Abstract
Background
The benefit of adding radiation therapy after excision of ductal carcinoma in situ (DCIS) is widely debated. Randomized clinical trials are underpowered to delineate long-term outcomes following radiation.
Methods
We constructed a Markov decision model to simulate the clinical course of DCIS in a 60 year-old woman treated with either of two breast-conserving strategies: excision alone or excision plus radiation therapy. Sensitivity analyses were used to study the influence of risk of local recurrence, likelihood of invasive disease at recurrence, surgical choice at recurrence, and patient age at diagnosis on treatment outcomes.
Results
The addition of radiation therapy was associated with slight improvements in invasive disease-free and overall survival. However radiation therapy decreased the chance of having both breasts intact over a patient’s lifetime. Radiation therapy improved survival by 2.1 months for women diagnosed with DCIS at age 60 but decreased the chance of having both breasts by 8.6% relative to excision alone. The differences in outcomes between the treatment strategies became smaller with increasing age at diagnosis. Sensitivity analyses revealed greater benefit for radiation with increased likelihood of invasive recurrence. The decrement in breast preservation with radiation therapy was mitigated with increased likelihood of mastectomy at time of recurrence or new breast cancer diagnosis.
Conclusion
Our analysis quantifies the benefits of radiation following excision of DCIS but also reveals that radiation therapy may increase the likelihood of eventual mastectomy. Patient age and preferences should therefore be considered when making the decision to add or forgo radiation for DCIS.
Keywords: breast cancer, ductal carcinoma in situ, radiation therapy, decision analysis
Introduction
With increasing adoption of mammography, the incidence of ductal carcinoma in situ (DCIS) has risen dramatically. DCIS currently represents up to one-third of the malignancies identified by mammograms1, 2 and over one-quarter of new breast cancer diagnoses.3, 4 While mastectomy was once the standard surgical procedure for treatment of DCIS, the majority of newly-diagnosed patients now receive breast-conserving surgery.3
The need for radiation therapy after breast-conserving surgery for DCIS is widely debated. Randomized trials studying the effect of adding radiation therapy for DCIS have demonstrated a reduction in local recurrence with radiation.5-9 However, some analyses have demonstrated low rates of recurrence with omission of radiation therapy in carefully selected patients,10, 11 while others have shown persistent high rates of local recurrence even among highly selected patients.12, 13
Differences in the perceived risk of recurrence without radiation therapy may contribute to the significant heterogeneity in management of DCIS. Population-based analyses show that among patients who receive breast-conserving surgery for DCIS, about half do not receive radiation, with significant regional variation in its use.2, 14, 15 The prevalence of DCIS and the marked variability in its patterns of care may explain the placement of DCIS management among the highest-priority topics in the Institute of Medicine’s list of areas in need of comparative effectiveness research.16
Radiation reduces the risk of local recurrence to about one-half of that without radiation therapy.5-9 When DCIS recurs, half of the recurrences are still DCIS but the other half are invasive breast cancers.5-8 Despite a reduction in invasive cancer recurrence, randomized trials fail to show a difference in survival between treatment arms.5-9 However, even in aggregate, these trials are underpowered to detect small differences in survival. In the invasive breast cancer setting, a meta-analysis of radiation after breast-conserving surgery did not show a survival advantage until 10-15 years after randomization.17 A survival benefit from radiation therapy for DCIS is likely to be even more difficult to detect, as the reduction in local recurrence with radiation is smaller than that for invasive disease, and only about half of recurrences represent invasive disease.
The decision about whether to add radiation therapy after excision for DCIS is not straightforward. Radiation is generally delivered to the whole breast and requires a commitment to daily treatments for 6 weeks. Although fairly well-tolerated, radiation therapy exposes patients to potential transient side-effects including fatigue and skin toxicity and a very slightly increased risk of secondary malignancies.17 Importantly, the use of radiation usually commits a patient to mastectomy should a local recurrence or new cancer develop in the same breast. Full dose radiation can be only given once to a single breast due to limits of normal tissue tolerance. On the other hand, radiation therapy reduces the risk of local recurrence, including invasive local recurrence. Not all local recurrences are amenable to breast-conserving surgery even if radiation had not been previously administered. Moreover, should a patient suffer an invasive local recurrence, there is the potential for spread to the nodes and/or distant metastases, which could ultimately compromise survival.
The decision about whether to add radiation to breast-conserving surgery for DCIS requires a weighing of its risks and benefits. To help clarify the nature of these tradeoffs, we developed a decision analysis to gain insights into the magnitude of benefits and disadvantages of adding radiation therapy after surgery for DCIS, using available data to model the downstream implications of recurrence.
Methods
Decision analytic model
We constructed a Markov decision-analytic model (Figure 1) to simulate the clinical history of 60-year-old women after breast-conserving surgery for DCIS using TreeAge Pro 2008 (TreeAge Software, Williamstown, MA). We separately examined outcomes for women diagnosed at age 45 and 75. This Markov model18 allows subjects to make transitions monthly among health states of being well, having recurrent local disease, having a new breast cancer, being well after diagnosis of a recurrence or new breast cancer, having metastatic cancer, and dying. We created separate states to keep track of the number of previously irradiated and non-irradiated breasts. The model compared the amount of time in the states after treatment with excision alone versus excision plus radiation therapy and was run until all subjects had died. Outcomes studied were: invasive disease-free survival; life expectancy; and probability of having both breasts intact over a lifetime (i.e., not having either unilateral or bilateral mastectomy). The impact of radiation therapy was determined by reducing the local-recurrence risk compared to management without radiation.
Figure 1. Simplified diagram of the Markov decision analysis model.
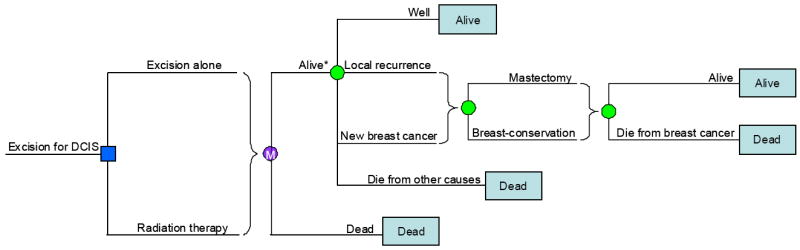
The square on the left is a decision node, representing the choice between excision alone and excision followed by radiation therapy. The brace signifies a subtree that occurs for all branches of the decision tree leading to that brace. A simulated patient enters the Markov process after each treatment choice where, if alive, the green circles are chance nodes, representing the various possible outcomes: remaining well without disease; having a local recurrence; having a new breast cancer; or dying from other causes. If a local recurrence or new breast cancer is diagnosed, another chance node is encountered representing treatment with mastectomy or breast-conserving therapy. Local recurrence or new breast cancer changes the chances of dying from breast cancer. The Markov cycle represents a single month during which a health state may change. At the end of each branch is a terminal node that describes the health state in which patients will begin the next 1-month cycle. The asterisk indicates that the actual model has separate “alive” states to keep track of the number of intact breasts and number of previously irradiated breasts. The probability estimates from the literature in Table 1 determine the likelihood of chance events. Although not indicated in this figure for simplicity, a women remains as risk of a new breast cancer for her entire lifetime even after undergoing radiation therapy for a recurrence.
Model assumptions
We assumed that risk of local recurrence was constant for 10 years and zero thereafter, and that the risk reduction with radiation therapy was constant over time. We did not include excess mortality from radiation therapy as newer techniques of delivering radiation in the breast-conservation setting are unlikely to increase mortality.17 We also assumed that DCIS itself without local recurrence carries no risk of breast-cancer mortality. A patient who developed a local recurrence or new primary cancer in a previously irradiated breast was assumed to undergo mastectomy, as is standard practice. Patients not previously treated with radiation could have breast-conserving therapy (excision and radiation) with a probability dependent on the stage of the recurrent or new disease. We assumed that patients simultaneously diagnosed with metastatic disease and local recurrence or new breast cancer would not undergo mastectomy. Once a patient was diagnosed with metastases, she remained in the metastatic-disease health state until death.
All recurrences in the ipsilateral breast within 10 years of diagnosis were assumed to be local recurrences. After 10 years, the ipsilateral breast carried the same risk of new breast cancer as the contralateral breast. A simulated patient was at risk for developing a new breast cancer during her entire lifetime at a rate dependent on the number of breasts. At time of local recurrence or new breast cancer diagnosis, stage of disease determined risk of death from breast cancer over the next 10 years.
Data sources
Table 1 presents the base case data estimates and ranges used in sensitivity analyses.
Table 1.
Model Parameters
| Variable | Baseline Value (range studied) | Reference |
|---|---|---|
| Local recurrence at 10-years without radiation | 0.2495 (0.15-0.35) | 5 |
|
| ||
| Reduction in local recurrence with radiation therapy | 0.46 (0.3-0.7) | 5 |
|
| ||
| Proportion of invasive local recurrences | 0.50 (0.3-0.7) | 5, 6 |
|
| ||
| Stage distribution of invasive local recurrences | 30 | |
| Stage I | 0.61 | |
| Stage II | 0.27 | |
| Stage III | 0.07 | |
| Stage IV | 0.05 | |
|
| ||
| Ten-year breast cancer-specific mortality | 20 | |
| Stage I | 0.08 | |
| Stage II | 0.27 | |
| Stage III | 0.52 | |
| Stage IV | 0.88 | |
|
| ||
| Contralateral-breast cancer/yr | 0.00812 (0.004-0.010) | 19 |
|
| ||
| Proportion of invasive new breast cancer | 0.687 (0.3-0.8) | 5, 19 |
|
| ||
| Stage distribution of invasive new breast cancers | 30 | |
| Stage I | 0.63 | |
| Stage II | 0.29 | |
| Stage III | 0.05 | |
| Stage IV | 0.03 | |
|
| ||
| Proportion mastectomy by Stage | 30 | |
| Stage 0 (noninvasive) | 0.25 | |
| Stage I | 0.28 | |
| Stage II | 0.49 | |
| Stage III | 0.73 | |
Likelihood of local recurrence with and without radiation therapy
The baseline rate of local recurrence and the effect of radiation on that rate were derived from the European Organisation for Research and Treatment of Cancer Trial 10853 that randomized patients with DCIS to radiation therapy or no further treatment after excision.5 This study provides recent randomized data with 10-year follow-up, and therefore techniques of margin assessment and radiation may be more similar to those performed currently. In sensitivity analyses, we assessed the influence of varying the rate of local recurrence, and used a range which included the higher rate observed in the National Surgical Adjuvant Bowel and Breast Project (NSABP) B-17 study.6 We assumed that the rate of local recurrence did not vary with age at diagnosis. The proportion of local recurrences and new contralateral breast cancers with invasive (as opposed to in situ) histology was derived from randomized data of DCIS patients (Table 1).5, 6, 19
Treatment and outcomes following local recurrence
The probability of undergoing mastectomy versus breast-conserving surgery for a local recurrence or new primary cancer in a breast that had not been previously irradiated was a function of the stage at which the new cancer was diagnosed and was derived from treatment patterns among newly-diagnosed women in the Surveillance Epidemiology and End Results (SEER) limited-use database, 1995-2005 (Table 1). The stage distribution of invasive local recurrence after DCIS was specified according to the stage distribution of invasive ipsilateral breast cancer diagnoses among women with a prior diagnosis of DCIS in this database.
Likelihood of and outcomes following new breast cancers in the contralateral breast
The stage distribution of invasive new breast cancers followed the distribution of invasive contralateral breast cancer diagnoses among women with prior DCIS in SEER. The baseline rate of developing a new breast cancer was determined by the rate of contralateral breast cancers reported in the no-tamoxifen arm of the NSABP B-24 study which randomized women undergoing excision and radiation for DCIS to tamoxifen or placebo.19
Breast cancer and non-breast cancer mortality
Stage-specific mortality rates associated with a new invasive breast cancer or local recurrence were taken from a population-based series of patients with newly-diagnosed breast cancer treated in British Columbia from 1989 to 1997.20 The likelihood of dying from other causes as a function of age was derived from 2004 US life tables for women.21
Sensitivity analyses
Variables with significant variation across data sources or those that might meaningfully influence model outcomes were tested with sensitivity analyses. These variables included local recurrence risk, reduction in recurrence with radiation, rate of developing new breast cancer, proportion of local recurrence or new cancer that is invasive, and likelihood of mastectomy upon recurrence or new cancer in a previously non-irradiated breast. These variables were tested over a wide range of values. We studied the effect of simultaneously varying the risk of local recurrence and likelihood of mastectomy upon recurrence or new cancer in a previously non-irradiated breast on lifetime breast preservation. We also examined the effect across age cohorts of simultaneously varying local recurrence risk and the proportion of local recurrence that is invasive disease on survival.
Results
Model results indicate that adding radiation therapy to excision alone increases invasive disease-free and overall survival. On average, for women aged 60 at diagnosis, radiation therapy increases invasive disease-free survival from 233.4 to 245.1 months, a difference of 11.7 months, and overall survival from 277.9 to 280.0 months, a difference of 2.1 months. Younger women experience greater benefits. For 45-year-old women, radiation increases invasive disease-free survival from 340.6 to 359.1 months, a difference of 18.5 months, and overall survival from 426.8 to 430.8 months, a difference of 4.0 months. For 75-year-old women the benefit from radiation therapy is 5.5 months and 0.7 months for invasive disease-free and overall survival, respectively.
However, the addition of radiation therapy decreases the likelihood of having intact breasts or being without mastectomy during a patient’s entire lifetime. For women diagnosed with DCIS at 60, the chance of maintaining both breasts (i.e., avoiding mastectomy) throughout their lifetime is 73.5% with radiation therapy and 82.1% with excision alone, a difference of 8.6%. For women diagnosed at the age of 45, this chance is 63.5% with radiation therapy and 74.8% with excision alone, a difference of 11.3%. For women diagnosed at age 75, the decrement in breast preservation with radiation is smaller at 5.3%.
Sensitivity analyses reveal model results to be consistent over a wide range of values for key variables (Table 2). Survival benefits with radiation therapy increase with greater risk of local recurrence, greater reduction in local recurrence with radiation, and greater proportion of local recurrence and new cancer diagnoses that is invasive disease. The effect of radiation therapy on breast preservation diminishes if the effectiveness of radiation in preventing recurrence increases or the likelihood of undergoing mastectomy at time of recurrence or new cancer diagnosis in a previously non-irradiated breast increases compared to baseline estimates.
Table 2.
One-way sensitivity analyses for addition of radiation therapy in women age 60
| Invasive disease-free survival | Overall survival | Percentage with both breasts during lifetime | |||
|---|---|---|---|---|---|
| Baseline Δ | 11.7 months | 2.1 months | -8.6% | ||
| Variable | Baseline | Range Studied | Δ of Range (months) | Δ of Range (months) | Δ of Range (%) |
| Local recurrence at 10-years | 0.2495 | 0.15 - 0.35 | 7.0 - 16.7 | 1.3 – 3.0 | -7.6 to -9.6 |
| Reduction in local recurrence with RT | 0.46 | 0.30 - 0.70 | 8.0 – 17.4 | 1.4 – 3.1 | -11.6 to -4.1 |
| Proportion of invasive LR | 0.50 | 0.30 - 0.70 | 9.0 - 15.9 | 1.4 - 2.9 | -9.0 to -8.2 |
| Proportion of invasive new cancer | 0.69 | 0.30 - 0.80 | 11.2 - 11.9 | 2.1 - 2.2 | -9.0 to -8.5 |
| Contralateral-breast cancer/yr | 0.008 | 0.004 - 0.010 | 12.1 – 11.6 | 2.1 – 2.2 | -10.0 to -8.1 |
| Proportion mastectomy at recurrence or new cancer if no prior RT | 0.32* | 0.20 – 0.48 | 11.7-11.5 | 2.1 – 2.1 | -11.7 to -4.3 |
RT = radiation therapy
LR = local recurrence
Δ = difference with the addition of radiation therapy
= in baseline analysis, this variable is a function of the stage of recurrence or new diagnosis; on average it is 0.32
Simultaneously varying the risk of local recurrence and the proportion of local recurrence that is invasive disease (Figures 2-3) demonstrates greater benefit from radiation therapy as the risk of invasive recurrence increases, due to either increasing the overall risk of recurrence or the likelihood that a recurrence is invasive. At the highest rates of invasive recurrence we examined, the survival benefit from radiation is 6–8 months for 45-year-old women, 3–4 months for 60-year-old women, and remains under 2 months for 75-year-old women.
Figure 2. Overall survival benefit with radiation therapy by age at diagnosis using baseline estimates, and maximum and minimum benefit estimates.
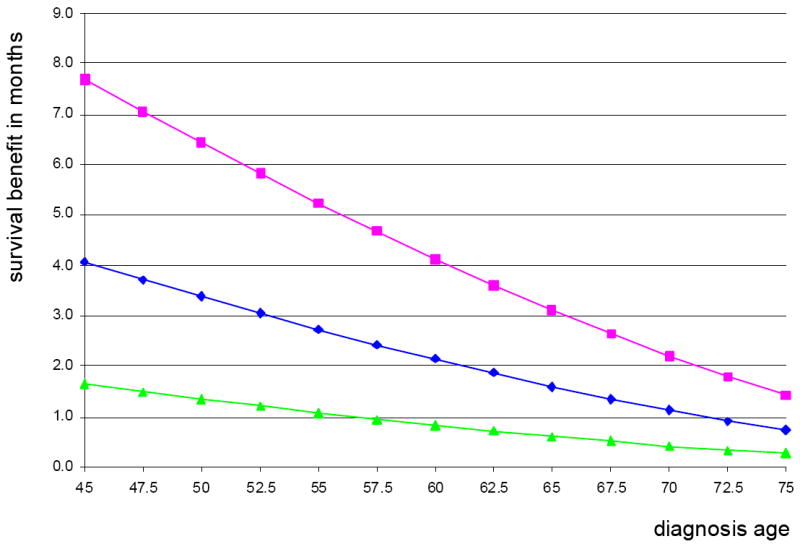
The benefit in survival with radiation therapy is plotted against age at diagnosis. The middle line (
 ) represents the benefit using baseline assumptions (10-year risk of local recurrence without radiation therapy of 0.2495 and 50% of recurrence consisting of noninvasive disease). The top line (
) represents the benefit using baseline assumptions (10-year risk of local recurrence without radiation therapy of 0.2495 and 50% of recurrence consisting of noninvasive disease). The top line (
 ) represents the maximum possible radiation benefit (10-year risk of local recurrence of 0.35 and 70% of recurrence consisting of invasive disease) and the bottom line (
) represents the maximum possible radiation benefit (10-year risk of local recurrence of 0.35 and 70% of recurrence consisting of invasive disease) and the bottom line (
 ) represents the minimum possible radiation benefit (10-year risk of local recurrence of 0.15 and 30% of recurrence consisting of invasive disease).
) represents the minimum possible radiation benefit (10-year risk of local recurrence of 0.15 and 30% of recurrence consisting of invasive disease).
Figure 3. Two-way sensitivity analyses for overall survival benefit with radiation therapy varying risk of local recurrence (LR) without radiation therapy and proportion of LR that is noninvasive disease (DCIS) for 3 age cohorts.
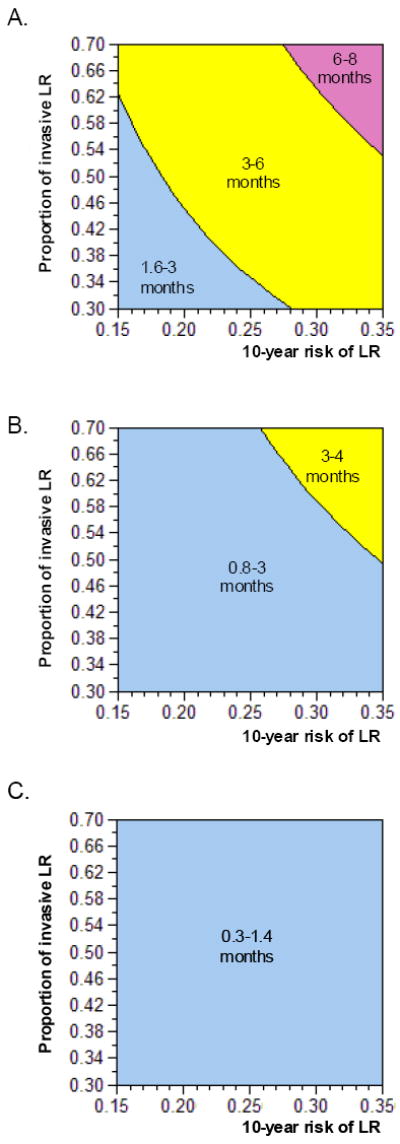
Panels A-C vary the proportion of LR that is invasive disease on the y-axis and the risk of LR at 10-years on the x-axis for 45-year-old (Panel A), 60-year-old (Panel B) and 75-year-old (Panel C) cohorts. In blue are the combinations of values where the survival benefit from radiation therapy is under 3 months, in yellow between 3 and 6 months, and in pink greater than 6 months. As the proportion of LR that is invasive disease and/or the risk of LR without radiation increases, the benefit increases. For the 60-year-old cohort, all possible combinations result in a benefit less than 4 months. For the 75-year-old cohort, all combinations lead to benefits under 2 months.
In a threshold analysis, we found that the radiation therapy strategy decreases the likelihood of lifetime breast preservation unless the chance of mastectomy after recurrence or a new cancer diagnosis exceeds 66% for 60-year old women (Figure 4). Two-way sensitivity analysis revealed that excision alone resulted in the higher likelihood of breast preservation unless the chance of mastectomy after recurrence or a new cancer diagnosis was high (Figure 5). The threshold mastectomy likelihood in a previously non-irradiated breast at which radiation therapy results in greater breast preservation was relatively insensitive to absolute recurrence risk above 0.10 at 10 years (Figure 5).
Figure 4. Sensitivity analysis for the likelihood of mastectomy at time of recurrence or new diagnosis on lifetime breast preservation for 60 year-old women at diagnosis.
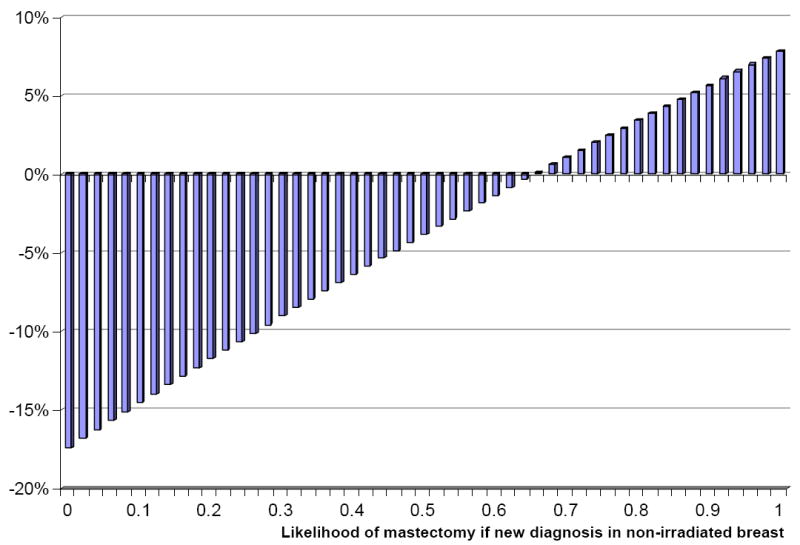
The percentage change in lifetime breast preservation with radiation therapy (y-axis) varies with the likelihood of mastectomy at time of recurrence or new breast cancer (x-axis) in a previously non-irradiated breast. Radiation therapy is associated with a reduction in lifetime breast preservation unless the likelihood of mastectomy at time of recurrence or new breast cancer exceeds 66% (baseline stage-weighted average 32%).
Figure 5. Two-way sensitivity analysis for maximizing lifetime breast preservation varying the likelihood of mastectomy at time of recurrence or new diagnosis and risk of recurrence without radiation therapy for 60 year-old women at diagnosis.
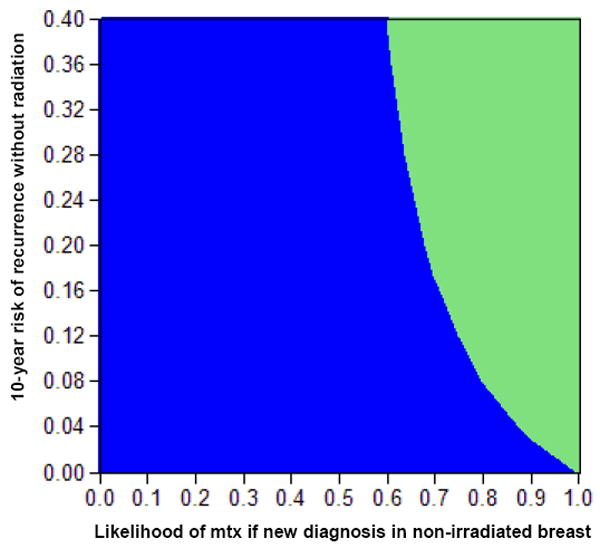
The 2-way sensitivity analysis for lifetime breast preservation demonstrates that the strategy that maximizes lifetime breast preservation varies with absolute likelihood of recurrence on the y-axis and the likelihood of needing mastectomy for a new diagnosis in a previously non-irradiated breast on the x-axis. The area in green indicates those combinations of the 2 likelihoods where radiation therapy maximizes breast preservation, and the area in blue, those where excision alone maximizes breast preservation
Discussion
Clinical trials of radiation therapy for DCIS have not been powered to detect survival differences between the treatment arms and their length of follow up is insufficient to evaluate lifetime breast preservation. Therefore, we used a decision analysis to model the downstream implications of recurrence after DCIS to gain insights into the tradeoffs associated with radiation. Our model suggests that the addition of radiation therapy after excision for DCIS results in modest improvements in invasive disease-free and overall survival, with declining benefits as age at diagnosis increases. However, the likelihood of long-term breast conservation is lower with radiation, since women who receive it are generally committed to mastectomy after any local recurrence or new cancer diagnosis in the treated breast.
In the model, the survival benefit with radiation therapy is a consequence of the reduced risk of an invasive local recurrence, and is a credible finding given the proven effects of radiation on the subsequent development of invasive recurrence, and of invasive cancer recurrence on survival.17 Our analysis shows that the magnitude of this benefit is very small, a finding consistent with the lack of difference observed in the randomized trials that have assessed radiation for DCIS22 and with prior DCIS modeling efforts.23
The key question raised by our results is whether an invasive disease-free and overall survival benefit is sufficient to justify the routine recommendation of radiation therapy for all women with DCIS. An improvement in invasive disease-free survival translates into a lower probability of a new cancer diagnosis, and the resulting need for further surgery and possibly adjuvant chemotherapy. And an overall survival advantage is an even more compelling potential benefit. But these benefits must be weighed against not only the inconvenience and potential side-effects of radiation, but also the reduced probability of maintaining intact breasts over a lifetime. The magnitude of the likely survival advantage is a critical consideration in weighing these risks and benefits. A prior modeling analysis estimated that prophylactic mastectomy among 30 to 60-year-old women without elevated breast cancer risk added approximately 6 months of additional life expectancy, a more substantial benefit than we found in any age group.24 Yet we do not routinely recommend prophylactic mastectomy to women with even markedly elevated breast cancer risk, and certainly not to average risk women. Instead, prophylactic mastectomy is uniformly considered to be a preference-sensitive decision. Our results suggest that the decision about whether to include radiation after excision of DCIS is also most appropriately considered a preference-sensitive decision. Women should be fully informed about the expected outcomes of the alternative treatment strategies, including the risks of both death from breast cancer and eventual mastectomy, and encouraged to choose the strategy that is most consistent with their own values and preferences.
Our analysis has limitations inherent to all modeling analyses. It was necessary to make a limited number of assumptions about the natural history and treatment of disease in order to specify a finite number of clinical states. However, most assumptions we used applied to both treatment arms, excision alone and excision plus radiation therapy, and were therefore unlikely to bias the analysis. We used estimates derived from the literature or databases to inform our baseline analysis, and found model results to be robust across a wide-range of values for key parameters in sensitivity analyses.
No population-based data are available on the proportion of women who choose mastectomy for recurrence or new cancer after prior DCIS treatment that did not include radiation. Therefore, we extrapolated the likelihood of mastectomy by stage from treatment patterns among patients newly diagnosed with cancer in a previously non-irradiated breast (average 32%). This average was close to that reported by a prospective study of excision alone for DCIS where the likelihood of mastectomy at time of recurrence was 31%.12 Our likelihood could actually overestimate the probability of mastectomy, if one of the primary reasons a woman would opt to forego radiation for DCIS is the desire to maximize the likelihood of breast preservation. In our sensitivity analysis, we included the 48% likelihood of mastectomy observed in an early report of treatment patterns for local recurrence in the excision-alone arm of the NSABP B-17 DCIS trial,25 and found that even at this rate, the radiation strategy decreased the likelihood of breast preservation. In fact, even if the probability were much higher than in our baseline analysis, as long is it did not exceed 66%, our sensitivity analysis indicates that the no radiation strategy would result in a higher rate of breast preservation.
However, if local control rates with radiation therapy in the setting of recurrent DCIS after excision alone are inferior to those achieved with new diagnosis of DCIS or if breast-preserving surgery is possible after new diagnosis in a previously irradiated breast,26, 27 the decrement in breast preservation with radiation therapy for DCIS would be mitigated. Moreover, if a patient is ineligible for repeat breast-conserving surgery due to anatomic constraints at time of new diagnosis in a previously non-radiated breast or if she would not elect breast preservation even if it were possible, then the radiation therapy arm would maximize her likelihood of breast preservation. However, our model also indicates, that for those patients who would not have experienced a local recurrence after breast-conserving surgery alone, radiation therapy comes at a cost – they are at risk of having a second primary breast cancer, and their history of prior radiation most often precludes breast conserving options.
We also did not study the effect of adding tamoxifen to radiation therapy.9, 19 However, unless tamoxifen eliminated all recurrences, it would not change the fundamental nature of the tradeoffs we describe. Finally, we did not model outcomes after mastectomy for DCIS, as our objective was to compare alternative breast-conserving surgical strategies. It is easy to extrapolate from our findings however, since mastectomy would result in even lower local recurrence rates, with resulting improvement in invasive-free survival, while reducing the likelihood of breast-preservation to zero.
Currently, about one-half of patients receive radiation after excision for DCIS with evidence of wide regional variation in practice patterns, suggesting the lack of consensus regarding use of radiation therapy.2, 15 Much of the debate surrounding the decision to add radiation for DCIS has focused on the absolute risk of recurrence without radiation. Our results suggest that this decision should be determined by patient preferences for women at average risk of recurrence. Our model also provides some insight into the role of radiation therapy in women whose constellation of clinical and biological factors places them at highest risk of recurrence.22, 28, 29 Our sensitivity analyses reveal that among the highest recurrence-risk group the survival benefits with radiation do indeed increase, while the harms of radiation therapy, in terms of lower rates of long-term breast preservation, remain relatively stable. However, even in this group, the magnitude of the benefit conferred by radiation remains quite modest, suggesting that a decision-making paradigm that emphasizes patient preferences is appropriate in all risk groups.
Our analysis demonstrates that the decision to add radiation therapy is essentially a toss-up, and suggests that more trials with longer follow-up will not change what can be learned from a relatively simple and transparent analysis of existing data. Radiation for DCIS is prophylactic; it reduces the risk of invasive recurrence, the only lethal form of breast cancer, while increasing the probability of eventual mastectomy. The absolute magnitude of both effects is modest, such that personal patient preferences should drive decision-making. Express delineation of the tradeoffs associated with radiation therapy may help guide treatment decisions that are consonant with patient preferences, with the result that variation in care reflects those individual preferences rather than the biases of treating physicians.
Acknowledgments
Funding: This research was supported by a Career Development Award from the National Institutes of Health (1K07 CA118629 to R.S.P.).
Footnotes
Presentation: Abstract at the American Society of Clinical Oncology, Chicago, IL, June 2010
No relevant financial disclosures from any of the authors.
References
- 1.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of Ductal Carcinoma In Situ in Women Undergoing Screening Mammography. Journal of the National Cancer Institute. 2002;94(20):1546–54. doi: 10.1093/jnci/94.20.1546. Available from http://jnci.oxfordjournals.org/content/94/20/1546.abstract. [DOI] [PubMed]
- 2.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–8. doi: 10.1093/jnci/djh069. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15026469. [DOI] [PubMed]
- 3.Sumner WE, 3rd, Koniaris LG, Snell SE, Spector S, Powell J, Avisar E, et al. Results of 23,810 cases of ductal carcinoma-in-situ. Ann Surg Oncol. 2007;14(5):1638–43. doi: 10.1245/s10434-006-9316-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17245612. [DOI] [PubMed]
- 4.American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 5.Bijker N, Meijnen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–7. doi: 10.1200/JCO.2006.06.1366. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16801628. [DOI] [PubMed]
- 6.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–18. doi: 10.1016/s0093-7754(01)90133-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11498833. [DOI] [PubMed]
- 7.Emdin SO, Granstrand B, Ringberg A, Sandelin K, Arnesson LG, Nordgren H, et al. SweDCIS: Radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45(5):536–43. doi: 10.1080/02841860600681569. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16864166. [DOI] [PubMed]
- 8.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. doi: 10.1016/s0140-6736(03)13859-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12867108. [DOI] [PubMed]
- 9.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. The Lancet Oncology. 2010;361(S1470-2045):70266–7. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilleard O, Goodman A, Cooper M, Davies M, Dunn J. The significance of the Van Nuys prognostic index in the management of ductal carcinoma in situ. World J Surg Oncol. 2008;6:61. doi: 10.1186/1477-7819-6-61. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18564426. [DOI] [PMC free article] [PubMed]
- 11.Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186(4):337–43. doi: 10.1016/s0002-9610(03)00265-4. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14553846. [DOI] [PubMed]
- 12.Wong JS, Kaelin CM, Troyan SL, Gadd MA, Gelman R, Lester SC, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24(7):1031–6. doi: 10.1200/JCO.2005.02.9975. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16461781. [DOI] [PubMed]
- 13.MacAusland SG, Hepel JT, Chong FK, Galper SL, Gass JS, Ruthazer R, et al. An attempt to independently verify the utility of the Van Nuys Prognostic Index for ductal carcinoma in situ. Cancer. 2007;110(12):2648–53. doi: 10.1002/cncr.23089. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17960606. [DOI] [PubMed]
- 14.Smith BD, Haffty BG, Buchholz TA, Smith GL, Galusha DH, Bekelman JE, et al. Effectiveness of radiation therapy in older women with ductal carcinoma in situ. J Natl Cancer Inst. 2006;98(18):1302–10. doi: 10.1093/jnci/djj359. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16985249. [DOI] [PubMed]
- 15.Rakovitch E, Pignol JP, Chartier C, Hanna W, Kahn H, Wong J, et al. The management of ductal carcinoma in situ of the breast: a screened population-based analysis. Breast Cancer Res Treat. 2007;101(3):335–47. doi: 10.1007/s10549-006-9302-0. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16838110. [DOI] [PubMed]
- 16.Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 17.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16360786. [DOI] [PubMed]
- 18.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10376613. [DOI] [PubMed]
- 20.Olivotto IA, Truong PT, Speers CH. Staging reclassification affects breast cancer survival. J Clin Oncol. 2003;21(23):4467–8. doi: 10.1200/JCO.2003.99.217. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14645447. [DOI] [PubMed]
- 21.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56(9):1–39. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18274319. [PubMed]
- 22.Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;41:162–77. doi: 10.1093/jncimonographs/lgq039. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20956824. [DOI] [PMC free article] [PubMed]
- 23.Hillner BE, Desch CE, Carlson RW, Smith TJ, Esserman L, Bear HD. Trade-offs between survival and breast preservation for three initial treatments of ductal carcinoma-in-situ of the breast. J Clin Oncol. 1996;14(1):70–7. doi: 10.1200/JCO.1996.14.1.70. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8558224. [DOI] [PubMed]
- 24.Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis--effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336(20):1465–71. doi: 10.1056/NEJM199705153362022. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9148160. [DOI] [PubMed]
- 25.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–52. doi: 10.1200/JCO.1998.16.2.441. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9469327. [DOI] [PubMed]
- 26.Kurtz JM, Amalric R, Brandone H, Ayme Y, Spitalier JM. Results of wide excision for mammary recurrence after breast-conserving therapy. Cancer. 1988;61(10):1969–72. doi: 10.1002/1097-0142(19880515)61:10<1969::aid-cncr2820611006>3.0.co;2-o. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3129175. [DOI] [PubMed]
- 27.Alpert TE, Kuerer HM, Arthur DW, Lannin DR, Haffty BG. Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys. 2005;63(3):845–51. doi: 10.1016/j.ijrobp.2005.02.035. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16199315. [DOI] [PubMed]
- 28.Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 102(9):627–37. doi: 10.1093/jnci/djq101. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20427430. [DOI] [PMC free article] [PubMed]
- 29.Rudloff U, Jacks LM, Goldberg JI, Wynveen CA, Brogi E, Patil S, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28(23):3762–9. doi: 10.1200/JCO.2009.26.8847. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20625132. [DOI] [PubMed]
- 30.National Cancer Institute: Surveillance Epidemiology and End Results (SEER) Http://www.seer.cancer.gov/about/


