Abstract
Sturge-Weber syndrome is a rare neurocutaneous disorder that often results in functional impairment caused by motor (typically hemiparesis) and cognitive deficits. A retrospective chart review of physiatric evaluation of 30 individuals, aged 4 mos to 55 yrs (median age, 2.4 yrs), with Sturge-Weber syndrome with brain involvement was conducted for the purpose of summarizing physiatric findings and recommendations in this cohort. Presence or absence of motor, cognitive, and behavioral concerns and need for orthoses, spasticity management, and therapy services were noted. Hemiparesis was common, but the need for intervention for spasticity was rare. Cognitive and behavioral concerns were noted frequently, meriting additional evaluation. Case vignettes are presented to highlight (1) a child with repeated functional setbacks in association with increased seizure frequency who, with seizure control, demonstrated return to functional baseline and subsequent further skill development and (2) a child with Sturge-Weber syndrome who made functional gains with constraint-induced movement therapy.
Keywords: Sturge-Weber Syndrome, Rehabilitation, Hemiparesis
Sturge-Weber syndrome (SWS) is a rare neurocutaneous disorder, estimated to affect one in 20,000 –50,000 individuals.1 SWS usually presents with a facial port-wine birthmark in the ophthalmic distribution of the trigeminal nerve and an ipsilateral occipital leptomeningeal angioma.2 Associated features may include glaucoma or vascular eye abnormalities, seizures, and migraines; functional deficits resulting from cerebral injury can include hemiparesis, visual field cuts, and cognitive dysfunction ranging from learning disabilities to intellectual disability.3 Brain injury in this population is thought to be related to ischemia resulting from abnormalities in cerebral vasculature,4 and significant functional decline can occur in association with “stroke-like episodes,” which often accompany seizure activity and may result from inadequate hemodynamic response to seizure.3,5
Cognitive and motor function is known to vary widely among individuals with SWS.6–9 Evidence supports that a larger extent of brain involvement (i.e., extension from the typically involved occipital lobes into the parietal, temporal, and frontal lobes), as demonstrated by neuroimaging, is associated with greater functional impairment.10,11 In youth with SWS, hemiparesis is associated with greater overall brain involvement, magnetic resonance imaging abnormalities in frontal cortices, and dysfunction in multiple domains of adaptive functioning.9 However, little has been reported about the physiatric needs of individuals with SWS. A single case study from Turkey reports that a young child with SWS made functional gains after hemispherectomy and intensive therapy.12
The purpose of this study was to summarize the physiatric findings and recommendations for a cohort of individuals with SWS through a retrospective review of clinical documentation.
METHODS
This project was approved by the local Institutional Review Board.
Participants
Data were included from all patients with confirmed SWS with brain involvement evaluated by a physiatrist at our institution's Sturge-Weber Center between 2003 and 2008. All individuals were offered physiatric evaluation as part of multidisciplinary evaluations, and individuals and their families selected whether to meet with a physiatrist. Data were collected retrospectively from chart review and a research database. For patients with more than one physiatric evaluation, data from the initial evaluation are reported, except where specifically noted.
For the purpose of comparison with those patients evaluated by physiatry, limited data (gender, age, and SWS-Neurological Rating Score) were extracted from a database for patients with SWS with brain involvement who were seen at the Center but not evaluated by a physiatrist.
Assessments
Sturge Weber Syndrome–Neurological Rating Score (SWS-NRS)
The SWS-NRS13 provides a clinically derived rating of visual field cut (0, none; 1, partial; and 2, full homonymous hemianopsia), frequency of seizures (0, none ever; 1, one or more controlled; 2, breakthrough; 3, monthly; and 4, weekly to regular), hemiparesis (0, normal; 1, slight [postures intermittently]; 2, mild [impaired fine motor]; 3, moderate [impaired gross and fine motor]; and 4, severe [little fine motor control and severe gross motor, poor helper arm function, walks with difficulty or not at all]), and cognitive functioning (score ranging from 0 to 5 based upon self- or parent-report of developmental and academic or vocational functioning or both). The final score is the sum of subscores and can range from zero to 15. A lower score represents better overall neurological functioning and fewer neurological indicators of SWS severity. The SWS-NRS was assigned by the treating neurologist in accordance with the published rating criteria13 without knowledge of results from the physiatric evaluation.
Statistical Analyses
Two-tailed Mann-Whitney U tests were used to evaluate for differences in age and SWS-NRS between individuals who did receive physiatric evaluation and those who did not.
RESULTS
Description of the Patient Population
Of 109 individuals with SWS with brain involvement evaluated at the Center, 30 (19 males) had a total of 43 physiatric evaluations; seven individuals had more than one physiatric evaluation. The age at the time of physiatric evaluation ranged from 4 mos to 55 yrs (mean, 8.5 yrs; median, 2.4 yrs). For individuals who did not receive physiatric evaluation, the age at the time of the only or last SWS Center evaluation ranged from 4 mos to 58 yrs (mean, 11.8 yrs; median, 7.4 yrs). The age in the cohort receiving physiatric evaluation was significantly younger than that in the cohort not receiving physiatric evaluation (P = 0.02).
Mean SWS-NRS at the time of first or only physiatry visit was 5.8, with mean subscale scores as follows: seizure score, 1.8; hemiparesis score, 1.9; visual field cut score, 1.0; and cognitive function score, 1.2. For the individuals who did not receive physiatric evaluation, the mean SWS-NRS score at last or only SWS Center neurology visit was 4.8, with mean subscale scores as follows: seizure score, 1.5; hemiparesis score, 1.2; visual field cut score, 0.7; and cognitive function score, 1.4. Statistically significant between-group differences were observed in hemiparesis score (P = 0.01) and total neuroscore (P = 0.05), with higher scores indicating more severe hemiparesis and greater global impairment in individuals receiving physiatric evaluation.
Physiatric Findings in Individuals with SWS
Given the wide age range of patients evaluated, data are presented by age-based subgroup: infants and toddlers (0–3 yrs, n = 19), school age (4–17 yrs, n = 6), and adult (21 yrs and older, n = 5). The frequency of functional concerns and physiatric needs is summarized in Table 1.
Table 1.
Frequency of functional concerns and physiatric needs of SWS patients, presented by age group
| 0–3 yrs Old(n = 19),% | 4–17 yrs Old(n = 6),% | 21–55 yrs Old(n = 5), % | |
|---|---|---|---|
| Cognitive concerns | 64a | 60 | 80 |
| Behavior concerns | 10 | 60 | 20 |
| Motor concerns | 95 | 80 | 100 |
| Orthotic recommendations | 32 | 33 | 60 |
| Spasticity management needs | 26 | 20 | 0 |
| Occupational,physical, and speech therapy needs | 95 | 80 | 60 |
Identification of cognitive concerns in the youngest subgroup was based on language skills and limited to 14 children aged 9 mos to 3 yrs.
SWS, Sturge-Weber syndrome.
Infants and Toddlers (Ages 0–3 yrs): 19 Initial Visits, Nine Follow-Up Visits
In these young children, parental report of child's language skills was used as a proxy for evaluation of cognitive development. Given that language milestones are more difficult to evaluate in children aged 8 mos and younger, data from five infants younger than 9 mos of age were excluded from consideration of cognitive function. Of the remaining 14 children, nine had delays in expressive language, raising concern for cognitive processes also mediated by the frontal lobe. No child had a clearly defined delay in receptive language. Two of the 19 children in this age group presented with behavioral concerns: one with features of pervasive developmental disorder, the other with low frustration tolerance and aggressive behaviors. Eighteen of 19 children had abnormal findings on motor examination, including 15 children with hemiparesis; for three of these children (ages 4 mos, 6 mos, and 10 mos), the family was not previously aware of their child's hemiparesis, which varied from mild-to-moderate in severity. The other three children with abnormal motor examinations demonstrated global delays in gross motor function: one 18-mo-old child had generalized hypotonia and sat only when propped, one 19-mo-old child had generalized dyscoordination and hypotonia, and one 32-mo-old child demonstrated delayed fine motor function bilaterally and had a history of delays in meeting gross motor milestones.
Six children in this age group had been prescribed bracing by other providers, and these six children were the only ones in this group felt to have orthotic needs at the time of evaluation. With the exception of a 2-yr-old child using in-shoe inserts, modifications were recommended to each child's bracing programs. A 3-yr-old child was using an articulated ankle-foot orthosis (AFO) only during school-based therapy sessions, and physiatric recommendations included extending brace wear to throughout the day. A 21-mo-old child had been using a nighttime splint to maintain dorsiflexion range of motion (ROM), and addition of an AFO for daytime wear was recommended. A 16-moold child had previously been provided with an AFO, which she was not using; no daytime AFO use was recommended by the physiatrist, but recommendations included consideration of a nighttime dorsiflexion stretching splint. A 3-yr-old child with mild right hemiparesis had used in-shoe inserts in the past; physiatric recommendations included a right supramalleolar orthosis, left in-shoe insert, and right thumb abduction splint. A 21-mo-old child with left hemiparesis had been prescribed a thumb abduction splint but was not using it; recommendations included a left AFO and reevaluation for a wrist-hand splint to improve functional positioning of the hand. During follow-up visits, AFOs were recommended for two additional children in this age range. Spasticity management recommendations were provided for five patients, consisting of a home-stretching program; for three children this was combined with use of an AFO. Fourteen of the 19 children were receiving therapy services at the time of evaluation, typically occupational therapy (OT) plus physical therapy (PT) and/or speech therapy 1–2 times weekly through early intervention programs. All 14 of those children had ongoing therapy needs. In addition, therapy needs were identified for four of five children who had not received therapy services at the time of evaluation; for three of these children, PT, OT, and speech evaluations through early intervention programs were recommended. For the fourth child, who was the child with features of pervasive developmental disorder, only outpatient speech therapy services were recommended.
Five children in this age group were followed up over time; one child had a total of six visits over 2 yrs, one child had three visits over 2 yrs, two children had two visits over 2 yrs, and one child had two visits over 6 mos. All of these children were noted to make functional progress over time; most notably, children with hemiparesis showed improvements in fine motor skills on the affected side and increased spontaneous incorporation of the affected arm and hand into bimanual activities over time. The child most frequently followed up presented with hemiparesis and global developmental delays in infancy; use of signs for expressive language emerged at approximately 18 mos of age, and independent ambulation emerged at age 2 yrs, 4 mos.
School-Age Children (Ages 4–17 yrs): Six Initial Visits, Two Follow-Up Visits
At initial evaluation, there were cognitive concerns for four of six children. The same four children presented with behavioral concerns: one child was withdrawn, one had crying and agitation with concern for depression, one had auditory hallucinations and aggressive behaviors, and one had aggressive and oppositional behaviors. Five of the six children had current motor impairments: four children had hemiparesis, and one child had bilateral fine motor delays. The sixth child had a history of hemiparesis that had resolved.
Of these six children, one had used AFOs in the past, and one was using an in-shoe insert. It was recommended that the latter child continue to use the in-shoe insert, and in-shoe orthoses were recommended for one additional child. One child had previously undergone bilateral heel cord releases, and this was the only child who required intervention for spasticity/ROM at the time of physiatric evaluation. A home-stretching program and local follow-up for botulinum toxin injection and/or serial casting were recommended to improve ankle dorsiflexion ROM. Five of six children were receiving therapy services at the time of evaluation: three were receiving school-based services, and two were receiving outpatient therapies. All of these children were receiving OT services: two were receiving OT and PT; two were receiving OT and speech therapy; and one was receiving OT, PT, and speech therapy. All five of these children had ongoing needs for the therapy services they were currently receiving, and additional services were recommended for three of the children. Direct outpatient OT was recommended for one child, and outpatient consultative OT, PT, and speech therapy were recommended for a second child, for the purpose of developing and monitoring home therapy programs. The third child was referred to a day-rehabilitation program for constraint-induced movement therapy (CIMT). The child in this age group who was not receiving therapy at the time of physiatric evaluation had a history of resolved hemiparesis, and no therapy needs were identified during the evaluation.
The two children in this age group who were followed up over time (one with two visits over 2 yrs and one with three visits over 3 yrs) were noted to make functional progress; as with the younger children, most notable gains were in functional use of the affected upper limb. In this age range, these improvements in upper limb use translated into increased independence with activities of daily living.
Adults (Ages 21–55 yrs): Five Initial Visits, Two Follow-Up Visits
Four of the five individuals in this age group had cognitive deficits, ranging from intermittent memory concerns associated with migraines to attention deficits to intellectual disability. Three individuals had participated in post– high school education; one of those individuals was in college at the time of examination, one had completed an associate's degree and was working part-time jobs, and one had completed a bachelor's degree but was not working because of debilitating seizures and migraines. The other two adults were unemployed, although one had previously worked in a sheltered workshop. Of the five adults, one lived independently at college, one lived in a group home, and three lived with family members who provided varying levels of assistance ranging from physical help with activities of daily living to oversight for managing finances. One of the five adults had behavioral issues with aggression and mood swings. All five adults had hemiparesis.
At the time of evaluation, one adult was wearing an AFO, and this was the only orthosis in use among the adult patients. Ongoing use of the AFO was recommended for that individual, and in-shoe orthoses were recommended for two additional adult patients. No individuals required additional recommendations for spasticity management. No individuals were receiving therapy at the time of evaluation. Therapy services were recommended for three individuals. For a 53-yr-old patient with left hemiparesis, OT was recommended for sensory perceptual reeducation and consideration of constraint-induced movement therapy. For a 21-yr-old man with mild right hemiparesis, OT was recommended to improve upper-limb endurance and fine motor skills and for adaptive equipment evaluation, and PT was recommended to address chronic back pain associated with mild scoliosis. For a 23-yr-old man with mild right hemiparesis, OT was recommended for a driver's evaluation, and PT was recommended for development of a home exercise program to address occasional back stiffness.
Case Vignettes
Case vignettes are provided to highlight two important considerations regarding rehabilitation in SWS: (1) occurrence of relatively rapid and dramatic fluctuations in function and (2) response to CIMT.
Case Vignette: Fluctuations in Function in Association with Seizures
Patient A is a boy with a facial port-wine birthmark whose early development was normal, including achievement of rolling and tripod sitting. At 5.5 mos of age, he experienced focal seizures lasting approximately 20 mins. Magnetic resonance imaging at that time revealed right hemisphere leptomeningeal angioma, consistent with SWS (Fig. 1A). During this episode, patient A developed left hemiparesis, with decreased movement of the left side, hypotonia, absent grasp, and left hemianopsia. Some functional improvement was noted over the course of days during hospitalization, and 3–6 wks after this episode left lower-limb strength was noted to be full.
FIGURE 1.
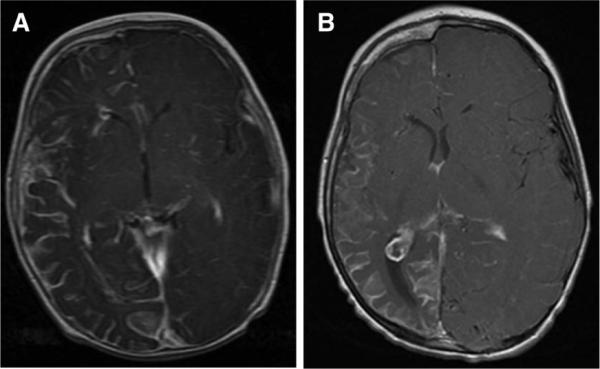
The MRI findings from patient with SWS. A, Contrast-enhanced T1-weighted axial MRI image from patient A obtained at age 5 mos demonstrating right-sided leptomeningeal angioma, consistent with SWS. All lobes of the right hemisphere are noted to be involved. B, Follow-up contrast-enhanced T1-weighted MRI at age 3 additionally reveals atrophy of the entire right hemisphere; a prominent choroid glomus within the right lateral ventricle is also demonstrated on this axial slice. SWS, Sturge-Weber Syndrome; MRI, magnetic resonance imaging.
He was seizure-free at his first SWS Center evaluation at 10 mos of age and was noted to have left upper-limb involvement such that he did not grasp toys and had mild shoulder weakness. He was only able to sit with support and had a dense left hemianopsia.
At 15 mos of age, he experienced multiple seizures, and left hemiparesis worsened. By 20 mos of age, his seizures were controlled; beginning at that time, use of the left upper and lower limbs was noted to improve, and babbling increased. At the time of his second SWS Center evaluation, at 21 mos of age, he had newly achieved independent ambulation with a mild hemiparetic pattern. He demonstrated increased spontaneous use of the left hand. Mild left shoulder weakness persisted. He was not talking but was felt to understand “no.” No definitive visual field cut was appreciated.
At 24 mos of age, the patient had recurrence of seizures in association with viral illness. Significant worsening of left hemiparesis was noted, and he was unable to walk because of increased weakness. However, after 2 wks, he quickly returned to his prior functional baseline. At the time of his third SWS Center evaluation, at 34 mos, seizures were well controlled, and he was beginning to run. He demonstrated further increase in spontaneous use of the left upper limb as a gross assist and had a raking grasp on the left. He continued to demonstrate significant delays in receptive and expressive language. Follow-up magnetic resonance imaging at age 3 revealed atrophy of the right hemisphere, consistent with typical progression of imaging findings in children with SWS (Fig. 1B).
Case Vignette: CIMT in SWS
Patient B is a girl with a facial port-wine birthmark who had seizures starting on day of life 2; head computed tomography demonstrated gyri-form calcifications and right frontal lobe atrophy, consistent with SWS. Her last seizure was at 1 mo of age. Left hemiparesis was appreciated at 3 mos of age, with increased fisting and decreased kicking on the left. Gross motor, fine motor, and self-help skills were delayed because of left hemiparesis; independent ambulation was achieved at 20 mos. Patient B participated twice in a CIMT program, at ages 4 and 6 yrs, with the goal of improving left upper-limb function. The CIMT program in which she participated was based in a day rehabilitation treatment setting; the child attended the rehabilitation facility from 8 a.m. to 3 p.m. on weekdays.
At admission to the CIMT program at age 4, she did not spontaneously use the left upper limb, even as an assist. She had scapular winging and increased tone at left biceps, wrist flexors, extensors, and pronators. Passive ROM was within normal limits; active ROM on the left was lacking five degrees of supination and 25 degrees of wrist extension. The right upper limb (unimpaired arm) was casted for 20 days to facilitate increased use of the hemiparetic left arm. While casted, patient B received 14 days of active treatment consisting of 6 hrs of direct therapy per day, provided by therapists. During the 6 weekend days for which she was casted, parents completed therapeutic activities at home as assigned by the treating therapists. Left upper-limb gains included decreased tone, increased active ROM, and improved reach, grasp, and release. She was discharged from the CIMT program to once weekly outpatient OT and PT and a home therapy program including structured play focusing on bimanual activities for 1–2 hrs daily and kinesiotaping and electrical stimulation.
At age 6, there was variability in maintenance of the gains achieved 2 yrs prior with CIMT; reduction in spasticity was sustained; however, scapular winging had increased, and functional use of the left upper limb was not maintained, at least in part because of limited active forearm supination. In her second course of CIMT, again performed at the day rehabilitation program, her right (unimpaired) arm was casted for 27 days, including 19 weekdays on which she received 6 hrs of direct therapy daily and 8 weekend days during which she participated in therapeutic activities at home as assigned by the treating therapists. In addition, after cast removal, she participated in 2 days of direct therapy (6 hrs per day) focused on bimanual skills. Goals met included improved grasp and release, which increased independence with dressing. At last follow-up, parents reported that gains in left hand control were sustained in the year after CIMT.
DISCUSSION
SWS is a rare disorder that can result in hemiparesis; the goal of this case series was to summarize physiatric findings in these patients. As would be expected, individuals for whom physiatric evaluation was requested had worse hemiparesis than individuals for whom physiatric care was not sought. Most individuals in this cohort evaluated by a physiatrist were younger than 3 yrs, which may reflect a bias of families of young children, for whom future function is uncertain, to seek physiatric care more frequently than families of older children and adults who typically have already attained speech and ambulation.
Of individuals receiving physiatric evaluation, approximately one third had need for orthoses, and fewer required other forms of spasticity management. In the authors' experience, these individuals required less bracing and spasticity management than would be anticipated for a cohort of similarly aged individuals with hemiparesis from other etiologies (e.g., middle cerebral artery stroke and traumatic brain injury). One reason for this difference could be that the cortical nature of brain involvement in SWS reduces the risk for spasticity in comparison with subcortical injuries. Most of these individuals with SWS had therapy needs. Six children who were evaluated more than once made functional gains in association with therapy services. Only one child in this cohort received formalized CIMT, described in the second vignette; she did not sustain all gains obtained with the first course of CIMT, but further benefit was achieved from an additional course of CIMT. The generaliz-ability of this child's experience with CIMT is unclear, especially because the content of pediatric CIMT programs is quite variable, including differences in duration and method for constraint of the dominant arm and duration and intensity of therapy.14 No data were available regarding response to therapy in adulthood.
The fluctuating functional course of some individuals with SWS, highlighted in the first case vignette, sets them apart from individuals with other etiologies of hemiparesis. Individuals with SWS are prone to significant functional setbacks, often associated with seizure activity, as noted previously by a number of authors.1,3,6,15,16 One hypothesis for this phenomenon is that impaired cerebral autoregulation in individuals with SWS during seizure activity results in inadequate delivery of oxygen and glucose to meet the metabolic demand, thereby increasing the risk of ischemic injury.1 The physiatrist must understand the importance of seizure control in optimizing overall function in this population. In addition, the physiatrist should recognize that relatively quick return to prior functional baseline may be possible with therapeutic intervention.
Cognitive concerns were identified for more than half of the individuals with SWS evaluated by a physiatrist. Our estimate of cognitive concerns must be interpreted with caution in the youngest (and largest) group of children, given our use of informal parent report of expressive language delays as a proxy for cognitive development. Although parent report of toddler's language skills using a formalized measure has been shown to correlate with child's cognitive function,17 it is unknown whether informal parent reporting is as reliable as use of a standardized measure. Our experience to date suggests that isolated expressive aphasia (without cognitive deficits) does not occur commonly in this population. In fact, given that expressive language is mediated by the frontal lobe, deficits in this domain are likely to be a marker for larger extent of cerebral abnormalities, and therefore broader cerebral dysfunction, in SWS in the same manner that hemiparesis in individuals with SWS is associated with greater generalized adaptive dysfunction.9 Furthermore, our estimate of cognitive concerns in two thirds of young children with SWS is consistent with previous reports of intellectual disability in 50%–60% of individuals with SWS,18 given that previous work did not account for cognitive deficits observed in individuals with SWS but without intellectual disability, such as specific learning disabilities6 and attention deficit hyperactivity disorder.7 The presence of cognitive concerns in 50% or more of individuals with SWS highlights the need for early and continued attention to cognitive function in these patients. Several risk factors for cognitive and adaptive deficits in SWS can be identified in clinical history or physiatric evaluation or both, including onset of seizures before 9 mos of age, poor seizure control, and the presence of hemiparesis.1,9 Neuropsychological testing is often useful before initiation of formal education and as needed over time based on disease-related functional decline and to optimize educational and vocational opportunities. Furthermore, the presence of adults in this cohort who were unable to live and work independently underscores the importance of early consideration of needs related to transition to adulthood, as is recommended for all children with disabilities.19,20 A number of behavioral concerns were identified in this cohort, which is consistent with a previous report of increased mood and compliance issues in children with SWS compared with their unaffected siblings.21 This highlights the importance of screening for symptoms of behavioral disorders among individuals with SWS and referring for additional care, if indicated.
Although this cohort provides a unique opportunity to examine the physiatric needs of individuals with SWS, several important biases must be recognized. Many individuals evaluated at the Center travel from out of state, reflecting a bias toward families with resources to identify and visit a specialty center. Based on severity of hemiparesis, individuals who sought physiatric care had worse function than those individuals with SWS who did not seek physiatric evaluation; thus, the findings reported here cannot be extrapolated to all individuals with SWS. Finally, the number of school age and adult individuals in this cohort is small, and in these groups in particular, the reported findings may not be representative of the needs and function of all individuals with SWS.
CONCLUSIONS
SWS is rare cause of hemiparesis, and the physiatric needs of individuals with SWS may differ from those with other causes of hemiparesis. Individuals with SWS are at risk for significant functional decline in association with seizures, but their rehabilitation potential can be good with seizure control. Spasticity management is often not needed in SWS, and orthotic needs may be relatively minimal. Thorough neuropsychological evaluation and educational and vocational planning are important.
ACKNOWLEDGMENTS
We thank Lisa Ferenc for her assistance with data management.
Footnotes
Disclosures: This study was supported by Hunter's Dream for a Cure Foundation, Sturge-Weber Foundation, NICHD grant K12HD001097, and NINDS grant K12NS01696. Presented at the 2009 Annual Assembly of the American Academy of Physical Medicine and Rehabilitation in Austin, TX. Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Comi AM. Sturge-Weber syndrome and epilepsy: An argument for aggressive seizure management in these patients. Expert Rev Neurother. 2007;7:951–6. doi: 10.1586/14737175.7.8.951. [DOI] [PubMed] [Google Scholar]
- 2.Bodensteiner JB, Roach ES. Sturge-Weber syndrome: Introduction and overview. In: Bodensteiner JB, Roach ES, editors. Sturge-Weber Syndrome. Sturge-Weber Foundation; Mount Freedom, NJ: 1999. pp. 1–10. [Google Scholar]
- 3.Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509–16. doi: 10.1177/08830738030180080701. [DOI] [PubMed] [Google Scholar]
- 4.Maria BL, Neufeld JA, Rosainz LC, et al. Central nervous system structure and function in Sturge-Weber syndrome: Evidence of neurologic and radiologic progression. J Child Neurol. 1998;13:606–18. doi: 10.1177/088307389801301204. [DOI] [PubMed] [Google Scholar]
- 5.Aylett SE, Neville BG, Cross JH, et al. Sturge-Weber syndrome: Cerebral haemodynamics during seizure activity. Dev Med Child Neurol. 1999;41:480–5. [PubMed] [Google Scholar]
- 6.Kramer U, Kahana E, Shorer Z, et al. Outcome of infants with unilateral Sturge-Weber syndrome and early onset seizures. Dev Med Child Neurol. 2000;42:756–9. doi: 10.1017/s0012162200001407. [DOI] [PubMed] [Google Scholar]
- 7.Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, et al. Sturge-Weber syndrome: Study of 55 patients. Can J Neurol Sci. 2008;35:301–7. doi: 10.1017/s0317167100008878. [DOI] [PubMed] [Google Scholar]
- 8.Juhasz C, Batista CE, Chugani DC, et al. Evolution of cortical metabolic abnormalities and their clinical correlates in Sturge-Weber syndrome. Eur J Paediatr Neurol. 2007;11:277–84. doi: 10.1016/j.ejpn.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reesman J, Gray R, Suskauer SJ, et al. Hemiparesis is a clinical correlate of general adaptive dysfunction in children and adolescents with Sturge-Weber syndrome. J Child Neurol. 2009;24:701–8. doi: 10.1177/0883073808329529. [DOI] [PubMed] [Google Scholar]
- 10.Marti-Bonmati L, Menor F, Mulas F. The Sturge-Weber syndrome: Correlation between the clinical status and radiological CT and MRI findings. Childs Nerv Syst. 1993;9:107–9. doi: 10.1007/BF00305319. [DOI] [PubMed] [Google Scholar]
- 11.Lin DD, Barker PB, Hatfield LA, et al. Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: Correlation with neurological symptoms. J Magn Reson Imaging. 2006;24:274–81. doi: 10.1002/jmri.20627. [DOI] [PubMed] [Google Scholar]
- 12.Kose N, Karakaya MG, Otman S. Rehabilitation results of a hemiparetic subject with Sturge-Weber syndrome and intractable epilepsy. Firat Tip Dergisi. 2004;9:130–3. [Google Scholar]
- 13.Kelley TM, Hatfield LA, Lin DD, et al. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–70. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 14.Huang HH, Fetters L, Hale J, et al. Bound for success: A systematic review of constraint-induced movement therapy in children with cerebral palsy supports improved arm and hand use. Phys Ther. 2009;89:1126–41. doi: 10.2522/ptj.20080111. [DOI] [PubMed] [Google Scholar]
- 15.Coley SC, Britton J, Clarke A. Status epilepticus and venous infarction in Sturge-Weber syndrome. Childs Nerv Syst. 1998;14:693–6. doi: 10.1007/s003810050299. [DOI] [PubMed] [Google Scholar]
- 16.Okudaira Y, Arai H, Sato K. Hemodynamic compromise as a factor in clinical progression of Sturge-Weber syndrome. Childs Nerv Syst. 1997;13:214–9. doi: 10.1007/s003810050070. [DOI] [PubMed] [Google Scholar]
- 17.Feldman HM, Dale PS, Campbell TF, et al. Concurrent and predictive validity of parent reports of child language at ages 2 and 3 years. Child Dev. 2005;76:856–68. doi: 10.1111/j.1467-8624.2005.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaroff CM, Isaacs K. Neurocutaneous syndromes: Behavioral features. Epilepsy Behav. 2005;7:133–42. doi: 10.1016/j.yebeh.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Stewart D. Transition to adult services for young people with disabilities: Current evidence to guide future research. Dev Med Child Neurol. 2009;51(suppl 4):169–73. doi: 10.1111/j.1469-8749.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics. American Academy of Family Physicians. American College of Physicians-American Society of Internal Medicine A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–6. [PubMed] [Google Scholar]
- 21.Chapieski L, Friedman A, Lachar D. Psychological functioning in children and adolescents with Sturge-Weber syndrome. J Child Neurol. 2000;15:660–5. doi: 10.1177/088307380001501004. [DOI] [PubMed] [Google Scholar]


