Abstract
The nuclear hormone receptor PPARγ has been shown to play an immuno-regulatory role in many immune-related cell types and activation of PPARγ has been reported to be an effective therapeutic approach in murine and human autoimmune disease. However, despite an association between lymphopenia and autoimmunity, there have been no prior studies of the role of T cell PPARγ in lymphopenia-associated autoimmunity. In the present studies we examined the role of PPARγ in CD4+ T cells in two murine models of lymphopenia-associated autoimmunity. Surprisingly, we find that PPARγ expression in CD4+ CD25− T cells (Teff) is actually required for development of autoimmunity under lymphopenic conditions. Mechanistically, the inability of PPARγ-deficient (T-PPAR) Teff to mediate lymphopenic autoimmunity is associated with a significant decrease in accumulation of Teff in the spleen, lymph nodes and tissues after adoptive transfer. This abnormal accumulation of T-PPAR Teff is associated with defects in both in vivo proliferation and survival. Additionally, T-PPAR Teff demonstrate decreased cytokine production in inflammatory sites and decreased expression of the homing receptor α4β7. Finally, these abnormalities in T-PPAR Teff function were not elicited by lymphopenia alone, but also required the additional activation involved in the mediation of autoimmunity. Thus, in contrast to its documented immunosuppressive role, we now identify an unexpected function for PPARγ in Teff, namely a role in Teff proliferation and survival in lymphopenia-associated autoimmunity. These findings highlight both the multifunctional role of PPARγ in T cells and the complexity of PPARγ as a potential therapeutic target in autoimmunity.
Introduction
PPARγ is a ligand-dependent nuclear hormone receptor that was originally described as a regulator of glucose homeostasis and fatty acid metabolism. Ligands for PPARγ, including the thiazolidinedione (TZD)3 class of drugs, are routinely used for the treatment of Type-2 diabetes. In addition to these synthetic ligands, endogenous ligands including fatty acid metabolites and unsaturated fatty acids have been identified (1). We and others have shown that PPARγ is expressed in and mediates important immuno-regulatory functions in conventional T cells, macrophages and dendritic cells (2–6). In conventional T cells, ligation of PPARγ results in down-regulation of IL-2, IFNγ, and IL-4 production (2, 7, 8). Interestingly, a recent report has also suggested that mice with a T cell-specific deletion in PPARγ show enhanced Th17 production in a murine model of autoimmunity (9). In addition, our lab and others have described a critical role for PPARγ in natural regulatory T cell (Treg) suppressive function and in the differentiation and stability of inducible Tregs (10–12).
Consistent with the immuno-regulatory effects of PPARγ, TZDs have been used to effectively treat a number of murine autoimmune and inflammatory models including NOD diabetes, experimental autoimmune encephalomyelitis (EAE), colitis, asthma, and allergic disease (13–18). In humans, TZDs have been shown to be effective in treating Crohn’s disease and Psoriasis, and initial studies in multiple sclerosis have suggested a beneficial effect in secondary progressive disease (19–22). In addition to the effects of TZDs on the suppression of immune responses, it has been demonstrated that PPARγ activation can influence immune cell survival. However, the role of PPARγ in T cell survival is controversial.
There is a strong associative link between lymphopenic proliferation and autoimmunity (23). Development of autoimmunity in multiple mouse models and some human diseases is associated with either a transient or sustained period of lymphopenic proliferation prior to development of autoimmune inflammation. Sjogren’s disease, Rheumatoid Arthritis, Celiac disease, and Crohn’s disease have all been associated with decreased peripheral blood lymphocytes prior to development or exacerbation of disease (23). Paradoxically, the lympho-depletion of CD4+ T cells by HIV has been associated with increased autoimmunity, including systemic lupus erythematosus, anti-phospholipid syndrome, vasculitis, and Graves' disease (24). Recovery from lymphopenia following highly active retroviral therapy (HAART) for HIV, or T cell depletion with Campath-1h in Multiple Sclerosis patients has been associated with increased autoimmunity, particularly Graves’ disease (24, 25). While prior studies have documented the immuno-regulatory role of T cell PPARγ in non-lymphopenic autoimmune models, in the present study we set out to test the relevance of T cell PPARγ-mediated immuno-regulation in lymphopenia-associated autoimmunity.
Surprisingly, we now find an unexpected role for T cell PPARγ in lymphopenic autoimmune models. In contrast to its well described function in down-regulating autoimmunity and enhancing apoptosis, we now find that in the absence of PPARγ, CD4+ T cells are unable to mediate lymphopenia-associated autoimmune disease.
Materials and Methods
Mice
RAG-1−/− and Bm12 mice were purchased from Jackson laboratories (Bar Harbor, ME). T cell-specific PPARγ-deficient (“T-PPAR”) mice were generated in our lab as previously described (11). In brief, these mice were generated by crossing mice expressing CRE recombinase behind the CD4 promoter with a mouse expressing a floxed PPARγ gene locus to generate the T-PPAR mice. As both parental strains were previously on a C57BL/6 background, this cross maintained the C57BL/6 lineage. As controls in all experiments, T-PPARfl/fl CREneg “littermate control” mice were used. T-PPAR mice show no observable clinical phenotype by 4 months of age and have normal frequencies and numbers of all splenic populations (11). All experiments were performed using either male or female mice that were less than 2 months old. All animals were bred and cared for according to the University of Connecticut Center for Animal Care regulations (Farmington, CT. USA).
Purification of CD4+CD25− effector T cells for adoptive transfer
For all adoptive transfer studies, CD4+CD25− effector T cells were purified from T-PPAR or Littermate Spleens using a Miltenyi Treg purification kit (Miltenyi Biotec). Briefly, CD4+ T cells are purified by magnetic-bead negative selection of CD8, CD11b, B220, DX5, and Ter-119 cells followed by a second negative selection column against CD25. The number of viable CD4+CD25− T cells was determined by trypan blue exclusion prior to adoptive transfer.
Graft-versus host disease
8–12 week old female Bm12 mice were sub-lethally irradiated with 600 Rads and allowed to rest for at least 5 hours prior to injecting cells. CD4+CD25− Teff were purified by magnetic beads (Miltenyi Biotec) from female littermate and T-PPAR Spleens. 5 × 104 CD4+CD25− T cells/mouse were injected intravenously. Survival was monitored daily for 40 days.
Colitis and RAG-1−/− cell transfers
CD4+CD25− T cells were purified by magnetic beads (Miltenyi Biotec) from littermate or T-PPAR mice and 0.75 – 1 × 106 cells were injected i.p. into RAG-1−/− mice. Weights were determined prior to injection and monitored weekly for 7–8 weeks. At the end of 7–8 weeks, the mice were sacrificed and the colons were fixed, sectioned, H&E stained and scored in a blinded fashion by a pathologist. Histological evidence of colitis was scored as a sum of acute and chronic inflammation as follows: acute inflammation score: 1) neutrophil infiltration with rare or absent cryptitis; 2) cryptitis with rare or no crypt abcesses; 3) severe crypt abcesses; 4) severe cryptitis with ulceration. chronic inflammation score: 1) mild inflammatory expansion of the colonic lamina propria; 2) moderate to severe LP infiltration with minimal transmural inflammation; 3) lifting of crypts and goblet cell depletion; 4) lymphoid follicles and intramural inflammation. The extent of inflammation was determined as a percent of the colon inflamed and the total scored was calculated by multiplying the extent by the sum of the scores of acute and chronic inflammation. For cellular evaluation, spleen, mesenteric lymph nodes, or colonic lamina propria were harvested at day 7, 14 or 56 after adoptive transfer as indicated. For CD8+ T cell studies, 1 × 106 CD8+ T cells, purified from littermate or T-PPAR spleens by magnetic bead purification (Miltenyi Biotec), were injected i.p. into RAG-1−/− mice. Spleens and mesenteric lymph nodes were harvested at day 7 or 14 after transfer. Naïve T-PPAR mice had normal numbers and viability (by trypan blue exclusion) of splenic CD8+ T cells (data not shown).
Ex-vivo analysis of adoptively transferred cell populations
Single cell suspensions were prepared from Spleens and Mesenteric lymph node cells, resuspended in RPMI media (containing 10% FBS, L-Glutamine, HEPES, Pen/Strep, gentamicin, and β-ME) counted by trypan blue and stained with various fluorescently-tagged antibodies as indicated. Single cell suspensions of colonic lamina propria cells were prepared by removing the colons below the cecum to the rectum and dissociating the tissue by sequential steps of dithioerithritol, EDTA, and dissociation buffer containing collagenase D (Roche), DNase (Sigma) and Dispase (Worthington). Lymphocytes were then purified from total lamina propria cells by percoll gradient separation (44%/67%).
Ex-vivo re-stimulation and intracellular cytokine analysis
The total number of viable spleen, mesenteric lymph nodes, and colonic lamina propria cells was determined by trypan blue exclusion and re-stimulated in vitro with 10 µg/ml plate-bound anti-CD3 ab (Clone 145-2C11, e-bioscience), 2 µg/ml anti-CD28 ab (Biolegend) and 5 µg/ml Brefeldin A (Sigma Aldrich) for 4 hours. Cells were then permeabilized with BD Permeabilization kit (BD Biosciences), anti-FcR blocked (anti-CD16/CD32 Fc Block (Biolegend), and stained for CD4 (intracellular), IL-17, and IFNγ. In some experiments cells were also stained with an anti-MHCII ab for use in a dump gate.
FACS analyses for surface markers and markers of proliferation and apoptosis
Cells were anti-FcR blocked prior to staining and stained with antibodies to: CD4 (FITC, PE-Cy7, PE, e450, e-bioscience- alexafluor700, Biolegend), MHCII (I-A/I-E PE-Cy7, Biolegend), IFNγ APC (Biolegend), CD69 Percp-Cy5.5 (BD Biosciences), CD62L efluor780 (e-bioscience), CD44 (APC, BD Biosciences- Pacific Blue, Biolegend), CD25 a700 (e-bioscience), Ki67 (Clone B56, BD biosciences), and CD127 PE (e-bioscience). For Ki67 staining, cells were initially fixed with 3.7% formaldehyde, anti-FcR blocked and surface stained. After surface staining, the cells were then permeabilized with the Foxp3 permeabilization kit (e-biosciences), anti-FcR blocked, and stained with Ki67 at room temperature in the dark for 1 hr. For staining with Annexin V, cells were stained using an Annexin V detection kit (BD Biosciences). Briefly, cells were stained for CD4 and Annexin V in Annexin binding buffer (BD Biosciences) for 15 minutes at room temperature, washed, and analyzed on either a FACscaliber or LSRII (BD Biosciences).
Recovery of CD4+T cells in irradiated mice
Female littermate or T-PPAR mice were sub-lethally irradiated with 600 Rads. Re-consitution of the endogenous T cells in the Spleen and Mesenteric lymph nodes was assessed at 7, 14, and 56 days after irradiation. Single cell suspensions were prepared from the Spleen and Mesenteric lymph nodes and stained for CD4 and Ki67 staining as above.
Statistics
P values were determined by Student’s unpaired t-Tests. All compilation graphs are shown as group means. Vertical bars are +/− s.e.m.
Results
PPARγ expression in CD4+CD25− T cells is required for development of GVHD
To determine the role of T cell PPARγ in lymphopenic models of immune-mediated inflammation, we utilized mice with a T cell-specific deletion of PPARγ (T-PPAR mice) (11). T cells from naïve T-PPAR mice show no statistically significant difference in expression of activation markers compared to littermate T cells (data not shown).
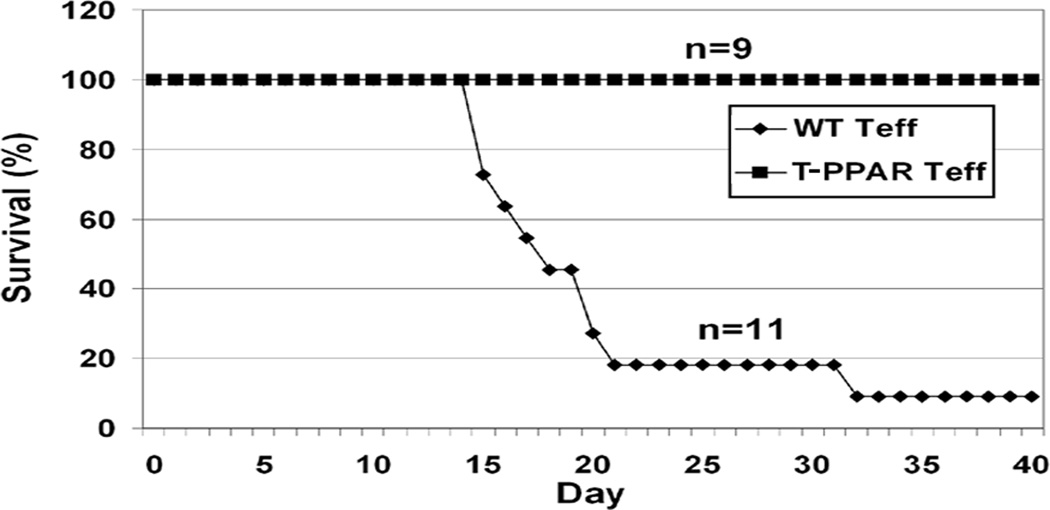
We first studied T-PPAR T cells using the adoptive transfer model of Bm12 graft-versus-host disease (GVHD) (26). In this model, splenic C57BL/6 (WT) CD4+CD25− effector T cells (Teff) are injected i.v. into a sub-lethally irradiated Bm12 mice. Bm12 mice express a mutant MHC II molecule recognized as foreign by the transferred WT Teff resulting in bone marrow failure and eventual death. Teff were purified from T-PPAR mice or from littermate controls. As expected, Teff from littermate controls mediated lethal GVHD when transferred into irradiated Bm12 mice, with the majority of mice dead by day 20 and all but 1/11 mice dead by day 31 (Fig 1). Surprisingly, all mice injected with T-PPAR Teff showed no signs of illness and remained healthy until the termination of the experiment at day 40 (Fig 1). Thus, in contrast to the well-documented immuno-suppressive function of PPARγ in T cells, these results indicate that expression of PPARγ in Teff actually plays a role in the development of Bm12 GVHD.
Figure 1. T-PPAR Teff are unable to mediate GVHD.
Splenic CD4+CD25− Teff were purified from littermate or T-PPAR mice by magnetic bead purification. 5 × 104 cells Teff were injected intravenously into sub-lethally (600 Rad) irradiated Bm12 mice. Survival was monitored daily for 40 days after adoptive transfer. Littermates (n=11), T-PPAR (n=9), (over four separate experiments).
PPARγ expression in Teff is required for the development of colitis
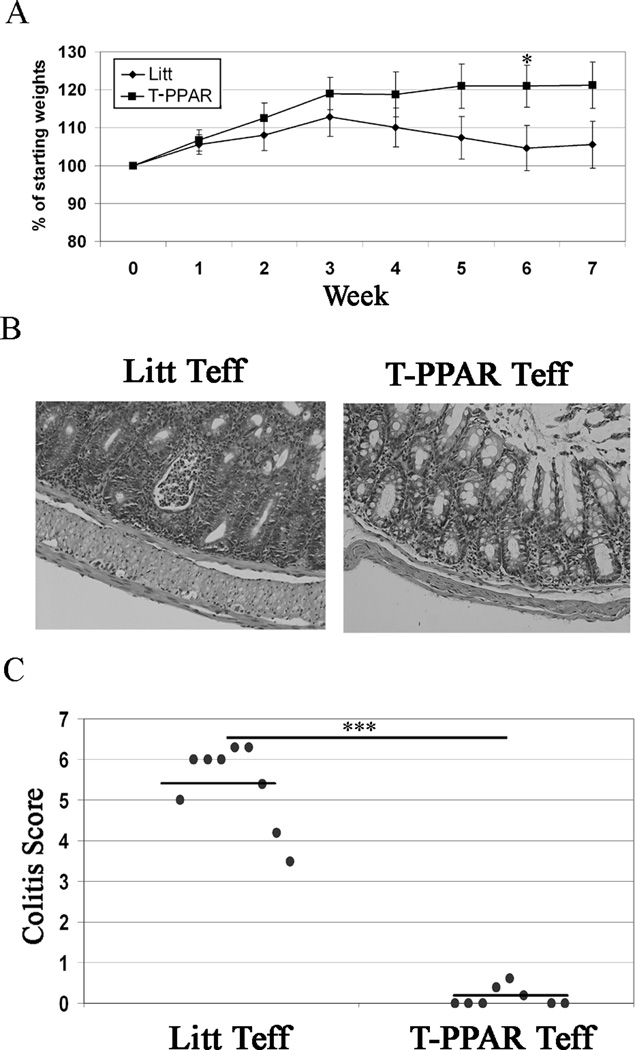
An inherent difficulty in utilizing the Bm12 GVHD model is that the fate of the Teff after transfer cannot be easily tracked. In order to be able to track the cells and determine the effect of PPARγ deletion on T cell activation, cytokine production, and proliferation in lymphopenic autoimmunity, we used an adoptive transfer model of colitis with a defined inflammatory site and draining lymph nodes (27). In this model, Teff are injected into RAG-1−/− mice and these mice begin developing progressive colitis within two weeks, characterized by weight loss, diarrhea, and inflammation developing along the entire length of the colon. To determine the requirement for PPARγ-expression in Teff for the mediation of this disease, Teff from littermate or T-PPAR mice were adoptively transferred into RAG-1−/− mice. Mice receiving littermate Teff began to lose weight by week 3 post-transfer. In contrast, mice receiving T-PPAR Teff continued to gain weight throughout the experiment (Fig 2A). Histological examination at seven weeks post-transfer revealed that littermate Teff caused severe colitis involving crypt abcesses, neutrophilic infiltration of crypts, goblet cell depletion, crypt elongation, and lamina propria expansion. In contrast, mice receiving T-PPAR Teff showed no histological signs of colitis. This difference in the mediation of colitis can be seen in typical histological examples (Fig 2B) and in the histological scores (Fig 2C). These findings confirmed the results seen with GVHD and indicated that expression of PPARγ in Teff also plays a role in the development of this adoptive transfer model of colitis.
Figure 2. T-PPAR Teff are unable to mediate colitis.
Splenic CD4+CD25− Teff were purified from littermate or T-PPAR mice by magnetic bead purification. 0.75–1 × 106 Teff/mouse were injected i.p. into RAG-1−/− mice. A) Mice were weighed once per week. Mean of weekly weights are shown as % of mean starting weights. Littermate, (n=14); T-PPAR, (n=13). B) Typical examples of colon histology at 7 weeks. C) Compilation and means of histological colitis scores. Error bars shown as s.e.m. Littermates (n=8), T-PPAR (n=8). *, p value <0.05; ***, p value <0.001.
T-PPAR Teff show decreased in vivo accumulation and cytokine production
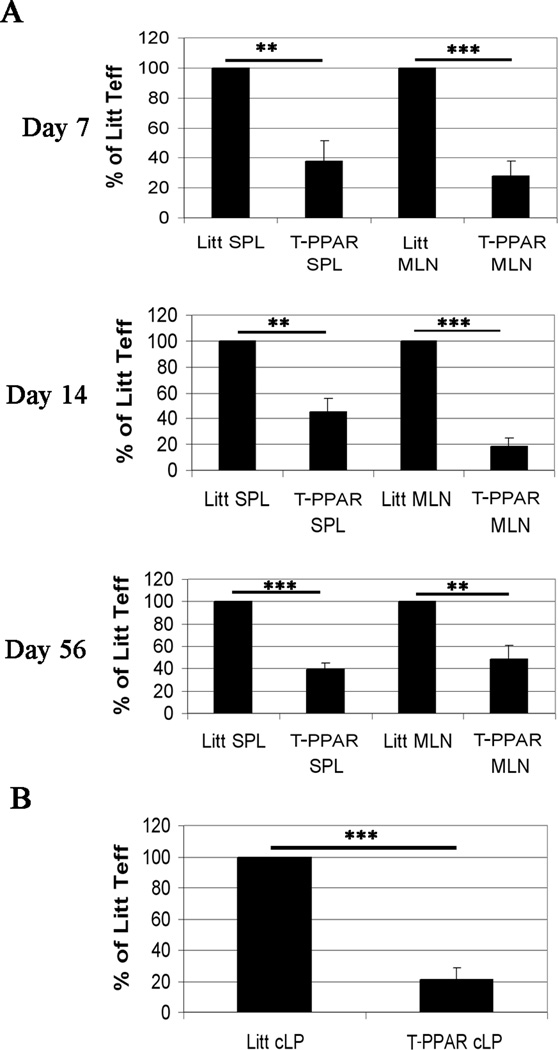
The colitis model allowed us to assess the accumulation and cytokine production of T-PPAR and littermate Teff in spleen (SPL), and mesenteric lymph nodes (MLN) at 7, 14, and 56 days post-transfer. In addition, the accumulation and cytokine production of the transferred Teff was determined in the colonic lamina propria (cLP) on day 14 (at the onset of colitis). Although there was significant variation from experiment to experiment in the total number of CD4+ T cells recovered from the SPL, MLN, and cLP, comparison of the total number of littermate versus T-PPAR CD4+ T cells in each experiment showed a consistent and dramatic decrease in the accumulation of T-PPAR CD4+ T cells in these tissues. (e.g., cLP at day 14: Littermate Teff: 0.39 × 106 CD4+ cells, +/− 0.194 s.e.m.; T-PPAR Teff: 0.07 × 106 CD4+ T cells, +/− 0.045 s.e.m.; MLN at day 7: Littermate Teff: 0.15 × 106, +/− 0.053 s.e.m; T-PPAR Teff: 0.023×106, +/− 0.01 s.e.m.). Normalizing the results from each individual experiment such that the littermate CD4+ T cell recovery is set at 100%, we found that the absolute number of T-PPAR CD4+ T cells was decreased 60–80% in the SPL, MLN, and cLP compared to littermate controls. The greatest decrease was noted in the MLN at day 7 and 14 (70% and 80% reduction in total CD4+ T cell numbers, Fig 3A) and in the cLP at day 14 (80% reduction in total CD4 cell numbers, Fig 3B).
Figure 3. T-PPAR CD4+ T cells are defective in accumulating in the SPL, MLN, or cLP.
Splenic CD4+CD25− Teff were purified from littermate or T-PPAR mice by magnetic bead purification. 1 × 106 Teff/mouse were injected i.p. into RAG-1−/− mice. On day 7, 14, and 56 post-injection, SPL and MLN were harvested, counted and stained for CD4+MHCII− cells. A) The total number of CD4+MHCII− T cells recovered in the SPL and MLN at days 7, 14, and 56 are normalized, with littermate Teff set at 100% in each individual experiment. B) The total number of CD4+MHCII− T cells recovered in the cLP at day 14 are normalized, with littermate Teff set at 100% in each individual experiment. Results of CD4+ T cell recovery in (A) and (B) are depicted as mean % of littermate Teff. For A, day 7, n=7; day 14, n=9; day 56, n=6. For B: n=5. Error bars shown as s.e.m. *, p<0.05; **, p<0.01; ***, p<0.001.
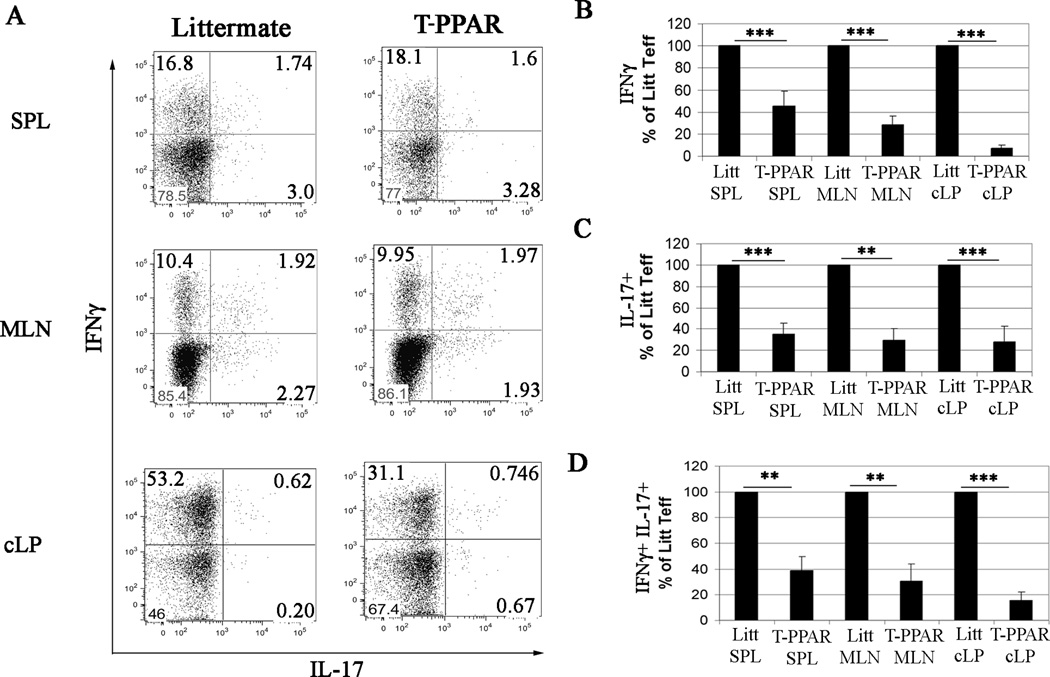
To determine cytokine production by the CD4+ T cells, SPL and MLN cells were harvested at day 14 after transfer, re-stimulated, and stained for intra-cellular IFNγ and IL-17. The frequency of CD4+ T cells that were IFNγ+, IL-17+, or IFNγ+IL-17+ in the SPL and MLN was similar between littermate and T-PPAR T cells (Fig 4A). The results were similar for SPL and MLN CD4+ T cells at days 7 and 56 after transfer (data not shown). Thus, the significantly fewer T-PPAR CD4+ T cells that are present in the SPL and MLN demonstrate normal frequencies and patterns of cytokine production. However, in contrast to the SPL and MLN, there was a 40–50% decrease in the frequency of T-PPAR CD4+ T cells that were IFNγ+ in the cLP (Figure 4A). Furthermore, despite a decrease in IFNγ-production by T-PPAR cLP CD4+ T cells, these cells showed only a slight increase in frequency of IL-17 production (Fig 4A). Thus, in contrast to a previous report utilizing a non-lymphopenic autoimmune model (9), we did not find an increase in Th17 T cells at the site of inflammation with PPARγ-deleted CD4+ T cells in our lymphopenic autoimmune model. Overall, when absolute numbers of littermate versus T-PPAR CD4+ T cells are compared, the T-PPAR T cells that are IFNγ+, IL-17+, or IFNγ+IL-17+ are 60–90% decreased in the SPL, MLN and the cLP at day 14 (Fig 4 B–D). These results suggest that T-PPAR Teff are defective in accumulating in both secondary lymphoid organs and tissues and that there is a specific defect in their ability to produce cytokines in inflammatory sites.
Figure 4. T-PPAR CD4+ T cell cytokine production.
Splenic CD4+CD25− Teff were purified from littermate or T-PPAR mice by magnetic bead purification. 1 × 106 Teff/mouse were injected i.p. into RAG-1−/− mice. On day 14, the SPL, MLN and cLP were harvested, restimulated with plate-bound anti-CD3 ab, anti-CD28 ab, and brefeldin A for 4 hours and stained for CD4, MHCII, IL-17, and IFNγ. A) Representative FACs plots, gated on CD4+MHCII− cells. B–D) Total number of cytokine producing cells, gated on CD4+MHCII− T cells, normalized, with littermate Teff set at 100% in each individual experiment. B) IFNγ+ ; C) IL-17+ ; D) IFNγ+ IL-17+. IFNγ: SPL (n=7); MLN (n=6); cLP (n=4). IL-17+: SPL, MLN and cLP (n=4). IFNγ+ IL-17+: SPL, MLN, and cLP (n=4). Error bars shown as s.e.m. *, p<0.05; **, p<0.01; ***, p<0.001.
T-PPAR CD4+ T cell expression of IL-7 receptor alpha and markers of activation
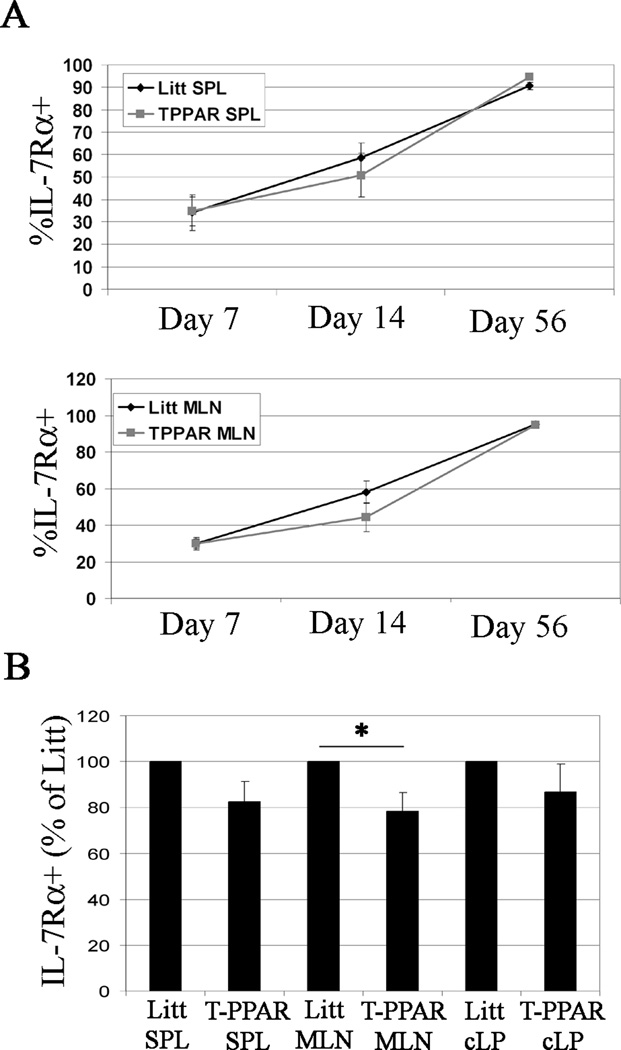
IL-7 is critical in driving and maintaining lymphopenic proliferation. Therefore, we assessed the expression of IL-7 receptor alpha (IL-7Rα) in SPL and MLN CD4+ T cells at days 7, 14, and 56 after adoptive transfer into RAG-1−/− mice. We found a decrease in the T-PPAR CD4+ T cell expression of IL-7Rα at day 14 in both the SPL and MLN when compared to littermate CD4+ T cells (Fig 5 A). Because of experiment to experiment variability in percent IL-7Rα expression, this decrease did not reach statistical significance when all the day 14 experiments were compiled. However, normalizing the results from each individual experiment such that the percent of littermate CD4+ T cells expressing IL-7Rα expression is set at 100% within individual experiments, demonstrates a statistically significant decrease in T-PPAR CD4+ T cell IL-7Rα-expression in the MLN at day 14 (Fig 5 B). A decrease in T-PPAR CD4+ T cell IL-7Rα-expression was not seen in the MLN either at day 7 or day 56 after adoptive transfer, nor was there a statistically significant decrease in T-PPAR CD4+ T cell IL-7Rα-expression in the SPL or cLP at any time point (Fig 5 A, B). These results suggest that a decreased expression of IL-7Rα is unlikely to be responsible for the abnormal accumulation of T-PPAR CD4+ T cells in this colitis model. However, we cannot completely rule out this possibility given the recent demonstration that even small decreases in IL7Rα expression can diminish the proliferative response of CD8+ T cells in vitro (28).
Figure 5. TPPAR CD4+ T cell expression of IL-7Rα.
Splenic CD4+CD25− Teff were purified from Littermate or T-PPAR mice by magnetic bead purification. 1 × 106 Teff/mouse were injected i.p. into RAG-1−/− mice. On the days indicated, SPL and MLN were harvested and stained for CD4, MHCII, and IL-7Rα. A) Mean % IL-7Rα expression, gated on CD4+MHCII− cells, on day 7 (n=5), day 14 (n=8), and day 56 (n=6) after adoptive transfer in the SPL and MLN. B) Mean % IL-7Rα expression at day 14, gated on CD4+MHCII− cells, in SPL, MLN and cLP, normalized, with littermate Teff set at 100% in each individual experiment. SPL, n=7; MLN, n=8; cLP, n=4. Error bars are shown as s.e.m. *, p<0.05;
Another mechanism potentially underlying the abnormal accumulation of T-PPAR CD4+ T cells is a defect in activation. To address this question, we analyzed the expression of activation markers by T-PPAR and littermate CD4+ T cells in the SPL and MLN at day 7 after adoptive transfer into RAG-1−/− mice (i.e., before the onset of colitis) as well as at day 14 and 56 (after the onset of colitis). We measured the expression of CD44, CD25, CD69, and CD62L and found no difference in expression of these markers on T-PPAR versus littermate CD4+ T cells at any time point (data not shown). These results suggest that there is no defect in the in vivo activation of T-PPAR Teff.
T-PPAR CD4+ T cells show decreased expression of the gut-homing molecule α4β7
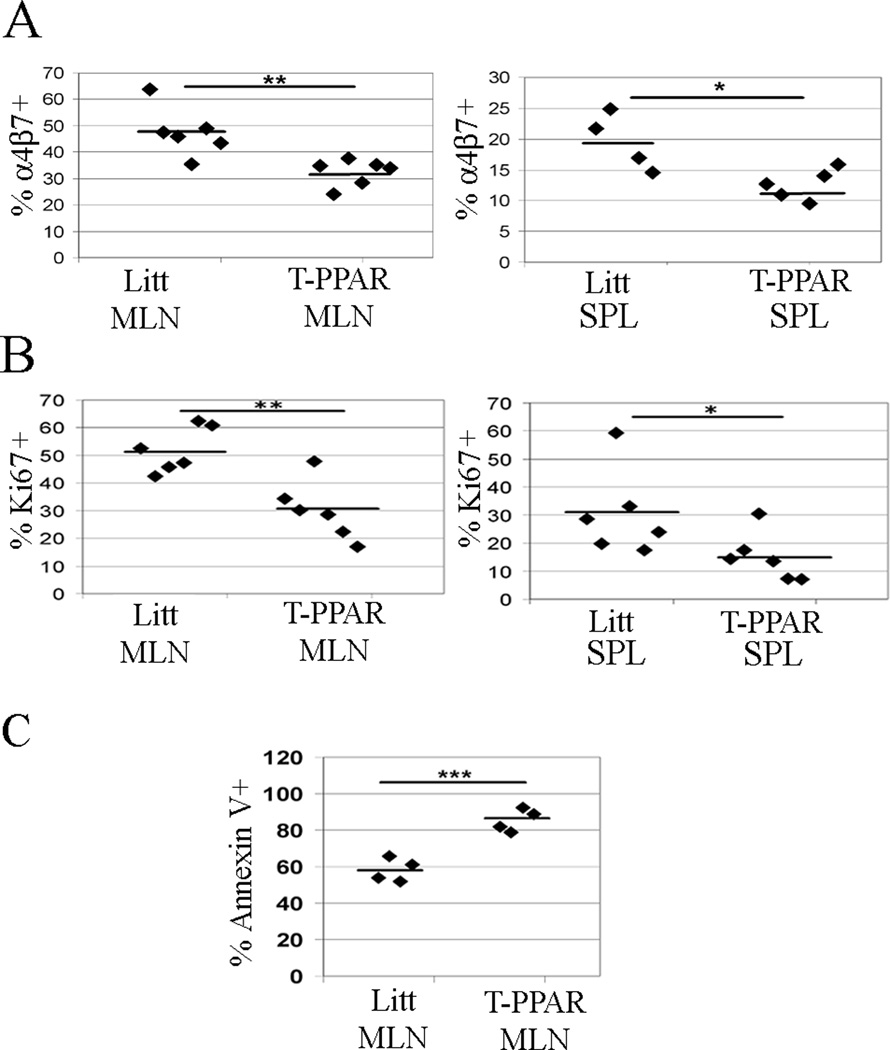
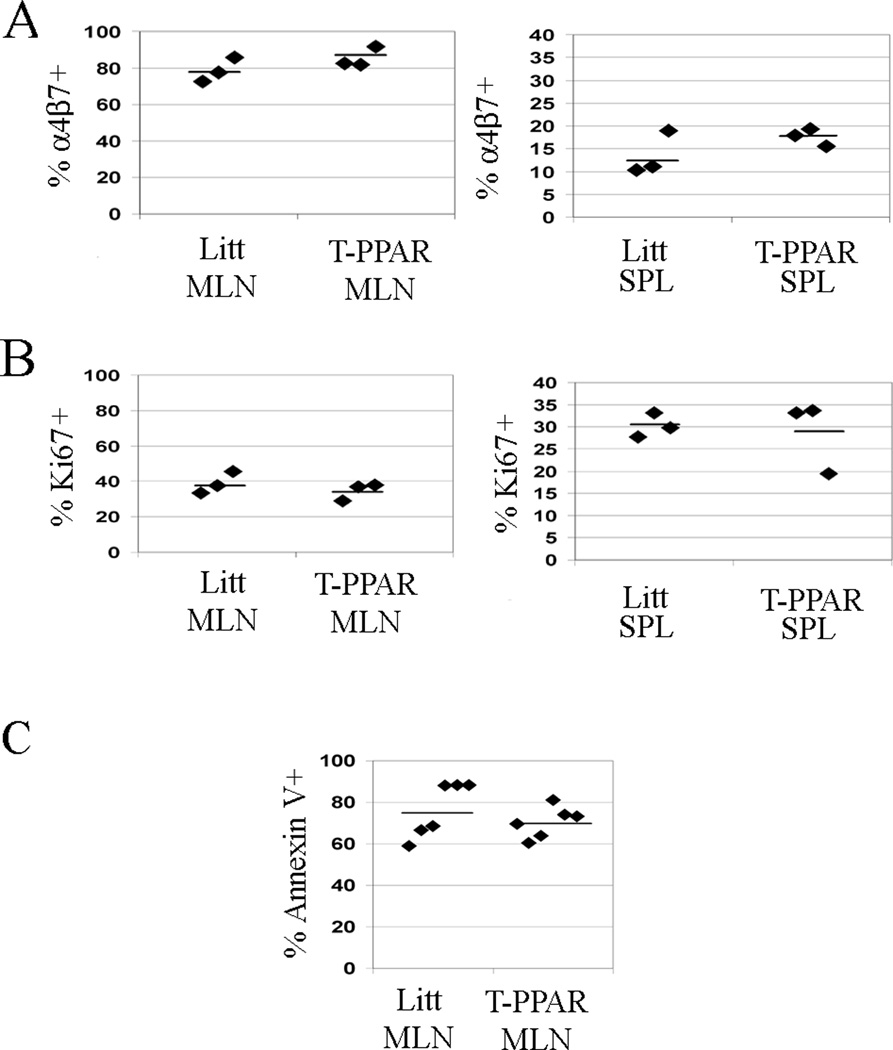
A potential mechanism by which the deletion of Teff PPARγ may control the development of colitis is by altering the ability of Teff to home to the gut. To address this possibility, CD4+ T cells in the MLN and SPL were stained for the gut-homing molecule α4β7 at day 7 after adoptive transfer. We found that there was a statistically significant decrease in expression of α4β7 in T-PPAR versus littermate CD4+ T cells in both the MLN (48% littermate, 32% T-PPAR) and SPL (20% littermate, 13% T-PPAR) at day 7 (Fig. 6A).
Figure 6. T-PPAR CD4+ T cells show decreased expression of α4β7, decreased proliferation, and increased apoptosis.
Splenic CD4+CD25− Teff were purified from littermate or T-PPAR mice by magnetic bead purification. 1 × 106 Teff/mouse were injected i.p. into RAG-1−/− mice. On day 7, SPL and MLN were harvested and stained for CD4 and also stained for MHCII as indicated. A) Expression of α4β7 on the CD4+MHCII− population in the SPL and MLN. B) Expression of Ki67 on the CD4+MHCII− population in the SPL and MLN. C) Expression of Annexin V on the CD4+ population in the MLN. *, p<0.05; **, p<0.01; ***, p<0.001.
While this decreased expression of α4β7 in T-PPAR CD4+ T cells could theoretically play a role in the decreased development of colitis after adoptive transfer of T-PPAR Teff, it is unlikely to explain the inability to mediate GVHD. The pathology and disease manifestations in the Bm12 model of GVHD are known to be related to bone marrow failure, with little if any involvement of colitis (29, 30). Furthermore, we have confirmed that Bm12 mice undergoing GVHD do not demonstrate histological changes of colitis (data not shown). The fact that T-PPAR Teff are incapable of mediating Bm12 GVHD, taken together with the concept that the decreased accumulation of T-PPAR CD4+ T cells in the SPL and MLN after transfer into RAG-1−/− mice cannot be accounted for by a decrease in α4β7-expression, suggests that the defect in α4β7-expression is not likely the primary abnormality underlying the inability of T-PPAR Teff to mediate lymphopenic autoimmune disease.
T-PPAR CD4+ T cells demonstrate decreased proliferation and increased cell death in vivo
To determine if there is a difference in proliferation between T-PPAR and littermate Teff after adoptive transfer into RAG-1−/− mice, we assessed the expression of the cell cycle marker Ki67 in SPL and MLN CD4+ T cells 7 days after transfer. We found that the percent of CD4+ T cells that were Ki67+ was significantly lower for T-PPAR than littermate cells in both the MLN (littermate: mean 52% Ki67+; T-PPAR: mean 30% Ki67+) and SPL (littermate: mean 30% Ki67+; T-PPAR: mean 15% Ki67+) (Fig 6B). These results suggest that T-PPAR Teff have a significant defect in their ability to proliferate in vivo (as measured by the expression of Ki67) in this model of autoimmunity.
To determine if there is a difference in cell survival between T-PPAR and littermate Teff after adoptive transfer into RAG-1−/− mice, 7 days after transfer we stained SPL and MLN for CD4 and Annexin V. We found a significant increase in the frequency of MLN T-PPAR CD4+ T cells that were Annexin V+, and thus apoptotic, compared to littermate CD4+ T cells (littermate: mean 58% Annexin V+; T-PPAR: mean 84% Annexin V+) (Fig 6C). We found no significant difference in the frequency of T-PPAR versus littermate Annexin V+ CD4+ T cells in the SPL (data not shown). Taken together, these results suggest that T cell PPARγ is involved in regulating both the proliferation and survival of CD4+ T cells in lymphopenic models of autoimmunity.
Both antigenic stimulation and lymphopenia are required for defective function of T-PPAR CD4+ T cells
The colitis and GVHD models require both lymphopenia and antigenic stimulation for disease development. In the GVHD model, in addition to the lymphopenia resulting from the sub-lethal irradiation, antigenic stimulation results from the alloantigen response. In the colitis model, in addition to the lymphopenia inherent in the RAG-1−/− mice, the disease requires the presence of colonic bacteria and is postulated to involve antigenic stimulation by these bacteria (31). Given the combination of lymphopenia and antigenic stimulus involved in these autoimmune models, we next asked whether the defect in T-PPAR Teff would be elicited by a lymphopenic environment alone. To address this question, littermate and T-PPAR mice were sub-lethally irradiated (600R) and the recovery and activation of the endogenous CD4+ T cell populations evaluated at day 7, 14, and 56 after irradiation.
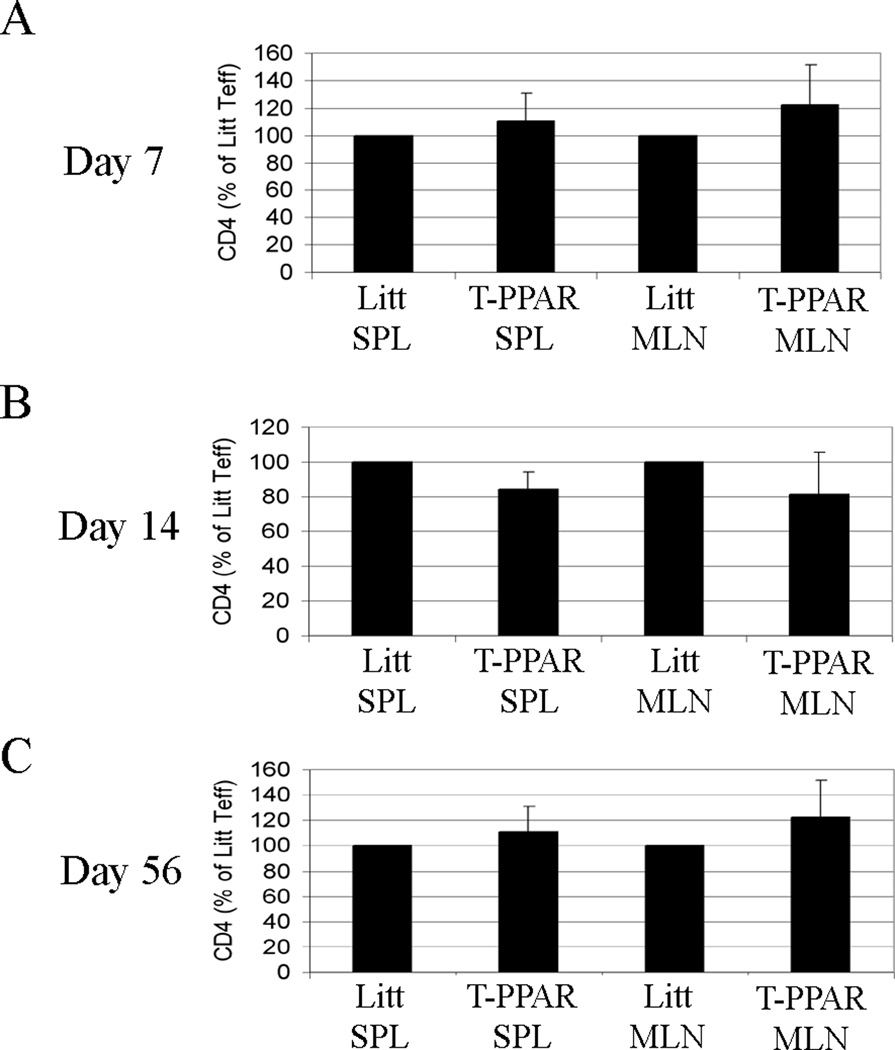
In contrast to the substantial reduction in T-PPAR CD4+ T cells recovered after transfer into RAG-1−/− mouse, there was no statistically significant difference in the number of CD4+ T cells recovered in the SPL or MLN from irradiated littermate versus T-PPAR mice at 7, 14, and 56 days post-irradiation (Fig 7 A–C). In addition, there were no differences observed in the proliferation (via Ki67 staining) of T-PPAR Teff compared to littermate controls at these time points (data not shown). These results suggest that lymphopenia alone is not sufficient to elicit the defects in proliferation and survival we found in T-PPAR Teff and that antigenic stimulation along with lymphopenia is required for the elicitation of these abnormalities.
Figure 7. Irradiated T-PPAR mice show no defect in CD4+ T cell accumulation.
Littermate and T-PPAR mice were sub-lethally irradiated (600 Rads) and SPL and MLN were harvested at A) day 7, B) day 14, C) day 56 after irradiation. Mean total T-PPAR CD4+MHCII− cells are shown normalized to littermate Teff. Error bars shown as s.e.m. For both littermate and T-PPAR: day 7, n=6; day 14, n=5; day 56, n=5.
CD8+ T cells from T-PPAR mice accumulate normally in RAG-1−/− mice
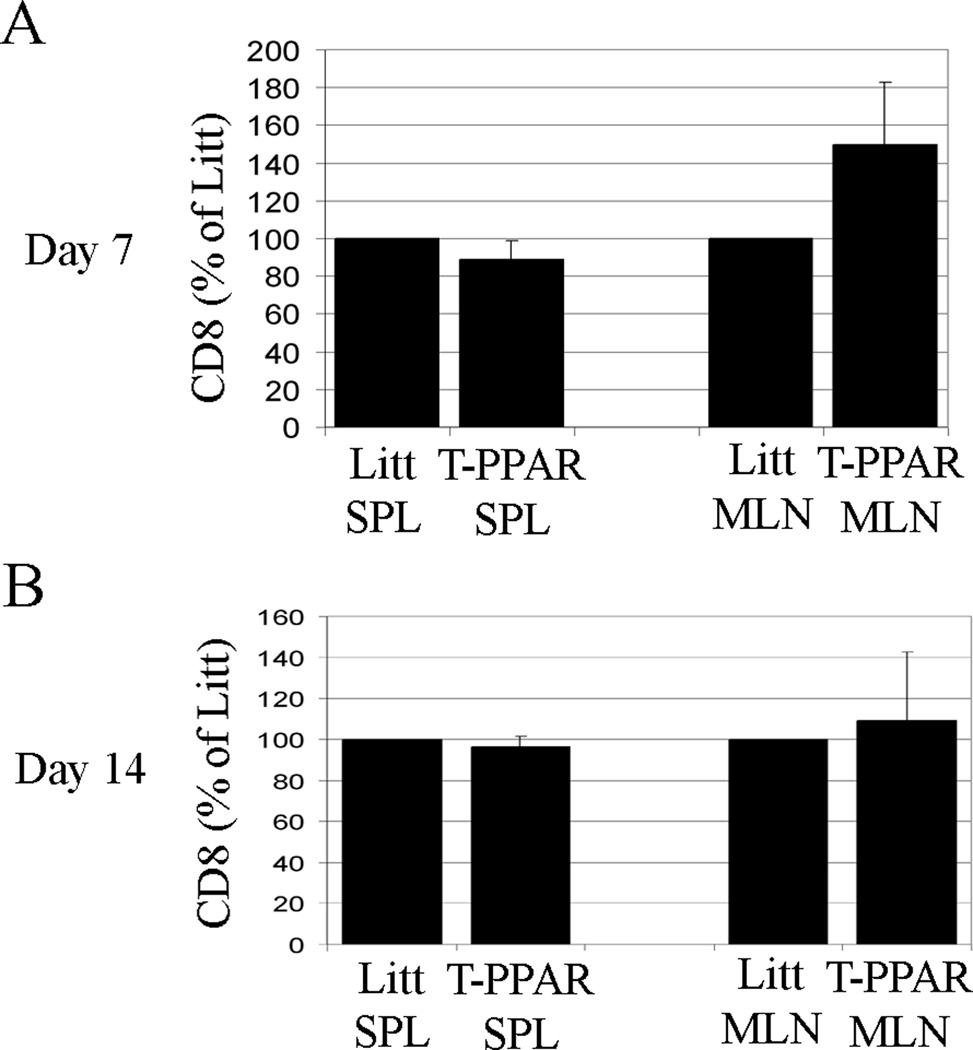
The transgenic CD4-Cre construct used in the generation of the T-PPAR mice (11) is first expressed in T cells at the double positive stage of thymic development and has been previously documented to be expressed in both mature CD4 and CD8 single positive T cells (32). Thus, CD8+ T cells as well as CD4+ T cells in T-PPAR mice are deficient in PPARγ expression. To determine whether T-PPAR CD8+ T cells would also have defects in proliferation and survival after adoptive transfer into RAG-1−/− mice. CD8+ T cells were purified from the SPL of littermate and T-PPAR mice and transferred into RAG-1−/− mice. At 7 and 14 days after adoptive transfer, the total number of CD8+ T cells in the SPL and MLN of the recipient RAG-1−/− mice was determined. Unlike T-PPAR CD4+ T cells, CD8+ T cells from T-PPAR mice showed no significant difference from littermate CD8+ T cells in accumulation in the SPL or MLN (Fig. 8 A, B). While not statistically significant, there was actually an increase in the total number of T-PPAR CD8 cells in the MLN at day 7, however this was not observed in the MLN at day 14, nor in the SPL at any time point. In addition, no differences were observed in the expression of activation markers by T-PPAR versus littermate CD8+ T cells in the SPL or MLN (data not shown).
Figure 8. CD8+ T cells from T-PPAR mice do not show a defect in accumulation.
Splenic CD8+ T cells were purified from littermate or T-PPAR mice by magnetic bead purification. 1 × 106 CD8+ T cells/mouse were injected i.p. into RAG-1−/− mice. MLN and SPL were harvested on day 7 and 14 after transfer and stained for CD8 and MHCII. A) Day 7 post-transfer (SPL, n=6; MLN, n=6). B) Day 14 post-transfer (SPL, n=3; MLN, n=4). Results shown are means of total CD8+ T cells normalized to total littermate CD8+ T cells. Error bars shown as s.e.m.
Finally, we also examined α4β7-expression and the percent Ki67+ and Annexin V+ cells among the SPL and MLN CD8+ T cells at day 7 after adoptive transfer. We found that T-PPAR CD8+ T cells did not differ from littermate CD8+ T cells in α4β7-expression or the percent of Ki67+ and Annexin V+ cells (Figure 9). Overall, these results suggest that PPARγ-deletion in CD4+ T cells, but not in CD8+ T cells, results in the abnormalities in proliferation and survival that we have found in lymphopenic models of autoimmunity.
Figure 9. CD8+ T cells from T-PPAR mice do not show abnormalities in expression of α4β7, proliferation, or apoptosis.
Splenic CD8+ cells were purified from littermate or T-PPAR mice by magnetic bead purification. 1 × 106 CD8+ T cells/mouse were injected i.p. into RAG-1−/− mice. MLN and SPL were harvested on day 7 and stained for CD8, MHCII and: A) α4β7; and B) Ki67. MLN were harvested on day 7 and stained for C) CD8 and Annexin V. Results shown are gated on CD8+, MHCII− cells (A,B), or CD8+ cells (C).
Discussion
We and others have previously demonstrated that PPARγ mediates regulatory functions in conventional T cells, natural Tregs, macrophages and dendritic cells and also enhances the generation and stability of inducible Tregs (2–6, 10–12, 33). Numerous studies have suggested that there is an important link between lymphopenic proliferation and autoimmunity (23). Despite the potential relevance of lymphopenia in the development of autoimmunity and the potential importance of PPARγ as an immunoregulator and therapeutic target in autoimmune diseases, there have been no prior studies of the role of PPARγ in regulating autoimmunity in lymphopenic conditions. In the present studies we investigated this issue and surprisingly find that, rather than having a down-regulatory role, T cell PPARγ is actually required for the mediation of autoimmunity.
Our current results demonstrate that T-PPAR Teff are unable to cause disease in two murine models of lymphopenic autoimmunity, GVHD and colitis. Using the adoptive transfer model of colitis, we find that T-PPAR Teff demonstrate a number of abnormalities, the most glaring of which is a markedly decreased accumulation in SPL, MLN, and cLP. Mechanistically, this decreased accumulation of adoptively transferred T-PPAR Teff appears to stem from both decreased proliferation (assessed by Ki67 staining) and increased apoptosis (assessed by Annexin V staining). We found no difference in littermate versus T-PPAR endogenous CD4+ T cell accumulation after sub-lethal irradiation of littermate and T-PPAR mice themselves. It is important to note that the colitis and GVHD models involve both lymphopenia and antigenic stimulation in disease development. Thus, the results with the directly irradiated T-PPAR mice suggest that the defect in accumulation in T-PPAR CD4+ T cells is not seen with lymphopenic stimulation alone, but requires a combination of lymphopenia and an additional strong antigenic stimulus.
Studies of PPARγ effects on survival of T cells in vitro have yielded conflicting results, with some studies showing a pro-apoptotic effect and others reporting a pro-survival effect (34). Harris et al., demonstrated that activation of T cells in vitro in the context of TZDs led to increased T cell apoptosis, but this effect may have resulted from effects not mediated through PPARγ (4). However, consistent with the concept of increased T cell apoptosis with PPARγ-activation, it has been reported that PPARγ can play a role in the T cell apoptosis seen in sepsis (35, 36). In contrast to these pro-apoptotic effects of PPARγ-activation in T cells, it has also been demonstrated that activation of PPARγ results in increased in vitro survival of T cells and T lymphoma cells under conditions of cytokine-withdrawal or serum starvation (34, 37, 38). Thus, our present findings may represent an in vivo equivalent to these in vitro cytokine-deprivation studies. It may be that the primary mechanism underlying the inability of PPARγ-deficient Teff to mediate lymphopenic autoimmunity is an increased sensitivity to the limiting levels of growth factors available under these highly activating and proliferative conditions in vivo.
Our current findings suggest that the few T-PPAR CD4+ T cells recovered in the SPL and MLN during the development of colitis show a similar pattern of cytokine production as the littermate CD4+ T cells. However, the few T-PPAR CD4+ T cells recovered in the cLP show a decrease in IFNγ-production. It was recently reported that PPARγ plays a role in regulating Th17 differentiation and that PPARγ-deficient T cells demonstrate enhanced early disease severity in EAE associated with increased Th17 T cells infiltrating the central nervous system. These results led to the conclusion that T cell PPARγ, interacting with endogenous ligands, may normally play a role in down-regulating T cell production of IL-17 (9). In the lymphopenic colitis model, we do not find a significant increase in IL-17 production from T-PPAR CD4+ T cells in any of the tissues tested. The differences in IL-17 production between the EAE and colitis models give further evidence of the multi-functional role of PPARγ in both driving and suppressing gene expression. Furthermore, this multi-functional role is likely at least partially dependent on the context in which the specific cells are functioning. As such, our results suggest a novel role for PPARγ specifically in the context of lymphopenia-associated autoimmunity.
We have identified a decreased expression of α4β7 by T-PPAR CD4+ T cells in vivo. This decreased expression, seen in both the MLN and SPL at day 7, could theoretically play a role in the decreased development of colitis after adoptive transfer of T-PPAR Teff. However, the fact that colitis does not play a significant role in the pathology of Bm12 GVHD (29, 30), taken together with the concept that the decreased accumulation of T-PPAR CD4+ T cells in the SPL and MLN cannot be accounted for by a decrease in α4β7-expression, indicates that the defect in α4β7-expression is not likely the primary abnormality underlying the inability of T-PPAR Teff to mediate lymphopenic autoimmune disease.
T-PPAR Teff also demonstrate a decreased expression of IL-7Rα at day 14 in the MLN. This decrease in T-PPAR CD4+ T cell IL-7Rα-expression was not seen either at day 7 or day 56 in the MLN and was not significantly decreased at any point in the SPL. These results suggest that a decreased expression of IL-7Rα (and a resulting decrease in IL-7-mediated proliferation and survival) is unlikely to be responsible for the decreased accumulation of T-PPAR CD4+ T cells in these lymphopenic autoimmune settings. However, we cannot completely rule out this possibility given that even small decreases in IL7Rα expression can diminish the in vitro proliferative response of CD8+ T cells in vitro (28).
In contrast to T-PPAR CD4+ T cells, we did not find an abnormality in accumulation, proliferation or apoptosis in T-PPAR CD8+ T cells. This suggests that these defects are specific for PPARγ-deficient CD4+ T cells. The reason for this difference in CD4+ versus CD8+ PPARγ-deficient T cell accumulation is unclear at this time. However, CD8+ T cells adoptively transferred into RAG-1−/− mice do not mediate colitis and therefore may not receive the strong antigenic stimulation seen by the colitis-mediating CD4+ T cells. Interestingly, these results may suggest that TZDs may be particularly effective at suppressing inflammatory conditions which are primarily mediated by CD8+ T cells as they would maintain the immuno-regulatory effects while not causing an increase in T cell survival. Future studies will further explore this issue.
Finally, the TZD class of PPARγ ligands been shown to be effective at treating a number of autoimmune conditions in both mice and humans and there is significant interest in using these drugs clinically in autoimmune and other inflammatory conditions (19–22, 39). While the immuno-regulatory effects of PPARγ activation are well-documented, our results now suggest a PPARγ paradox in autoimmunity; namely, while ligation of PPARγ may ameliorate autoimmune inflammation under non-lymphopenic conditions, CD4+ T cell PPARγ may also allow for increased survival of auto-reactive T cells in lymphopenic conditions. Our results highlight the complexity of PPARγ function in T cells and may suggest a new caveat in the potential role of PPARγ-activation as a therapeutic modality in autoimmunity.
Footnotes
The authors declare no financial conflict of interest in this work.
This work was supported by National Institutes of Health (NIH) training grant 2T32AI007080 (W.J.H.), NIH grant IR56 AI072533-01 A1 (R.B.C.), and a National Multiple Sclerosis Society Grant RG 4070-A-6 (R.B.C.).
Abbreviations:
Thiazolidinedione (TZD); PPARγ: Peroxisome Proliferator-activated Receptor gamma; Teff: T effector; Treg: Regulatory T cell; SPL: Spleen; MLN: mesenteric lymph nodes MLN; cLP: Colonic Lamina Propria; GVHD: Graft-versus-Host disease.
References
- 1.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends in immunology. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 3.Faveeuw C, Fougeray S, Angeli V, Fontaine J, Chinetti G, Gosset P, Delerive P, Maliszewski C, Capron M, Staels B, Moser M, Trottein F. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-12 production in murine dendritic cells. FEBS Lett. 2000;486:261–266. doi: 10.1016/s0014-5793(00)02319-x. [DOI] [PubMed] [Google Scholar]
- 4.Harris SG, Phipps RP. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. European journal of immunology. 2001;31:1098–1105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 6.Housley WJ, O'Conor CA, Nichols F, Puddington L, Lingenheld EG, Zhu L, Clark RB. PPARgamma regulates retinoic acid-mediated DC induction of Tregs. Journal of leukocyte biology. 2009;86:293–301. doi: 10.1189/jlb.1208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung SW, Kang BY, Kim TS. Inhibition of interleukin-4 production in CD4+ T cells by peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands: involvement of physical association between PPAR-gamma and the nuclear factor of activated T cells transcription factor. Molecular pharmacology. 2003;64:1169–1179. doi: 10.1124/mol.64.5.1169. [DOI] [PubMed] [Google Scholar]
- 8.Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, Langhans B, Klockgether T, Waisman A, Eberl G, Schultze J, Famulok M, Kolanus W, Glass C, Kurts C, Knolle PA. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei J, Hasegawa H, Matsumoto T, Yasukawa M. Peroxisome proliferator-activated receptor alpha and gamma agonists together with TGF-beta convert human CD4+CD25− T cells into functional Foxp3+ regulatory T cells. J Immunol. 2011;185:7186–7198. doi: 10.4049/jimmunol.1001437. [DOI] [PubMed] [Google Scholar]
- 11.Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and - independent mechanisms. J Immunol. 2007;178:4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 12.Hontecillas R, Bassaganya-Riera J. Peroxisome Proliferator-Activated Receptor {gamma} Is Required for Regulatory CD4+ T Cell-Mediated Protection against Colitis. J Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 13.Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Beales PE, Liddi R, Giorgini AE, Signore A, Procaccini E, Batchelor K, Pozzilli P. Troglitazone prevents insulin dependent diabetes in the non-obese diabetic mouse. Eur J Pharmacol. 1998;357:221–225. doi: 10.1016/s0014-2999(98)00574-3. [DOI] [PubMed] [Google Scholar]
- 15.Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- 16.Feinstein DL, Galea E, Gavrilyuk V, Brosnan CF, Whitacre CC, Dumitrescu-Ozimek L, Landreth GE, Pershadsingh HA, Weinberg G, Heneka MT. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- 17.Mueller C, Weaver V, Vanden Heuvel JP, August A, Cantorna MT. Peroxisome proliferator-activated receptor gamma ligands attenuate immunological symptoms of experimental allergic asthma. Arch Biochem Biophys. 2003;418:186–196. doi: 10.1016/j.abb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J Exp Med. 2003;198:411–421. doi: 10.1084/jem.20021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. PARgamma as a new therapeutic target in inflammatory bowel diseases. Gut. 2006;55:1341–1349. doi: 10.1136/gut.2006.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pershadsingh HA. Peroxisome proliferator-activated receptor-gamma: therapeutic target for diseases beyond diabetes: quo vadis? Expert Opin Investig Drugs. 2004;13:215–228. doi: 10.1517/13543784.13.3.215. [DOI] [PubMed] [Google Scholar]
- 21.Pershadsingh HA, Heneka MT, Saini R, Amin NM, Broeske DJ, Feinstein DL. Effect of pioglitazone treatment in a patient with secondary multiple sclerosis. J Neuroinflammation. 2004;1:3. doi: 10.1186/1742-2094-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drew PD, Xu J, Racke MK. PPAR-gamma: Therapeutic Potential for Multiple Sclerosis. PPAR research. 2008;2008 doi: 10.1155/2008/627463. 627463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunology letters. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmunity reviews. 2002;1:329–337. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 25.Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, Weetman A, Hale G, Chatterjee VK, Waldmann H, Compston A. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691–1695. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie IF, Morgan GM, Sandrin MS, Michaelides MM, Melvold RW, Kohn HI. B6.C-H-2bm12. A new H-2 mutation in the I region in the mouse. J Exp Med. 1979;150:1323–1338. doi: 10.1084/jem.150.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Housley WJ, Adams CO, Nichols FC, Puddington L, Lingenheld EG, Zhu L, Rajan TV, Clark RB. Natural but Not Inducible Regulatory T Cells Require TNF-{alpha} Signaling for In Vivo Function. J Immunol. 2011;186:6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 28.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nature immunology. 2011;12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blazar BR, Sharpe AH, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Korngold R, Vallera DA. Infusion of anti-B7.1 (CD80) and anti-B7.2 (CD86) monoclonal antibodies inhibits murine graft-versus-host disease lethality in part via direct effects on CD4+ and CD8+ T cells. J Immunol. 1996;157:3250–3259. [PubMed] [Google Scholar]
- 30.Sprent J, Surh CD, Agus D, Hurd M, Sutton S, Heath WR. Profound atrophy of the bone marrow reflecting major histocompatibility complex class II-restricted destruction of stem cells by CD4+ cells. J Exp Med. 1994;180:307–317. doi: 10.1084/jem.180.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida M, Watanabe T, Usui T, Matsunaga Y, Shirai Y, Yamori M, Itoh T, Habu S, Chiba T, Kita T, Wakatsuki Y. CD4 T cells monospecific to ovalbumin produced by Escherichia coli can induce colitis upon transfer to BALB/c and SCID mice. International immunology. 2001;13:1561–1570. doi: 10.1093/intimm/13.12.1561. [DOI] [PubMed] [Google Scholar]
- 32.Rahman AH, Zhang R, Blosser CD, Hou B, Defranco AL, Maltzman JS, Wherry EJ, Turka LA. Antiviral memory CD8 T-cell differentiation, maintenance, and secondary expansion occur independently of MyD88. Blood. 2011;117:3123–3130. doi: 10.1182/blood-2010-11-318485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark RB. The role of PPARs in inflammation and immunity. Journal of leukocyte biology. 2002;71:388–400. [PubMed] [Google Scholar]
- 34.Wang YL, Frauwirth KA, Rangwala SM, Lazar MA, Thompson CB. Thiazolidinedione activation of peroxisome proliferator-activated receptor gamma can enhance mitochondrial potential and promote cell survival. J Biol Chem. 2002;277:31781–31788. doi: 10.1074/jbc.M204279200. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt MV, Paulus P, Kuhn AM, Weigert A, Morbitzer V, Zacharowski K, Kempf VA, Brune B, von Knethen A. PPAR{gamma}-Induced T-Cell Apoptosis Reduces Survival During Polymicrobial Sepsis. American journal of respiratory and critical care medicine. 2011 doi: 10.1164/rccm.201010-1585OC. [DOI] [PubMed] [Google Scholar]
- 36.Soller M, Tautenhahn A, Brune B, Zacharowski K, John S, Link H, von Knethen A. Peroxisome proliferator-activated receptor gamma contributes to T lymphocyte apoptosis during sepsis. Journal of leukocyte biology. 2006;79:235–243. doi: 10.1189/jlb.0205058. [DOI] [PubMed] [Google Scholar]
- 37.Jo SH, Yang C, Miao Q, Marzec M, Wasik MA, Lu P, Wang YL. Peroxisome proliferator-activated receptor gamma promotes lymphocyte survival through its actions on cellular metabolic activities. J Immunol. 2006;177:3737–3745. doi: 10.4049/jimmunol.177.6.3737. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Jo SH, Csernus B, Hyjek E, Liu Y, Chadburn A, Wang YL. Activation of peroxisome proliferator-activated receptor gamma contributes to the survival of T lymphoma cells by affecting cellular metabolism. The American journal of pathology. 2007;170:722–732. doi: 10.2353/ajpath.2007.060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaturvedi RK, Beal MF. PPAR: a therapeutic target in Parkinson's disease. Journal of neurochemistry. 2008;106:506–518. doi: 10.1111/j.1471-4159.2008.05388.x. [DOI] [PubMed] [Google Scholar]