Abstract
Background. This study evaluated whether large venous-arterial CO2 gap (PCO2 gap) preoperatively is associated to poor outcome. Method. Prospective study which included adult high-risk surgical patients. The patients were pooled into two groups: wide [P(v-a)CO2] versus narrow [P(v-a)CO2]. In order to determine the best value to discriminate hospital mortality, it was applied a ROC (receiver operating characteristic) curve for the [P(v-a)CO2] values collected preoperatively, and the most accurate value was chosen as cut-off to define the groups. Results. The study included 66 patients. The [P(v-a)CO2] value preoperatively that best discriminated hospital mortality was 5.0 mmHg, area = 0.73. Preoperative patients with [P(v-a)CO2] more than 5.0 mmHg presented a higher hospital mortality (36.4% versus 4.5% P = 0.004), higher prevalence of circulatory shock (56.8% versus 22.7% P = 0.01) and acute renal failure postoperatively (27.3% versus 4.5% P = 0.02), and longer hospital length of stays 20.0 (14.0–30.0) versus 13.5 (9.0–25.0) days P = 0.01. Conclusions. The PCO2 gap values more than 5.0 mmHg preoperatively were associated with worse postoperatively outcome.
1. Introduction
Although every year millions of surgeries are performed all around the world, few patients that undergo major surgeries are judged to be of high risk for postoperative complications and death [1]. An observational study reported that only 12.5% of all surgical procedures are considered high risk, but over 80% of the deaths which occurred were in the high-risk group [1]. Despite the high mortality rate, less than 15% of those patients are admitted to intensive care units (ICU) [1], which shows that the individual risk may easily be underestimated and that high-risk patients may not be recognized.
The mechanisms by which inflammatory response and tissue hypoperfusion occur in major surgery are still not entirely clear, but evidence indicates that oxygen requirements increase significantly as a result of the injury and metabolic response to the trauma caused by the surgery. However, very often high-risk patients are not able to increase their cardiac output and thus the oxygen delivery [2]. Therefore, those patients will more likely develop tissue hypoperfusion and severe systemic inflammatory response and death due to the organ dysfunction [3].
Surgical patients with a high risk for complications admitted to an ICU frequently die as a primary or secondary consequence of hypoperfusion-related organ dysfunction or severe infections [4, 5]. However, predictors of death caused by tissue hypoperfusion are still not studied very much in high-risk surgery patients. The [P(v-a)CO2] has been reversely correlated to cardiac output in surgical patients [6–8]. In a recent review, Lamia et al. [9] showed that PCO2 gap is a blood flow marker to remove total CO2 produced by the peripheral tissue. Cuschieri et al. [10] have found a reverse correlation between [P(cv-a)CO2] and cardiac index with a central venous blood sample and suggested that, for this purpose, a sample of central venous blood could be used instead of blood from the pulmonary artery.
In the literature, we find only very few prospective clinical studies with surgical patients, which have evaluated the real impact from [P(v-a)CO2] as complications and death preoperative marker in this population. Therefore, the objective of this study was to evaluate the role of PCO2 gap as a predictor for complications and death in high-risk surgical patients.
2. Methods
The study was performed in a tertiary hospital. It was approved by the local Ethics and Research Committees, and a written postinformed consent was obtained from each patient or legal responsible. The study design was a post hoc analysis applying a cut point derived from the data, with the following inclusion criteria: (1) patients aged 18 years or older and (2) surgeries which required a central venous catheter in intraoperative and intensive care in postoperative phase. Patients were selected during the preanesthetic evaluation. High-risk surgeries were patients undergoing surgeries with requested ICU postoperative stay and at least one of the following:
severe cardiorespiratory comorbidities (coronary insufficiency, chronic obstructive pulmonary disease, previous stroke),
surgery scheduled for neoplasm resection (esophagectomy, total gastrectomy) longer than 8 hours,
above 70 years-old, with evidence of physiological reserve impairment involving at least one vital organ,
acute renal failure (blood urea nitrogen > 100 mg/dL or creatinine > 3 mg/dL),
advanced vascular disease, or aortal involvement,
massive acute intraoperative blood loss predicted,
severe nutritional disorders patients.
Patients undergoing palliative surgery, with low life expectancy, liver failure (Child B or C), patients with a functional class IV heart failure or an echocardiogram-measured ejection fraction of less than 30%, and those who did not accept participating in the study were excluded. The patients with a functional class IV heart failure or ejection fraction of less than 30% could present flow alteration due to underlying disease, which is not related to surgery procedure.
At the time of the inclusion, the Multiple-Organ Dysfunction Syndrome (MODS) [11] and Acute Physiology And Chronic Health Evaluation (APACHE II) [12] scores were also evaluated by taking the worst values of their variables. An echocardiogram, which is an integral part of preoperative evaluation of high-risk surgical patients in our institution, was performed to measure the ejection fraction and the presence or absence of systolic or diastolic ventricular dysfunction, considering the increasingly clear literature data on their similarities regarding mortality and morbidity [13–16].
At the day of the surgery, patients were equipped with central venous (positioned with the tip within the superior vena cava) and arterial catheters. A chest X-ray confirmed central venous catheter position. Before anesthesia induction, in intraoperatively and postoperatively central venous and arterial blood samples were collected to measure [P(v-a)CO2].
The surgical team decided on which therapy should be used during the intraoperative period. In the postoperative phase, the intensive care physician's goal was to improve the perfusion parameters according to standard in local institution; he had no knowledge about preoperative [P(v-a)CO2].
The primary endpoint was hospital mortality; all patients were therefore monitored until they were discharged from hospital. The secondary endpoint was to check for the presence of an organ dysfunction, shock (need of vasoactive drugs for over 1 hour, despite volemic resuscitation), acute pulmonary dysfunction (PaO2/FiO2 ratio < 200), renal failure (increase of creatinine by 50% or urinary output of less than 400 mL in 24 hours), confusion (change in behavior, memory lapses, or psychomotor agitation), and platelet dysfunction (platelets reduced by 30% compared to the basal value) in up to 24 hours in the postoperative period. Diagnosis of postoperative infection was based on international consensus guidelines and/or use of antibiotic [17]. The infections incidence during ICU length of stay and the time of mechanical ventilation were verified, besides the hospital length of stay.
Based on the best [P(v-a)CO2] value which discriminated hospital mortality, the patients were allocated in two groups: narrow [P(v-a)CO2] (group 1) and wide [P(v-a)CO2] (group 2).
2.1. Statistical Analysis
Initially, we described the demographic, clinical, and physiological characteristics from patients that were included in the study. Frequencies were calculated to describe categorical variables. Quantitative variables were described by using measures of central tendency and dispersion.
The statistical method to be used in the assessment of each variable was chosen based on its distribution. Categorical variables were analyzed through the Chi-square test, and continuous variables through the mean with the Student's t-test for normal distribution and continuous variables with irregular distribution were analyzed by means of the Mann-Whitney Test. P values < 0.05 (two-tailed) were considered significant. ROC curves were used to test the discrimination power of the groups in predicting clinical evolution (the capacity of correctly classifying survivors and nonsurvivors) and to determine the best cut-off value of tissue perfusion in connection with hospital mortality. SPSS 13.0 was used to analyze those calculations. Group 01 patients were compared to those of group 02. Box plots were created to compare survivors and nonsurvivors in [P(v-a)CO2] in different time points through Mann-Whitney test.
To determine the risk of hospital death we developed a multivariable Cox proportional hazard model in the population. Variables were included in the model if they reached a significance level of P < 0.05 in univariate analysis or were clinically relevant. Mortality estimative curve was created using the Cox method and compared by Cox regression.
3. Results
Over a period of 6 months, the study included 66 patients, of which 37 males and 29 females, with an average age of 65.6 years old. Elective surgeries were more frequent (Table 1).
Table 1.
Patient characteristics and comparison between patients with [P(v-a) CO2] < 5.0 and [P(v-a)CO2] ≥ 5.0.
| Variables | All patients (n = 66) |
[P(v-a)CO2] < 5.0 mmHg (n = 22) |
[P(v-a)CO2] ≥ 5.0 mmHg (n = 44) |
P |
|---|---|---|---|---|
| Age | 65.6 ± 12.2 | 64.6 ± 13.5 | 66.0 ± 11.7 | 0.67 |
| Males (%) | 56.1 | 50.0 | 59.1 | 0.48 |
| APACHE II | 16.9 ± 5.6 | 15.8 ± 5.1 | 17.4 ± 5.8 | 0.29 |
| MODS | 3 (1.0–4.0) | 3.0 (1.0–4.0) | 3.5 (1.0–4.5) | 0.46 |
| ASA (%) | 0.65 | |||
| I | 6.6 | 9.5 | 5.0 | |
| II | 68.9 | 71.4 | 67.5 | |
| III | 24.6 | 19.0 | 27.5 | |
| Elective surgery (%) | 95.5 | 95.5 | 95.5 | 1.00 |
| Emergency surgery (%) | 4.5 | 4.5 | 4.5 | 1.00 |
| Gastrointestinal surgery (%) | 78.8 | 81.8 | 73.3 | 0.38 |
| [P (v-a) Co2] (mmHg) | 5.4 ± 2.0 | 3.3 ± 1.2 | 6.5 ± 1.4 | 0.00 |
| Lactate (mmol/L) | 1.5 ± 0.8 | 1.4 ± 0.6 | 1.6 ± 0.9 | 0.35 |
| Base excess (mmol/L) | −0.7 (−3.0–0.6) | −1.6 (−3.4–0.7) | −0.5 (−2.4–0.6) | 0.35 |
| ScvO2 (%) | 74.1 ± 7.6 | 77.0 ± 6.2 | 72.7 ± 7.9 | 0.03 |
| Ventricular dysfunction (%) | 77.3 | 54.5 | 88.6 | 0.002 |
| Ejection fraction (%) | 63.7 ± 9.5 | 63.5 ± 9.9 | 63.9 ± 92 | 0.93 |
| Hemoglobin (g/dL) | 11.4 ± 1.7 | 11.3 ± 1.7 | 11.5 ± 1.7 | 0.70 |
| Glucose (mg/dL) | 120.0 ± 54.2 | 120.7 ± 61.7 | 120.3 ± 50.8 | 0.98 |
ScvO2-central venous oxygen saturation; ASA: American Society of Anesthesiologists; values between brackets represent the median and percentile 25–75%.
During surgery, 50% of patients received blood transfusions, 32.5% vasoactive drugs, and 54.5% of them had complications, where circulatory shock was most prevalent (Table 2).
Table 2.
Patient characteristics and comparison between patients with [P(v-a)CO2] < 5.0 and [P(v-a)CO2] ≥ 5.0 in intraoperative.
| Variables | All patients (n = 66) |
[P(v-a)CO2] < 5.0 mmHg (n = 22) |
[P(v-a)CO2] ≥ 5.0 mmHg (n = 44) |
P |
|---|---|---|---|---|
| Transfusion (%) | 50.0 | 40.9 | 54.5 | 0.29 |
| Crystalloids (mL) | 7000 (4625–8875) | 5500 (4500–8650) | 7000 (5500–8875) | 0.44 |
| Colloids (mL) | 1000 (500–1000) | 500 (500–1250) | 1000 (500–1000) | 0.26 |
| Fluid balance (ml) | 800 (175–1420) | 400 (−250–875) | 800 (262–1750) | 0.13 |
| Vasopressors (%) | 32.5 | 23.5 | 40.5 | 0.27 |
| Length of surgery (hours) | 6.9 ± 2.4 | 7.0 ± 3.1 | 6.8 ± 1.9 | 0.83 |
Values between brackets represent the median and percentile 25–75%.
A ROC curve was created in order to establish the best [P(v-a)CO2] value, which discriminated hospital mortality in this population, where [P(v-a)CO2] = 5.0 mmHg was the cut-off between narrow [P(v-a)CO2] and wide [P(v-a)CO2], presenting sensitivity of 93.3% and specificity of 50.2%, ROC area of 0.73, P(area = 0.5) = 0.0006, 95% CI = 0.61 to 0.84 (Figure 1). Moreover, comparative ROC curves from lactate, BE and ScvO2 can demonstrate that [P(v-a)CO2] had the greatest AUC, respectively, 0.53 (0.41 to 0.67), 0.56 (0.43 to 0.69), and 0.71 (0.59 to 0.82).
Figure 1.
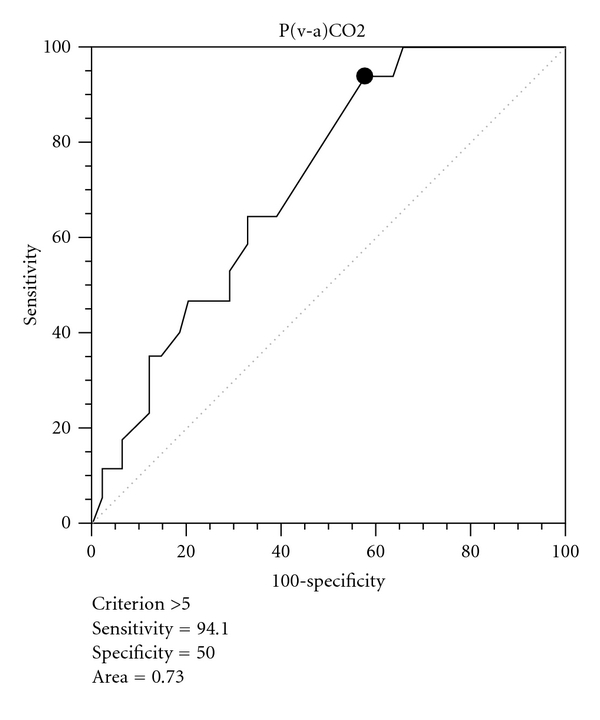
Roc curve from [P(v-a)CO2] and hospital mortality.
A Box plot graphic has compared survivors and non survivors in pre-, intra-, and postoperatively; it could show that only the preoperatively period presented statistical significance between them (Figure 2).
Figure 2.
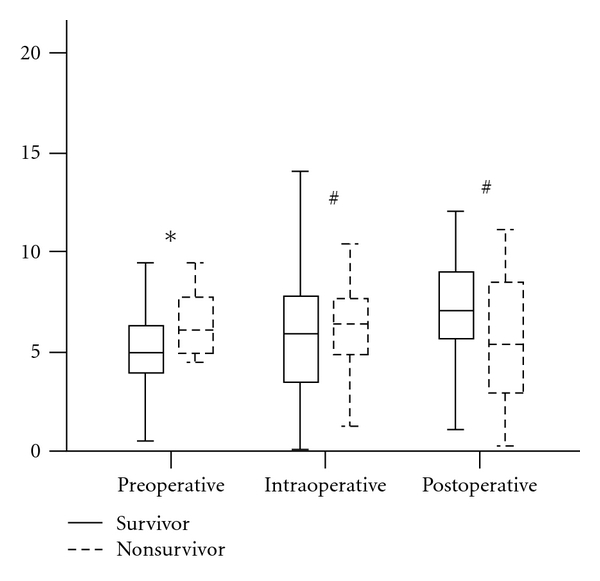
Box plot between [P(v-a)CO2] value from survivors and nonsurvivors (*P < 0.05; # P > 0.05).
Thus, when comparing the groups with narrow [P(v-a)CO2] and wide [P(v-a)CO2], although they present the same pre- and intraoperative demographic and clinical data, except for a lower ScvO2 and a higher ventricular dysfunction in patients with [P(v-a)CO2] ≥ 5.0 mmHg, we find that patients with [P(v-a)CO2] ≥ 5.0 mmHg had a higher hospital mortality and a higher incidence of complications in the postoperative period, mainly circulatory shock, renal failure, and ICU infection. (Tables 1, 2, 3, and 4)
Table 3.
Patient characteristics and comparison between patients with adequate [P(v-a)CO2] < 5.0 and [P(v-a)CO2] ≥ 5.0 in postoperative.
| Variables | All patients (n = 66) |
[P(v-a)CO2] < 5.0 mmHg (n = 22) |
[P(v-a)CO2] ≥ 5.0 mmHg (n = 44) |
P |
|---|---|---|---|---|
| Transfusion (%) | 18.2 | 18.2 | 18.2 | 1.00 |
| Crystalloids (mL) | 1900 (1000–2500) | 1500 (1000–2875) | 2000 (1000–2500) | 0.77 |
| Colloids (mL) | 500 (500–875) | 500 (500–750) | 500 (500–1000) | 1.00 |
| Duration of mechanical ventilation (hours) | 24 (12–24) | 12 (12–24) | 24 (12–30) | 0.07 |
Values between brackets represent the median and percentile 25–75%.
Table 4.
Outcomes.
| Variables | All patients (n = 66) |
[P(v-a)CO2] < 5.0 mmHg (n = 22) |
[P(v-a)CO2] ≥ 5.0 mmHg (n = 44) |
RR | P |
|---|---|---|---|---|---|
| Postoperative complications (%) | 54.5 | 40.9 | 61.4 | 1.73 | 0.09 |
| Shock | 45.5 | 22.7 | 56.8 | 2.83 | 0.01 |
| ARF | 19.7 | 4.5 | 27.3 | 5.15 | 0.02 |
| Platelet dysfunction | 19.7 | 13.6 | 22.7 | 1.55 | 0.38 |
| Infection | 16.7 | 4.5 | 22.7 | 4.20 | 0.05 |
| Acute pulmonary dysfunction | 10.6 | 9.1 | 11.4 | 1.18 | 0.77 |
| Confusional state | 7.6 | 0.0 | 11.4 | 0.64 | 0.10 |
| Length of ICU stay (days) | 3.0 (2.0–4.0) | 2.5 (1.0–4.0) | 3.0 (2.0–4.5) | 0.12 | |
| Hospital length of stay (days) | 20.0 (12.5–27.5) | 13.5 (9.0–25.0) | 20.0 (14.0–30.0) | 0.01 | |
| Hospital mortality (%) | 25.8 | 4.5 | 36.4 | 2.10 | 0.004 |
ARF: acute renal failure; ICU: intensive care unit; values between brackets represent the median and percentile 25–75%; RR: relative risk.
When patients with [P(v-a)CO2] < 5.0 mmHg and [P(v-a)CO2] ≥ 5.0 mmHg were compared, the group with [P(v-a)CO2] ≥ 5.0 mmHg had a lower survival probability, even when it was adjusted by MODS, ScvO2, ventricular dysfunction heart. HR = 2.07 IC95% 1.14–3.77 (Figure 3).
Figure 3.
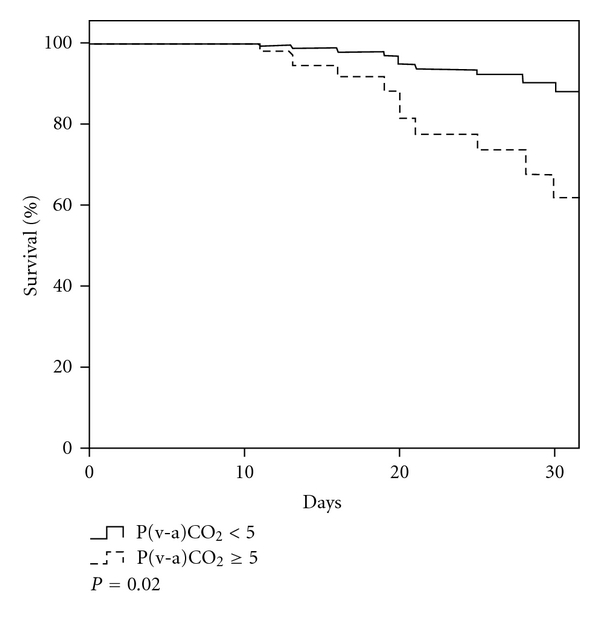
Kaplan Meier curve.
4. Discussion
Despite the large quantity of resources that are directed towards assessing perioperative risk and of cardiovascular complications, this study shows that a simple analysis of PCO2 gap ≥ 5.0 mmHg in the preoperative period is an important marker for postoperative complications, mainly shock, renal failure, infection, increased length hospital stay, and hospital mortality. Currently, few parameters are used at bed side to assess tissue hypoxia, such as urinary output, base differences, and blood lactate, but those parameters show that hypoperfusion is already installed and may be late to guide the onset of a hemodynamic resuscitation [18]. However, in this study, [P(v-a)CO2] has proven to be an early parameter to identify worse outcome in surgery patients.
The worse outcome of these patients can be explained by previous reports that have suggested the PCO2 gap as a marker for tissue hypoxia [19, 20]. In an animal model for acute hemorrhage, Van der Linden et al. [21] found a significant correlation between blood lactate and PCO2 gap. A progressive increase of [P(v-a)CO2] was observed during the dependence period of oxygen consumption and delivery (VO2/DO2) in another experimental model with progressive flow reduction [22]. High [P(v-a)CO2] values have been reported during cardiac arrest and cardiopulmonary resuscitation [19, 23]. However, in all those studies, tissue hypoxia was secondary to the reduction of blood flow.
Circulatory failure is followed by tissue CO2 accumulation. Tissue CO2 increases as a consequence of an accumulation of the carbon dioxide produced by the aerobic metabolism and by the tissue bicarbonate buffer effect, which is necessary to correct the excess of hydrogen ions during the anaerobic metabolism. When the cell concentration of bicarbonate ion (HCO3 −) and hydrogen ion (H+) rises, HCO3 − diffuses out of red blood cells into the plasma, but H+ cannot diffuse easily because the cell membrane is relatively impermeable to cations. Some of H+ liberated are bound to hemoglobin (Hb). This reaction occurs because reduced Hb is a better acceptor of H+ than the oxygenated Hb. In the peripheral blood the loading of CO2 is facilitated by the presence of reduced Hb (Haldane effect) [9].
Low blood flow can result in tissue hypercapnia. Under anaerobic metabolism conditions, it is expected that the production of CO2 (VCO2) decreases less than the consumption of O2 (VO2). In other words, the VCO2/VO2 ratio (respiratory quotient) should increase. According to the Fick equation, a low blood flow may result in an increase of [P(v-a)CO2], even without any additional production of CO2. This can be explained by the CO2-stagnation phenomenon [24] due to the slower microcirculation blood flow, which is larger than normal CO2 production; hypercapnia tends to be generated in the venous circulation.
Therefore, [P(v-a)CO2] is proportional to CO2 production and inversely related to cardiac output (Fick equation). Anaerobic CO2 production occurs when tissue hypoxia is present, mainly to protect bicarbonate ions against excess protons, produced as a secondary consequence of adenosine triphosphate hydrolysis [25].
In this respect, an increased [P(v-a)CO2] value has been reported in patients with low cardiac output and no global tissue hypoxia, as demonstrated by the normal lactate levels that were found [26]. This emphasizes the lack of specificity of [P(v-a)CO2] in detecting tissue hypoxia. In this study, we observed that even patients with inadequate [P(v-a)CO2] values presented normal lactate or base difference values. Serum lactate has been traditionally accepted like an indicator of anaerobic metabolism and of tissue hypoxia, but it has to be emphasized that, under normal conditions, the liver is capable of increasing the metabolism of the produced lactate; this means that, in hypoxia and anaerobic metabolism situations, a few hours may elapse between the onset of the phenomenon and the detection of elevated lactate levels in the blood [27], which explains why the lactate may not be as early as of the [P(v-a)CO2].
Otherwise, a normal [P(v-a)CO2] value may be associated with global tissue hypoxia in normal or hyperdynamic states, which was demonstrated by some authors [28]. This fact explains why an elevated venous flow is sufficient to clear the CO2 that is produced by the hypoxic cell, even if the production of CO2 is higher than normal due to the anaerobiosis that generates CO2 [24]. Indeed, Vallet and colleagues evidenced that PCO2 gap increased during low blood flow-induced tissue hypoxia (ischemic hypoxia) while it remained unchanged during hypoxemia-induced hypoxia (hypoxic hypoxia) [29]. We emphasize once again the poor sensitivity of [P(v-a)CO2] to detect tissue hypoxia in some cases. However, in this study, patients with an inadequate [P(v-a)CO2] had a lower ScvO2, which may be explained by the fact that ScvO2 is influenced by different variables [30], such as low blood flow; this might be occurring with the sample in question. This hypothesis is substantiated by a higher percentage of ventricular dysfunction in this group.
The prevalence of ventricular dysfunction in this study was evaluated, considering both the systolic and diastolic ventricular dysfunction, since their evolution as for morbidity and mortality is similar [13–16]. A greater prevalence of ventricular dysfunction was found in the inadequate [P(v-a)CO2] group. The EF—close to 65% in both groups—reflects a predominant diastolic dysfunction, which substantiates current data, which present diastolic dysfunction as a more relevant prognosis predictor than EF per se. [31–34].
However, Vallée et al. [35] found that a ScvO2 of over 70% in septic patients was not enough to reach adequate resuscitation, and that [P(v-a)CO2] ≥6.0 mmHg could be helpful to identify patients that were not adequately resuscitated. Mekontso-Dessap et al. [24] demonstrated that, under conditions of tissue hypoperfusion, defined as a blood lactate level of >2.0 mmol/L, a PvCO2-PaCO2/Ca-vO2 ratio of >1.4 was the best predictor of hyperlactatemia and a good prognostic index.
Thus, it is important to detect early a risk factor for low oxygen delivery due to low blood flow evaluated by PCO2 gap ≥5.0 mmHg in surgery patients, because changes in clinical management may be made in order to avoid an unfavorable outcome in this population. Several clinical studies have documented that and aggressive early resuscitation using defined protocols improve the patient's outcome [36, 37]. These studies used therapy strategies to increase the cardiac function and keep organ perfusion, which reduced the ICU length of stay, accelerated the recovery of the gastrointestinal function, and reduced the mortality rate in high-risk surgery patients [38]. Therefore, [P(v-a)CO2] could help identify patients that require such optimizations in the preoperative phase and specific measures taken in the preoperative or intraoperative period to ensure that the increase of blood flow could bring benefits to the postoperative period and improve the prognosis.
However, in this study, the [P(v-a)CO2] value that best discriminated mortality was lower than of those described previously in the literature. This can be probably explained by the fact that those studies discussed more severe patients with septic conditions that required high oxygen delivery, contrary to what was shown in the study in question, since their patients were in the preoperative period of elective surgeries, with low APACHE II values, most of them in ASA II physical condition. In addition, values of the inadequate [P(v-a)CO2] found in this evaluation may eventually mean that the patients were not well enough to undergo major surgeries, but tolerable for basal activities, which are not comparable to the stress experienced in a surgical procedure.
Another aspect that has to be discussed is related to the fact that inadequate [P(v-a)CO2] was found only in the preoperative and not in the intraoperative phase; it is important to highlight that in the intraoperative period some variables may interfere in the measurement of [P(v-a)CO2], such as the anesthesia and hypothermia that might occur during surgery; they may reduce cell respiration and, thus, CO2 production. Hypothermia reduces metabolism by 7% per grade, with reduction of ATP formation and reduction of cellular oxygen and cerebral glucose requirements, in addition, decrease in CO2 production and O2 consumption [39].
Other aspects can bring impact to change in [P(v-a)CO2], due to CO2 dissociation curve, which is curvilinear more than oxygen dissociation curve, and temperature, hematocrit, oxygen saturation, and pH influence the PCO2/CO2 content relationship an example is showed when metabolic acidosis worsens; the relationship is shifted to the right; it means that the same CO2 content value can determinate superior PCO2 value [9].
Besides, although in most studies that evaluated [P(v-a)CO2] patients were monitored by means of a pulmonary artery catheter [24], absence of this tool in this study does not limit the results that were found. By the other hand, the gastric tonometry has also been utilized as carbon dioxide monitoring [40], but this method assesses the regional tissue dysoxia, differently of the proposal from present study which has aimed to measure the systemically problem. Therefore, since the study's objective does not require the measures that are evaluated by these devices, this way can be cheaper.
The complications, except infection, were evaluated in 24 hours only, which makes a limitation, but the length of ICU stay of all patients was short, that is, median 3.0 days.
The sample sizes as well as the observational character of this study are limiting factors. Future researches are needed to validate this finding.
Therefore, patients who underwent major surgeries and that—in the preoperative period—presented a PCO2 gap ≥5 mmHg had a worse prognosis in the postoperative. This marker may turn out to be important to stratify risk and suggests a useful additional tool for perioperative management.
Conflict of Interests
The authors declare that there is no conflict of interests.
Authors' Contribution
J. M. S. Jr. conceived of this study, participated in the design of the study, performed the statistical analysis, and drafted the paper. A. M. R. R. Oliveira participated in the design of the study, performed the collection of data and the statistical analysis, and drafted the paper. J. L. Segura, M. H. Ribeiro, and C. N. Sposito performed the collection of data. D. O. Toledo, E. Rezende, and L. M. S. Malbouisson helped in revising of the draft, the paper and helped in the final revision of writing the paper. All authors read and approved the final paper.
References
- 1.Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia. 2008;63(7):695–700. doi: 10.1111/j.1365-2044.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- 2.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 3.Older P, Smith R, Courtney P, Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993;104(3):701–704. doi: 10.1378/chest.104.3.701. [DOI] [PubMed] [Google Scholar]
- 4.Mayr VD, Dünser MW, Greil V, et al. Causes of death and determinants of outcome in critically ill patients. Critical Care. 2006;10(6, 154) doi: 10.1186/cc5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran DD, Groeneveld AB, van der Meulen J, Nauta JJ, van Schijndel RJS, Thijs LG. Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Critical Care Medicine. 1990;18(5):474–479. doi: 10.1097/00003246-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Groeneveld AB, Vermeij CG, Thijs LG. Arterial and mixed venous blood acid-base balance during hypoperfusion with incremental positive end-expiratory pressure in the pig. Anesthesia and Analgesia. 1991;73(5):576–582. [PubMed] [Google Scholar]
- 7.Durkin R, Gergits MA, Reed JF, III, Fitzgibbons J. The relationship between the arteriovenous carbon dioxide gradient and cardiac index. Journal of Critical Care. 1993;8(4):217–221. doi: 10.1016/0883-9441(93)90005-6. [DOI] [PubMed] [Google Scholar]
- 8.Brandi LS, Giunta F, Pieri M, Sironi AM, Mazzanti T. Venous-arterial PCO2 and pH gradients in acutely ill postsurgical patients. Minerva Anestesiologica. 1995;61(9):345–350. [PubMed] [Google Scholar]
- 9.Lamia B, Monnet X, Teboul JL. Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiologica. 2006;72(6):597–604. [PubMed] [Google Scholar]
- 10.Cuschieri J, Rivers EP, Donnino MW, et al. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Medicine. 2005;31(6):818–822. doi: 10.1007/s00134-005-2602-8. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Critical Care Medicine. 1995;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 13.Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. Journal of Cardiology. 2010;55(1):13–22. doi: 10.1016/j.jjcc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. New England Journal of Medicine. 2006;355(3):260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. Journal of the American College of Cardiology. 2007;50(8):768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD) Circulation Journal. 2009;73(10):1893–1900. doi: 10.1253/circj.cj-09-0254. [DOI] [PubMed] [Google Scholar]
- 17.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Critical Care Medicine. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 18.Rezende E, Silva JM, Jr., Isola AM, Campos EV, Amendola CP, Almeida SL. Epidemiology of severe sepsis in the emergency department and difficulties in the initial assistance. Clinics. 2008;63(4):457–464. doi: 10.1590/S1807-59322008000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weil MH, Rackow EC, Trevino R, Grundler W, Falk JL, Griffel MI. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. New England Journal of Medicine. 1986;315(3):153–156. doi: 10.1056/NEJM198607173150303. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BA, Weil MH. Redefining ischemia due to circulatory failure as dual defects of oxygen deficits and of carbon dioxide excesses. Critical Care Medicine. 1991;19(11):1432–1438. doi: 10.1097/00003246-199111000-00021. [DOI] [PubMed] [Google Scholar]
- 21.van der Linden P, Rausin I, Deltell A, et al. Detection of tissue hypoxia by arteriovenous gradient for PCO2 and pH in anesthetized dogs during progressive hemorrhage. Anesthesia and Analgesia. 1995;80(2):269–275. doi: 10.1097/00000539-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Vincent JL. Arteriovenous differences in PCO2 and pH are good indicators of critical hypoperfusion. American Review of Respiratory Disease. 1993;148(4):867–871. doi: 10.1164/ajrccm/148.4_Pt_1.867. [DOI] [PubMed] [Google Scholar]
- 23.Adrogue HJ, Rashad MN, Gorin AB, Yacoub J, Madias NE. Assessing acid-base status in circulatory failure. Differences between arterial and central venous blood. New England Journal of Medicine. 1989;320(20):1312–1316. doi: 10.1056/NEJM198905183202004. [DOI] [PubMed] [Google Scholar]
- 24.Mekontso-Dessap A, Castelain V, Anguel N, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Medicine. 2002;28(3):272–277. doi: 10.1007/s00134-002-1215-8. [DOI] [PubMed] [Google Scholar]
- 25.Randall HM, Jr., Cohen JJ. Anaerobic CO2 production by dog kidney in vitro. The American Journal of Physiology. 1966;211(2):493–505. doi: 10.1152/ajplegacy.1966.211.2.493. [DOI] [PubMed] [Google Scholar]
- 26.Teboul JL, Mercat A, Lenique F, Berton C, Richard C. Value of the venous-arterial Pco2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Critical Care Medicine. 1998;26(6):1007–1010. doi: 10.1097/00003246-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Critical Care Medicine. 2004;32(8):1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 28.Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101(2):509–515. doi: 10.1378/chest.101.2.509. [DOI] [PubMed] [Google Scholar]
- 29.Vallet B, Teboul JL, Cain S, Curtis S. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. Journal of Applied Physiology. 2000;89(4):1317–1321. doi: 10.1152/jappl.2000.89.4.1317. [DOI] [PubMed] [Google Scholar]
- 30.Silva JM, Jr., Toledo DO, Magalhães DD, et al. Influence of tissue perfusion on the outcome of surgical patients who need blood transfusion. Journal of Critical Care. 2009;24(3):426–434. doi: 10.1016/j.jcrc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Little WC, Oh JK. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation. 2009;120(9):802–809. doi: 10.1161/CIRCULATIONAHA.109.869602. [DOI] [PubMed] [Google Scholar]
- 32.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Journal of the American Society of Echocardiography. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Brucks S, Little WC, Chao T, et al. Relation of anemia to diastolic heart failure and the effect on outcome. American Journal of Cardiology. 2004;93(8):1055–1057. doi: 10.1016/j.amjcard.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 34.Sturgess DJ, Marwick TH, Joyce C, et al. Prediction of hospital outcome in septic shock: a prospective comparison of tissue Doppler and cardiac biomarkers. Critical Care. 2010;14(2):p. R44. doi: 10.1186/cc8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallée F, Vallet B, Mathe O, et al. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Medicine. 2008;34(12):2218–2225. doi: 10.1007/s00134-008-1199-0. [DOI] [PubMed] [Google Scholar]
- 36.Fenwick E, Wilson J, Sculpher M, Claxton K. Pre-operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost-effectiveness analysis. Intensive Care Medicine. 2002;28(5):599–608. doi: 10.1007/s00134-002-1257-y. [DOI] [PubMed] [Google Scholar]
- 37.Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Archives of Surgery. 1995;130(4):423–429. doi: 10.1001/archsurg.1995.01430040085019. [DOI] [PubMed] [Google Scholar]
- 38.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Critical Care Medicine. 2002;30(8):1686–1692. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Aslami H, Juffermans NP. Induction of a hypometabolic state during critical illness—a new concept in the ICU? Netherlands Journal of Medicine. 2010;68(5):190–198. [PubMed] [Google Scholar]
- 40.Marik PE. Regional carbon dioxide monitoring to assess the adequacy of tissue perfusion. Current Opinion in Critical Care. 2005;11(3):245–251. doi: 10.1097/01.ccx.0000158091.57172.f9. [DOI] [PubMed] [Google Scholar]


