Abstract
Sulfotransferases (SULTs) are important phase II drug-metabolizing enzymes. SULTs regulation by hormones and other endogenous molecules is relatively well understood, while xenobiotic induction of SULTs is not well studied. Caffeine is one of the most widely consumed psychoactive substances. However, SULTs regulation by caffeine has not been reported. In this report, male and female rats were treated with different oral doses of caffeine (2, 10, 50 mg/kg/day) for 7 days. Western blot and real-time RT-PCR were used to investigate the changes of SULT protein and mRNA expression following the caffeine treatment. Caffeine induced both rat aryl sulfotransferase (rSULT1A1, AST-IV) and rat hydroxysteroid sulfotransferase (rSULT2A1, STa) in the liver and intestine of female rats in a dose-dependent manner. Caffeine induction of rSULT1A1 and rSULT2A1 in the female rat intestine was much stronger than that in the liver. Although caffeine induced rSULT1A1 significantly in the male rat liver, it did not significantly induce rSULT2A1. In male rat intestine, caffeine significantly induced rSULT2A1. The different SULTs induction patterns in male and female rats suggest that the regulation of rat SULTs by caffeine may be affected by different hormone secretion patterns and levels. Our results suggest that consumption of caffeine can induce drug metabolizing SULTs in drug detoxification tissues.
Keywords: sulfotransferase, SULT1A1, SULT2A1, caffeine, regulation, induction
Introduction
Sulfotransferases (SULTs) comprise one of the major families of phase II drug-metabolizing enzymes (Glatt, Engelke et al. 2000; Pacifici 2004; Gamage, Barnett et al. 2006; Runge-Morris and Kocarek 2009; Suzuki, Miki et al. 2010). They catalyze the sulfation of hydroxyl-containing compounds. The co-substrate for sulfation for all SULTs is adenosine 3'-phosphate 5'-phosphosulfate (PAPS). The substrate specificities of some SULTs, such as SULT1A1 and SULT2A1, are very broad. Most hydroxyl-containing compounds (phenols and alcohols) are substrates of SULT isoforms. SULTs contribute significantly for xenobiotic detoxification and drug metabolism. Many drugs or their phase I metabolites can be metabolized by SULTs. SULT induction will increase xenobiotics detoxification; however, it can also cause drug resistance.
Sulfation is also widely observed in various biological processes. The biological activities of various biological signaling molecules—including neurotransmitters; hydroxysteroid, glucocorticoid, and thyroid hormones; heparan; peptides; and proteins—can be altered through sulfation. Sulfation usually leads to the inactivation of biological signaling molecules, as the sulfated forms are usually unable to bind to receptors. SULT-catalyzed sulfation regulates the biological activities and functions of many biological signaling molecules. Thus, improper regulation of SULTs can cause these signaling molecules to act improperly and therefore may cause cancer and other diseases. In special cases, sulfation reactions can also lead to the bioactivation of procarcinogens, causing carcinogenic responses.
Caffeine (1,3,7-trimethylxanthine) is the most widely consumed psychoactive substance and is the most commonly used legal stimulant in sports (Morelli, Carta et al. 2010; Prediger 2010; Yang, Palmer et al. 2010). Extensive studies have been done on the effect of caffeine on various biological processes in humans and other mammals, such as brain motor activity, cognitive functions, sleep, and hypertension. In humans, caffeine is a central nervous system (CNS) and metabolic stimulant, having the effect of temporarily warding off drowsiness and restoring alertness. Like alcohol and nicotine, caffeine readily crosses the blood-brain barrier that separates the bloodstream from the interior of the brain. Once in the brain, caffeine's principal mode of action is as an antagonist of adenosine receptors (Shapiro 2007), mainly A1 and A2A receptors. In addition, caffeine is known to be a competitive inhibitor of the enzyme cAMP phosphodiesterase (cAMP-PDE), which converts cyclic AMP (cAMP) in cells to its noncyclic form. Caffeine thus causes cAMP to accumulate in cells (Jones 2008; Chen, Yu et al. 2010), which leads to metabolic and hormone-related responses (Murosaki, Lee et al. 2007; Riddoch, Brown et al. 2007).
SULTs induction by hormones and other endogenous molecules is relatively well studied (Klaassen, Liu et al. 1998; Chapman, Best et al. 2004; Glatt and Meinl 2004). Recent data suggest that SULTs can be induced by xenobiotics, although the mechanisms for xenobiotic induction are less understood (Glatt, Engelke et al. 2000; Coughtrie 2002; Chen, Baker et al. 2005; Runge-Morris and Kocarek 2005; Nimmagadda, Cherala et al. 2006; Chen, Zhang et al. 2007; Chen, Huang et al. 2010). Our previous results indicate that the anti-cancer drug methotrexate (Maiti and Chen 2003; Chen, Baker et al. 2005; Chen, Maiti et al. 2006; Chen, Zhang et al. 2007; Dutta, Maiti et al. 2008) and all-trans-retinoic acids (Maiti, Chen et al. 2005) show induction activities toward various human and rat SULTs. Isoflavones (Chen, Huang et al. 2008; Chen, Huang et al. 2010) and methamphetamine (Zhou, Huang et al. 2010) have also been shown to regulate rat SULTs expression.
To the best of our knowledge, there are no reports on the regulation of SULTs by purified caffeine in vivo. In the present study, we investigated the regulation of rSULT1A1 and rSULT2A1 by caffeine in male and female rat liver and intestine. SULT1A1 and SULT2A1 have broad substrate specificity and wide distribution in tissues. They are important drug-metabolizing enzymes. Liver and intestine are the most important detoxification tissues. It is important to understand how caffeine consumption will affect xenobiotic detoxification and drug metabolism.
Materials and methods
Materials
Caffeine was purchased from Sigma-Aldrich (St. Louis, MO). SDS-polyacrylamide gel electrophoresis reagents were obtained from Bio-Rad (Hercules, CA). Western blot chemiluminescence reagent kits (Super Signal West Pico Stable Peroxide and Super Signal West Pico Luminol/Enhancer solutions) were purchased from Pierce Chemical (Rockford, IL). PVDF membranes used for Western blotting analyses were purchased from Millipore Corporation (Bedford, MA). TRI Reagent for total RNA extraction was purchased from MRC (Cincinnati, OH). M-MLV Reverse Transcriptase was obtained from Promega (Madison, WI); and qPCR MasterMix Plus with SYBR® Green I dNTP was purchased from Eurogentec (San Diego, CA). Antibodies against AST-IV (rSULT1A1) and STa (rSULT2A1) were provided by Dr. Michael W. Duffel (Division of Medicinal and Natural Products Chemistry, College of Pharmacy, the University of Iowa, Iowa City, IA). Protein assay reagent was purchased from Bio-Rad. All other reagents and chemicals were of the highest analytical grade available.
Animals and drug treatments
Male and female Sprague-Dawley rats (Harlan, Indianapolis, IN), 10 to 11 weeks old and 200–300g body weight, were used in this investigation. Rats were housed in a temperature- and humidity-controlled room and supplied with rodent chow and water for at least one week before use. Rats were divided randomly into three groups, with four rats/group. Caffeine was dissolved in sterilized water and administered by gavage at 2, 10, and 50 mg/kg/day for 7 days to the three separate groups of both male and female rats. The control rats received only sterilized water. Rats were sacrificed 24 h after the final drug treatment. Livers were collected, washed with sterile, ice-cold NaCl (0.9%, w/v) solution, and snap frozen. Intestinal lumens were carefully washed with sterile NaCl (0.9%, w/v) solution. Luminal cavities were opened and fat particles and small blood vessels were trimmed out. The mucosal cells from all small intestines were collected by scraping and snap freezing. Samples were stored at −80°C until use.
Cytosol preparation
Both liver and intestine tissues were homogenized in 50 mM Tris buffer containing 0.25 M sucrose, 3 mM β-mercaptoethanol, and 0.02% (v/v) Tween-20, pH 7.4. All homogenates were centrifuged at 100,000g for 1 h at 4°C. Cytosol aliquots were collected and preserved at −80°C for Western blotting analyses.
Western blot analysis
Cytosol protein from liver (10 μg) and intestine (50 μg) was subjected to electrophoresis (Novex, San Diego, CA) on a 12% polyacrylamide gel. After running at 200V, the protein bands were transferred onto PVDF membranes overnight at 35V. Membranes were blocked for 1 h with 5% (w/v) nonfat dry milk in Tris-buffered saline (TBS). For both liver and intestine cytosol proteins, membranes were incubated with either rabbit anti-rat AST-IV (rSULT1A1) or rabbit anti-rat STa (rSULT2A1) (1:1000) diluted in TBST (50 mM Tris [pH 7.5], 150 mM NaCl, and 0.05% [v/v] Tween 20) containing 5% (w/v) nonfat dry milk on a shaker overnight at 4°C. After incubation, all membranes were washed with TBST for 3 × 10 min and incubated in secondary antibody (horseradish peroxidase-conjugated Immuno-Pure goat anti-rabbit IgG; H + L) at a dilution of 1:8000 in the same buffer for at least 1 h. The membranes were washed with TBST for 3 × 10 min. Immunofluorescent bands were developed with 3 ml of substrate containing the same volume each of Super Signal West Pico Luminol Enhancer solution and Super Signal West Pico Stable Peroxidase solution at room temperature for 2 min. Fluorescence images were obtained using a VersaDoc Imaging System 5000MP (Bio-Rad, Hercules, CA). Densitometric quantification of protein bands was obtained using Quantity One 4.6.5 software of the VersaDoc imaging system. All the Western blot experiments were repeated at least two times, representative blot results are shown in the figures.
Quantitative real-time PCR
Total RNA was extracted from liver and intestinal mucosal cells using TRI Reagent from MRC according to the supplier's guidelines. The concentration and purity of the extracted RNA were checked spectrophotometrically by measuring 260/280 absorption ratios. M-MLV Reverse Transcriptase (Promega) with 1 μg of total RNA was used to synthesize cDNA, and 1 μl of reverse-transcribed product served as the template in polymerase chain reactions. Real-time PCR was performed using the qPCR MasterMix Plus with SYBR® Green I kit (Eurogentec), following the manufacturer's instructions. Primers were designed with Primer Express as follows: rActin-F320: 5'-AGGCCCCTCTGAACCCTAAG-3'; rActin-R435: 5'-AGAGGCATACAGGGACAACACA-3'; (GI NM_031144) rSULT1A1-F530: 5'-AGCTGAGACACACTCACCCTGTT-3'; rSULT1A1-R651: 5'-ATCCACAGTCTCCTCGGGTAGA-3'; (GI L19998) rSULT2A1-F496: 5'-ATCCGTGCCTGGCTGTCTAT-3'; rSULT2A1-R642: 5'-GAGGACCAAATCCAGCTCATCT-3'(GI M33329).
Real-time PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Initially, regular PCR products were purified with GENECLEAN Turbo (Qbiogene, Carlsbad, CA) for constructing standard curves (10–108 copies). A standard curve was plotted with the threshold cycle (CT) versus the logarithmic value of the gene copy number. The gene copy number of unknown samples was generated directly from the standard curve by Sequence Detector 1.7 software. At least two duplications were run for each standard or unknown sample. All gene copy numbers were normalized to rat β-actin mRNA.
Data analysis
One-way ANOVA followed by the Dunnett's test was used to calculate the statistical significance of the difference between the control group means and treated group means. In all cases, P<0.05 was considered significant and P<0.01 was considered very significant. Data presented in the figures are means ± standard deviation (SD).
Results
Caffeine induction of rSULT1A1 and rSULT2A1 in rat liver
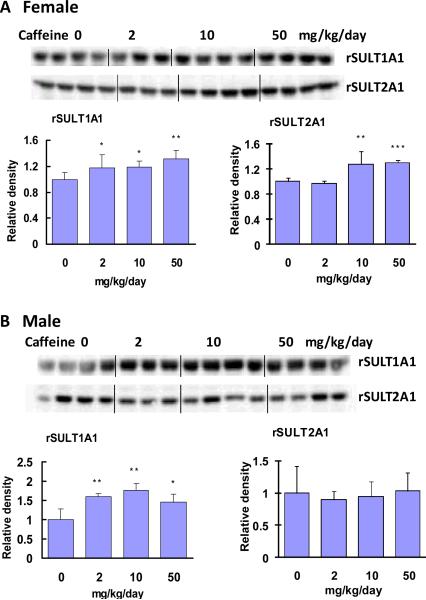
Rat SULT1A1 (rSULT1A1, AST-IV) and rat SULT2A1 (rSULT2A1, STa) protein expression was assessed through Western blot and densitometric analyses (Fig. 1). Caffeine treatment at a dose range of 2 to 50 mg/kg/day significantly up-regulated rSULT1A1 protein expression in both female (Fig. 1A) and male (Fig. 1B) rats. Moreover, rSULT1A1 induction in male rat liver was slightly greater than that in female rat liver. The caffeine induction of rSULT2A1, however, showed a different induction pattern than rSULT1A1. In female rat liver (Fig. 1A), mid- to high-dose caffeine treatment induced rSULT2A1, whereas in male rat liver (Fig. 1B) none of the three doses of caffeine treatments induced rSULT2A1. The levels of rSULT1A1 and rSULT2A1 mRNA induction were consistent with those of protein induction.
Figure. 1.
Representative Western blot and densitometric analyses of rSULT1A1 and rSULT2A1 in the liver of female (A) and male (B) rats treated with varying doses of caffeine for one week. Values were divided by the smallest densitometric value obtained from the blot. The division factors are plotted and expressed as relative densities. *p<0.05; ** p <0.01; and *** p <0.001.
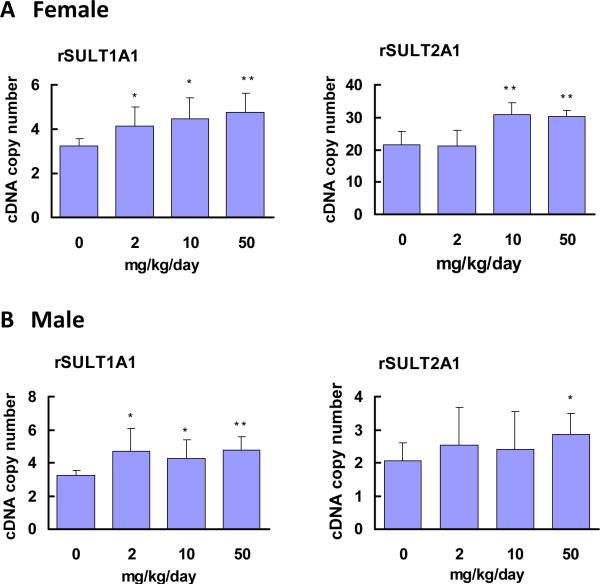
Real-time PCR results (Fig. 2) suggest that caffeine treatment up-regulated rSULT1A1 mRNA expression, both in female (Fig. 2A) and male (Fig. 2B) rat livers. In female rat liver (Fig. 2A), rSULT2A1 mRNA expression was much greater than that in male rat liver, which was consistent with rSULT2A1 protein expression results. In male rat liver, induction was observed only in the group of rats treated with the highest dose of caffeine (Fig. 2B).
Figure. 2.
Expression of rSULT1A1 and rSULT2A1 mRNA in the livers of female (A) and male (B) rats treated with varying doses of caffeine for one week.
Rat β-actin was used as an internal control for real-time PCR. The relative copy numbers of rSULT1A1 and rSULT2A1 mRNA were standardized by using rat β-actin mRNA. Induction fold was calculated by dividing the copy number of rSULT mRNA in caffeine-treated rats by the copy number of the corresponding rSULT mRNA in control rats. The division factors were plotted and expressed as relative densities. *p<0.05 and ** p <0.01.
Caffeine induction of rSULT1A1 and rSULT2A1 in rat intestine
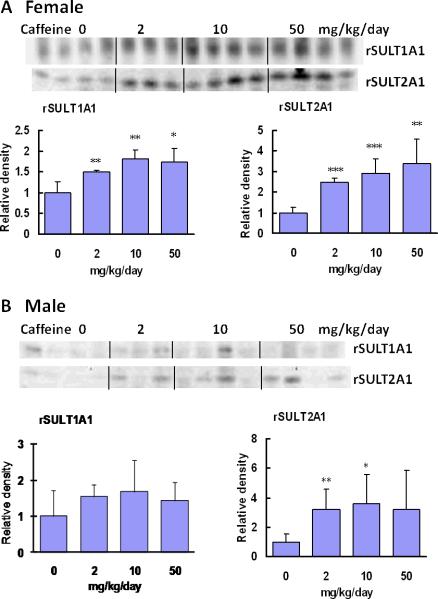
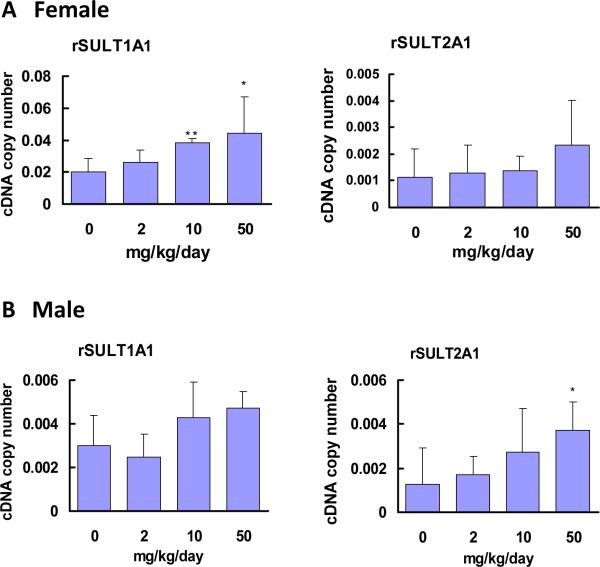
The Western blot and densitometric analyses of intestinal rSULT1A1 and rSULT2A1 revealed that caffeine treatment at a dose range of 2 to 50 mg/kg/day significantly up-regulated rSULT1A1 and rSULT2A1 protein expression in female rat intestine (Fig. 3A). Caffeine induction of rSULT1A1 and rSULT2A1 in female rat intestine was much stronger than that in liver (Fig. 1A). In male rat intestine, caffeine up-regulated rSULT2A1 significantly (Fig. 3B). Although caffeine also increased rSULT1A1, induction was not statistically significant. The real-time PCR results of caffeine induction of rSULT1A1 and rSULT2A1 in rat intestine are in agreement with our Western blot results.
Figure. 3.
Representative Western blot and densitometric analysis of rSULT1A1 and rSULT2A1 in intestinal mucosa cells of female (A) and male (B) rats treated with varying doses of caffeine for one week.
Values were divided by the smallest densitometric value obtained from the blot. The division factors are plotted and expressed as relative densities. *p<0.05; ** p <0.01; and*** p <0.001.
Discussion
Although caffeine is the most widely consumed psychoactive substance in the world, many of its biological effects are not well studied, such as its regulation of drug-metabolizing enzymes. Most studies on caffeine on drug-metabolizing enzymes have been focused on its metabolism by cytochrome P450 isoforms (Kot and Daniel 2008; Kot and Daniel 2008; Kot and Daniel 2008). Caffeine is metabolized in the liver by the cytochrome P450 enzyme system into three metabolic dimethylxanthines: paraxanthine, theobromine, and theophylline. The most important P450 isoform for caffeine metabolism is CYP1A2 (Kotsopoulos, Ghadirian et al. 2007; Kotsopoulos, Vitonis et al. 2009). Caffeine has been used as a popular marker substrate for assessing the activity of CYP1A2, CYP2C, and other isoforms (Kot and Daniel 2008). However, there are very few studies that have examined how caffeine regulates the expression of drug-metabolizing enzymes. One study showed that caffeine treatment increases its own metabolism in a dose-dependent manner and induces CYP1A1/1A2 expression in rat liver and kidney (Goasduff, Dreano et al. 1996). In another study, caffeine was found to induce two cytochrome P450 genes, Cyp6a2 and Cyp6a8, of Drosophila melanogaster in adult flies and in cell culture (Bhaskara, Dean et al. 2006; Bhaskara, Chandrasekharan et al. 2008).
To the best of our knowledge, there are no reports on the regulation of SULTs by purified caffeine. In the present study, we investigated the regulation of rSULT1A1 and rSULT2A1 by caffeine in both the liver and intestine of male and female rats. Caffeine induced both rat aryl sulfotransferase (rSULT1A1, AST-IV) and rat hydroxysteroid sulfotransferase (rSULT2A1, STa) in the liver of female rats at both protein and mRNA levels in a dose-dependent manner. Moreover, Western blot results indicated that in female rats caffeine-mediated induction of intestinal rSULTs was greater than that of liver. For female rats, protein expression of rSULT1A1 was induced 1.2-, 1.2-, and 1.3-fold in liver versus 1.5-, 1.8-, and 1.7-fold in intestine after 2, 10, and 50 mg/kg/day caffeine treatment, respectively. Our results indicate that in female rats intestinal rSULTs (rSULT1A1 and rSULT2A1) are more inducible than liver. This finding agrees with our previous report on methotrexate induction of rSULTs in female rats (Maiti and Chen 2003), which suggested that the gastrointestinal tract, like liver, is an important tissue for toxicant detoxification and drug metabolism. The overall expression level of rSULTs in intestine, however, is much lower than that in liver.
As in female rats, caffeine also induced rSULT1A1 in the liver and intestine of male rats, both at protein and mRNA expression levels. Intestinal induction, however, was not statistically significant. Since the overall level of intestinal rSULT1A1 expression is low, variation among different rats was high. This may be the reason that SULT1A1 intestinal induction results were not statistically significant. The Western blot results showed that, in all three caffeine-treated groups, caffeine did not induce rSULT2A1 protein in male rat liver. The induction of mRNA expression occurred only in the liver of male rats treated with the highest dose of caffeine, suggesting that induction first occurred at the transcriptional level. The expression of liver or intestinal rSULT2A1 both at protein and mRNA levels also varied widely among the different male rats.
In the 1990s, Dr. Curtis Klaassen's research team systematically studied the hormonal regulation and mRNA expression of six isoforms of rat SULTs using male and female rat livers (Liu and Klaassen 1996; Liu and Klaassen 1996; Liu, LeCluyse et al. 1996; Klaassen, Liu et al. 1998). They found that rSULT1A1 expression is not regulated by growth hormone (GH), in male rats rSULT1C1 expression is controlled by the secretory pattern of male growth hormone, and in female rats rSULT1E2 expression is suppressed by the secretory pattern of female growth hormone. Hydroxysteroid SULTs are primarily expressed in adult female rats. Synthetic glucocorticoid hormones, such as dexamethasone (DEX) and pregnenolone-16-alpha-carbonitrile (PCN), can induce some rat hepatic SULT isoforms. These results indicate that one hormone (such as GH) may differentially affect the regulation of different SULT isoforms or any given SULT isoform in different sexes, whereas different hormones may differentially affect any given isoform SULT isoform. This may be underlying the reason for the different induction patterns we have observed in male and female rats.
Caffeine is known to be a competitive inhibitor of the enzyme cAMP-PDE, which converts cAMP in cells to its noncyclic form, thus causing cAMP to accumulate in cells (Colas, James et al. 2008; Crain and Shen 2008). In turn, cAMP accumulation leads to metabolic and hormone-related responses. High doses of caffeine have been shown to activate the hypothalamo-pituitary-adrenocortical (HPA) axis (Patz, Day et al. 2006), which appears to play a significant role in the regulation of individual SULT genes (Runge-Morris 1997). A low caffeine dose of 2 mg/kg significantly increased plasma adrenocorticotropic hormone (ACTH) and corticosterone concentration (Welsch 1994; Welsch and VanderPloeg 1994). Moreover, caffeine was shown to enhance the secretion of parathyroid hormone (PTH) in sheep brain tissue in vitro; and like other xanthine phosphodiesterase inhibitors, caffeine was demonstrated to stimulate growth hormone secretion by directly affecting pituitary cells (Hochberg, Hertz et al. 1984). The molecular mechanisms underlying the caffeine-mediated induction of rSULT1A1 and rSULT2A1 need to be further investigated.
Our results suggest that drug metabolizing SULTs are significantly induced by caffeine in drug detoxification tissues. Caffeine induction of rSULTs is gender-dependent. Consumption of caffeine can enhance xenobiotics detoxification, it can also increase drug metabolism rate, which could cause drug resistance.
Figure. 4.
Expression of rSULT1A1 and rSULT2A1 mRNA in intestinal mucosa cells of female (A) and male (B) rats treated with varying doses of caffeine for one week. Rat β-actin was used as a control for real-time PCR.
The relative copy numbers of rSULT1A1 and rSULT2A1 mRNA were standardized by using rat β-actin mRNA. Induction fold was calculated by dividing the copy number of rSULT mRNA in caffeine-treated rats by the copy number of corresponding rSULT mRNA in control rats. The division factors were plotted and expressed as relative densities. *p<0.05; and ** p <0.01.
Acknowledgment
This work was supported in part by NIH grant GM078606 (G.C.), American Cancer Society grant RSG-07-028-01-CNE (G.C.), USDA grant 2006-35200-17137 (G.C.), and Oklahoma Center for the Advancement of Science and Technology (OCAST) grant HR05-015 (G.C.).
Abbreviations
- SULT
sulfotransferase
- rSULT1A1
rat aryl sulfotransferase IV (AST IV)
- rSULT2A1
rat hydroxysteroid sulfotransferase a (STa)
- PAPS
adenosine 3'-phosphate 5'-phosphosulfate
References
- Bhaskara S, Chandrasekharan MB, et al. Caffeine induction of Cyp6a2 and Cyp6a8 genes of Drosophila melanogaster is modulated by cAMP and D-JUN protein levels. Gene. 2008;415(1–2):49–59. doi: 10.1016/j.gene.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Bhaskara S, Dean ED, et al. Induction of two cytochrome P450 genes, Cyp6a2 and Cyp6a8, of Drosophila melanogaster by caffeine in adult flies and in cell culture. Gene. 2006;377:56–64. doi: 10.1016/j.gene.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Chapman E, Best MD, et al. Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew Chem Int Ed Engl. 2004;43(27):3526–3548. doi: 10.1002/anie.200300631. [DOI] [PubMed] [Google Scholar]
- Chen JF, Yu L, et al. What knock-out animals tell us about the effects of caffeine. J Alzheimers Dis. 2010;20(Suppl 1):S17–24. doi: 10.3233/JAD-2010-1403. [DOI] [PubMed] [Google Scholar]
- Chen X, Baker SM, et al. Methotrexate induction of human sulfotransferases in Hep G2 and Caco-2 cells. J Appl Toxicol. 2005;25:354–360. doi: 10.1002/jat.1071. [DOI] [PubMed] [Google Scholar]
- Chen X, Maiti S, et al. Nuclear receptor interactions in methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1) J Biochem Mol Toxicol. 2006;20(6):309–317. doi: 10.1002/jbt.20149. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang J, et al. Human constitutive androstane receptor mediated methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1) Toxicology. 2007;231(2–3):224–233. doi: 10.1016/j.tox.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang C, et al. Genistein induction of human sulfotransferases in HepG2 and Caco-2 cells. Basic Clin Pharmacol Toxicol. 2008;103(6):553–559. doi: 10.1111/j.1742-7843.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang C, et al. Biochanin A induction of sulfotransferases in rats. J Biochem Mol Toxicol. 2010;24(2):102–114. doi: 10.1002/jbt.20318. [DOI] [PubMed] [Google Scholar]
- Colas C, James P, et al. Cyclic-AMP initiates protein tyrosine phosphorylation independent of cholesterol efflux during ram sperm capacitation. Reprod Fertil Dev. 2008;20(6):649–658. doi: 10.1071/rd08023. [DOI] [PubMed] [Google Scholar]
- Coughtrie MW. Sulfation through the looking glass--recent advances in sulfotransferase research for the curious. Pharmacogenomics J. 2002;2(5):297–308. doi: 10.1038/sj.tpj.6500117. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Low doses of cyclic AMP-phosphodiesterase inhibitors rapidly evoke opioid receptor-mediated thermal hyperalgesia in naive mice which is converted to prominent analgesia by cotreatment with ultra-low-dose naltrexone. Brain Research. 2008;1231:16–24. doi: 10.1016/j.brainres.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Dutta SM, Maiti S, et al. Effect of folic acid on methotrexate induction of sulfotransferases in rats. Drug Metab Lett. 2008;2(2):115–119. doi: 10.2174/187231208784040997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage N, Barnett A, et al. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci. 2006;90(1):5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- Glatt H, Engelke CE, et al. Sulfotransferases: genetics and role in toxicology. Toxicology Letters. 2000;112–113:341–348. doi: 10.1016/s0378-4274(99)00214-3. [DOI] [PubMed] [Google Scholar]
- Glatt H, Meinl W. Pharmacogenetics of soluble sulfotransferases (SULTs) Naunyn Schmiedebergs Arch Pharmacol. 2004;369(1):55–68. doi: 10.1007/s00210-003-0826-0. [DOI] [PubMed] [Google Scholar]
- Goasduff T, Dreano Y, et al. Induction of liver and kidney CYP1A1/1A2 by caffeine in rat. Biochem Pharmacol. 1996;52(12):1915–1919. doi: 10.1016/s0006-2952(96)00522-9. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Hertz P, et al. Caffeine stimulates growth hormone secretion by cultured rat pituitary cells. J Endocrinol Invest. 1984;7(1):59–60. doi: 10.1007/BF03348378. [DOI] [PubMed] [Google Scholar]
- Jones G. Caffeine and other sympathomimetic stimulants: modes of action and effects on sports performance. Essays Biochem. 2008;44:109–123. doi: 10.1042/BSE0440109. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu L, et al. Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chemico-Biological Interactions. 1998;109(1–3):299–313. doi: 10.1016/s0009-2797(97)00141-5. [DOI] [PubMed] [Google Scholar]
- Kot M, Daniel WA. Caffeine as a marker substrate for testing cytochrome P450 activity in human and rat. Pharmacol Rep. 2008;60(6):789–797. [PubMed] [Google Scholar]
- Kot M, Daniel WA. The relative contribution of human cytochrome P450 isoforms to the four caffeine oxidation pathways: an in vitro comparative study with cDNA-expressed P450s including CYP2C isoforms. Biochem Pharmacol. 2008;76(4):543–551. doi: 10.1016/j.bcp.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Kot M, Daniel WA. Relative contribution of rat cytochrome P450 isoforms to the metabolism of caffeine: the pathway and concentration dependence. Biochem Pharmacol. 2008;75(7):1538–1549. doi: 10.1016/j.bcp.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos J, Ghadirian P, et al. The CYP1A2 genotype modifies the association between coffee consumption and breast cancer risk among BRCA1 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2007;16(5):912–916. doi: 10.1158/1055-9965.EPI-06-1074. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos J, Vitonis AF, et al. Coffee intake, variants in genes involved in caffeine metabolism, and the risk of epithelial ovarian cancer. Cancer Causes & Control. 2009;20(3):335–344. doi: 10.1007/s10552-008-9247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Klaassen CD. Ontogeny and hormonal basis of male-dominant rat hepatic sulfotransferases. Molecular Pharmacology. 1996;50(3):565–572. [PubMed] [Google Scholar]
- Liu L, Klaassen CD. Regulation of hepatic sulfotransferases by steroidal chemicals in rats. Drug Metabolism & Disposition. 1996;24(8):854–858. [PubMed] [Google Scholar]
- Liu L, LeCluyse EL, et al. Sulfotransferase gene expression in primary cultures of rat hepatocytes. Biochemical Pharmacology. 1996;52(10):1621–1630. doi: 10.1016/s0006-2952(96)00569-2. [DOI] [PubMed] [Google Scholar]
- Maiti S, Chen G. Methotrexate is a novel inducer of rat liver and intestinal sulfotransferases. Arch Biochem Biophys. 2003;418(2):161–168. doi: 10.1016/j.abb.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Maiti S, Chen X, et al. All-trans retinoic acid induction of sulfotransferases. Basic Clin Pharmacol Toxicol. 2005;96(1):44–53. doi: 10.1111/j.1742-7843.2005.pto960107.x. [DOI] [PubMed] [Google Scholar]
- Morelli M, Carta AR, et al. Pathophysiological roles for purines: adenosine, caffeine and urate. Progress in Brain Research. 2010;183:183–208. doi: 10.1016/S0079-6123(10)83010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murosaki S, Lee TR, et al. A combination of caffeine, arginine, soy isoflavones, and L-carnitine enhances both lipolysis and fatty acid oxidation in 3T3-L1 and HepG2 cells in vitro and in KK mice in vivo. J Nutr. 2007;137(10):2252–2257. doi: 10.1093/jn/137.10.2252. [DOI] [PubMed] [Google Scholar]
- Nimmagadda D, Cherala G, et al. Cytosolic sulfotransferases. Indian J Exp Biol. 2006;44(3):171–182. [PubMed] [Google Scholar]
- Pacifici GM. Inhibition of human liver and duodenum sulfotransferases by drugs and dietary chemicals: a review of the literature. Int J Clin Pharmacol Ther. 2004;42(9):488–495. doi: 10.5414/cpp42488. [DOI] [PubMed] [Google Scholar]
- Patz MD, Day HE, et al. Modulation of the hypothalamo-pituitary-adrenocortical axis by caffeine. Psychoneuroendocrinology. 2006;31(4):493–500. doi: 10.1016/j.psyneuen.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD. Effects of caffeine in Parkinson's disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis. 2010;20(Suppl 1):S205–220. doi: 10.3233/JAD-2010-091459. [DOI] [PubMed] [Google Scholar]
- Riddoch FC, Brown AM, et al. Changes in functional properties of the caffeine-sensitive Ca2+ store during differentiation of human SH-SY5Y neuroblastoma cells. Cell Calcium. 2007;41(3):195–206. doi: 10.1016/j.ceca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek TA. Regulation of sulfotransferases by xenobiotic receptors. Curr Drug Metab. 2005;6(4):299–307. doi: 10.2174/1389200054633871. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek TA. Regulation of Sulfotransferase and UDP-Glucuronosyltransferase Gene Expression by the PPARs. PPAR Res. 2009;2009:728941. doi: 10.1155/2009/728941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge-Morris MA. Regulation of expression of the rodent cytosolic sulfotransferases. FASEB Journal. 1997;11(2):109–117. doi: 10.1096/fasebj.11.2.9039952. [DOI] [PubMed] [Google Scholar]
- Shapiro RE. Caffeine and headaches. Neurol Sci. 2007;28(Suppl 2):S179–183. doi: 10.1007/s10072-007-0773-5. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miki Y, et al. Steroid sulfatase and estrogen sulfotransferase in human carcinomas. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Welsch CW. Caffeine and the development of the normal and neoplastic mammary gland. Proceedings of the Society for Experimental Biology and Medicine. 1994;207(1):1–12. doi: 10.3181/00379727-207-43782a. [DOI] [PubMed] [Google Scholar]
- Welsch CW, VanderPloeg LC. Enhancement by caffeine of mammary gland lobulo-alveolar development in mice: a function of increased corticosterone. Journal of Environmental Pathology, Toxicology and Oncology. 1994;13(2):81–88. [PubMed] [Google Scholar]
- Yang A, Palmer AA, et al. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology. 2010;211(3):245–257. doi: 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou TY, Huang CQ, et al. Methamphetamine Regulation of Sulfotransferases in Rat Liver and Brain. American Journal of Pharmacology and Toxicology. 2010;5(3):125–132. [Google Scholar]