).
Experience-Dependent Plasticity
Ocular dominance of binocular cortex is a classic model system to study experience-dependent plasticity. Recording from single units in binocular cortex, monocular deprivation of the contralateral eye induces a shift in responsiveness of the cortex to the non-deprived, ipsilateral eye[95]. This shift can only be induced during a critical period of development[95], demonstrating the role of experience in modifying innate wiring of the visual system. While the regulation of brain derived neurotrophic factor (BDNF) [96], the maturation of the inhibitory circuitry [97] and cholinergic activity [98, 99] have demonstrated roles in critical period closure, recent evidence suggests that consolidation of anatomic circuitry may play an important role in gating the critical period. Based on their role in limiting CNS regeneration[9, 36, 100], CSPGs were hypothesized to close the critical period by consolidating the anatomy of visual cortex circuitry in the developing rodent brain [101]. Condensation of CSPGs around the somas and dendrites of cortical neurons into perineuronal nets correlates with the closure of the critical period. When CSPGs are enzymatically digested in adult rats, shifts in ocular dominance can be induced well into adulthood[101]. These results suggest that cortical plasticity in the adult brain is dependent on anatomical rearrangements, and that a common system inhibits both injury-induced and experience-dependent plasticity.
Parallel studies have been pursued with myelin inhibitors[83, 102]. The end of the critical period coincides with an increase of myelination in the visual cortex[102], suggesting a role for myelin in consolidating cortical circuitry and thus limiting shifts in ocular dominance in the adult brain. In the absence of Nogo-A or NgR1, monocular deprivation results in significant shifts in ocular dominance well into adulthood, demonstrating an absolute role for Nogo-A and NgR1 in critical period closure[102]. The mechanism by which these MAIs limit ocular dominance plasticity remains unclear. Since the absence of Nogo-A or NgR1 did not affect the maturation of inhibitory circuitry or levels of BDNF[102], ongoing studies are examining the possibility that this system limits ocular dominance plasticity by limiting the anatomic plasticity of the visual cortex in the adult brain. PirB has similarly been demonstrated to limit ocular dominance plasticity in the adult rodent[83]. A common system of growth inhibitors therefore limits both cortical plasticity in the intact CNS and axonal growth after an SCI. Together, these results suggest that MAIs limit experience-dependent plasticity as part of a developmentally tuned process to consolidate circuitry in the adult cortex.
Anatomic Plasticity
Although the mechanism by which MAIs limit experience-dependent plasticity remains unclear, it is clear that MAIs limit anatomic plasticity in both the developing and intact CNS. In testing whether a similar system limits anatomical plasticity in the intact and injured brain, Schwab and colleagues acutely applied the IN-1 antibody to uninjured cerebella and demonstrated that IN-1 treatment leads to axonal sprouting from intact Purkinje cells that is detectable within 2 days of injection but absent a month after treatment[103]. These results suggest that IN-1 acutely and reversibly removes a homeostatic brake on anatomic growth, tipping the balance towards growth in the intact brain. Schwab and colleagues extended these studies to assess the role of Nogo-A and NgR1 in limiting anatomic plasticity using organotypic hippocampal cultures[104]. Application of an anti-Nogo-A antibody leads to significant changes in dendritic and axonal complexity as measured by Scholl analysis. Genetic deletion of NgR1 leads to significant effects of dendritic and axonal complexity while deletion of Nogo-A only causes a mild affect on dendritic architecture[104]. Together, these studies suggest that the physiologic role of MAI-mediated growth inhibition is to consolidate circuitry in the intact brain.
Although we focus on the deleterious effects of circuit consolidation in the wake of an SCI, circuit consolidation is an important developmental and regulatory process in the CNS. A hyperplastic brain with aberrant connectivity may be at an evolutionary disadvantage due to an inability to appropriately consolidate circuitry. For instance, loss of function mutations in NgR1 have been associated with schizophrenia in humans, and mice deficient for NgR1 show deficits in working memory[105]. Thus Nogo-A and NgR1 may serve an important physiological role in limiting anatomic plasticity in the intact CNS in order to consolidate necessary circuitry and thus, finely tune the CNS network.
Activity Dependent Plasticity
Macro-neuroanatomical reorganization is not the only substrate for activity dependent plasticity; activity can additionally modify pre-existing circuitry by regulating synaptic strength. Increasing evidence suggests that activity and anatomy are intimately linked[106], and recent studies have begun to explore the role of MAIs in activity-dependent plasticity as a possible substrate for the plasticity observed in injury and sensory deprivation models. In adult hippocampal slices, application of Nogo-66 or OMgp suppress long-term potentiation (LTP) in an NgR1 dependent manner[107]. Deletion of NgR1 does not affect LTP[19, 107], although long-term depression (LTD) is impaired in mice lacking NgR1 [19, 107, 108]. Neither LTP nor LTD are affected by the absence of PirB, although it may play a role in OMgp mediated suppression of LTP[107]. How MAIs exert their effects on activity-dependent plasticity in the acute setting remains to be clarified, but the fact that MAIs modulate synaptic strength provides another potential substrate for limiting plasticity and functional recovery after a SCI.
These results also suggest a novel role for MAIs in learning and memory. To date, the only MAI demonstrated to have a memory phenotype is NgR1. Deletion of NgR1 is associated with impaired working memory[109] but normal acquisition of a spatial memory task[109, 110]. In mice over-expressing NgR1, acquisition of a spatial memory task is intact while the long-term consolidation of that memory is impaired[108]. NgR1 therefore limits a wide spectrum of plasticity in the intact brain, suggesting a broad regulatory role for MAIs in circuit consolidation that affects a wide spectrum of phenotypes in the injured and intact CNS.
INJURY, PLASTICITY, AND MAIs
The potential for recovery after a SCI is limited by the inhibitory environment of the mature CNS. The identification of these inhibitors and their receptors has led to the understanding that regeneration is a point along a continuum of anatomic growth that includes sprouting and plasticity. This spectrum represents distinct but fundamentally related forms of cytoskeletal rearrangements; the principle difference is magnitude. Given their demonstrated roles in regulating cytoskeletal dynamics[23, 48], it is not surprising then that MAIs (and myelin independent inhibitors of growth) limit this spectrum of growth in both the injured and naïve CNS. In the injured and naïve CNS, the same system limits anatomical rearrangements as nuanced as dendritic spine turnover or as dramatic as the elaboration of collateral branches from an axon (Figure 3). MAIs therefore represent a point of convergence for plasticity: regardless of the stimulus (injury, experience, etc), a common system of MAIs functions to limit plasticity and consolidate existing circuitry.
The history of MAI research therefore represents the potential of cooperation between translational and basic neuroscience. Original inquiries into spinal cord regeneration uncovered a novel system of myelin inhibitors that limit both axonal regeneration and functional recovery. The observation that recovery outpaces regeneration, however, led to the description of regeneration-independent mechanisms for repair, greatly expanding and complicating our understanding of basic spinal cord plasticity. Basic research on these substrates for recovery led to the description of novel regulators of experience and activity-dependent plasticity, which in turn, suggested novel substrates for SCI recovery. Characterizing the role of MAIs in CNS remodeling has therefore blurred the distinction between basic plasticity research and translational injury studies by advancing our understanding of basic mechanisms of plasticity while simultaneously broadening the therapeutic horizon for SCI repair and recovery.
Acknowledgments
F.A. is supported by an Institutional Medical Scientist Training Program from the N.I.H, NIH MSTP TG 2T32GM07205. This work was supported by the grants from the Christopher and Dana Reeve Foundation, the Wings for Life Foundation and the Dr. Ralph and Marion Falk Medical Research Trust to S.M.S., and from the National Institutes of Health to W.B.J.C. and S.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: S.M.S. is a co-founder of Axerion Therapeutics seeking to develop PrP and NgR therapies.
REFERENCES
- 1.Burns SP, et al. Recovery of ambulation in motor-incomplete tetraplegia. Archives of Physical Medicine and Rehabilitation. 1997;78(11):1169–1172. doi: 10.1016/s0003-9993(97)90326-9. [DOI] [PubMed] [Google Scholar]
- 2.Geisler FHC, William P, Giacinto Grieco, Devinder Poonian the Sygen Study Group. Measurements and Recovery Patterns in a Multicenter Study of Acute Spinal Cord Injury. Spine. 2001;26(24S):S68–S86. doi: 10.1097/00007632-200112151-00014. [DOI] [PubMed] [Google Scholar]
- 3.Cajal SRy. Cajal's Degeneration and Regeneration of the Nervous System. Oxford Univeristy Press; 1991. p. 976. [Google Scholar]
- 4.David S, Aguayo A. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284(5753):264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 6.Savio T, Schwab M. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J. Neurosci. 1989;9(4):1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandtlow C, Zachleder T, Schwab M. Oligodendrocytes arrest neurite growth by contact inhibition. J. Neurosci. 1990;10(12):3837–3848. doi: 10.1523/JNEUROSCI.10-12-03837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coumans JV, et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21(23):9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 10.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. The Journal of Cell Biology. 1988;106(4):1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343(6255):269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen MS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403(6768):434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 13.GrandPre T, et al. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403(6768):439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 14.Prinjha R, et al. Neurobiology: Inhibitor of neurite outgrowth in humans. Nature. 2000;403(6768):383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. Localization of Nogo-A and Nogo-66 Receptor Proteins at Sites of Axon-Myelin and Synaptic Contact. J. Neurosci. 2002;22(13):5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber AB, et al. Patterns of Nogo mRNA and Protein Expression in the Developing and Adult Rat and After CNS Lesions. J. Neurosci. 2002;22(9):3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephson A, et al. NOGO mRNA Expression in Adult and Fetal Human and Rat Nervous Tissue and in Weight Drop Injury. Experimental Neurology. 2001;169(2):319–328. doi: 10.1006/exnr.2001.7659. [DOI] [PubMed] [Google Scholar]
- 18.Richard M, et al. Neuronal expression of Nogo-A mRNA and protein during neurite outgrowth in the developing rat olfactory system†. European Journal of Neuroscience. 2005;22(9):2145–2158. doi: 10.1111/j.1460-9568.2005.04418.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, et al. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28(11):2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409(6818):341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 21.Oertle T, et al. Nogo-A Inhibits Neurite Outgrowth and Cell Spreading with Three Discrete Regions. J. Neurosci. 2003;23(13):5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu F, Strittmatter SM. The N-Terminal Domain of Nogo-A Inhibits Cell Adhesion and Axonal Outgrowth by an Integrin-Specific Mechanism. The Journal of Neuroscience. 2008;28(5):1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322(5903):967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 24.Bregman BS, et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378(6556):498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 25.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417(6888):547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 26.Kim J-E, et al. Axon Regeneration in Young Adult Mice Lacking Nogo-A/B. Neuron. 2003;38(2):187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 27.Simonen M, et al. Systemic Deletion of the Myelin-Associated Outgrowth Inhibitor Nogo-A Improves Regenerative and Plastic Responses after Spinal Cord Injury. Neuron. 2003;38(2):201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 28.Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord–injured rats. Annals of Neurology. 2005;58(5):706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 29.Fouad K, Klusman I, Schwab ME. Regenerating corticospinal fibers in the Marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur J Neurosci. 2004;20(9):2479–2482. doi: 10.1111/j.1460-9568.2004.03716.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim JE, et al. Nogo-C is sufficient to delay nerve regeneration. Mol Cell Neurosci. 2003;23(3):451–459. doi: 10.1016/s1044-7431(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 31.Pot C, et al. Nogo-A expressed in Schwann cells impairs axonal regeneration after peripheral nerve injury. The Journal of Cell Biology. 2002;159(1):29–35. doi: 10.1083/jcb.200206068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolf CJ. No Nogo: Now Where to Go? Neuron. 2003;38(2):153–156. doi: 10.1016/s0896-6273(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 33.Zheng B, et al. Lack of Enhanced Spinal Regeneration in Nogo-Deficient Mice. Neuron. 2003;38(2):213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 34.Cafferty WB, et al. Response to correspondence: Kim et al., "axon regeneration in young adult mice lacking Nogo-A/B." Neuron 38, 187–199. Neuron. 2007;54(2):195–199. doi: 10.1016/j.neuron.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimou L, et al. Nogo-A-Deficient Mice Reveal Strain-Dependent Differences in Axonal Regeneration. J. Neurosci. 2006;26(21):5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cafferty WB, et al. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27(9):2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freund P, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12(7):790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 38.Freund P, et al. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates – re-examination and extension of behavioral data. European Journal of Neuroscience. 2009;29(5):983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freund P, et al. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. The Journal of Comparative Neurology. 2007;502(4):644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- 40.Brosamle C, et al. Regeneration of Lesioned Corticospinal Tract Fibers in the Adult Rat Induced by a Recombinant, Humanized IN-1 Antibody Fragment. J. Neurosci. 2000;20(21):8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai C, et al. Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal neural development, are produced by alternative splicing. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(12):4337–4341. doi: 10.1073/pnas.84.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trapp BD. Myelin-Associated Glycoprotein Location and Potential Functionsa. Annals of the New York Academy of Sciences. 1990;605(1):29–43. doi: 10.1111/j.1749-6632.1990.tb42378.x. [DOI] [PubMed] [Google Scholar]
- 43.Bartsch U, Kirchhoff F, Schachner M. Immunohistological localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. The Journal of Comparative Neurology. 1989;284(3):451–462. doi: 10.1002/cne.902840310. [DOI] [PubMed] [Google Scholar]
- 44.McKerracher L, et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13(4):805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay G, et al. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13(3):757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 46.Vargas ME, Barres BA. Why Is Wallerian Degeneration in the CNS So Slow? Annual Review of Neuroscience. 2007;30(1):153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- 47.Bartsch U, et al. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15(6):1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 48.Cafferty WBJ, et al. MAG and OMgp Synergize with Nogo-A to Restrict Axonal Growth and Neurological Recovery after Spinal Cord Trauma. J. Neurosci. 2010;30(20):6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li M, et al. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. Journal of Neuroscience Research. 1996;46(4):404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 50.Ng WP, et al. Myelin from MAG- deficient mice is a strong inhibitor of neurite outgrowth. NeuroReport. 1996;7(4):861–864. doi: 10.1097/00001756-199603220-00005. [DOI] [PubMed] [Google Scholar]
- 51.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417(6892):941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 52.Mikol DD, Stefansson K. A phosphatidylinositol-linked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes. The Journal of Cell Biology. 1988;106(4):1273–1279. doi: 10.1083/jcb.106.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habib AA, et al. Expression of the Oligodendrocyte-Myelin Glycoprotein by Neurons in the Mouse Central Nervous System. Journal of Neurochemistry. 1998;70(4):1704–1711. doi: 10.1046/j.1471-4159.1998.70041704.x. [DOI] [PubMed] [Google Scholar]
- 54.Huang JK, et al. Glial Membranes at the Node of Ranvier Prevent Neurite Outgrowth. Science. 2005;310(5755):1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- 55.Kottis V, et al. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. Journal of Neurochemistry. 2002;82(6):1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- 56.Chang K-J, et al. Oligodendrocyte Myelin Glycoprotein Does Not Influence Node of Ranvier Structure or Assembly. J. Neurosci. 2010;30(43):14476–14481. doi: 10.1523/JNEUROSCI.1698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji B, et al. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Molecular and Cellular Neuroscience. 2008;39(2):258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JK, et al. Assessing Spinal Axon Regeneration and Sprouting in Nogo-, MAG-, and OMgp-Deficient Mice. Neuron. 2010;66(5):663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu BP, et al. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor.[comment] Science. 2002;297(5584):1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 60.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417(6892):941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 61.Barton WA, et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. Embo J. 2003;22(13):3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fournier AE, et al. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22(20):8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatesh K, et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25(4):808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7(3):221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 65.Park JB, et al. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45(3):345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 66.Shao Z, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45(3):353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 67.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23(4):1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsieh SH-K, Ferraro GB, Fournier AE. Myelin-Associated Inhibitors Regulate Cofilin Phosphorylation and Neuronal Inhibition through LIM Kinase and Slingshot Phosphatase. J. Neurosci. 2006;26(3):1006–1015. doi: 10.1523/JNEUROSCI.2806-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montani L, et al. Neuronal Nogo-A Modulates Growth Cone Motility via Rho-GTP/LIMK1/Cofilin in the Unlesioned Adult Nervous System. Journal of Biological Chemistry. 2009;284(16):10793–10807. doi: 10.1074/jbc.M808297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niederost B, et al. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22(23):10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chivatakarn O, et al. The Nogo-66 Receptor NgR1 Is Required Only for the Acute Growth Cone-Collapsing But Not the Chronic Growth-Inhibitory Actions of Myelin Inhibitors. J. Neurosci. 2007;27(27):7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huebner EA, et al. A multi-domain fragment of nogo-a is a potent inhibitor of cortical axon regeneration via nogo receptor 1. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M110.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JE, et al. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44(3):439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 74.Robak LA, et al. Molecular basis of the interactions of the Nogo-66 receptor and its homolog NgR2 with myelin-associated glycoprotein: development of NgROMNI-Fc, a novel antagonist of CNS myelin inhibition. J Neurosci. 2009;29(18):5768–5783. doi: 10.1523/JNEUROSCI.4935-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S, et al. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29(1):26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S, et al. Blockade of nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24(46):10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58(5):706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, et al. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60(5):540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(10):5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takai T, Ono M. Activating and inhibitory nature of the murine paired immunoglobulin-like receptor family. Immunological Reviews. 2001;181(1):215–222. doi: 10.1034/j.1600-065x.2001.1810118.x. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura A, Kobayashi E, Takai T. Exacerbated graft-versus-host disease in Pirb−/− mice. Nat Immunol. 2004;5(6):623–629. doi: 10.1038/ni1074. [DOI] [PubMed] [Google Scholar]
- 82.Omoto S, et al. Genetic Deletion of Paired Immunoglobulin-Like Receptor B Does Not Promote Axonal Plasticity or Functional Recovery after Traumatic Brain Injury. J. Neurosci. 2010;30(39):13045–13052. doi: 10.1523/JNEUROSCI.3228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Syken J, et al. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313(5794):1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 84.Science, A.I.f.B. Allen Spinal Cord Atlas [Internet] 2009. [Google Scholar]
- 85.Nakamura Y, et al. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. Journal of Biological Chemistry. 2010 doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cafferty WBJ, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends in Neurosciences. 2008;31(5):215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kakulas B. The clinical neuropathology of spinal cord injury. A guide to the future. Paraplegia. 1987;25(3):212–216. doi: 10.1038/sc.1987.37. [DOI] [PubMed] [Google Scholar]
- 88.Thallmair M, et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1(2):124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- 89.Z'Graggen WJ, et al. Functional Recovery and Enhanced Corticofugal Plasticity after Unilateral Pyramidal Tract Lesion and Blockade of Myelin-Associated Neurite Growth Inhibitors in Adult Rats. J. Neurosci. 1998;18(12):4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 91.Schwab M, Schnell L. Channeling of developing rat corticospinal tract axons by myelin-associated neurite growth inhibitors. J. Neurosci. 1991;11(3):709–721. doi: 10.1523/JNEUROSCI.11-03-00709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23(10):4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee JK, et al. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24(27):6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seymour AB, et al. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25(10):1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 95.Crair MC, Gillespie DC, Stryker MP. The Role of Visual Experience in the Development of Columns in Cat Visual Cortex. Science. 1998;279(5350):566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang ZJ, et al. BDNF Regulates the Maturation of Inhibition and the Critical Period of Plasticity in Mouse Visual Cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 97.Hensch TK, Stryker MP. Columnar Architecture Sculpted by GABA Circuits in Developing Cat Visual Cortex. Science. 2004;303(5664):1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Higley MJ, Strittmatter SM. Neuroscience. Lynx for braking plasticity. Science. 2010;330(6008):1189–1190. doi: 10.1126/science.1198983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morishita H, et al. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moon LD, et al. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4(5):465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 101.Pizzorusso T, et al. Reactivation of Ocular Dominance Plasticity in the Adult Visual Cortex. Science. 2002;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 102.McGee AW, et al. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buffo A, et al. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci. 2000;20(6):2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zagrebelsky M, et al. Nogo-A Stabilizes the Architecture of Hippocampal Neurons. J. Neurosci. 2010;30(40):13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Budel S, et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28(49):13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saneyoshi T, Fortin DA, Soderling TR. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Current Opinion in Neurobiology. 2010;20(1):108–115. doi: 10.1016/j.conb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raiker SJ, et al. Oligodendrocyte-Myelin Glycoprotein and Nogo Negatively Regulate Activity-Dependent Synaptic Plasticity. J. Neurosci. 2010;30(37):12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karlen A, et al. Nogo receptor 1 regulates formation of lasting memories. Proceedings of the National Academy of Sciences. 2009;106(48):20476–20481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Budel S, et al. Genetic Variants of Nogo-66 Receptor with Possible Association to Schizophrenia Block Myelin Inhibition of Axon Growth. J. Neurosci. 2008;28(49):13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park JH, et al. Subcutaneous Nogo Receptor Removes Brain Amyloid-{beta} and Improves Spatial Memory in Alzheimer's Transgenic Mice. J. Neurosci. 2006;26(51):13279–13286. doi: 10.1523/JNEUROSCI.4504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]