Abstract
Radiation and other types of DNA damaging agents induce a plethora of signaling events simultaneously originating from the nucleus, cytoplasm, and plasma membrane. As a result, this presents a dilemma when seeking to determine causal relationships and provide better insight into the intricacies of stress signaling. ATM plays critical roles in both nuclear and cytoplasmic signaling, of which, the DNA damage response (DDR) is the best characterized. We have recently created experimental conditions where the DNA damage signal alone can be studied while minimizing the influence from the extranuclear compartment. We have been able to document pro-survival and growth promoting signaling (via ATM-AKT-ERK) resulting from low levels of DSBs (equivalent to •2 Gy). More extensive DSBs (>2 Gy eq.) result in phosphatase-mediated ERK dephosphorylation, and thus shutdown of ERK signaling. In contrast, radiation does not result in such dephosphorylation even at very high doses. We propose that phosphatases are inactivated perhaps as a result of reactive oxygen species, which does not occur in response to ‘pure’ DNA damage. Our findings suggest that clinically relevant radiation doses, intended to halt tumor growth and induce cell death, are unable to inhibit tumor pro-survival signaling via ERK dephosphorylation.
Keywords: ATM, AKT, DNA repair, EGFR, ERK, MAP kinase, phosphatase, ROS
Introduction
We have reported in several recent studies that DNA DSB (double-strand break) repair (homologous recombination repair (HRR), as well as nonhomologous end joining (NHEJ)) is modulated by the EGFR-RAF-MEK-ERK and AKT pro-survival signaling axes. Our data suggest that ATM plays a central role in monitoring growth and well-being of the cell via a regulatory loop with ERK and AKT, which also influences DSB repair. This review summarizes our recent work on ATM-dependent pro-survival signaling and DSB repair, which we formulate into a model providing a new perspective on how radiation-induced nuclear and cytoplasmic signaling might be coordinated.
Background
ATM is the principal regulator of the DNA damage response
The most toxic DNA damage lesion generated by a wide variety of endogenous and exogenous sources is the DNA double-strand break (DSB). DSBs resulting from ionizing radiation (IR) and radiomimetic drugs activate the phosphoinositide 3-kinase-related kinase (PIKK) ataxia telangiectasia (A–T) mutated (ATM), a member of a family of proteins also including ATM and RAD3-related protein (ATR) and DNA-dependent protein kinase (DNA-PK). Together, the PIKKs serve to transduce and amplify the DNA damage signal by localizing to DNA breaks, activating cell cycle checkpoints, modulating survival and apoptotic/death signaling pathways, and directly participating in DSB repair (reviewed in [1]). This process is referred to as the DNA damage response (DDR). ATM phosphorylates more than 700 proteins, many directly involved in DSB repair and DNA damage checkpoints such as p53, CHK2, and the histone isoform H2AX to mention only a few [2].
The repair of DSBs in mammalian cells occurs primarily via two pathways - nonhomologous end joining (NHEJ) and homologous recombination repair (HRR). If left unrepaired, DSBs result in cell lethality [3]. The two pathways have distinct mechanisms; HRR requires a homologue, to be found either in a sister chromatid, homologous chromosome, or repeat sequence, from which the HRR machinery can duplicate an intact copy of the damaged DNA and ensure high DNA repair fidelity. NHEJ can be an error-free or error-prone process that is able to rejoin double-stranded ends with no or little sequence homology [1]. Both HRR and NHEJ are regulated by PIKKs [1]. In human cells, ATM participates in both HRR and NHEJ through cell cycle control and chromatin-associated processes [4–6]. Furthermore, NHEJ is coordinated by the direct actions of DNA-PK, a holoenzyme comprised of the regulatory KU hetero-dimer, KU70 and KU80, and the large catalytic subunit of DNA-PK (DNA-PKcs) [1]. DNA-PKcs binds to damaged DNA directly and functions both structurally and as a kinase to synapse the two broken ends of DNA and help target other NHEJ factors to the site of damage (reviewed in [7]). Misrepair of DSBs can lead to mutagenic events such as chromosome translocations, deletions, duplications, or DNA loss that lead to cancer and other diseases (reviewed in [8,9]). The extent to which any of the PIKKs (ATM, ATR, or DNA-PK) may substitute for another is incompletely understood and is complicated by substantial evidence for cross-talk between the three PIKKs [10–12]. Furthermore, DSB repair displays differential reliance on each kinase during the cell cycle, over the course of development, and whether the DSB resides in eu- or heterochromatin [6,13,14].
To monitor the impact of ATM signaling on DSB repair, we have employed engineered DNA repair cassettes harboring a unique restriction endonuclease site to introduce DNA breaks at defined locations. This technology uses the rare-cutting endonuclease, I-SceI, which cleaves an 18-bp long DNA recognition sequence [15]. The DSB ends formed after I-SceI cleavage have 5 -phosphate and 3 -OH groups suitable for ligation, whereas DSBs formed after exposure to IR, radiomimetic drugs, or oxidative damage have chemically heterogeneous ends that require processing by nucleases or other DNA modifying enzymes prior to repair by HRR or NHEJ. The I-SceI-based technology has a number of advantages relative to other methods aimed at measuring DSB repair. First, the integrated repair cassettes are subject to changes in chromatin structure and therefore the DSB is repaired under more physiological conditions than, for example, episomal DNA substrates. Second, this method allows for the direct monitoring of repair at a single DSB rather than overwhelming the cellular machinery with the excessive DNA damage required to demonstrate cleavage and re-sealing by pulsed-field electrophoresis or other methods. Finally, the DNA sequence spanning the DSB is known, so PCR-based detection is possible, which is not the case with damage resulting from radiation. Stably integrated repair cassettes expressing fluorescent reporter proteins have been used quite successfully by our group to monitor HRR and NHEJ events, and we have shown critical involvement of ATM in both HRR and NHEJ [4,16,17]. However, it is important to remember that these artificial constructs are only surrogates, and critical findings need confirmation by repair foci assays, radiosurvival, and other means.
In addition to canonical repair factors, DSB repair is also modulated by EGFR and MAPK signaling (reviewed in [18]). We have demonstrated that EGFR-RAF-MEK-ERK and JUN signaling are important for efficient HRR, whereas p38 signaling seems to negatively modulate this type of repair [17]. Similarly, NHEJ is also affected by EGFR-MEK-ERK signaling since γ-H2AX foci removal is impaired or promoted when dominant negative EGFR is expressed or stimulated with EGFRvIII, respectively [16]. The ligand-independent and, thus, constitutively active EGFRvIII enhances AKT signaling relative to wild type EGFR [19]. Furthermore, EGFR needs to heterodimerize with ERBB2 to activate AKT signaling in response to radiation but not EGF [20]. AKT activity is critical for radiation-induced phosphorylation of DNA-PKcs at T2609 and S2056 and for NHEJ [21,22]. Results similar to ours regarding EGFR, DNA-PK and DSB repair have also been reported by others [23]. In addition, ERK signaling, partly under control of ATM and EGFR-MEK-ERK signaling, is also important for IR-induced p(S1981)-ATM formation [17]. This suggests that a regulatory loop exists between ATM and MEK/ERK that regulates the DDR, DSB repair, and the choice between cell survival and death (Fig. 1). In support of this notion, an important role of MEK-ERK signaling in modulating ATM function in response to the DNA damaging agent etoposide was revealed in a recent study where ERK was depleted [24].
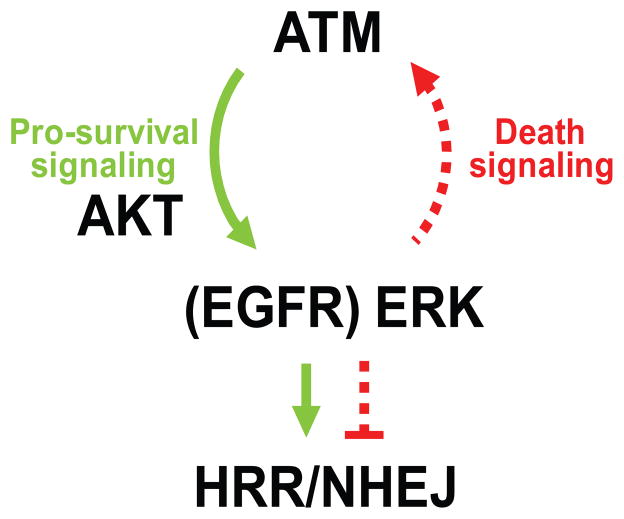
Fig. 1.
Regulatory loop between ATM and AKT-ERK signaling. A dynamic relationship between survival and death pathways is regulated by ATM, AKT and ERK that together control DSB repair (HRR – homologous recombination repair, and NHEJ – nonhomologous end joining). When damage is low and the cell can manage to repair its DNA, cell survival ensues. When severe damage occurs, the cell shuts down repair and the cell dies. Green arrows and text; survival signaling, red arrows and text; death signaling. This schema summarizes findings from several of our recent publications [12,16,17].
Cytoplasmic role of ATM
A number of recent reports have found that ATM also plays important roles outside of the nucleus by regulating reactive oxygen species (ROS) signaling [25]. In response to ROS, ATM activates TSC2 in a p53-independent manner to suppress mTORC1 signaling, which is distinct from the DDR. Furthermore, oxidation of ATM at a critical cysteine residue (C2991) directly induces ATM activation in the absence of DSBs and the MRN complex [26]. Auto-phosphorylation of ATM at S1981, phosphorylation of p53 (S15), and CHK2 (T68) occurred in response to hydrogen peroxide, whereas H2AX and KAP1 both residing in the nucleus were not phosphorylated. Thus, only a subset of ATM targets that are phosphorylated during the DDR are also phosphorylated in response to ROS. The observation that the ATM R3047X mutation generates an ataxia phenotype in A–T patients while retaining a normal DDR suggests that most of the clinical manifestations of A–T may result from an inability to effectively regulate the response to ROS [26]. Since radiation effectively generates ROS, as well as DNA damage, it seems as if multiple, parallel ATM signaling pathways are triggered in separate cellular compartments that are somehow co-regulated.
Radiation-induced growth factor receptor-mediated signaling
Epidermal growth factor receptor (EGFR) is a member of the receptor tyrosine kinase superfamily. In response to the binding of epidermal growth factor or other ligands, EGFR oligomerizes at the plasma membrane, which stimulates its intrinsic protein tyrosine kinase activity to trans-phosphorylate tyrosine residues on the intracellular portion of the receptor. In addition, clinically relevant, low dose radiation exposure (1–2 Gy) activates EGFR by homo- and hetero-dimerization with other members of the EGFR/ERBB receptor family (ERBB2, ERBB3, and ERBB4) (reviewed in [27]). EGFR auto-phosphorylation triggers downstream signaling events channeled into different pathways through activating proteins that bind to phosphorylated tyrosine moieties via their SH2 domains. These signaling pathways include the ERK, JNK, and phosphatidylinositol-3-kinase (PI3K)/AKT pathways which regulate a wide variety of cellular processes, including growth, migration, and senescence. Radiation-induced EGFR activation is believed to result from alterations in the plasma membrane and/or inactivation of (a) phosphatase(s), which signals into the cell (“outside-in”) to regulate gene expression and cell growth. However, radiation also produces DSBs and other types of DNA damage that signal from the nucleus to the cytoplasm (“inside-out”) (reviewed in [28]). Other growth factor receptors, such as the insulin receptor (Ins-R) and the insulin-like growth factor 1 receptor (IGF-1R), also modulate cell survival after radiation (Fig. 2). ATM seems to regulate signaling processes from all three of these receptors [12,29–31]. For example, insulin signaling is impaired in A–T cells and small molecule inhibitors of the ATM kinase reduce AKT phosphorylation in cells stimulated with insulin [12]. Furthermore, the radioresistance of A–T cells increases to normal levels when IGF-1R is over-expressed [30]. Along that same line, when IGF-1R is down-regulated normal cells become more radiosensitive [31]. Altogether, ATM regulates nuclear as well as cytoplasmic signaling responses with documented crosstalk between the two compartments.
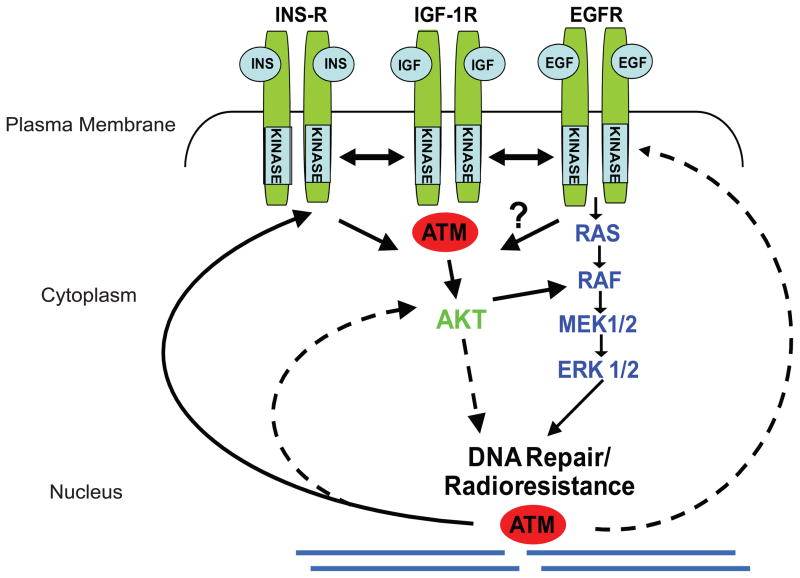
Fig. 2.
ATM separately regulates DNA damage responses in the nucleus and cytoplasm. Cross-talk exists and is referred to as “inside-out” and “outside-in” signaling [28]. Growth factor receptors, the insulin receptor (Ins-R), the insulin growth factor 1 receptor (IGF-1R), and the epidermal growth factor receptor (EGFR), residing in the plasma membrane are regulated by ATM and transmit growth and radioresistance signaling through the RAS-RAF-MEK-ERK and AKT signaling pathways. ATM has well-established nuclear function and binds to or close to the DSB after DNA damage and mediates the DDR. ATM plays a separate role in the cytoplasm where it regulates ROS signaling [25,26]. The schema merely provides a basic overview of functions and interactions, and does not reflect the specific characteristics of the receptors and mediators depicted.
Low levels of DSBs trigger pro-survival signaling
To address the origin of the signal that activates pro-survival responses - either nuclear via DNA damage or cytoplasmic through plasma membrane events or ROS - we first generated DSBs by BrdU photolysis [32]. We demonstrated that low levels of DSBs (equivalent to •2 Gy) resulted in increased ERK and AKT phosphorylation. This prosurvival signaling was dependent on ATM for transmitting the signal to ERK, via AKT and MEK. Greater levels of DSBs (>2 Gy eq.) resulted in ERK dephosphorylation which was ATM-independent, suggesting that excessive DNA damage shuts down prosurvival signaling. Similar results were seen after transient expression of a restriction endonuclease from a plasmid or by electroporation of the same endonuclease into cells [32]. Altogether, this bi-phasic ERK response likely plays an important role in determining cellular outcomes in response to DNA damage and the balance between cell survival and death.
Whereas ERK phosphorylation was bi-phasic and AKT-dependent, γ-H2AX levels increased in linear fashion with increasing DSBs. Interestingly, low levels of DSBs introduced by a restriction endonuclease actually increased cell proliferation over several days. Contrary to the observed bi-phasic effect on ERK and the threshold effect on p53 signaling [32], neither MKK6-p38 or c-Jun signaling increased after photolysis (Fig. 3). Ultraviolet light, and to some extent IR, activated both signaling pathways, and all three treatments increased p53 phosphorylation. The increased p53 phosphorylation observed after treatment with either photolysis, ultraviolet light, or IR indicate that each treatment successfully induced DNA damage. However, while phosphorylated MKK6, Jun, and p38 can be seen after treatment with ultraviolet light or IR, each are absent in the samples generated from photolysis, suggesting that MKK6-p38 and JNK-Jun signaling are triggered by non-DNA damage events. A likely candidate for the induction of MKK6-p38 and JNK-Jun signaling is the generation of ROS that accompanies both IR and ultraviolet light treatment [33,34]. All combined, our findings suggest that the low levels of DSBs, which the cell is able to handle and repair, result in ATM- and AKT-dependent MEK-ERK pro-survival signaling. Extensive DSBs trigger an ATM-independent phosphatase(s) that prevent ERK signaling, and, thus, inhibits proliferation and growth. Conversely, radiation increases ERK phosphorylation in a dose- and time-dependent manner but dephosphorylation of ERK is not observed [32].
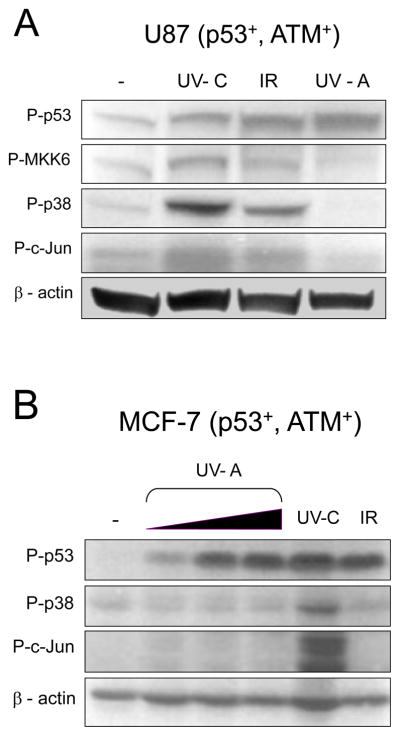
Fig. 3.
Nuclear signaling from DSBs does not activate the p38 and Jun signaling pathways. (A) U87 cells were treated with IR (20 Gy), UV-C (20 J/m2), or labeled with BrdU, treated with Hoechst dye and UV-A irradiated with 1500 J/m2 [32]. Cells were collected after 1 h and samples processed for western blot analysis. (B) MCF-7 cells were treated with IR (20 Gy), UV-C (20 J/m2), or treated with BrdU as in (A). UV-A was applied at 150, 500 or 1500 J/m2. Cells were harvested after 1 h and samples processed for western blot analysis [32]. Membrane was probed with anti-phospho antibodies for p53 (S15), MKK6 (S189/207), p38 (T180/Y182), and c-Jun (S63). Anti- -actin antibody was used to normalize protein loading.
Phosphatases in the DNA damage response
Inactivation of negative regulatory components, such as protein tyrosine phosphatases (PTPs), is one mechanism that results in increased protein phosphorylation [35]. For example, ROS inactivates a PTP that results in EGFR activation [36]. However, most key proteins involved in the DDR are controlled by serine-threonine (S–T) phosphatases including ATM (reviewed in [37]). This type of phosphatase, including PP1, PP2A, and MKP3, can also be inactivated by ROS, which is indeed what occurs during cellular senescence [38].
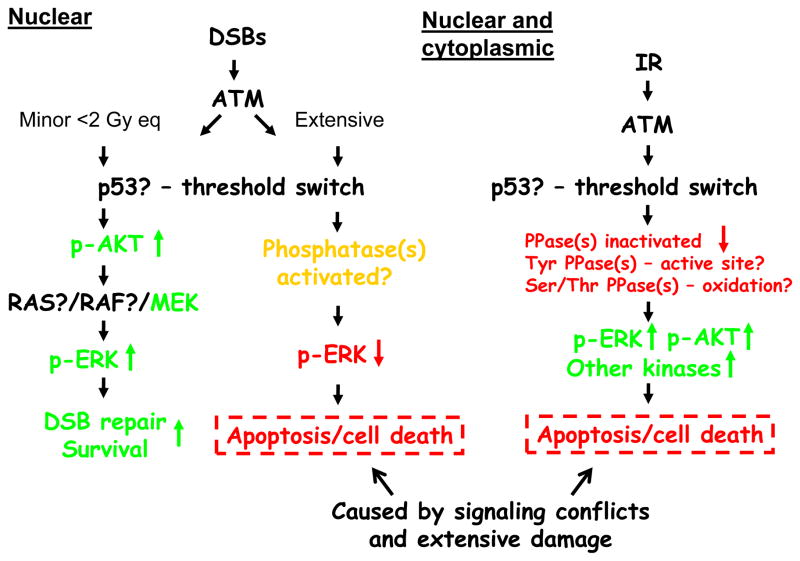
Thus, rather than being controlled by kinases, protein phosphatases could regulate the DSB-induced pro-survival signaling we observe. For example, we recently reported on the possibility that a phosphatase might negatively control the phosphorylation of AKT at S473 in an ATM-dependent manner [12]. Protein phosphatase 2A has strong links with ATM, AKT, and ERK signaling, and could control a multitude of these DSB-induced responses [12]. It is also tempting to speculate on the role of inactivated phosphatases and activation of ATM in states of redox stress. Oxidation of a cysteine-disulfide bond (C2991) in the FRAP/ATM/TRRAP C-terminal (FATC) domain of ATM could very well occur after IR as it does after hydrogen peroxide or bleomycin treatment [26]. A summary of the signaling occurring from nuclear and cytoplasmic compartments in response to BrdU photolysis at low and high levels of DSBs as well as radiation is shown in Fig. 4. Photolysis and restriction endonucleases trigger nuclear signaling that can be divided into minor (•2 Gy eq.) or extensive (>2 Gy eq.) DNA damage. Minor DNA damage activates ATM, ERK and AKT signaling, promotes DSB repair, and increases cell survival. Extensive DNA damage activates ATM and ATM-independent phosphatases, which abrogate ERK phosphorylation and cause apoptosis/cell death by signaling conflicts and extensive damage. The tumor suppressor p53 is activated at very low levels of DSBs and acts as a threshold switch for the DDR. On the other hand, radiation activates ATM in the nucleus and separately in the cytoplasm (via ROS) in a dose-dependent manner. ROS inactivates phosphatases by oxidation preventing dephosphorylation of p-ERK and possibly many other phosphorylated proteins. Based on our recent findings, this inactivation might start occurring at about 2 Gy since at 2 Gy eq. of DSBs ERK dephosphorylation is seen [32]. Sustained phosphorylation of ERK, AKT, and other proteins results in proliferative responses and senescence at low doses, and apoptosis/cell death at higher doses.
Fig. 4.
Summary of “inside-out” and “outside-in” signaling and the importance of phosphatases. See text for explanation. Green arrows and text denote survival signaling, and red arrows and text denote growth inhibitory and death signaling. Yellow denotes unique effects.
Why is the regulation of phosphatases relevant?
Most clinical protocols deliver radiation in 2 Gy fractions. The case has been made that this relatively low dose, under protracted fractionation, could possibly result in accelerated repopulation in certain types of tumors [39–41]. We and others have demonstrated that radiation at low and clinically relevant doses increases ERK signaling. However, ERK dephosphorylation does not occur at higher doses. The reason behind this could be that critical phosphatases are inactivated. Sustained ERK signaling could lead to growth and/or tumor senescence, both undesirable effects of radiotherapy. Our studies have revealed an interesting observation that is worthy of further investigation. Of course, explaining tumor repopulation by a single mechanism is too simplistic since many responses aside from ERK and AKT signaling also occur and play a role in determining cell fate. Yet, targeting and manipulating phosphatases for clinical benefit could perhaps hold the key to better constraining tumor repopulation.
Acknowledgments
This work was supported in part by NIH P01CA72955, R21ES016636, R01NS064593 (K.V.), and T32CA085159 and American Brain Tumor Association (S.E.G.). The work presented here is an overview of the work in the Valerie laboratory during the past few years and was not meant to be a thorough review on the topic. We apologize to all those researchers whose important studies were not cited in this review. We thank members of the Valerie lab for providing insight and useful discussions.
Footnotes
Conflict of interest
The authors have no conflicts to declare.
Author contributions
A.J.H, S.E.G., and A.K. carried out experiments. A.J.H. and K.V. wrote the manuscript. All authors read, commented, and approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka S, Ballif BA, Smogorzewska A, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 3.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 4.Golding SE, Rosenberg E, Khalil A, et al. Double strand break repair by homologous recombination is regulated by cell cycle-independent signaling via ATM in human glioma cells. J Biol Chem. 2004;279:15402–15410. doi: 10.1074/jbc.M314191200. [DOI] [PubMed] [Google Scholar]
- 5.Riballo E, Kuhne M, Rief N, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Goodarzi AA, Noon AT, Deckbar D, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Meek K, Gupta S, Ramsden DA, Lees-Miller SP. The DNA-dependent protein kinase: the director at the end. Immunol Rev. 2004;200:132–141. doi: 10.1111/j.0105-2896.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson LH, Schild D. Recombinational DNA repair and human disease. Mutat Res. 2002;509:49–78. doi: 10.1016/s0027-5107(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 9.Weinstock DM, Richardson CA, Elliott B, Jasin M. Modeling oncogenic translocations: distinct roles for double-strand break repair pathways in translocation formation in mammalian cells. DNA Repair (Amst) 2006;5:1065–1074. doi: 10.1016/j.dnarep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM Phosphorylates Histone H2AX in Response to DNA Double-strand Breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 11.Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 12.Golding SE, Rosenberg E, Valerie N, et al. Improved ATM kinase inhibitor KU- 60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol Cancer Ther. 2009;8:2894–2902. doi: 10.1158/1535-7163.MCT-09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams BR, Golding SE, Rao RR, Valerie K. Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants. PLoS One. 2010;5:e10001. doi: 10.1371/journal.pone.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouet P, Smih F, Jasin M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci U S A. 1994;91:6064–6068. doi: 10.1073/pnas.91.13.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Prosurvival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 18.Meyn RE, Munshi A, Haymach JV, Milas L, Ang KK. Receptor signaling as a regulatory mechanism of DNA repair. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:316–322. doi: 10.1016/j.radonc.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammering G, Hewit TH, Valerie K, et al. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene. 2003;22:5545–5553. doi: 10.1038/sj.onc.1206788. [DOI] [PubMed] [Google Scholar]
- 20.Toulany M, Minjgee M, Kehlbach R, Chen J, Baumann M, Rodemann HP. ErbB2 expression through heterodimerization with erbB1 is necessary for ionizing radiation- but not EGF-induced activation of Akt survival pathway. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;97:338–345. doi: 10.1016/j.radonc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. International journal of radiation biology. 2007;83:781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 22.Toulany M, Kehlbach R, Florczak U, et al. Targeting of AKT1 enhances radiation toxicity of human tumor cells by inhibiting DNA-PKcs-dependent DNA doublestrand break repair. Molecular cancer therapeutics. 2008;7:1772–1781. doi: 10.1158/1535-7163.MCT-07-2200. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee B, McEllin B, Camacho CV, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei F, Xie Y, Tao L, Tang D. Both ERK1 and ERK2 kinases promote G2/M arrest in etoposide-treated MCF7 cells by facilitating ATM activation. Cellular signalling. 2010;22:1783–1789. doi: 10.1016/j.cellsig.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Alexander A, Cai SL, Kim J, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 27.Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Valerie K, Yacoub A, Hagan MP, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 29.Gueven N, Keating KE, Chen P, et al. Epidermal growth factor sensitizes cells to ionizing radiation by down-regulating protein mutated in ataxia-telangiectasia. J Biol Chem. 2001;276:8884–8891. doi: 10.1074/jbc.M006190200. [DOI] [PubMed] [Google Scholar]
- 30.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macaulay VM, Salisbury AJ, Bohula EA, Playford MP, Smorodinsky NI, Shiloh Y. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene. 2001;20:4029–4040. doi: 10.1038/sj.onc.1204565. [DOI] [PubMed] [Google Scholar]
- 32.Khalil A, Morgan RN, Adams BR, et al. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle. 2011;10:481–491. doi: 10.4161/cc.10.3.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. The Journal of biological chemistry. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 34.Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Molecular and cellular biology. 2001;21:6913–6926. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Molecular cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type proteintyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. The Journal of biological chemistry. 2006;281:27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng A, Maller JL. Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene. 2010;29:5977–5988. doi: 10.1038/onc.2010.371. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. The Journal of biological chemistry. 2003;278:37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- 39.Withers HR. Cell cycle redistribution as a factor in multifraction irradiation. Radiology. 1975;114:199–202. doi: 10.1148/114.1.199. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Ullrich RK, Contessa JN, Dent P, et al. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Investig. 1999;7:321–330. doi: 10.1002/(SICI)1520-6823(1999)7:6<321::AID-ROI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]