Abstract
Antibody detection is a safely applied method at wide scale in diagnosis of Visceral Leishmaniasis (VL). Towards further advancement of serodiagnosis, rK28 antigen has been recently introduced as a candidate for diagnosis of VL. We evaluated sensitivity and specificity of rK28 antigen in a micro-ELISA format in comparison to rk39 antigen. The test was conducted on 252 parasitologically confirmed VL cases, 103 endemic healthy controls, 95 non endemic healthy controls, 88 other infectious disease and 53 follow-up cases. Of 252 parasitologically confirm VL cases, 251 cases were reported positive by rK28 antigen yielding 99.6% sensitivity (95% CI, 0.97–0.99) which was similar to sensitivity of rK39 ELISA (99.6%) (95% CI, 0.97–0.99). Specificity of rK28 antigen in non-endemic and endemic healthy controls was 100% (95%CI 0.96–1) and 94.17% (95% CI, 0.88–0.97), respectively. In 88 different diseases, specificity was 95.45% (95% CI, 0.84–0.96). With rK39 antigen, specificity of non-endemic and endemic controls, and different diseases was 100% (95%CI 0.96–1) and 92.23% (95% CI 0.85–0.96), and 96.59% (95% CI 0.90–0.98) respectively. Our results show that rK39 and rK28 antigens have similar sensitivity and specificity and rK28 can also be used as a serodiagnostic tool in endemic population of Bihar.
Keywords: Visceral Leishmaniasis, rk-28, serodiagnosis, ELISA
INTRODUCTION
Visceral Leishmaniasis is commonly known as kala-azar in endemic areas of Indian subcontinent, where definitive diagnosis and treatment still needs attention. The demonstration of amastigotes in splenic and/or bone marrow aspirates remains gold standard for accurate and definitive diagnosis of VL. Though this procedure is most specific but its sensitivity widely depends on smear preparation, proper staining and appropriate working of microscope [1]. Spleen aspirates are risky, and therefore require technical expertise. Serodiagnosis based on IFA, Western blot, DAT and ELISA are poorly adapted in field conditions of endemic region. Immunochromatographic tests (ICT) are ready to use and require low maintenance in field settings. In current scenario, rK39 ICT assay is used as a reference standard for the diagnosis of VL but its inability to discriminate between clinical and subclinical infection in endemic population drew our attention towards more specific and sensitive novel antigens. Many other L. donovani specific antigens have been characterized demonstrating variable specificity and sensitivity [2, 3]. rK9, rK26, rKRP42 and other antigens have been largely evaluated on symptomatic VL of endemic population but diagnostic accuracy of rK39 antigen is leader worldwide [4]. The sensitivity of rK26 antigen developed from Leishmania chagasi is only 20–40% in India [5] [6]. K9 antigen which posses 11 copies of a 14-amino-acid repeat in the open reading frame of K26 [5] yields only 78% sensitivity [7]. Important drawbacks of rK39 in any format was its low sensitivity in Africa [8–10], which to some extent has improved with the rapid tests manufactured by DiaMed Cressier, Switzerland and a large proportion (up to 32%) of asymptomatic healthy individuals from endemic area testing positive [11]. Thus to resolve these drawbacks, a new generation fusion antigen rK28 has been design which can perform better [12]. K28 is a recombinant synthetic gene of kinesin region, consisting multiple tandem repeat sequences of the L. donovani haspb1 and k39 kinesin genes to the complete open reading frame of haspb2, increasing antigen epitope density [12]. Though rK28 RDT has shown improved performance in Sudan [12], its evaluation in Indian subcontinent is yet to be done. Thus our aim of this study is to evaluate diagnostic accuracy of novel rK28 antigen in endemic population of Bihar.
MATERIALS AND METHODS
This prospective study was conducted at Department of Medicine, Institute of Medical Sciences, Banaras Hindu University and at its field site at Kala-azar Medical Research Center, Muzaffarpur, Bihar. The study was approved by ethical committee of Institute of Medical Sciences, BHU and KAMRC, Muzaffarpur. Written informed consent was obtained from all subjects.
In this study 591 subjects were enrolled during January 2010 to August 2010, including 252 parasitologically confirmed patients with VL, 103 endemic healthy controls (EHC) selected from VL endemic region of Muzaffarpur, 95 non endemic healthy controls were recruited from Varanasi. All these individuals never had past history Kala-azar. In panel of 88 other diseases, there was Malaria (n=15), Tuberculosis (n=10), amoebic liver abscess (n=17), dengue (n=15), leprosy (n=15) and typhoid (n=16) patients. These subjects were recruited and treated at SS Hospital, Banaras Hindu University, Varanasi. Patients either below the age of 2 years, with past history of kala-azar, positive HIV serology or positive pregnancy tests were excluded. Median age of patients was 25 years. Among the recruited patients, 48% subjects were male and 52% were female.
Serum Sample collection
Serum was collected from patients with VL before the onset of treatment. Serum was separated from 1 ml of blood collected from different groups of controls and confirmed VL patients in cryovials and stored at −20°C. rK39 antigen was used as a comparator in this study.
ELISA
rK28 and rK39 antigens were received as a kind gift from SG Reed, Seattle, USA. ELISA was carried out as described earlier [13]. Briefly, flat-bottom 96-well micro titer plates were coated with 25ng/well (100μl) of rK28 and rK39 antigen in coating buffer and incubated overnight at 4°C. The plates were then blocked with blocking buffer (1% BSA in 0.05 M Phosphate buffer) for 2 hours at room temperature. Plates were loaded with serum sample diluted 1: 400 and incubated at room temperature for 1 hr. The plate were washed five times with PBS containing 0.1% Tween-20 (pH 7.4) and incubated with peroxidase-conjugated goat anti-human IgG (1: 32,000 dilution in serum dilution buffer) at 37°C for 1 hr, the plates were incubated with TMB substrate (GeNeiTM) for 5 minutes at room temperature in dark. Finally the reaction was stopped with 0.1 N H2SO4.
The optical density was measured at 450 nm. Each sample was assayed in duplicate. Serum pools of pretreated VL patients were used as positive control and pooled non endemic controls were used as negative control in each plate.
Statistical analysis
Determination of sample size for the evaluation of new antigen rK28 in endemic population was done by Mc Nemar’s test. The cut off values for anti rK39 antibodies was set as the mean plus 3 standard deviation of the healthy nonendemic controls. The serological data thus obtained were analyzed with EPIInfo (version-6) and SPSS 16 softwares. The generated predicted values were used to construct a receiver-operator characteristic (ROC) curve that plots the true-positive rate against the false positive rate for different potential cutoff points. Data from the repeatability experiment were used to estimate the kappa value for the ELISA method for both the antigens. The kappa coefficient of agreement quantifies reproducibility, correcting for agreement expected by chance. A kappa value of 1 represents perfect agreement.
RESULTS
Pool of sera from VL patients was serially diluted from 100 to 4 × 105 times to find out baseline of reactivity of anti rK39 antibodies and anti rK28 antibody responses with their respective antigens. Optical density at 4 × 105 dilution lies just above the cutoff range of nonendemic healthy controls. 1:400 dilution of serum from a parasitologically confirmed VL patient reacted strongly with rK28 antigens at concentration as low as 25ng/well.
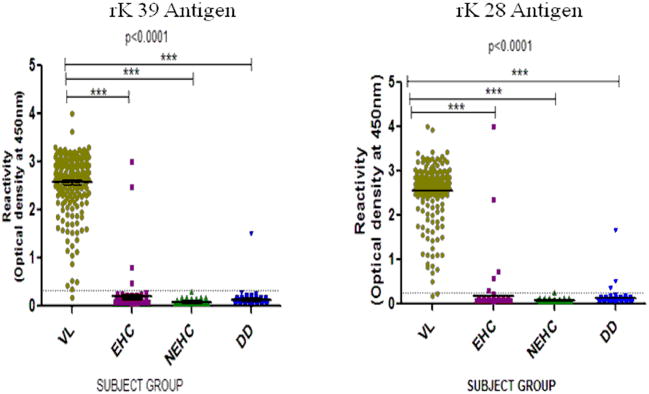
To determine the serodiagnostic performance of rK28 antigen, sensitivity and specificity of rK28 ELISA was compared to sensitivity and specificity of rK39 ELISA. In symptomatic VL cases, mean titer of antibodies against rK28 and rK39 is 31 times higher than the mean titer of nonendemic controls (Figure 1).
Figure 1.
Anti rK39 and anti rK28 antibody levels in serum determined by ELISA in parasitologically confirmed cases (VL) and three groups of controls viz nonendemic healthy (NEHC) , endemic healthy (EHC) and different diseases (DD). Results are expressed as optical density at 450nm. Dotted line represents cut-off values between cases and control groups. Each dot represent an individual subject, bars display median value.
Of 252 VL patients, 251 were positive by rK28 antigen yielding sensitivity of 99.6% which was exactly similar to sensitivity of rK39 ELISA (99.6%) (Table 1). There was excellent agreement between both the assays (kappa 0.97, P<0.001) for serodiagnosis. The data for specificity is given in table 2. The specificity for rk28 was very high in all groups, being 100% in non-endemic controls, 94.17% and 95.45% for endemic controls and different diseases (Table 2). The data was comparable between rK28 and rK39.
Table 1.
Comparative performance of rK39 and rK28 antigens in Visceral Leishmaniasis patients.
| Subjects | rK 39 antigen | rK 28 antigen | Kappa | ||
|---|---|---|---|---|---|
| VL (N=252) | Positive (%) | 95% CI | Positive (%) | 95% CI | |
| 251 (99.6%) | 97.3–99.9 | 251 (99.6%) | 97.3–99.9 | 0.97 | |
Table 2.
Comparative specificity of rK39 and rK28 antigen in different control groups.
| Subjects | rK 39 antigen | rK 28 antigen | Kappa | ||
|---|---|---|---|---|---|
| Negative (%) | 95% CI | Negative(%) | 95% CI | ||
| Endemic healthy (N=103) | 95 (92.2%) | 0.85–0.96 | 97 (94.2%) | 0.87–0.97 | 0.86 |
| Non-endemic healthy(N=95) | 95(100%) | 0.96–1.0 | 95(100%) | 0.96–1.0 | 1 |
| Different diseases ( N= 88) | 85 (96.6%) | 0.90–0.98 | 84 (95.5%) | 0.84–0.96 | 0.86 |
Area under curve of ROC plot by both the antigens was 0.995, which shows the excellent performance and diagnostic accuracy of rK28 and rK39 antigens.
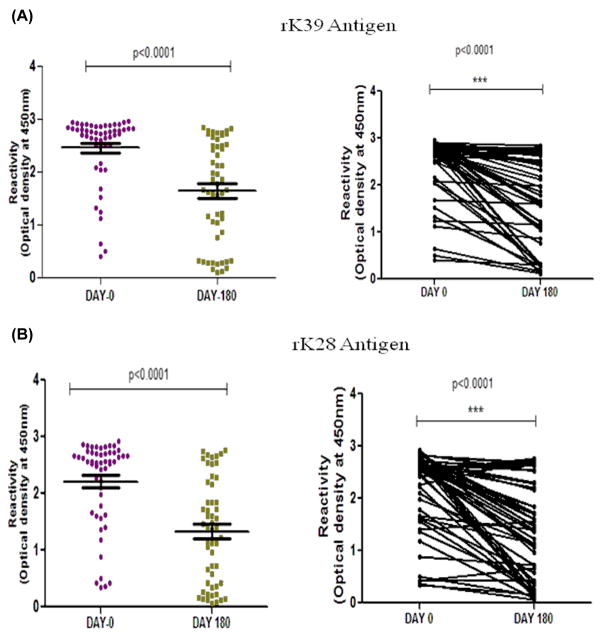
For 53 patients with VL, sera at six month follow up were also available. There was 3.6 and 2.98 folds decline in the titer of antibodies against rK28 and rK39 antigens, respectively (Figure 3). However, no significant decline in titers was observed at day 30 (p > 0.05) in both antigens. 5 out of 8 relapse cases showed same value of antibodies titer persisting till six months of follow-up. Whereas in rest 45 cases, only 4 cases were as such in which there was no significant decrease in antibodies titer.
DISCUSSION
In this study, sensitivity of rK28 antigen was excellent as out of 252 only one was negative in symptomatic VL cases. The only rK28 antigen negative patient was positive with rK39 though the OD was low for this patient. VL sera with border line/low ELISA reactivity against rK39 antigen could be detected with similar accuracy with rK28 antigen. This can be clearly demonstrated with the fact that there was no significant difference between mean optical densities of ELISA against both the antigens in case of VL. Hence sensitivity of rK28 was similar (99.6%) to rK39 antigen. Area under ROC curve also determines excellent diagnostic accuracy of rK28 antigen, which was similar to rK39 antigen. Specificity of rK28 antigens was slightly higher (approximately 2%) than rK39 antigens for healthy subjects of endemic population. In panel of patients with different diseases, all 15 malaria patients were negative and one tuberculosis patient was positive with rK39. The situation was just opposite for rK28 antigen i.e. one was positive in malaria group and all were negative with tuberculosis. None subjects were positive in group of patients suffering from dengue and leprosy, while one individual was positive in typhoid group with both the antigens. In group of amoebic liver abscess one subject was positive with rk 39 and two were positive by rK 28 antigen. Hence, there was only 4.5% cross reactivity with rK28 which was quite similar to rK39 antigen. Overall specificity in control group against both antigen was quite similar (>94%) and agreement between both the assay was also excellent (Kappa 0.86).
In 53 follow up cases, there was similar decrease in antibody level at follow up with both the antigens, though the titers remained high with both antigens. Thus we can conclude that synthetic new generation rK28 antigen shows excellent sensitivity and specificity similar to rK39 antigen in ELISA format, and is an additional tool in the armamentarium of the VL diagnosis.
Figure 2.
Trend of anti rK39 antibody (A) and anti rK28 antibody (B) titer in pretreated (day 0) and post treated (day 180) subjects.( p = < 0.001)
Acknowledgments
This work was supported by National Institute of Allergy and Infectious disease (NIAID), DMID funding mechanism: Tropical Medicine Research Center Grant number: P50AI074321. We thank to Prof R.N. Mishra (Department of Community Medicine ,Institute of Medical Sciences ,Banaras Hindu University ,Varanasi,India) for the help in statistical Analysis.
Footnotes
Conflict of interest: No conflict
References
- 1.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.Maalej IA, Chenik M, Louzir H, et al. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am J Trop Med Hyg. 2003;68:312–20. [PubMed] [Google Scholar]
- 3.Rosati S, Ortoffi M, Profiti M, et al. Prokaryotic expression and antigenic characterization of three recombinant Leishmania antigens for serological diagnosis of canine leishmaniasis. Clin Diagn Lab Immunol. 2003;10:1153. doi: 10.1128/CDLI.10.6.1153-1156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333:723. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia A, Daifalla NS, Jen S, Badaro R, Reed SG, Skeiky YA. Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasi. Mol Biochem Parasitol. 1999;102:249–61. doi: 10.1016/s0166-6851(99)00098-5. [DOI] [PubMed] [Google Scholar]
- 6.Sundar S, Singh RK, Bimal SK, et al. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Trop Med Int Health. 2007;12:284–9. doi: 10.1111/j.1365-3156.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- 7.Mohapatra TM, Singh DP, Sen MR, Bharti K, Sundar S. Compararative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J Infect Dev Ctries. 4:114–7. doi: 10.3855/jidc.544. [DOI] [PubMed] [Google Scholar]
- 8.Zijlstra EE, Nur Y, Desjeux P, Khalil EA, El-Hassan AM, Groen J. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. Trop Med Int Health. 2001;6:108–13. doi: 10.1046/j.1365-3156.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- 9.Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan. Visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95 (Suppl 1):S27–58. doi: 10.1016/s0035-9203(01)90218-4. [DOI] [PubMed] [Google Scholar]
- 10.Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan. Post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95 (Suppl 1):S59–76. doi: 10.1016/s0035-9203(01)90219-6. [DOI] [PubMed] [Google Scholar]
- 11.Sundar S, Maurya R, Singh RK, et al. Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti-K39 antibody. J Clin Microbiol. 2006;44:251–3. doi: 10.1128/JCM.44.1.251-253.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattabhi S, Whittle J, Mohamath R, et al. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis. 4(9):e822. doi: 10.1371/journal.pntd.0000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badaro R, Benson D, Eulalio MC, et al. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–61. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 14.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]