Abstract
Rationale
Methylphenidate (Ritalin®) is commonly prescribed for behavioral problems associated with attention deficit/hyperactivity disorder (ADHD). The results of previous studies suggest that methylphenidate increases cigarette smoking in participants without psychiatric diagnoses. Whether methylphenidate increases cigarette smoking in participants diagnosed with ADHD is unknown.
Objective
In this within-subjects, repeated measures experiment, the acute effects of a range of doses of methylphenidate (10, 20, and 40 mg) and placebo were assessed in nine cigarette smokers who were not attempting to quit and met diagnostic criteria for ADHD but no other Axis I psychiatric disorders other than nicotine dependence.
Methods
Each dose of methylphenidate was tested once while placebo was tested twice. One hour after ingesting drug, participants were allowed to smoke ad libitum for 4 h. Measures of smoking included total cigarettes smoked, total puffs, and carbon monoxide levels. Snacks and decaffeinated drinks were available ad libitum; caloric intake during the 4-h smoking session was calculated.
Results
Methylphenidate increased the total number of cigarettes smoked, total number of puffs, and carbon monoxide levels. Methylphenidate decreased the number of food items consumed and caloric intake.
Conclusions
The results of this experiment suggest that acutely administered methylphenidate increases cigarette smoking in participants with ADHD, which is concordant with findings from previous studies that tested healthy young adults. These data indicate that clinicians may need to consider non-stimulant options or counsel their patients before starting methylphenidate when managing ADHD-diagnosed individuals who smoke.
Keywords: Methylphenidate, Smoking, ADHD, Subjective effects, Humans
Introduction
Methylphenidate is commonly prescribed and effective for the treatment of attention deficit/hyperactivity disorder (ADHD) symptoms in children and adults (Brown et al. 2005; Faraone et al. 2006; Spencer et al. 2005). ADHD is characterized by abnormal levels of impulsivity, inattention, and hyperactivity, the primary cause for which is not fully understood. Results of single-photon emission computed tomography (SPECT) studies suggest that alterations in dopamine transporter (DAT) availability may, in part, contribute to the pathophysiology of ADHD (Cheon et al. 2003; Dougherty et al. 1999; Krause 2008; Krause et al. 2000). Methylphenidate is a central nervous system stimulant that blocks the dopamine transporter, preventing the reuptake of dopamine, resulting in increased extracellular dopamine. It is through the DAT mechanism that methylphenidate likely exerts its therapeutic effects in ADHD-diagnosed individuals (Krause et al. 2000).
Results of previous studies from our laboratory demonstrate that methylphenidate, regardless of formulation type (i.e., sustained or immediate-release), increases ad libitum cigarette smoking in non-ADHD adults (Rush et al. 2005; Vansickel et al. 2007, 2009). In those studies, participants were administered sustained or immediate-release methylphenidate and were allowed to smoke ad libitum for 4 h during the peak effects of the medication. Sessions were videotaped and scored for various smoking behaviors. In all studies, at least one dose of methylphenidate increased the total number of puffs significantly compared to placebo (Rush et al. 2005; Vansickel et al. 2007, 2009). In another study from our laboratory, methylphenidate was shown to increase the choice of cigarettes over money (Stoops et al. 2011). Participants in that study were administered methylphenidate (0, 10, 20, and 40 mg) and then made a series of choices between half of a cigarette and US $0.25. Methylphenidate increased choice of cigarettes over money, suggesting that methylphenidate increases the reinforcing efficacy of cigarettes. Results of several other human laboratory studies suggest that d-amphetamine, a psychostimulant medication also used to treat ADHD, increases cigarette smoking when administered acutely (Cousins et al. 2001; Henningfield and Griffiths 1981; Sigmon et al. 2003; Tidey et al. 2000). Taken together, results of these studies demonstrate a clear relationship between the acute effects of stimulant drugs and increased cigarette smoking behavior in non-ADHD adult cigarette smokers.
Individuals with ADHD are at increased risk to smoke relative to individuals without psychiatric disorders (Lambert and Hartsough 1998; Molina and Pelham 2003; Milberger et al. 1997: McClave et al. 2010). ADHD-diagnosed individuals also initiate smoking earlier and have a greater severity of tobacco abstinence symptoms and greater difficulty quitting than people without psychiatric disorders (Pomerleau et al. 1995, 2003; McClernon et al. 2008; Milberger et al. 1997; Lambert and Hartsough 1998; Gray et al. 2010). The reason for the increased prevalence and severity of smoking and nicotine dependence in persons with ADHD is unknown. Some researchers have speculated that individuals with ADHD self-medicate by abusing stimulant-like drugs such as nicotine in order to enhance attention or reduce symptoms of ADHD (Gehricke et al. 2007; Khantzian 1997; Milberger et al. 1997). Consistent with that notion, the nicotine patch and nicotinic agonists have been shown to ameliorate some ADHD symptoms (Gehricke et al. 2006, 2009; Levin et al. 1996; Wilens et al. 1999, 2006). This hypothesis would indicate that smoking would be reduced in individuals with ADHD whose symptoms were properly controlled through treatment with psychostimulants such as methylphenidate (Winhusen et al. 2010). Other researchers have suggested that treatment with psychostimulants may actually increase the risk of later tobacco smoking dependence (Lambert and Hartsough 1998; Lambert 2002).
To the best of our knowledge, the direct effects of methylphenidate on cigarette smoking have not been examined in adults with ADHD. The purpose of the current investigation was to determine the acute effects of methylphenidate (0, 10, 20, and 40 mg) on cigarette smoking behavior in ADHD-diagnosed, non-medicated daily cigarette smokers. Nine otherwise healthy adults participated in this study. Outcome measures included number of cigarettes, total puffs, expired air carbon monoxide, caloric intake, cardiovascular indices, and subjective effects. Caloric intake was included as a secondary measure of the effects of methylphenidate on consumptive behavior and to highlight the specificity of the effect of methylphenidate on cigarette smoking behavior. We hypothesized that acute methylphenidate administration would increase smoking and decrease caloric intake in these non-medicated ADHD-diagnosed participants.
Methods
Participants
Nine adult cigarette smokers (four males, five females) who met diagnostic criteria for ADHD were recruited via newspaper ads, flyers, and word-of-mouth to participate in this experiment. In order to be included, participants had to: (1) report smoking 10–20 cigarettes daily; (2) not be attempting to quit smoking; (3) be diagnosed with adult ADHD following a clinical evaluation along with the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID; Multi-Health Systems 2001), which was completed by a boarded adult and child psychiatrist with expertise in diagnosing adult ADHD (PEAG); (4) report not having used physician prescribed psychotropic medications in the past 2 months; (5) have no significant medical or psychiatric disorders, other than ADHD, which in the opinion of the study physician would interfere with participation; (6) have no medical contraindications to stimulant drugs; and (7) provide serum (at screening) and urine (during sessions) samples that were negative for pregnancy if female. Participants were compensated $480 for completing the study.
General procedures
The Institutional Review Board of the University of Kentucky Medical Center approved this study and the informed consent document. Participants enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology at the University of Kentucky Medical Center Monday through Friday for six experimental sessions. Participants were informed that, during their participation, they would receive various drugs and these could include placebo and medications indicated for ADHD. Participants were told that the purpose of the study was to see how these drugs affect mood and behavior. Other than receiving this general information, participants were blind to the type of drug administered and were given no instructions regarding what they were “supposed” to do or what outcomes might be expected.
Prior to initiating medication testing, participants completed one “practice” session, which was used to familiarize them with the drug-effect questionnaires and daily laboratory routine. No medications were administered during practice sessions.
Participants were requested to refrain from using all illicit psychoactive drugs throughout the study, caffeine and solid food for 4 h prior to a scheduled experimental session, and alcohol for 12 h prior to a scheduled experimental session. On each experimental session day, participants arrived at the laboratory and had to provide a urine sample negative for amphetamine, barbiturates, benzodiazepines, cocaine, and opioids in order to begin session (OnTrak Teststik, Varian Inc., Lake Forest, CA). If a participant provided a urine sample positive for THC, he/she was still allowed to complete session if his/her CO level was below 10 parts per million (ppm) and he/she passed the field sobriety test. As noted above, female subjects also had to provide a urine sample negative for pregnancy (Mainline Confirm HCG, Mainline Technology, Ann Arbor, MI). Participants also provided an expired air specimen, which had to be negative for the presence of alcohol using a handheld breathalyzer (Alco-Sensor, Intoximeters, Inc., St. Louis, MO) and pass a standard field sobriety test.
Participants generally arrived at the Laboratory of Human Behavioral Pharmacology at 0800 hours. Participants were instructed to abstain from smoking for at least 4 h before arriving at the Laboratory of Human Behavioral Pharmacology. Immediately after arriving, participants provided an expired breath sample that was used to determine their carbon monoxide level using a handheld carbon monoxide meter (Smokerlyzer, Bedfont Scientific, Medford, NJ). Carbon monoxide levels had to be <10 ppm for session to begin. If an acceptable carbon monoxide level could not be obtained within 1 h of arrival, the experimental session was canceled and rescheduled. After meeting the carbon monoxide criterion, generally between 0815 and 0830 hours, participants were allowed to smoke one cigarette of their preferred brand in order to reduce the possibility of testing the effects of methylphenidate during acute nicotine withdrawal. Between 0830 and 0845 hours, participants were provided a standard low-fat breakfast. At approximately 0845 hours, participants completed the drug-effect questionnaires. Between 0830 and 1000 hours, participants were alone in the experimental testing room, but they were not allowed to smoke. During this time, participants were allowed to engage in sedentary recreational activities (e.g., read, watch television), but they could not sleep.
Experimental medications were administered at 0900 hours. At 1000 hours, participants were provided with a pack of their preferred brand of cigarettes and an assortment of snacks and decaffeinated drinks. Subject-rated drug-effect questionnaires and carbon monoxide and physiological measures were completed at regular intervals following drug administration (see below).
Smoking procedures
Participants were tested individually in a 3×3-m room that contained a lounge chair, a television and digital-video disc (DVD) player, a computer (iMac, Apple Computer Inc., Cupertino, CA) for completing the subject-rated drug-effect questionnaires, and an assortment of reading material. During each session, participants remained seated in the lounge chair. One hour after ingesting drug, participants were allowed to smoke ad libitum for 4 h.
Outcome measures used to assess smoking included total puffs per session, exhaled carbon monoxide (see physiological measures below), and total cigarettes smoked per session. All experimental sessions were digitally recorded. All smoking within each session was double scored from a digital recording by a primary and a secondary observer, both of whom were blind to the dose conditions. Dividing the number of agreements between observers by the number of possible agreements and multiplying by 100 determined the interobserver reliability (Interobserver Reliability 2004). If the interobserver reliability was ≥85%, data from the primary observer were used for data analysis. If the interobserver reliability was <85%, the session was re-scored by both observers.
Food intake
Food and beverage intake following drug administration was measured to further characterize the behavioral effects of methylphenidate in ADHD-diagnosed adults. An assortment of food items and decaffeinated beverages, which were individually selected by participants based upon their preference, were available ad libitum during each experimental session. The available food items and beverages remained the same across all experimental sessions for each participant. Both the number of items consumed and the total caloric intake were determined. The number of items consumed was calculated at the end of each experimental session by counting the number of food packages and beverage containers opened by the volunteer. To calculate caloric intake, the available food items and beverages were weighed prior to being served. At the end of the session, if a food item or beverage was not completely consumed, it was reweighed, and the proportion consumed was multiplied by the caloric content of the entire food item. If a food or beverage item was completely consumed, the caloric content for the entire item was recorded. The number of calories consumed for each food item and beverage was then summed to calculate the total caloric intake for the experimental session.
Subject-rated drug-effect questionnaires
Subject-rated drug-effect questionnaires were administered on a computer or completed using paper-and-pencil forms. The subject-rated drug-effect questionnaires were completed in fixed order. Subject-rated drug-effect questionnaires included a locally developed drug-effect questionnaire that contains 20 items rated on a likert-type scale (zero [not at all] to four [extremely]) and an adjective rating scale that contains two subscales: sedative and stimulant (Rush et al. 2005; Oliveto et al. 1992). These questionnaires were completed approximately 30 min before drug administration and 1, 2, 3, 4, and 5 h after drug administration. Approximately 5 h after drug administration, participants completed a five-item cigarette rating scale as well as a five-item food rating scale. Other than the words “cigarettes” and “food,” these scales were identical in wording. The items rated were: (1) Did you “ENJOY” your cigarettes/food more than usual during today’s session?; (2) Did you “CRAVE” cigarettes/food more than usual during today’s session?; (3) Did your cigarettes/food “TASTE” better than usual during today’s session?; (4) Did you “LIKE” your cigarettes/food more than usual during today’s session?; and (5) Did you get more “PLEASURE” from your cigarettes/food during today’s session? Participants responded to these questions using five options: Not At All, A Little Bit, Moderately, Quite A Bit, and Extremely (scored numerically from 0 to 4). Scores from each item were summed to create a total score.
Physiological measures
Blood pressure and heart rate were recorded using an automated blood pressure monitor. Blood pressure and heart rate were monitored for approximately 30 min before drug administration and 0.5, 1, 1.5, 2, 2.5, 3, 4, and 5 h after drug administration. Expired carbon monoxide was measured 30 min before drug administration and 1, 2, 3, 4, and 5 h after drug administration. When measured at the same time point, physiological measures were recorded immediately before participants completed the subject-rated drug-effect questionnaires.
Drug administration
The drug conditions were placebo, 10, 20, and 40 mg methylphenidate. The standard adult daily dose of methylphenidate ranges from 20 to 40 mg/day, though some adults require higher (up to 60 mg) doses. Therefore, the doses of methylphenidate selected for the current study are within the therapeutic range. Each active dose of methylphenidate was tested once, while placebo was tested twice. Doses were administered in mixed order with the exception that the highest dose was never administered during the first experimental session. All dose conditions were administered in a double-blind fashion. Commercially available drug (10 mg, methylphenidate, CelTech, Rochester, NY) was over-encapsulated in a size zero capsule to prepare the doses. Cornstarch was used to fill the remainder of these capsules. Placebo capsules contained only cornstarch.
During each experimental session participants ingested four capsules. Administering the appropriate number of active and placebo capsules varied dose. Capsules were taken orally with approximately 150 ml of water. Drug administration procedures were designed to ensure that participants swallowed the capsules. To accomplish this, the research assistant: (a) watched the participant to ensure that he/she swallowed the capsules and did not remove them from his/her mouth, (b) conducted a brief oral examination to ensure that the participant was not hiding the capsules under his/her tongue, and (c) spoke with the participant to determine if he/she had anything in their mouth. At least 24 h separated all drug administrations.
Data analysis
One-tailed t tests (Statview, Cary, NC) were used to compare data from each of the active dose conditions to the average from the placebo conditions. Data were analyzed statistically as raw scores for most measures, with the exception that data from the placebo sessions were averaged. Effects were considered significant for p≤ 0.05. For carbon monoxide levels, change from baseline was calculated and averaged across the 4-h smoking period. For the adjective rating scale, drug-effect questionnaire, heart rate, and blood pressure, data collected after the first hour were considered uninterpretable because participants determined the amount they smoked (i.e., they smoked varying numbers of cigarettes with different nicotine contents). For this reason, only data collected 1 h after drug administration were analyzed from these measures.
Results
Participants
Participants ranged in age from 19 to 30 years (mean=24) and in weight from 54 to 102 kg (mean=71). Participants reported smoking 10 to 20 cigarettes/day (mean=14). All participants met criteria for childhood and adult ADHD. Six were diagnosed with combined-type (e.g., hyperactive–inattentive) adult ADHD, and three were diagnosed with inattentive-type adult ADHD by the study physician (PEAG). Participants had an average of 8.4 adult inattentive symptoms (range, 7–9; SD, 0.73), 5.8 adult hyperactive symptoms (range, 1–9; SD, 2.8), 8.3 childhood inattentive symptoms (range, 7–9; SD, 1), and 6.4 childhood hyperactive symptoms (range, 3–9; SD, 2.4) on the CAADID. Two participants had received stimulant treatment for ADHD in the past; one had received methylphenidate and the other had received Adderall XR®, but neither participant had received stimulant treatment within 2 months of study participation.
Smoking
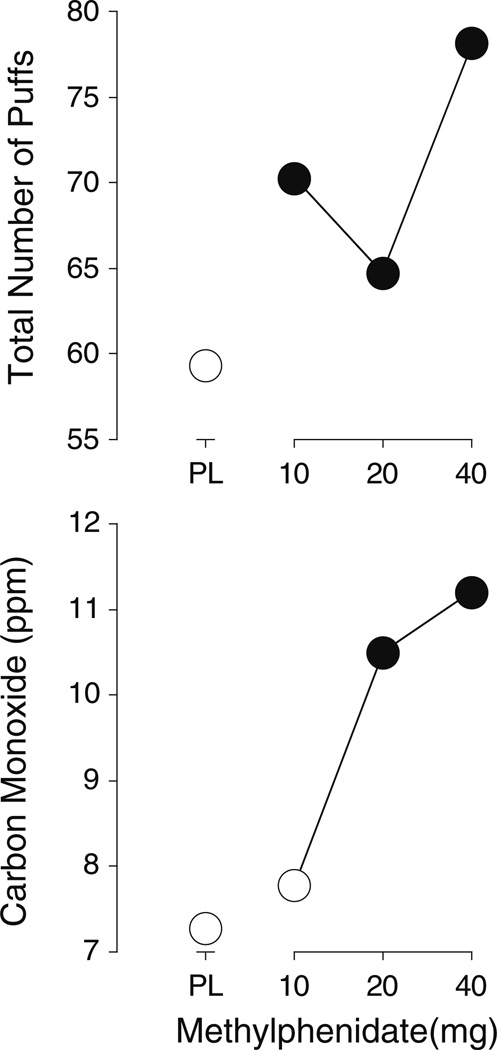
Each of the active doses of methylphenidate increased the total number of puffs (t8=2.5, 2.0, and 2.4, respectively, p<0.05), while the two higher doses increased CO levels significantly above placebo levels (t8=2.8 and 2.1, p≤0.03). Methylphenidate, 10 and 40 mg, increased the total number of cigarettes smoked relative to placebo (t8=2.0 and 2.2, respectively, p≤0.04). See Table 1 for means of measures (not represented in figures) in which at least one dose of methylphenidate was significantly different from placebo. Figure 1 shows the methylphenidate dose–response function for total number of puffs and CO levels.
Table 1.
Means for measures not presented graphically in which at least one dose of methylphenidate was significantly different from placebo
| Measure | Placebo | 10 mg | 20 mg | 40 mg |
|---|---|---|---|---|
| Smoking | ||||
| Total cigarettes | 5.7 | 6.4a | 6.2 | 7.7a |
| Drug-effect questionnaire | ||||
| Active/Alert/Energetic | 0.2 | 0.2 | 0.8a | 0.8 |
| Like Drug | 0.4 | 0.3 | 1.2a | 0.8 |
| Shaky/Jittery | 0.1 | 0.2 | 0.1 | 0.4a |
| Stimulated | 0.2 | 0.2 | 0.8a | 0.4 |
| Talkative/Friendly | 0.1 | 0.1 | 0.3a | 0.3 |
| Willing to Take Again | 0.3 | 0.6 | 0.9a | 0.8 |
Dose is significantly different from placebo
Fig. 1.
Dose–response functions for total number of puffs (top panel) and carbon monoxide levels (bottom panel). X-axes methylphenidate dose in milligrams; data points above PL designate placebo values. Data points show the means of nine participants. Filled symbols indicate those values that are significantly different from the placebo value
Food intake
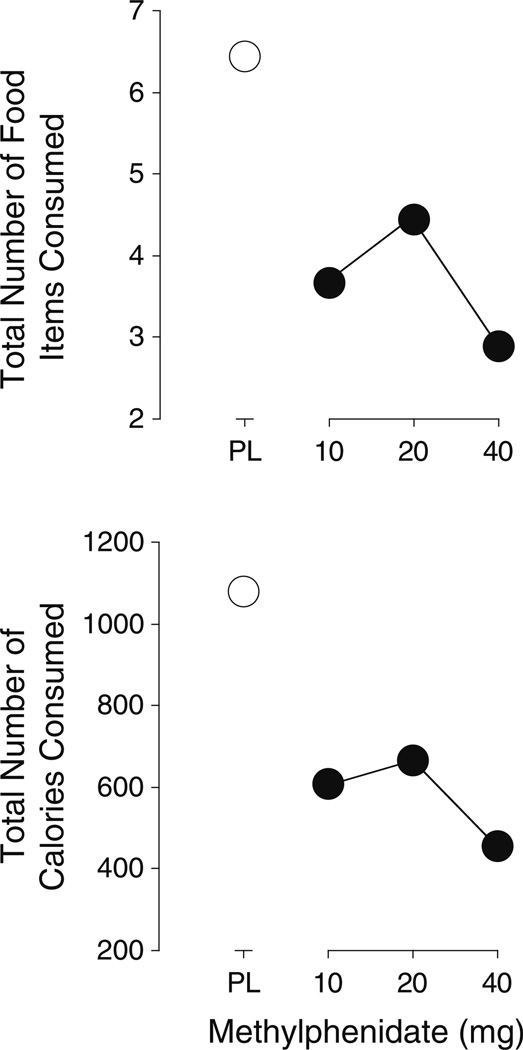
Each of the active doses of methylphenidate significantly decreased the number of items consumed (t8=5.1, 3.2, and 5.4, respectively, p<0.01) (Fig. 2). Each of the active doses of methylphenidate also significantly decreased the total number of calories consumed (t8=6.2, 3.7, and 5.1, respectively, p<0.01) (Fig. 2).
Fig. 2.
Dose–response functions for number of food items (top panel) and calories consumed (bottom panel). All other details are the same as for Fig. 1
Cigarette rating scale
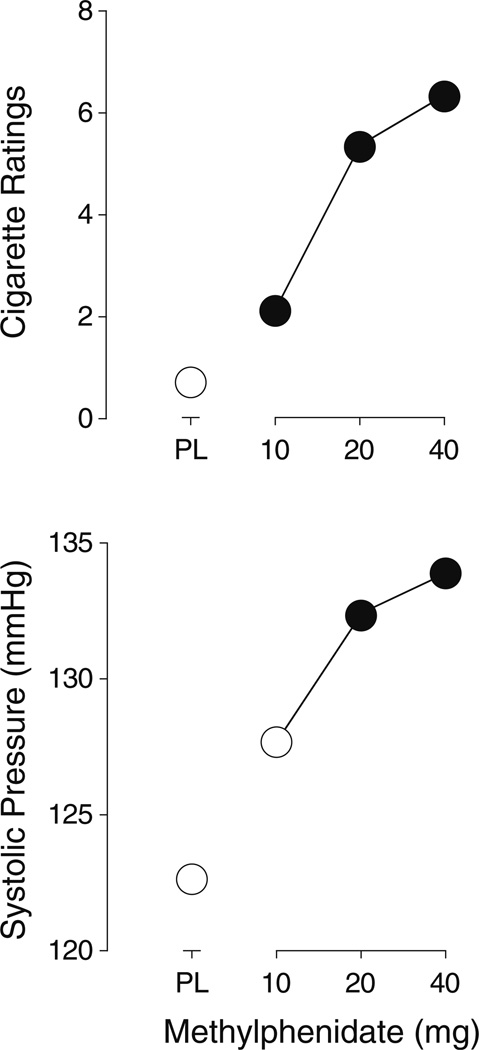
Each of the active doses of methylphenidate increased responses on the cigarette rating scale significantly above placebo levels (t8=2.0, 2.3, and 2.9, respectively, p≤0.04) (Fig. 3).
Fig. 3.
Dose–response functions for responses on the cigarette rating scale (top panel) and systolic blood pressure (bottom panel). All other details are the same as for Fig. 1
Food and adjective rating scales
Methylphenidate did not significantly affect responses on the food rating scale nor responses on the stimulant and sedative subscales of the adjective rating scale.
Drug-effect questionnaire
The intermediate dose of meth-ylphenidate, 20 mg, increased ratings significantly above placebo level on five items from the drug-effect questionnaire: Active/Alert/Energetic, Like Drug, Stimulated, Willing to Take Again, and Talkative/Friendly (t8=2.0, 2.3, 2.4, 1.9, and 1.9, respectively, p<0.05), while the high dose, 40 mg, increased ratings of Shaky/ Jittery (t8=1.9, p<0.05) (Table 1). There were no other significant effects of methylphenidate on the drug-effect questionnaire.
Heart rate and blood pressure
The two higher doses of methylphenidate, 20 and 40 mg, increased systolic blood pressure significantly above placebo levels (t8=2.5 and 3.7, p<0.02) (Fig. 3). Methylphenidate did not significantly change heart rate or diastolic blood pressure.
Discussion
Acute methylphenidate administration increased cigarette smoking in ADHD-diagnosed adults. Findings from this study could have important clinical implications for the pharmacologic treatment of persons with ADHD who also smoke cigarettes. ADHD affects approximately 2.5–4% of adults (Kessler et al. 2006; McClave et al. 2010; Simon et al. 2009). A recent analysis of findings from the National Health Interview Survey revealed that 37.2% of persons suffering from ADD or hyperactivity were current smokers compared to 18.3% of individuals that reported no lifetime history of mental illness (McClave et al. 2010). Stimulant medications such as methylphenidate are generally the first-line treatment options for persons with ADHD (Faraone and Glatt 2010; Peterson et al. 2008). Potential increases in cigarette smoking following stimulant treatment may increase overall exposure to smoke-related toxicants such as cardiotoxic carbon monoxide and carcinogenic tobacco specific nitrosamines, potentially altering the risk for smoking related disease.
Methylphenidate was administered acutely in the current study whereas, in a clinical setting, it would be taken chronically. Chronic exposure to stimulants and other drugs has been shown to affect the morphology and function of neuronal systems, suggesting that the interaction between methylphenidate and cigarette smoking and/or nicotine may be altered following chronic treatment (Russo et al. 2009). Although methylphenidate was not administered chronically in this study, as would be used in the clinical setting, the results do suggest that clinicians may wish to consider non-stimulant treatment options or counsel their patients before starting methylphenidate in patients with ADHD who also smoke. Cognitive behavioral therapy, for example, has been shown to improve ADHD symptoms in adults (Knouse and Safron 2010). Atomoxetine, a non-stimulant medication indicated for the treatment of ADHD, also improves ADHD symptoms in adults (Adler et al. 2009). Furthermore, in a previous laboratory study, atomoxetine did not increase smoking when administered acutely to non-ADHD adults, suggesting that it may be an effective alternative for smokers (Vansickel et al. 2007). The mechanisms driving methylphenidate-induced increases in smoking are still unclear. It now seems possible that the mechanisms underlying stimulant-induced smoking increases in non-ADHD adults are the same for adults with ADHD.
Methylphenidate may increase the reinforcing efficacy of smoking. In a previous study, methylphenidate was shown to increase choice of cigarettes over money in non-ADHD adults (Stoops et al. 2011). Similar findings have been observed for other stimulant medications. Results of two studies demonstrate that d-amphetamine, another medication prescribed for ADHD, increases the reinforcing effects of smoking in non-ADHD participants (Sigmon et al. 2003; Tidey et al. 2000). In the first study, d-amphetamine (0, 7.5, and 15 mg/70 kg) dose-dependently increased choice of smoking (i.e., two puffs per choice) over money (i.e., $0.25 per choice) (Tidey et al. 2000). In the later study, d-amphetamine (0, 7.5, and 15 mg/70 kg) administration increased breakpoints on a progressive ratio schedule of reinforcement when participants were responding for cigarettes but not money (Sigmon et al. 2003). Whether methylphenidate increases the reinforcing efficacy of cigarettes in adults with ADHD is unknown and should be examined in future studies.
Consistent with the notion that methylphenidate increased the reinforcing efficacy of cigarettes in this sample of adults with ADHD, methylphenidate dose-dependently increased scores on the cigarette rating scale. This questionnaire poses such questions as, “Did you enjoy your cigarettes more than usual during today’s session?” Again, this finding is similar to findings from previous studies that used the same questionnaire (Vansickel et al. 2007, 2009). While subjective ratings are not considered a direct measure of reinforcement, they often align with behavioral measures of reinforcement.
The reinforcing efficacy of cigarettes following methylphenidate administration might be enhanced due to an additive or synergistic interaction of methylphenidate and nicotine which results in increased extracellular dopamine concentrations in areas of the brain known to be involved in reward processes (Gerasimov et al. 2000; Vansickel et al. 2007). Results of several SPECT studies suggest that individuals with ADHD have abnormal dopamine functioning in these and other areas of the brain compared to non-ADHD controls (Cheon et al. 2003; Dougherty et al. 1999; Krause 2008; Krause et al. 2000). The results of the present study demonstrate that despite probable differences in baseline dopaminergic function, acute methylphenidate similarly affects smoking behavior in ADHD and non-ADHD adults. Future studies should be designed to better determine the pharmacologic interaction of methylphenidate and nicotine in ADHD and non-ADHD participants.
Similar to previous findings, methylphenidate also decreased caloric intake (Rush et al. 2005; Vansickel et al. 2007, 2009). This finding is consistent with the notion that dopaminergic mechanisms regulate appetite and eating (Berridge 1996; Leddy et al. 2004). In our previous studies that examined the effects of methylphenidate on caloric intake, methylphenidate (40 mg) decreased caloric consumption by up to 50% (Vansickel et al. 2007) while the same dose reduced caloric intake by approximately 70% in the current study. Available pre-packaged food items were similar across studies and remained the same across sessions for each participant. Individual differences in baseline appetite may have contributed to the enhanced effect of methylphenidate on caloric intake in the current study.
Methylphenidate (20 mg) increased significantly ratings of Active/Alert/Energetic, Like Drug, Stimulated, Willing to Take Again, and Talkative/Friendly, and methylphenidate (40 mg) increased ratings of Shaky/Jittery from the drug-effect questionnaire in the first hour following drug administration. These findings are similar to those found in previous studies that examined the effects of methylphenidate across a longer observation period (Rush and Baker 2001; Stoops et al. 2004, 2005). The subjective effects of methylphenidate observed 1-h after medication administration in the current study are discordant with findings from previous studies that used similar procedures and identical doses of methylphenidate (Rush et al. 2005; Vansickel et al. 2007, 2009). Fewer statistically significant changes in subjective effects were observed 1-h after methylphenidate administration in those previous studies. The reason for the difference in subjective effects across similarly designed studies is unknown. Perhaps the effects of methylphenidate peak sooner in non-medicated ADHD adults than in non-ADHD adults, suggesting potential differences in the pharmacokinetics of methylphenidate across groups. Future studies should examine differences in the distribution and metabolism of methylphenidate in ADHD and non-ADHD adults. This finding may also suggest that the non-medicated ADHD adults were more sensitive to the effects of methylphenidate than non-ADHD participants.
Methylphenidate (20 and 40 mg) significantly increased systolic pressure within the first hour of administration. The cardiovascular effects of methylphenidate in the current study were not as pronounced as we have seen in previous studies that assessed the cardiovascular effects of methylphenidate across a number of hours (Rush and Baker 2001; Stoops et al. 2004, 2005). This finding is consistent, however, with findings from similarly designed studies in which cardiovascular data were analyzed only for the first hour following medication administration (Rush et al. 2005; Vansickel et al. 2007, 2009).
Some methodological considerations of the current investigation include the small sample size, acute dosing procedure, and the formulation of methylphenidate used. Nine subjects completed the current protocol, potentially limiting the overall interpretation of these data. However, the use of a within-subject, repeated measures design decreased the potential for spurious results. Future studies should examine the effects of methylphenidate on cigarette smoking behavior in a larger sample of ADHD cigarette smokers. Also, methylphenidate was administered acutely in the current study, whereas the drug would be administered chronically in the natural environment. Whether the effects of methylphenidate on cigarette smoking in ADHD-diagnosed individuals would be similar following chronic treatment is unknown and should be examined prospectively. Finally, the immediate-release formulation of methylphenidate was administered in the current study while sustained or extended-release preparations are often prescribed. Results of a previous human laboratory study demonstrate that stimulant-induced increases in smoking are probably dependent on drug concentration rather than the rate-of-onset of drug action in non-ADHD participants (Vansickel et al. 2009). Whether the same is true for non-medicated ADHD-diagnosed individuals is not known.
Despite these limitations, findings from this study are consistent with results of previous studies, suggesting that acute methylphenidate administration increases cigarette smoking and decreases caloric intake in both non-ADHD and ADHD adults alike (Rush et al. 2005; Stoops et al. 2011; Vansickel et al. 2007, 2009). The true impact that methylphenidate might have on smoking and subsequent health of adults with ADHD that smoke and are maintained on methylphenidate cannot be fully determined from the results of this study, however. A placebo-controlled, double-blind, randomized, multi-site trial of methylphenidate for the treatment of ADHD-diagnosed individuals that includes observational and biochemically verified measures of smoking behavior would be ideal for addressing this potentially serious effect of an often-prescribed medication. Indeed, methylphenidate is effective for the treatment of ADHD, and the results of this study should not be used to imply that clinicians should be reluctant to prescribe this medication. Results of this and other studies simply indicate that until a better understanding of the interaction between stimulant treatment and smoking can be reached, smoking should be considered prior to stimulant treatment.
Acknowledgments
The authors wish to thank Frances P. Wagner, R. N. for her expert nursing assistance and Amanda Bucher, Kathryn Hays, Shawn England, Michelle Gray, Erika Pike, and Sarah Veenema for their expert technical assistance. The National Institute on Drug Abuse Grants R01 DA 012665 (CRR) and T32 DA 007027-34 as well as the National Cancer institute grant R21 CA 124881 (WWS) supported this project. Research professorship funds from the University of Kentucky to CRR, startup funds from the University of Kentucky Department of Behavioral Science to WWS, and pilot grant funds from the University of Kentucky Department of Behavioral Science to ARV also supported this project.
Contributor Information
Andrea R. Vansickel, Department of Behavioral Science, College of Medicine, University of Kentucky, Lexington, KY 40536, USA Department of Psychology, College of Arts and Science, University of Kentucky Lexington, KY 40536, USA.
William W. Stoops, Department of Behavioral Science, College of Medicine, University of Kentucky, Lexington, KY 40536, USA Department of Psychology, College of Arts and Science, University of Kentucky Lexington, KY 40536, USA.
Paul E. A. Glaser, Department of Psychiatry, College of Medicine, University of Kentucky, Lexington, KY 40536, USA Department of Anatomy and Neurobiology, College of Medicine, University of Kentucky, Lexington, KY 40536, USA; Department of Pediatrics, College of Medicine, University of Kentucky, Lexington, KY 40536, USA.
Megan M. Poole, Department of Psychology, College of Arts and Science, University of Kentucky, Lexington, KY 40536, USA
Craig R. Rush, Email: crush2@uky.edu, Department of Behavioral Science, College of Medicine, University of Kentucky, Lexington, KY 40536, USA; Department of Psychiatry, College of Medicine, University of Kentucky, Lexington, KY 40536, USA; Department of Psychology, College of Arts and Science, University of Kentucky Lexington, KY 40536, USA; Department of Behavioral Science, University of Kentucky Medical Center, 140 Medical Behavioral Science Building, Lexington, KY, USA.
References
- Adler LA, Spencer T, Brown TE, Holdnack J, Saylor K, Schuh K, Trzepacz PT, Williams DW, Kelsey D. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29:44–50. doi: 10.1097/JCP.0b013e318192e4a0. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Brown RT, Amler RW, Freeman WS, Perrin JM, Stein MT, Feldman HM, Pierce K, Wolraich ML. American Academy of Pediatrics Committee on Quality Improvement, American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder (2005) Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 115(6):749–757. [Google Scholar]
- Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter availability in the basal ganglia assessed with [123]IPT SPECT in children with attention deficit hyperactivity disorder. Eur J Nucl Med. 2003;30:306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Cousins M, Stamat H, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology. 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter availability in patients with attention deficit hyperactivity disorder. Lancet. 1999;354:2132–2133. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71:754–763. doi: 10.4088/JCP.08m04902pur. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. Med Gen Med. 2006;8(4):4. [PMC free article] [PubMed] [Google Scholar]
- Gehricke JG, Whalen CK, Jamner LD, Wigal TL, Steinhoff K. The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: a preliminary examination. Nicotine Tob Res. 2006;8(1):37–47. doi: 10.1080/14622200500431619. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, Belluzzi JD, Leslie FM. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine Tob Res. 2007;9(S4):S523–S536. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Hong N, Whalen CK, Steinhoff K, Wigal TL. Effects of transdermal nicotine on symptoms, moods, and cardiovascular activity in the everyday lives of smokers and nonsmokers with attention-deficit/hyperactivity disorder. Psychol Addict Behav. 2009;23(4):644–655. doi: 10.1037/a0017441. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse. 2000;38:432–437. doi: 10.1002/1098-2396(20001215)38:4<432::AID-SYN8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gray KM, Baker NL, Carpenter MJ, Lewis AL, Upadhyaya HP. Attention-deficit/hyperactivity disorder confounds nicotine withdrawal self-report in adolescent smokers. Am J Addict. 2010;19(4):325–331. doi: 10.1111/j.1521-0391.2010.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR. Cigarette smoking and subjective response: effects of d-amphetamine. Clin Pharmacol Ther. 1981;30:497–505. doi: 10.1038/clpt.1981.194. [DOI] [PubMed] [Google Scholar]
- Interobserver Reliability. http://www.ccny.cuny.edu/bbpsy/modules/interob_reliability.htm. Accessed 15 Jul 2004. [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Knouse LE, Safron SA. Cognitive behavioral therapy for adult attention-deficit hyperactivity disorder. Psychiatr Clin N Am. 2010;33:497–509. doi: 10.1016/j.psc.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J. SPECTand PETofthe dopamine transporter in attention-deficit hyperactivity disorder. Expert Rev Neurother. 2008;8(4):611–625. doi: 10.1586/14737175.8.4.611. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dressel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285:107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- Lambert N. Stimulant treatment as a risk factor for nicotine use and substance abuse. In: Jensen, Cooper, editors. Attention deficit hyperactivity disorder: state of the science-best practices. Kingston: Civic Research Institute; 2002. Chapter 18. [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Leddy JJ, Epstein LH, Jaroni JL, Roemmich JN, Paluch RA, Goldfield GS, Lerman C. Influence of methylphenidate on eating in obese men. Obes Res. 2004;12:224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Pub Health. 2010;100(12):2464–2472. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology. 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Academic Child Adol Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology. 2008;197:1–11. doi: 10.1007/s00213-007-0996-4. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Downey KK, Stelson FW, Pomerleau OF. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Downey KK, Snedecor SM, Mehringer AM, Marks JL, Pomerleau OF. Smoking patterns and abstinence effects in smokers with no ADHD, childhood ADHD, and adult ADHD symptomatology. Addict Behav. 2003;28:1149–1157. doi: 10.1016/s0306-4603(02)00223-x. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW. Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Exp Clin Psychopharmacol. 2001;9:59–73. doi: 10.1037/1064-1297.9.1.59. [DOI] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PEA. Methylphenidate increases cigarette smoking. Psychopharmacology. 2005;181:781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robinson MS, Ables JL, Nestler EJ. Neuro-trophic factors and structural plasticity in addiction. Neurophar-macology. 2009;56(S9):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology. 2003;167:393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J, Mick E, Aleardi M, Herzig K, Faraone S. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456–463. doi: 10.1016/j.biopsych.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Fillmore MT, Glaser PEA, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Rush CR. Discriminative-stimulus and self-reported effects of methylphenidate, d-amphetamine and triazolam in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2005;13:56–64. doi: 10.1037/1064-1297.13.1.56. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Poole MM, Vansickel AR, Hays K, Glaser PEA, Rush CR. Methylphenidate increases choice of cigarettes over money. Nicotine Tob Res. 2011;13(1):29–33. doi: 10.1093/ntr/ntq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. d-Amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology. 2000;153:85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PE, Rush CR. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology. 2007;193:305–313. doi: 10.1007/s00213-007-0786-z. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Poole MM, Stoops WW, Hays KE, Upchurch MB, Glaser PE, Rush CR. Stimulant-induced changes in smoking and caloric intake: influence of rate of onset. Pharmacol Biochem Behav. 2009;92:597–602. doi: 10.1016/j.pbb.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, Croghan IT, Adler LA, Weiss RD, Leimberger JD, Lewis DF, Dorer EM. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(12):1680–1688. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]