Abstract
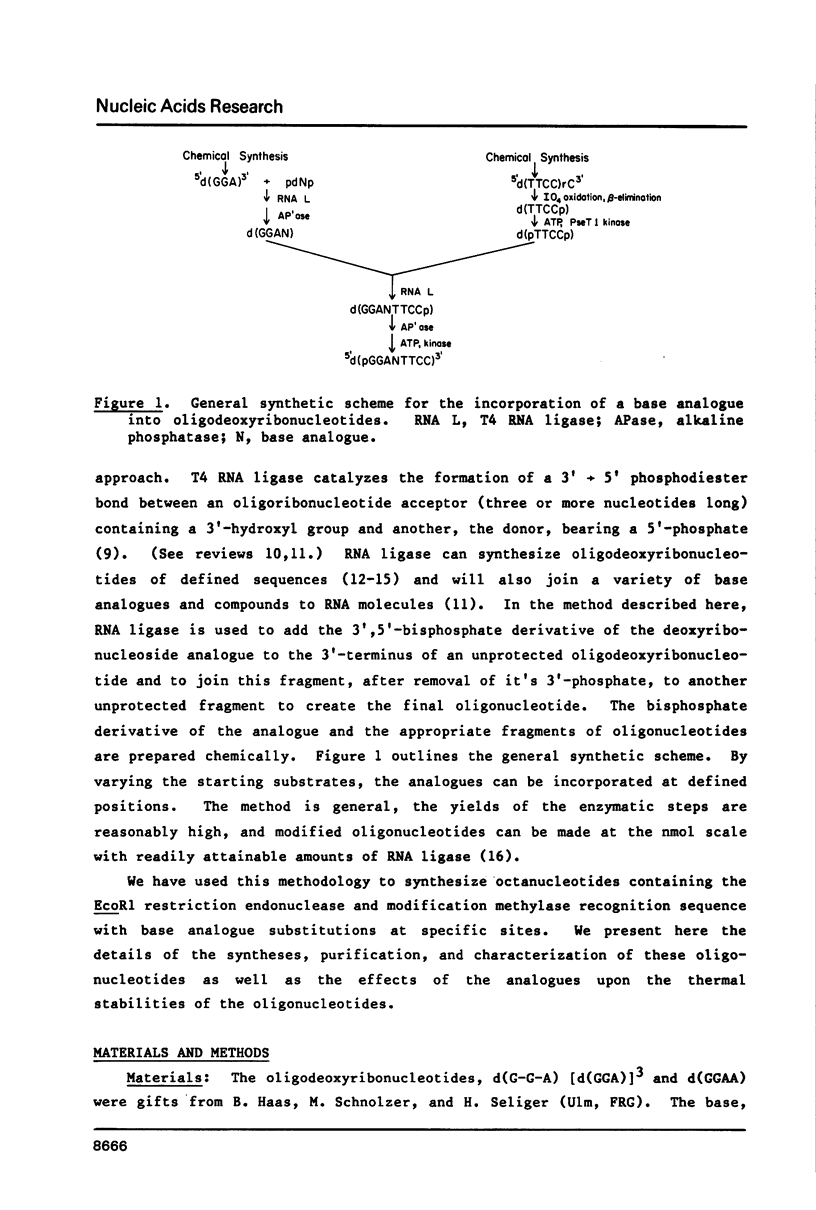
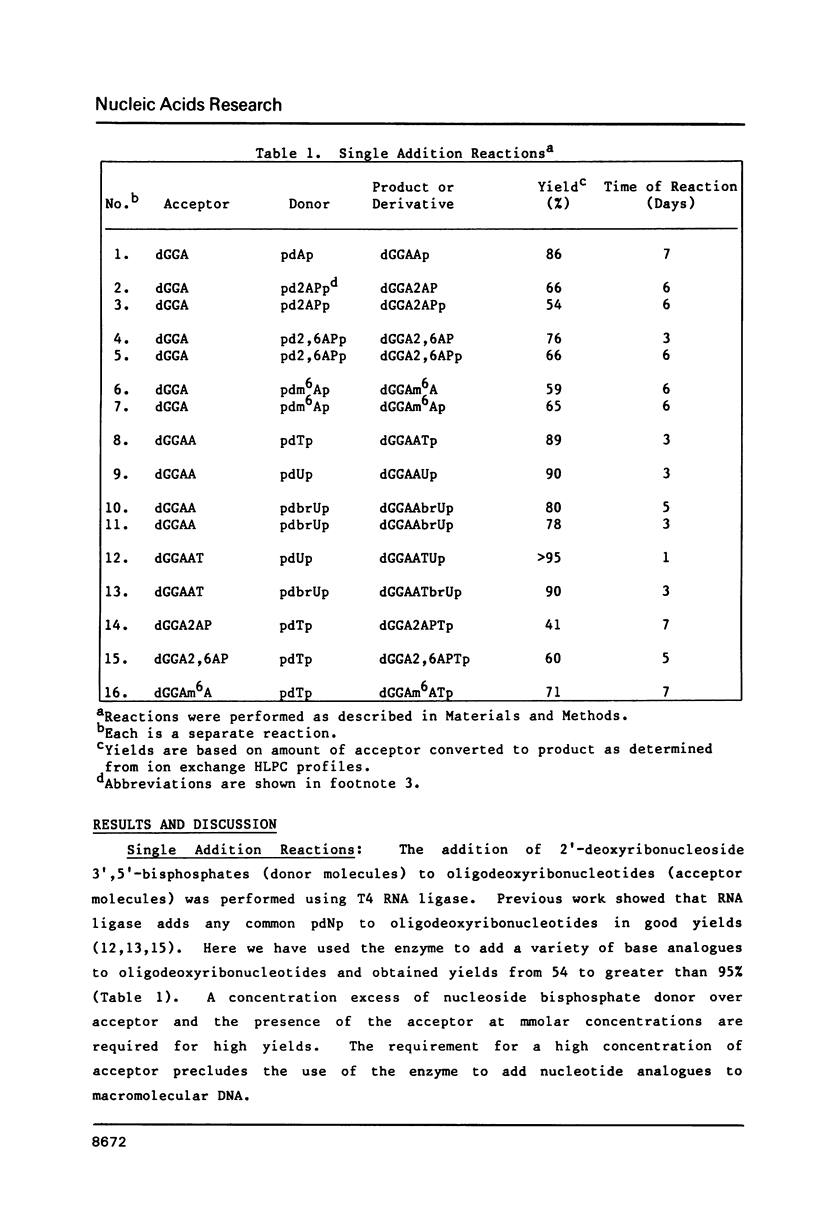
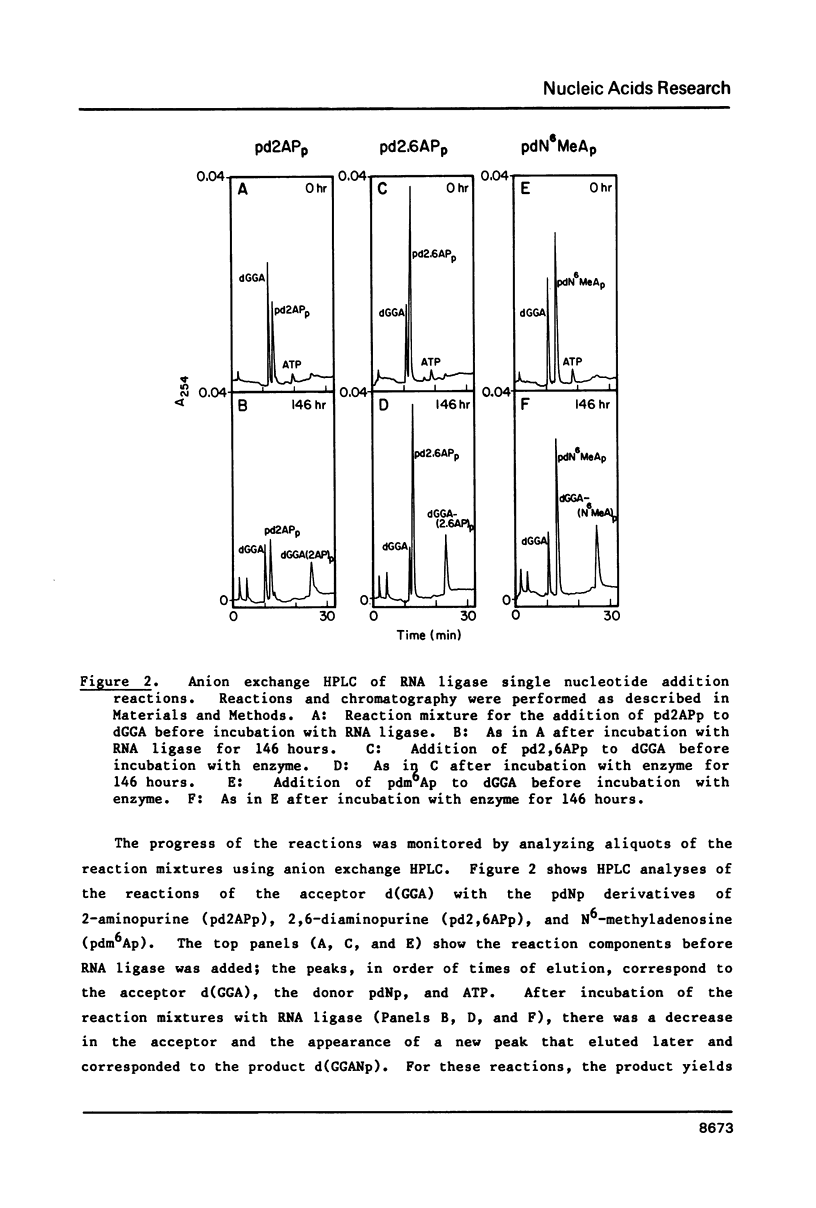
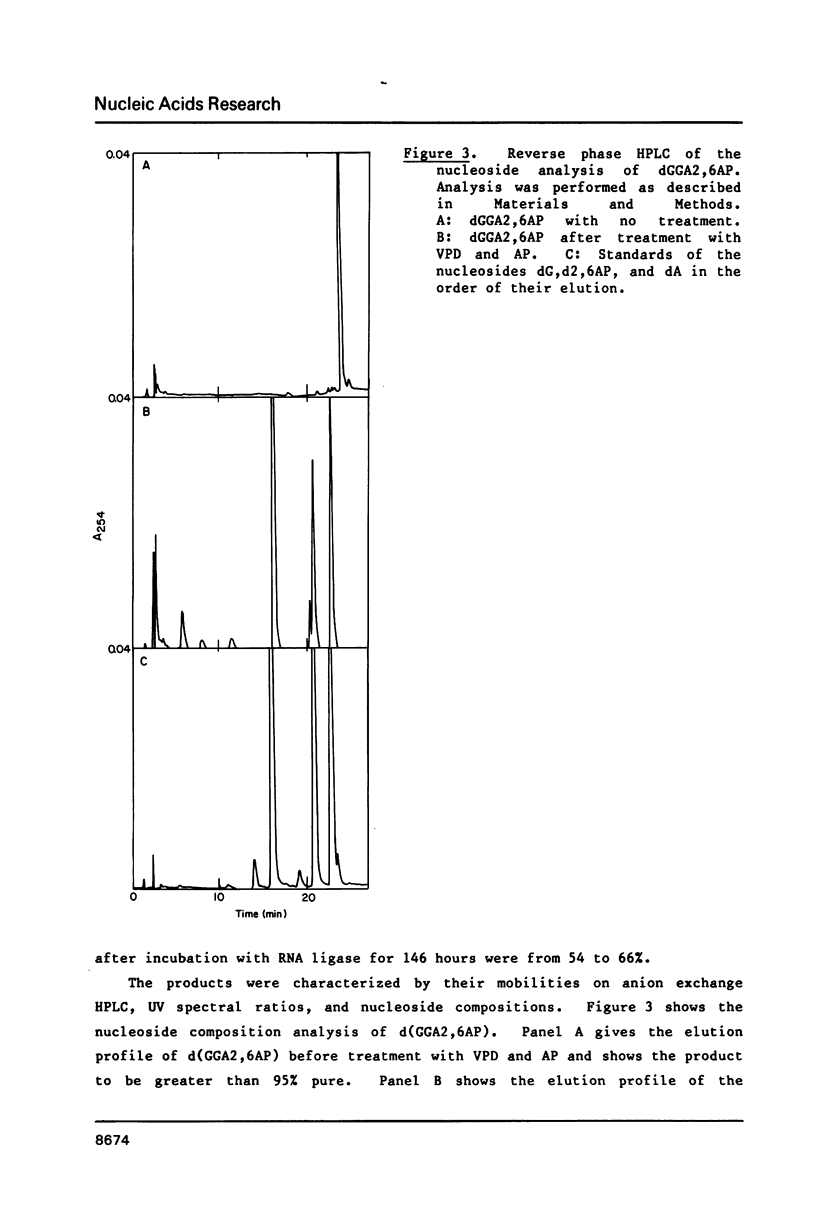
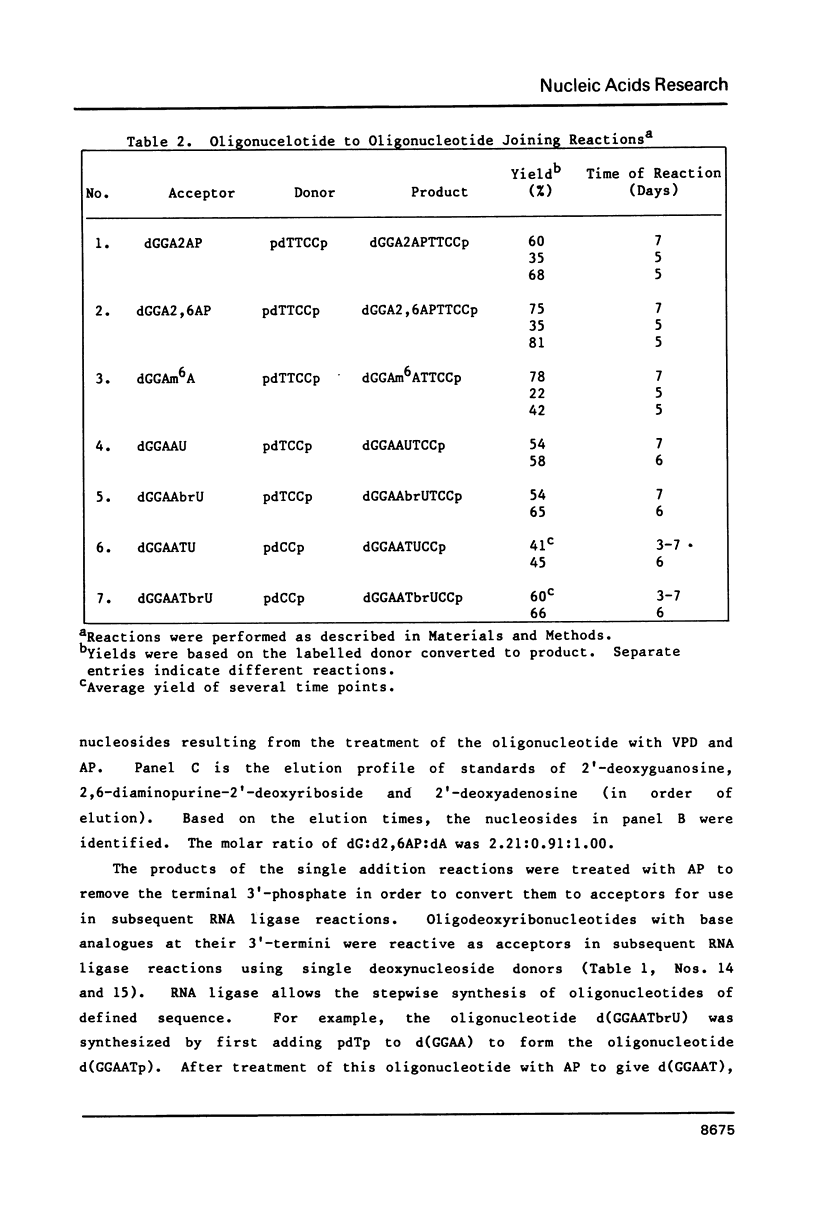
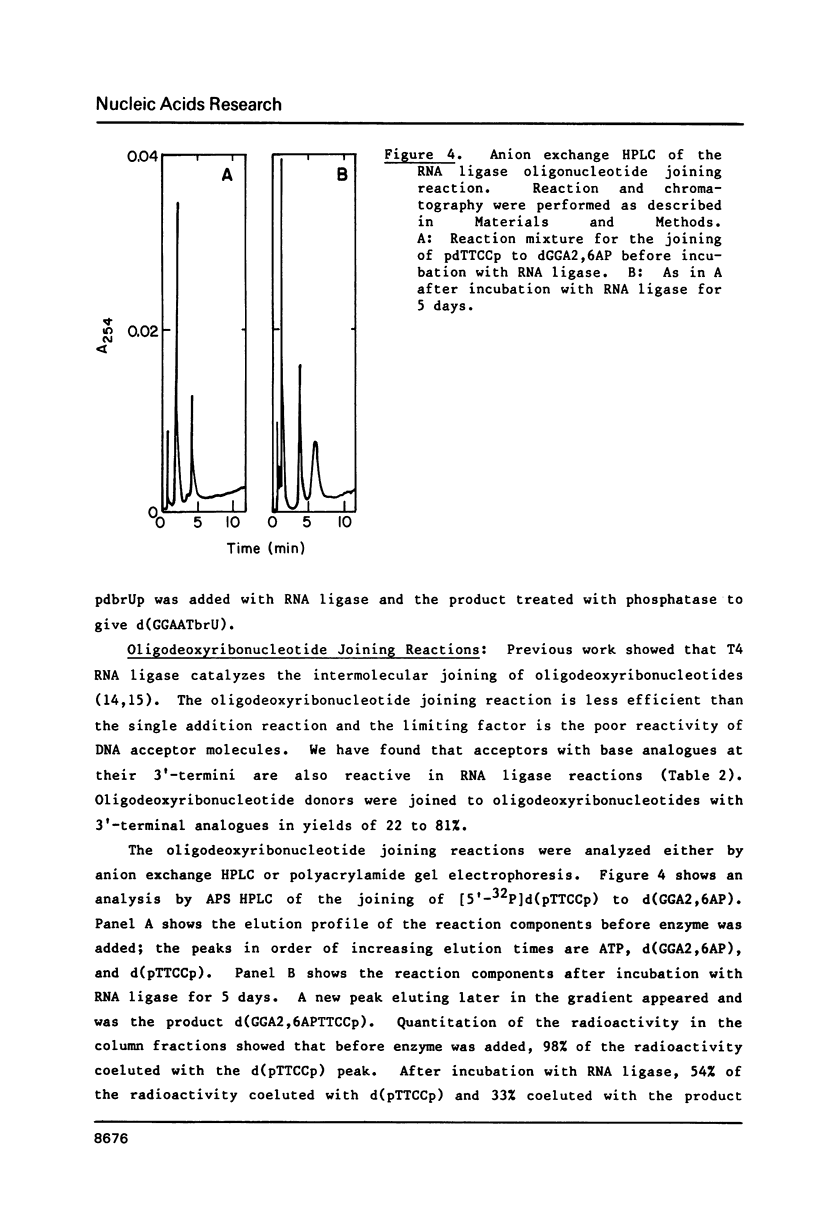
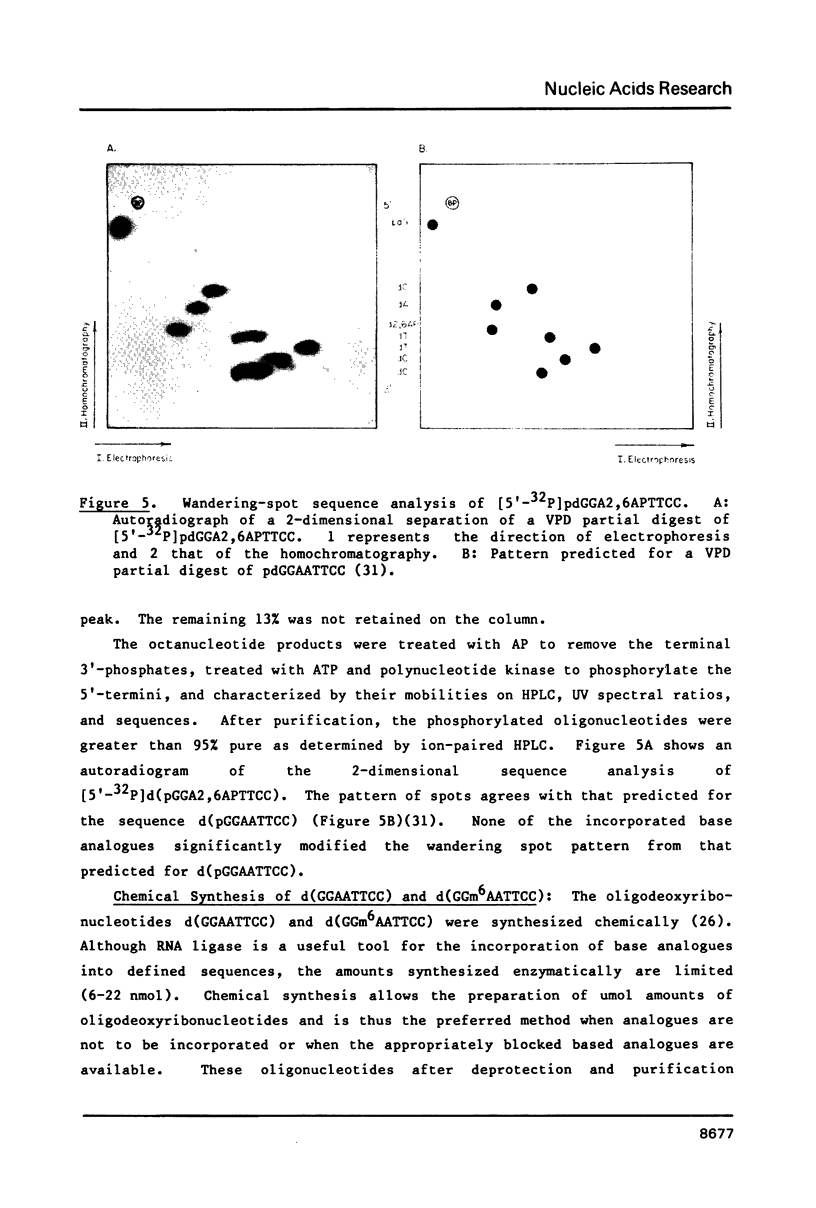
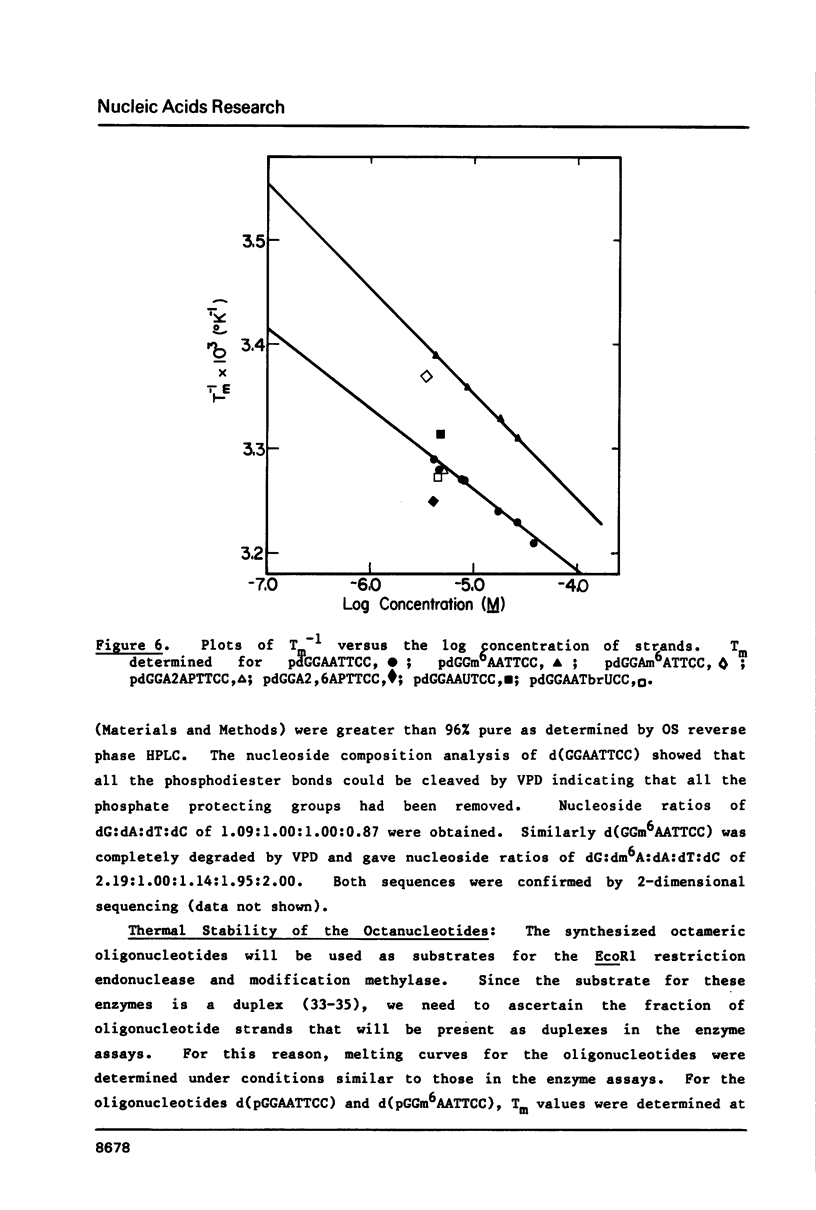
Self-complementary oligodeoxyribonucleotides containing the base analogues 2-aminopurine, 2,6-diaminopurine, N6-methyladenine, uracil, and 5-bromouracil were synthesized by a general method that allows incorporation of the analogues at specific positions. The method uses chemically synthesized partial sequences but circumvents the need for protected base analogues by incorporating their unprotected 3',5'-bisphosphate derivatives enzymatically. T4 RNA ligase was used to add the analogues to the oligodeoxyribonucleotides with yields from 54 to greater than 95 percent. Oligodeoxyribonucleotides were joined to the oligodeoxyribonucleotides containing the analogues at their 3'-termini in yields from 22 to 81 percent. The high yields obtained in these joinings suggest that RNA ligase should be of general use for the specific incorporation of other deoxyribonucleotide analogues into oligodeoxyribonucleotides. The oligodeoxyribonucleotides containing the base analogues were characterized by their mobilities during HPLC, nucleoside compositions, sequences, and thermal stabilities.
Full text
PDF





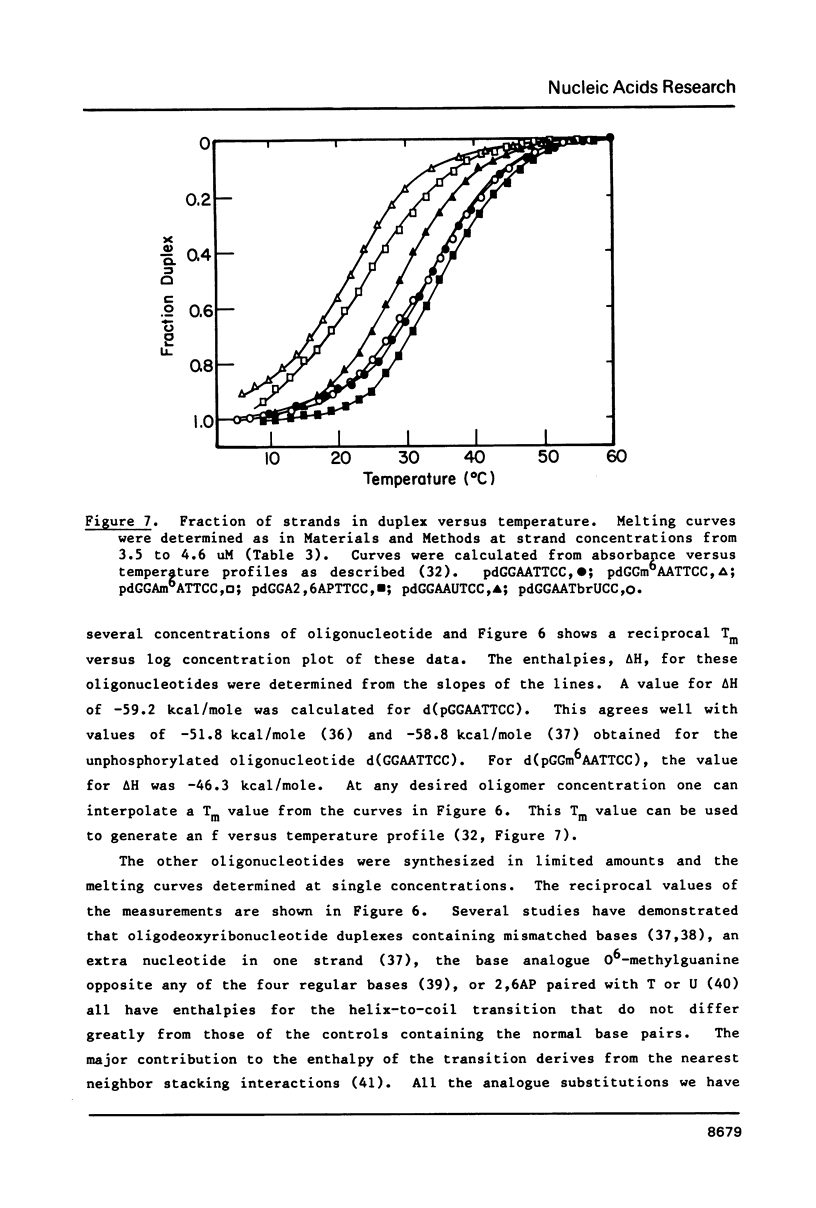
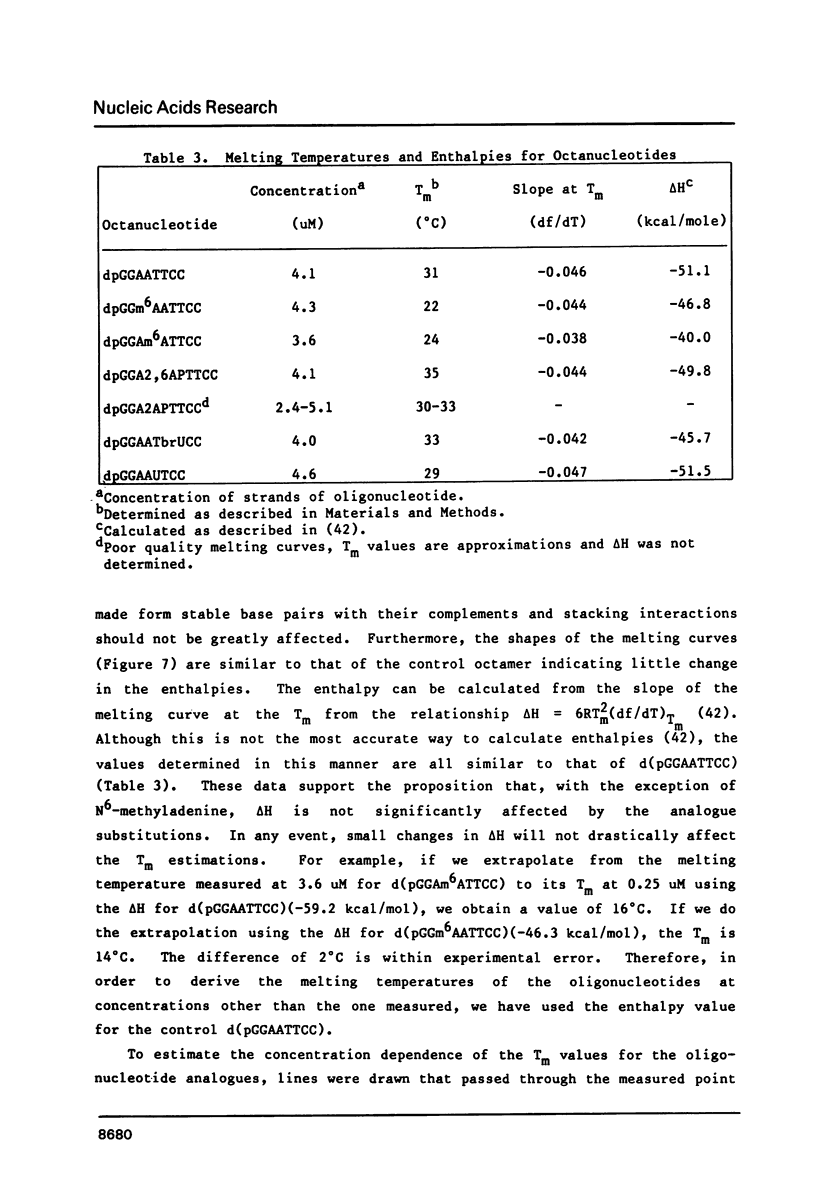




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albergo D. D., Marky L. A., Breslauer K. J., Turner D. H. Thermodynamics of (dG--dC)3 double-helix formation in water and deuterium oxide. Biochemistry. 1981 Mar 17;20(6):1409–1413. doi: 10.1021/bi00509a001. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Barrio M. C., Leonard N. J., England T. E., Uhlenbeck O. C. Synthesis of modified nucleoside 3',5'-bisphosphates and their incorporation into oligoribonucleotides with T4 RNA ligase. Biochemistry. 1978 May 30;17(11):2077–2081. doi: 10.1021/bi00604a009. [DOI] [PubMed] [Google Scholar]
- Bodnar J. W., Zempsky W., Warder D., Bergson C., Ward D. C. Effect of nucleotide analogs on the cleavage of DNA by the restriction enzymes AluI, DdeI, HinfI, RsaI, and TaqI. J Biol Chem. 1983 Dec 25;258(24):15206–15213. [PubMed] [Google Scholar]
- Brennan C. A., Manthey A. E., Gumport R. I. Using T4 RNA ligase with DNA substrates. Methods Enzymol. 1983;100:38–52. doi: 10.1016/0076-6879(83)00044-0. [DOI] [PubMed] [Google Scholar]
- Cameron V., Soltis D., Uhlenbeck O. C. Polynucleotide kinase from a T4 mutant which lacks the 3' phosphatase activity. Nucleic Acids Res. 1978 Mar;5(3):825–833. doi: 10.1093/nar/5.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. C., Herman G., Modrich P. Extent of equilibrium perturbation of the DNA helix upon enzymatic methylation of adenine residues. J Biol Chem. 1985 Jan 10;260(1):191–194. [PubMed] [Google Scholar]
- Dwyer-Hallquist P., Kézdy F. J., Agarwal K. L. Interaction of the HpaI endonuclease with synthetic oligonucleotides. Biochemistry. 1982 Sep 14;21(19):4693–4700. doi: 10.1021/bi00262a027. [DOI] [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations. Studies at the polymer level. J Biol Chem. 1978 Feb 10;253(3):927–934. [PubMed] [Google Scholar]
- Fisher E. F., Caruthers M. H. Studies on gene control regions XII. The functional significance of a lac operator constitutive mutation. Nucleic Acids Res. 1979 Sep 25;7(2):401–416. doi: 10.1093/nar/7.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. Synthesis and characterization of a set of four dodecadeoxyribonucleoside undecaphosphates containing O6-methylguanine opposite adenine, cytosine, guanine, and thymine. Biochemistry. 1984 Nov 20;23(24):5686–5691. doi: 10.1021/bi00319a004. [DOI] [PubMed] [Google Scholar]
- Goppelt M., Pingoud A., Maass G., Mayer H., Köster H., Frank R. The interaction of the EcoRI restriction endonuclease with its substrate. A physico-chemical study employing natural and synthetic oligonucleotides and polynucleotides. Eur J Biochem. 1980 Feb;104(1):101–107. doi: 10.1111/j.1432-1033.1980.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Gough G. R., Collier K. J., Weith H. L., Gilham P. T. The use of barium salts of protected deoxyribonucleoside-3' p-chlorophenyl phosphates for construction of oligonucleotides by the phosphotriester method: high-yield synthesis of dinucleotide blocks. Nucleic Acids Res. 1979 Dec 11;7(7):1955–1964. doi: 10.1093/nar/7.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough G. R., Singleton C. K., Weith H. L., Gilham P. T. Protected deoxyribonucleoside-3' aryl phosphodiesters as key intermediates in polynucleotide synthesis. Construction of an icosanucleotide analogous to the sequence at the ends of Rous sarcoma virus 35S RNA. Nucleic Acids Res. 1979 Apr;6(4):1557–1570. doi: 10.1093/nar/6.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. H., Poonian M. S., Nussbaum A. L., Tobias L., Garfin D. E., Boyer H. W., Goodman H. M. Restriction and modification of a self-complementary octanucleotide containing the EcoRI substrate. J Mol Biol. 1975 Dec 5;99(2):237–261. doi: 10.1016/s0022-2836(75)80143-4. [DOI] [PubMed] [Google Scholar]
- Gumport R. I., Uhlenbeck O. C. T4 RNA ligase as a nucleic acid synthesis and modification reagent. Gene Amplif Anal. 1981;2:313–345. [PubMed] [Google Scholar]
- Hinton D. M., Baez J. A., Gumport R. I. T4 RNA Ligase joins 2'-deoxyribonucleoside 3',5'-bisphosphates to oligodeoxyribonucleotides. Biochemistry. 1978 Nov 28;17(24):5091–5097. doi: 10.1021/bi00617a004. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Brennan C. A., Gumport R. I. The preparative synthesis of oligodeoxyribonucleotides using RNA ligase. Nucleic Acids Res. 1982 Mar 25;10(6):1877–1894. doi: 10.1093/nar/10.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton D. M., Gumport R. I. The synthesis of oligodeoxyribonucleotides using RNA ligase. Nucleic Acids Res. 1979 Sep 25;7(2):453–464. doi: 10.1093/nar/7.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calorimetric determination of base-stacking enthalpies in double-helical DNA molecules. Biopolymers. 1982 Nov;21(11):2185–2194. doi: 10.1002/bip.360211107. [DOI] [PubMed] [Google Scholar]
- McCoy M. I., Gumport R. I. T4 ribonucleic acid ligase joins single-strand oligo(deoxyribonucleotides). Biochemistry. 1980 Feb 19;19(4):635–642. doi: 10.1021/bi00545a005. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. W., Cramer F., Sprinzl M. Rapid analysis of modified tRNAphe from yeast by high-performance liquid chromatography: chromatography of oligonucleotides after RNase T1 digestion on aminopropylsilica and assignment of the fragments based on nucleoside analysis by chromatography on C18-silica. Anal Biochem. 1981 Mar 15;112(1):60–69. doi: 10.1016/0003-2697(81)90260-8. [DOI] [PubMed] [Google Scholar]
- Modrich P., Rubin R. A. Role of the 2-amino group of deoxyguanosine in sequence recognition by EcoRI restriction and modification enzymes. J Biol Chem. 1977 Oct 25;252(20):7273–7278. [PubMed] [Google Scholar]
- Ono A., Sato M., Ohtani Y., Ueda T. Synthesis of deoxyoligonucleotides containing 7-deazaadenine: recognition and cleavage by restriction endonuclease Bgl II and Sau 3AI (nucleosides and nucleotides Part 55). Nucleic Acids Res. 1984 Dec 11;12(23):8939–8949. doi: 10.1093/nar/12.23.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Helix-coil transition of the self-complementary dG-dG-dA-dA-dT-dT-dC-dC duplex. Eur J Biochem. 1979 May 15;96(2):267–276. doi: 10.1111/j.1432-1033.1979.tb13037.x. [DOI] [PubMed] [Google Scholar]
- Riley M., Paul A. Properties of synthetic polydeoxyribonucleotide complexes containing adenine and bromouracil. Biochemistry. 1971 Oct 12;10(21):3819–3825. doi: 10.1021/bi00797a003. [DOI] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. Transcription of T7 DNA containing modified nucleotides by bacteriophage T7 specific RNA polymerase. J Biol Chem. 1978 Jul 25;253(14):4951–4959. [PubMed] [Google Scholar]
- Tibanyenda N., De Bruin S. H., Haasnoot C. A., van der Marel G. A., van Boom J. H., Hilbers C. W. The effect of single base-pair mismatches on the duplex stability of d(T-A-T-T-A-A-T-A-T-C-A-A-G-T-T-G) . d(C-A-A-C-T-T-G-A-T-A-T-T-A-A-T-A). Eur J Biochem. 1984 Feb 15;139(1):19–27. doi: 10.1111/j.1432-1033.1984.tb07970.x. [DOI] [PubMed] [Google Scholar]
- Trip E. M., Smith M. Enzymatic synthesis of oligodeoxyribonucleotides of defined sequence. Polynucleotide phosphorylase catalysed synthesis using pyrimidine analog-containing deoxyribonucleoside 5'-diphosphates. Nucleic Acids Res. 1978 May;5(5):1539–1549. doi: 10.1093/nar/5.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Borer P. N., Dengler B., Tinoco I., Jr Stability of RNA hairpin loops: A 6 -C m -U 6 . J Mol Biol. 1973 Feb 5;73(4):483–496. doi: 10.1016/0022-2836(73)90095-8. [DOI] [PubMed] [Google Scholar]
- Ward D. C., Reich E., Stryer L. Fluorescence studies of nucleotides and polynucleotides. I. Formycin, 2-aminopurine riboside, 2,6-diaminopurine riboside, and their derivatives. J Biol Chem. 1969 Mar 10;244(5):1228–1237. [PubMed] [Google Scholar]
- Winter G., Brownlee G. G. 3'End labelling of RNA with 32P suitable for rapid gel sequencing. Nucleic Acids Res. 1978 Sep;5(9):3129–3139. doi: 10.1093/nar/5.9.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Bahl C. P. Synthetic oligodeoxynucleotides for analyses of DNA structure and function. Prog Nucleic Acid Res Mol Biol. 1978;21:101–141. doi: 10.1016/s0079-6603(08)60268-8. [DOI] [PubMed] [Google Scholar]




