Abstract
Bronchiolitis obliterans syndrome (BOS) is a progressive, insidious, and often fatal lung allo-reaction that can occur following allogeneic hematopoietic stem cell transplantation (HCT) or allogeneic lung transplantation. Current estimates in the literature suggest that approximately 2–3% of all allogeneic HCT recipients and 6% of patients who develop chronic GVHD will develop this syndrome. However, based on newer data it is likely that the true incidence of BOS is higher. Unfortunately, the survival and treatment of patients with BOS after HCT has not improved over the last 20 years. Attempts at clinical trials have been hindered by the lack of uniform diagnostic criteria and inability to detect the syndrome at a reversible stage in its natural history. Recently, the NIH consensus project for criteria in chronic GVHD has made recommendations regarding the diagnosis of BOS and monitoring of lung disease among long term survivors. Although a rare and poorly understood manifestation of chronic GVHD, BOS occurs commonly after lung transplantation and is similar in pathology, clinical presentation, radiographic presentation, and presumed immunologic pathogenesis. This review describes the current understanding of the epidemiology and pathogenesis of BOS and presents information on evaluations and therapies for patients with BOS after HCT.
Keywords: Bronchiolitis obliterans, chronic graft-versus-host, allogeneic transplantation
INTRODUCTION
The significant decrease in early transplant related mortality after allogeneic hematopoietic cell transplantation (HCT) increased the number of patients living long enough to experience late complications of transplantation. Chronic GVHD is the most common late complication of allogeneic HCT and when lungs are affected by chronic GVHD bronchiolitis obliterans syndrome (BOS) ensues. Unfortunately, the survival and treatment of patients with BOS have not improved over the last 20 years.[1,2] Attempts at clinical trials have been hindered by the lack of uniform diagnostic criteria. Recently, the NIH consensus project for criteria in chronic GVHD has made recommendations regarding the diagnosis of BO and monitoring of lung disease among long term survivors.[3] Challenges to the progress in medical management of BOS include little knowledge about pathogenesis to direct effective therapies and delay in diagnosis. This article provides an overview of the epidemiology, diagnosis and treatment of BOS and the immunobiology of BOS after lung transplantation, a better understood disease similar to BOS after HCT pathologically, clinically and radiographically.
EPIDEMIOLOGY AND PATHOGENESIS OF BRONCHOLITIS OBLITERANS AFTER ALLOGENEIC CELL TRANSPLANTATION
BOS is a rare complication of allogeneic HCT, characterized by the new development of fixed airflow obstruction after allogeneic HCT. Depending upon the definition used to define BOS, the prevalence of this syndrome ranges widely from approximately 2–3% among all allogeneic HSCT recipients to 6% of patients who develop chronic GVHD.[1,4–6] Studies that have used more relaxed definitions have suggested that the prevalence may be even higher. We hypothesized that because this syndrome is characterized by airflow obstruction, early rapid airflow decline, defined as a an annualized rate of one-second forced expiratory volume (FEV1) decline of 5% per year, might identify a population at higher risk for poor outcomes.[7] This study revealed that among all patients who receive an allogeneic transplant, 26% developed significant airflow decline, and among patients who developed chronic GVHD, 30% had significant airflow decline after transplant. Rapid airflow decline was also associated with significant attributable mortality rates of 9% at 3 years, 12% at 5 years, and 18% at 10 years after transplant. Mortality was much higher for the subpopulation of patients with chronic GVHD (22% at 3 years, 27% at 5 years, and 40% at 10 years). These results suggested that the prevalence of airflow obstruction after allogeneic HCT may have been underestimated previously, and the presence of rapid early airflow decline within the first year after transplant is associated with significantly increased risk for mortality even among long term survivors. As an effort to provide a uniformed definition of BOS for clinical management and clinical trials, the NIH recently provided new guidelines for the diagnosis of non-pathologically confirmed BOS (see below).[3] Using this stringent definition, we conducted an analysis that revealed an overall BOS prevalence of approximately 5.5%, with 10% in patients surviving at least one year after transplant and 16% of all patients with chronic GVHD (Jason Chien, personal communication). Again, these data suggest that BOS is likely under recognized in the transplant community.
Many risk factors have been found to be associated with the development of BOS after allogeneic HCT. These include low IgG levels, the use of peripheral blood stem cells, the use of busulfan or methotrexate during the transplant process, the intensity of the conditioning employed, poor pre-transplant lung function and respiratory infection during the first 100 days post transplantation.[4,6–10] The clinical factor most commonly associated with the development of BOS is the presence of GVHD at another site.[11–16] In study by Dudek et al, 81% of all of the BOS cases was diagnosed with GVHD prior to the onset of BOS.[1] Even in our study assessing a milder definition of airflow obsruction, all of the patients who had significant early airflow decline had some form of GVHD at a site other than the lung.[7] In the same study, older age at transplant, poor lung function at baseline, and respiratory viral infection within the first 100 days after transplant were also identified as risk factors for early airflow decline. Additional analysis of the early respiratory viral infections revealed that most respiratory viral infections during the first 100 days after HCT result in fixed airflow obstruction at one year and lower respiratory tract parainfluenza virus infection was associated with the highest risk for fixed airflow obstruction at one year (odds ratio, 17.9 [95% confidence interval, 2.0–160]; P=.01).[17]
Examination or pathologic samples have contributed to our understanding of BOS. Biopsy specimens usually demonstrate bronchiolitis involving the small airways and fibrinous obliteration of the lumen of the respiratory bronchioles, with or without associated interstitial pneumonia, fibrosis, or diffuse alveolar damage; inflammatory cell infiltrates consisting of neutrophils and mononuclear cells, the former more often in the lumens of the affected bronchioles, are prominent early in the disease process.[18,19] In the chronic phase, there are variable degrees of intralumenal or peribronchiolar fibrosis, ranging from proliferation of fibroblasts and myofibroblasts to collagen scarring. This leads to progressive circumferential fibrosis and ultimate cicatrization of the small terminal airways, manifesting as new fixed airflow obstruction.
Despite all the advances in stem cell transplantation over the last two decades, the cause of BOS is still unknown. Allorecognition of lung antigens is the suspected etiology of this disease because the two clinical situations associated with BOS involve alloimmunity, lung transplantation—host (hematopoietic cells)-versus-graft (lung) disease, and HSCT—graft (hematopoietic cells)-versus-host (lung) disease. Additionally, when BOS occurs after HSCT, it is typically accompanied by alloimmune manifestations in other organs, e.g. liver, eyes, or skin. Indeed, the lung epithelium may be the target of immune mediated-injury induced by donor cytotoxic T cells in chronic GvHD[6], supporting the hypothesis that BOS is a manifestation chronic GvHD in the lung. Unfortunately, there are no direct data to support this statistical correlation; animal models of graft vs. host reactions in the lungs primarily result in alveolitis and interstitial infiltration with lymphocytes and associated chemokines and cytokines such as tumor necrosis factor alpha. Unfortunately, all of these studies have been conducted using mouse models of host versus graft disease, which is more representative of lung transplantation.[18,20–24]
There is also substantial evidence from studies in other airway diseases associated with chronic airway inflammation that endotoxin-induced cellular activation and other components of the innate immune system may play an important role in the pathogenesis, and that the innate immune response is likely under genetic control.[25–28] To test the hypothesis in the BOS setting, we preformed a study using a gene-wide approach to determine if genetic variation in the innate immune pathway is associated with the development of AFO following HCT.[29] Single marker tagSNP analysis of 15 genes in the innate immunity pathway demonstrated significant associations with the patients BPI genotype for rs 33065, p=0.038; and rs 36045, p=0.025. Multimarker haplotype analysis of the BPI tagSNPs demonstrated that 4 haplotypes in the patient and donor samples were significantly associated with a two to three-fold increase in risk for developing airflow decline (p-values 0.004 to 0.038). In a validation study carried out in an independent cohort of patients, an association with 9 haplotypes in the BPI gene was detected (p-values 0.013 to 0.043), suggesting that genetic variants on the BPI gene or in linkage disequilibrium with the BPI tagSNP haplotypes identified may influence the risk for developing HCT-related AFO. Hildebrant et al conducted an analysis of the NOD2/CARD15 variants (SNP8 [Arg702Trp], SNP12 [Gly908Arg], and SNP13 [Leu1007fsinsC]) and found that the cumulative incidence of BO rose from 1.3% in donor-recipient pairs without mutation to 18.7% in pairs with donor or recipient NOD2/CARD15 variants (P < .001), and the recipient NOD2/CARD15 variants alone led to BO in 22.3% (P < .001), whereas donor variants alone associated with BO in 13.2% (P = .04).[30] Similar studies in lung transplantation have also found genetic associations between CD14 variants and lung transplant outcomes.[31]
BRONCHIOLITIS OBLITERANS SYNDROME IN LUNG ALLOGRAFT RECIPIENTS
Bronchiolitis obliterans syndrome (BOS) that occurs after allogeneic lung transplantation shares a number of common features with the BOS of allogeneic HCT recipients. The histological features and clinical manifestations of BOS are virtually indistinguishable among these transplantation populations. In the case of pulmonary transplantation, experimental evidence affirms BOS is a sequela of recipient (host) adaptive immune processes that are triggered by T-cells with reactivity for allogeneic donor lung (graft) epitopes.[32–36] BOS among HCT recipients results from grafted (donor) hematologic cell responses that target allogeneic lung epitopes (in addition to other organs) of the hosts (recipients).[37] Thus, the immunopathogenesis of BOS in lung transplant patients is a “mirror image” of the abnormality among HCT recipients. The requisite feature of both cases is the presence of immune effector cells, particularly T-cells, which mount chronic rejection responses against lung alloantigens. As such, insights relevant to the pathogenesis of BOS in either transplant population could have useful application in the other patient group.
BOS eventually occurs in >50% of long-surviving lung transplant recipients[38], and thus is far more prevalent than after HCT. The increased BOS frequency of lung transplantation is probably a reflection of greater donor:recipient alloantigen disparities. Unlike HCT, lung allografts are only rarely and fortuitously HLA matched with recipients, since the logistics of these donor organ procurements are so problematic. BOS is overwhelmingly the single most frequent serious complication of lung transplantation, and the prevalence of this complication has not appreciably diminished for more than two decades.[38,39]
Contributing factors for the development of BOS
Multiple factors are known to increase risks for BOS after lung transplantation. The number of HLA donor:recipient mismatches are correlated with increased incidences of BOS.[40] Acute rejection (i.e., lymphocytic infiltrations) is near ubiquitous in transplant recipients, and the severity and number of these episodes are also correlated with risks for later BOS.[41,42] Anti-allo-HLA IgG antibodies are produced among many (albeit not all) lung transplant recipients, and the presence and quantity of these immunoglobulins are also associated with development of BOS.[43] However, the specific contribution of these humoral responses to BOS pathogenesis is uncertain, given that analogous chronic rejection injuries also occur among B-cell knock-out animals.
BOS is also more frequent among lung transplant recipients who had ischemia-reperfusion injuries of the newly implanted donor lung[44], developed pulmonary infections, particularly with cytomegalovirus (CMV)[45], or have certain other co-morbidities, including gastroesophageal reflux disease (GERD).[40] Although often described as “allo-independent” factors, these co-morbidities almost certainly exert their deleterious effects by enhancing immunologic recognition of the allograft[39]. Nonspecific injuries (e.g., lung ischemia, gastric content aspiration, etc.) cause increased production of pro-inflammatory mediators and up-regulation of donor organ HLA. CMV and other infections also have many potent pro-inflammatory effects, including productions of viral-specific proteins that mimic HLA or act as superantigens and promiscuously (non-specifically) activate large proportions of recipient T-cells[46,47]. Lung ischemia and GERD are both common among normal humans, as well as recipients of other solid organ transplantations, and does not cause BOS in either populations. CMV pneumonia has also been implicated in chronic rejection of other transplanted solid organs, but without concurrent development of BOS[46,48].
Alloantigen recognition
It has long been known that recognition of alloantigens by recipient T-cells is a critical initial step in the cascade of events leading to chronic allograft rejection[49]. Solid organ allografts persist indefinitely in T-cell depleted experimental animals, despite complete donor:recipient MHC disparities. On the other hand, these same allografts undergo inexorable graft rejection after adoptive transfers of T-cells that are allogeneic to the donor organ.[32–36] In addition, manipulations that result in recipient T-cell tolerance for donor alloantigens obviate BOS[34].
Sequential intrathymic positive and negative selections limit survival and maturation of nascent thymocytes to only those cells that bear antigen receptors (TCR) with low avidity for self-HLA-peptide complexes.[50] However, since recipients have not undergone prior negative selection of thymocytes that are reactive with donor alloantigens, their T-cell populations include unusually high proportions of precursors with often considerable allo-avidity. Conventional peptide antigens (e.g., influenza hemagglutinin epitopes) are typically recognized by 1 out of 10,000–100,000 T-cells. However, the initial precursor frequency for allo-antigen responses is 100-to-1000 times greater.[49,50] Moreover, these responses are also magnified by clonal proliferations of the allo-antigen reactive T-cells.[51,52] Thus, immune responses to alloantigens are far more brisk and intense than those evoked by conventional peptide antigens, which accounts for the difficulty of allograft rejection prevention (or treatment) by medical therapies.
Inflammatory responses to allografts
Inflammatory infiltrations into experimental allografts follow a staged, sequential time course. These animal models include heterotopic tracheal implantations into mice, orthotopic lung transplantations in higher species, and recent development of human-murine chimeras.[32–36,53,54] These models generally show that recipient T-cells are present in the allograft within a few days, followed by successive waves of macrophages, neutrophils, and lesser numbers of other leukocytes, including B-cells.
Interestingly, T-cell numbers, and eventually other inflammatory cell infiltrations, within the allograft later tend to diminish, particularly after extensive progression of airway fibroproliferation. It is not uncommon in both humans and animal models for fully developed BOS histological lesions to appear nearly bereft of inflammatory cells.[55]
Diagnosis of BOS after lung transplant
The Gold Standard for diagnosis of bronchiolitis obliterans (BO) is demonstration of typical fibroproliferative lesions on histological evaluations of lung specimens.[55] Transbronchial biopsies (TBB) obtained by bronchoscopy are often used as the initial diagnostic procedure. The diagnostic sensitivity of TBB is notably limited (~20–50%), however, due to the heterogeneity of BO, and small biopsy size.[38,40,56] Open lung biopsy or video assisted thorascopic (VAT) procedures are far more sensitive, but also far more invasive, and are generally reserved for unusual or confusing diagnostic problems. In most cases, a surrogate diagnosis of BO is established in lung transplant recipients by spirometric findings of expiratory airflow obstruction (i.e., the term bronchiolitis obliterans syndrome or BOS).[39,56] Although pulmonary function tests are clinically expedient, the concordance between BOS and histologically proven BO has not really be established, and by some indications spirometry has far from perfect sensitivity and specificity.[42]
The biggest problem with physiologic testing, however, is the inability of these measures to detect impending or even very early allograft injury. The development of overt expiratory airflow obstruction indicates BOS is widespread and advanced. It seems likely that by the time the airway lesions are detectible by spirometry, the fibroproliferative abnormality has already progressed to irreversibility, which probably accounts for the refractoriness of BOS to medical treatment.
Biomarkers of BOS
The identification of biomarkers that accurately predict imminent BOS, or detect very early disease at a stage when the allo-injury may be more amenable to treatment, could represent a significant advance. The clinical utilization of assays based on global markers of immunologic activity or nonspecific lung injury is usually limited by their inability to distinguish BOS from infections, acute rejection or, in the case of single lung transplantations, underlying processes of the remaining native, diseased lung. No biomarkers for BOS have yet been validated in large prospective cohorts.
We have shown in cross-sectional studies that peripheral T-cell clonal expansions, particularly among CD4 T-cells, are unusually frequent and extreme in lung transplant recipients with BOS. These clonal expansions were also evident in some patients before they developed expiratory airflow obstruction.[51,52]
We have lately examined surrogate markers of CD4 T-cell clonality, in order to develop a more facile assay for monitoring the intensity of chronic allo-rejection.[57] Nearly all normal human CD4 T-lymphocytes express the co-stimulatory molecule CD28 on their cell surfaces. However, CD28 becomes down-regulated on T-cells that have undergone repeated (clonal) divisions, and proportions of CD28null T-cells are often increased among patients with chronic adaptive immune diseases.[58] In addition to loss of cell surface CD28, the CD4+CD28null cells also develop unusual and potentially pathogenic phenotypes that include expression of killer immunoglobulin-like receptors (KIR), autonomous production of IFN-γ and TNF-α, as well as production of cytotoxic mediators perforin and granzyme B, which are otherwise never seen among “normal” CD4 T-cells. We hypothesized CD4+CD28null cells may also be present in the circulation of lung transplant recipients, especially among those with particularly intense chronic allograft responses.
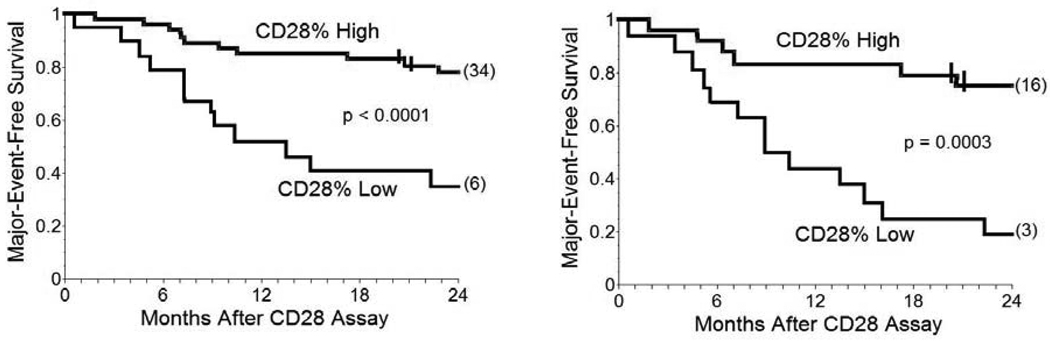
The CD4+CD28null lymphocytes we found in lung transplant recipients also had many pathogenic characteristics, including relative resistance to anti-proliferative effects of cyclosporine.[57] Most importantly, the presence of increased proportions of CD4+CD28null cells was associated with worse prognoses among individual lung transplant recipients, irrespective of their status at the time of the T-cell assays. Thus, CD28 quantitation was able to predict many of the subsequent clinical deteriorations among recipients with normal spirometry at the time of testing, as well as identify most of the lung transplant recipients who were destined for poor outcomes (Figure 1). Neither acute rejection nor pulmonary infections confounded these CD28 determinations.
Figure 1. Survival curves showing cumulative freedom from major adverse events (death or necessity for re-transplantation) of lung transplant recipients stratified by their CD28 expressions.
Left panel: Major adverse event-free survival of CD28% High (n = 46) and CD28% Low (n = 19) among all recipients (including subjects with either BOS and no evidence of rejection). CD28% High denotes subjects with CD4+CD28+/CD4+Total ≥0.90. CD28% Low denotes subjects with CD4+CD28+/CD4+Total <0.90. Values for CD28% among non-transplanted healthy, age-matched normal individuals are ~0.98 ± 0.02. Tick marks denote interval censored events, and numbers in parenthesis at end of the survival curves denote remaining, unafflicted subjects that were censored at 24 months of observation. Comparisons were made by log-rank. Right panel: Survival curves showing cumulative freedom from major adverse events of CD28% High (n = 24) and CD28% Low (n = 16) among the recipients with BOS. Reprinted from reference[56] with permission.
Selected assays of peripheral T-cells may provide insights into the pathogenesis of BOS and/or have clinical utility to predict imminent allograft injuries. A longitudinal prospective study of CD28 expression among a lung transplant recipient cohort is ongoing, as are investigations using a humanized mouse model for in vivo generation and characterization of human CD4+CD28null T-cells[53], and further work on methods to induce specific tolerance to donor alloantigens.[59]
CLINICAL ASSESSMENT OF BOS AFTER HCT
Because patients are often asymptomatic in the early stages of BOS, diagnosis is often delayed until significant air flow obstruction has occurred leading to dyspnea on exertion and poor exercise tolerance.[60] Other signs and symptoms may include hyperinflation on examination or chest x-ray, crackles or wheeze by auscultation and a nonproductive cough by history. Both because patients with BOS are at particular risk for infections and because infections may mimic BOS signs and symptoms, a thorough infectious disease work-up is crucial. Importantly, BOS patients should be distinguished from those with cryptogenic organizing pneumonia (COP), also known as bronchiolitis obliterans organizing pneumonia (BOOP). COP typically presents with fever, productive cough, consolidated infiltrates on chest CT, and has an excellent response rate to steroid therapy. In contrast, BOS is rarely associated with fever and infiltrates and is often progressive despite immunosuppressive modulations. Thus, the initial work-up for a patient with new onset air flow obstruction should include: pulmonary function tests (PFTs), inspiratory and high resolution expiratory chest CT, and comprehensive infectious disease evaluation (including bronchoalveolar lavage with stains and cultures for viral, bacterial, fungal, and mycobacterial pathogens, blood cultures, CMV PCR, and nasopharyngeal wash for respiratory viruses), and a thorough chronic GVHD evaluation. We also recommend an echocardiogram to assess pulmonary artery pressures and a 6-minute walk evaluation for oxygenation status in patients with likely BOS. For patients diagnosed with infections, a follow-up pulmonary evaluation should be done after resolution to evaluate for BOS.
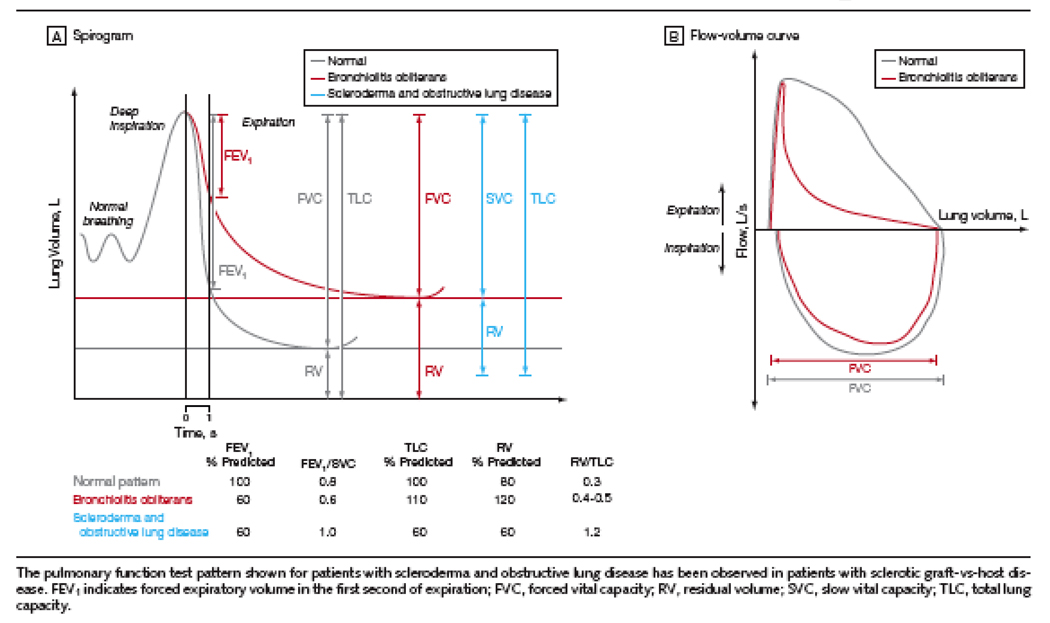
The early diagnosis of BOS remains a challenge; this probably contributes to the poor prognosis of this disease since optimal therapies are likely to be most effective in the earliest stages of disease. By pulmonary function test, Forced Expiratory volume at 1 second (FEV1) is the most sensitive marker of emerging obstructive disease and severity of BOS[1] (Figure 2). Thus, FEV1 decline in a given patient post-HCT is likely the best way to identify patients at risk to develop significant BOS disease[7], using an annualized decline in FEV1 of greater than >5%/ year with an FEV1/FVC ratio < 0.8 to alert physicians to the potential for progression to BOS. Frequent monitoring of PFTs in the post-HCT period may ultimately improve diagnosis of patients in early stages of BOS, although this has yet to be tested and would commend monetary considerations.
Figure 2.
Hypothetical Spirogram and Flow Volume Curves for Bronchiolitis Obliterans Syndrome. Copied with permission from Journal of American Medical Association[70]
Recently, the NIH chronic GVHD consensus project developed a definition of BOS[3] (Table 1) in an effort to standardize definitions, thereby enhancing comparisons among publications on chronic GVHD manifestations between trial centers. This definition required demonstration of the characteristic findings of airflow obstruction by: 1) decreased FEV1 (FEV1<75% predicted), AND 2) evidence of airway obstruction with FEV1/FVC <0.7, and air trapping: 3) elevated residual volume of air (RV>120%) and 4) air trapping on expiratory CT or lung biopsy[3], with lung biopsy or 5) another manifestation of chronic GVHD[3].
Table 1.
NIH Consensus BOS Definition and proposed modification
| NIH Consensus BOS Clinical Definition | Proposed Amended NIH BOS Clinical Definition |
|---|---|
| Absence of Infection | Absence of Infection |
| Another chronic GVHD manifestation | Another chronic GVHD manifestation |
| FEV1 < 75% predicted | FEV1 < 75% predicted OR Decline >10% |
| FEV1/FVC < 0.7 (ratio) | Sign of Obstruction: FEV1/SVC < 0.7 (ratio) OR RV OR RV/TLC > 120% AND CT: air trapping |
| RV > 120% | |
| CT: air trapping or bronchiectasis |
Retrospective evaluation of a cohort of BOS patients at NIH with clinically identified (n=15) or clinically identified with biopsy proven disease (n=7) revealed only 18% (4/22) of patients met the NIH consensus definition for a clinical diagnosis of BOS, all of which had an FEV1≤ 55% suggestive of severe disease. This low capture rate highlighted the need for thoughtful evaluation of diagnostic approaches to BOS. While lung biopsy is definitive for diagnosis, the complication rate of 13% (including persistent air leak and death) has diminished enthusiasm for this approach[61–64]. Review of the literature suggested that patients may have falsely elevated ratios of FEV1/FVC due to dynamic airway obstruction (early collapse of airways during forced expiration) and that slow vital capacity (SVC) may better reflect the true vital capacity and reveal ‘hidden’ obstruction in most BOS patients. Despite this, there was evidence that patients with myositis or manifestations of GVHD similar to scleroderma might still evade detection due to external restriction of air movement. This decrease in excursion could further reduce the vital capacity, masking obstructive disease.[65,66] The ratio of elevated Residual Volume over Total Lung Capacity (RV/TLC) could be used to reveal air trapping in these patients similar to patients with scleroderma and obstructive lung disease.[67,68] In combination with CT evidence of air trapping, a specific finding of BOS[69], these parameters may be able to identify patients with early obstruction even in the presence of severe restrictive diseases of myositis or sclerotic GVHD. In a recent publication, we proposed that these criteria be modified to capture patients with earlier disease for interventions with minimal risk, reserving more stringent criteria for diagnosis and therapies with greater risks (Table 1)[70].
Treatment of BOS
The prognosis for BOS is poor with an overall survival rate of 44% at 2 years and 13% at 5 years[1,7,71,72]. Despite advances in immunosuppressive regimens and supportive care, these dismal outcomes are unchanged between reports from the earliest and recent publications on BOS after HCT: 1989 to 2003.[1,60] Nonetheless, due to the presumed alloimmune pathogenesis of disease, immunosuppressive agents still comprise the backbone of BOS treatment including: calcineurin inhibitors, sirolimus, corticosteroids, azathioprine, and antithymocyte globulin. Data from lung transplantation literature suggests that these agents are likely to be beneficial by decreasing the rate of progression of BOS.[73,74] Current recommendations for BOS therapy include: high dose systemic corticosteroids (1mg/kg/day) for a protracted course with expected “improvements” in 8–20%, of which most are likely transient given the poor overall survival.[1,75–77] Azithromycin and inhaled steroids have been tested in small clinical trials of patients with BOS after HCT with evidence for some benefit by pulmonary function tests.[78,79] Anecdotal reports of efficacy for the stabilization of BOS include: extracorporeal photopheresis (6/7 patients stable disease), tumor necrosis factor blockade (1/1 patient stable disease), imatinib (1/1 patient improved)[75,80–83]. Finally, novel agents such as leukotriene inhibitors and statins have emerged as possible therapies.[84,85] Human and murine studies have demonstrated high levels of leukotrienes with BOS after lung transplant and pulmonary fibrosis[86–88] and a pilot study with montelukast (a leukotriene inhibitor) showed benefit in 3 of 5 patients with BOS after HCT[85].
In addition to immunosuppressive therapies, supportive care is critical for this very ill population. Hypoxia with exertion may be missed with spot check saturations in a waiting room after rest; saturation after a 6 minute walk will reveal the necessity of supplemental oxygen. Consideration of contributing factors to poorer pulmonary status should include: methemoglobinemia (PJP prophylaxis agents), beta-blockers (vasoconstriction), and Ace-inhibitors (cough). Most importantly, investigation should be frequent for active and potential pulmonary infections in patients with BOS. Of the 34 cases of BOS reported after death, 50% succumbed to infectious complications rather than pulmonary progression[1,66]. Risk factors are likely to include immunosuppression (especially protracted steroid therapy), severity of disease, and other complicating factors that occur with chronic GVHD such as aspiration, poor nutrition, and lymphopenia.
Given the poor prognosis of BOS after HCT, clinical trials are currently underway to test novel therapies including: montelukast (NCI and FHCRC, Seattle) and symbicort (Paris). A U54 grant by the Office of rare disease was recently funded to develop trials for BOS after HCT. Outcomes from these trials and future studies will hopefully provide insight into the mechanisms of this rare complication of HCT and provide avenues for better identification of patients at risk for BOS disease, those who have developed early disease, and which therapies are most likely to benefit these patients, to ultimately improve survival and quality of life.
Acknowledgement
This work was supported in part by the NIH grants HL088201; 1RO1HL073241; and the National Cancer Institute, NIH, Intramural Research Program, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The opinions expressed here are those of the authors and do not represent the official position of the National Institutes of Health or the US government.
References
- 1.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biology of Blood & Marrow Transplantation. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 2.Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3:444–449. doi: 10.1513/pats.200601-007AW. [DOI] [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Santo Tomas LH, Loberiza FR, Jr, Klein JP, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Marras TK, Chan CK, Lipton JH, et al. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplantation. 2004;33:509–517. doi: 10.1038/sj.bmt.1704377. [DOI] [PubMed] [Google Scholar]
- 6.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 7.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 8.Watkins TR, Chien JW, Crawford SW. Graft versus host-associated pulmonary disease and other idiopathic pulmonary complications after hematopoietic stem cell transplant. Seminars in respiratory and critical care medicine. 2005;26:482–489. doi: 10.1055/s-2005-922031. [DOI] [PubMed] [Google Scholar]
- 9.Yoshihara S, Tateishi U, Ando T, et al. Lower incidence of Bronchiolitis obliterans in allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning compared with myeloablative conditioning. Bone Marrow Transplant. 2005;35:1195–1200. doi: 10.1038/sj.bmt.1704985. [DOI] [PubMed] [Google Scholar]
- 10.Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 11.Chien JW, Madtes DK, Clark JG. Pulmonary function testing prior to hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:429–435. doi: 10.1038/sj.bmt.1704783. [DOI] [PubMed] [Google Scholar]
- 12.Curtis DJ, Smale A, Thien F, Schwarer AP, Szer J. Chronic airflow obstruction in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;16:169–173. [PubMed] [Google Scholar]
- 13.Duncker C, Dohr D, Harsdorf S, et al. Non-infectious lung complications are closely associated with chronic graft-versus-host disease: a single center study of incidence, risk factors and outcome. Bone Marrow Transplant. 2000;25:1263–1268. doi: 10.1038/sj.bmt.1702429. [DOI] [PubMed] [Google Scholar]
- 14.Schwarer AP, Hughes JM, Trotman-Dickenson B, Krausz T, Goldman JM. A chronic pulmonary syndrome associated with graft-versus-host disease after allogeneic marrow transplantation. Transplantation. 1992;54:1002–1008. doi: 10.1097/00007890-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Beinert T, Dull T, Wolf K, et al. Late pulmonary impairment following allogeneic bone marrow transplantation. Eur J Med Res. 1996;1:343–348. [PubMed] [Google Scholar]
- 16.Tait RC, Burnett AK, Robertson AG, et al. Subclinical pulmonary function defects following autologous and allogeneic bone marrow transplantation: relationship to total body irradiation and graft-versus-host disease. Int J Radiat Oncol Biol Phys. 1991;20:1219–1227. doi: 10.1016/0360-3016(91)90231-r. [DOI] [PubMed] [Google Scholar]
- 17.Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein-Streilein J, Lipscomb MF, Hart DA, Darden A. Graft-versus-host reaction in the lung. Transplantation. 1981;32:38–44. doi: 10.1097/00007890-198107000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Epler GR, Colby TV. The spectrum of bronchiolitis obliterans. Chest. 1983;83:161–162. doi: 10.1378/chest.83.2.161. [DOI] [PubMed] [Google Scholar]
- 20.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aris RM, Walsh S, Chalermskulrat W, Hathwar V, Neuringer IP. Growth factor upregulation during obliterative bronchiolitis in the mouse model. Am J Respir Crit Care Med. 2002;166:417–422. doi: 10.1164/rccm.2102106. [DOI] [PubMed] [Google Scholar]
- 22.Boehler A, Bai XH, Liu M, et al. Upregulation of T-helper 1 cytokines and chemokine expression in post-transplant airway obliteration. Am J Respir Crit Care Med. 1999;159:1910–1917. doi: 10.1164/ajrccm.159.6.9806035. [DOI] [PubMed] [Google Scholar]
- 23.Boehler A, Chamberlain D, Kesten S, et al. Lymphocytic airway infiltration as a precursor to fibrous obliteration in a rat model of bronchiolitis obliterans. Transplantation. 1997;64:311–317. doi: 10.1097/00007890-199707270-00023. [DOI] [PubMed] [Google Scholar]
- 24.Neuringer IP, Chalermskulrat W, Aris R. Obliterative bronchiolitis or chronic lung allograft rejection: a basic science review. J Heart Lung Transplant. 2005;24:3–19. doi: 10.1016/j.healun.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Michel O, Kips J, Duchateau J, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154:1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 26.Michel O, Ginanni R, Duchateau J, et al. Domestic endotoxin exposure and clinical severity of asthma. Clin Exp Allergy. 1991;21:441–448. doi: 10.1111/j.1365-2222.1991.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 27.Kline JN, Cowden JD, Hunninghake GW, et al. Variable airway responsiveness to inhaled lipopolysaccharide. Am J Respir Crit Care Med. 1999;160:297–303. doi: 10.1164/ajrccm.160.1.9808144. [DOI] [PubMed] [Google Scholar]
- 28.Savov JD, Brass DM, Lawson BL, et al. Toll-like receptor 4 antagonist (E5564) prevents the chronic airway response to inhaled lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2005;289:L329–L337. doi: 10.1152/ajplung.00014.2005. [DOI] [PubMed] [Google Scholar]
- 29.Chien JW, Zhao LP, Hansen JA, et al. Genetic variation in bactericidal/permeability-increasing protein influences the risk of developing rapid airflow decline after hematopoietic cell transplantation. Blood. 2006;107:2200–2207. doi: 10.1182/blood-2005-06-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrandt GC, Granell M, Urbano-Ispizua A, et al. Recipient NOD2/CARD15 variants: a novel independent risk factor for the development of bronchiolitis obliterans after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:67–74. doi: 10.1016/j.bbmt.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Palmer SM, Klimecki W, Yu L, et al. Genetic regulation of rejection and survival following human lung transplantation by the innate immune receptor CD14. Am J Transplant. 2007;7:693–699. doi: 10.1111/j.1600-6143.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 32.Chalermskulrat W, Neuringer IP, Brickey WJ, et al. Hierarchical contributions of allorecognition pathways in chronic lung rejection. Am J Respir Crit Care Med. 2003;167:999–1007. doi: 10.1164/rccm.200209-1099OC. [DOI] [PubMed] [Google Scholar]
- 33.Richards DM, Dalheimer SL, Ehst BD, et al. Indirect minor histocompatibility antigen presentation by allograft recipient cells in the draining lymph node leads to the activation and clonal expansion of CD4+ T cells that cause obliterative airways disease. J Immunol. 2004;172:3469–3479. doi: 10.4049/jimmunol.172.6.3469. [DOI] [PubMed] [Google Scholar]
- 34.Duncan SR, Capetanakis NG, Lawson BR, Theofilopoulos AN. Thymic dendritic cells traffic to thymi of allogeneic recipients and prolong graft survival. J Clin Invest. 2002;109:755–764. doi: 10.1172/JCI12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation. 1998;66:764–771. doi: 10.1097/00007890-199809270-00011. [DOI] [PubMed] [Google Scholar]
- 36.Neuringer IP, Mannon RB, Coffman TM, et al. Immune cells in a mouse airway model of obliterative bronchiolitis. American journal of respiratory cell and molecular biology. 1998;19:379–386. doi: 10.1165/ajrcmb.19.3.3023m. [DOI] [PubMed] [Google Scholar]
- 37.Sakaida E, Nakaseko C, Harima A, et al. Late-onset noninfectious pulmonary complications after allogeneic stem cell transplantation are significantly associated with chronic graft-versus-host disease and with the graft-versus-leukemia effect. Blood. 2003;102:4236–4242. doi: 10.1182/blood-2002-10-3289. [DOI] [PubMed] [Google Scholar]
- 38.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 40.Knoop C, Estenne M. Acute and chronic rejection after lung transplantation. Seminars in respiratory and critical care medicine. 2006;27:521–533. doi: 10.1055/s-2006-954609. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins PM, Aboyoun CL, Chhajed PN, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2004;170:1022–1026. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 42.Verleden GM, Dupont LJ, Van Raemdonck DE. Is it bronchiolitis obliterans syndrome or is it chronic rejection: a reappraisal? Eur Respir J. 2005;25:221–224. doi: 10.1183/09031936.05.00057404. [DOI] [PubMed] [Google Scholar]
- 43.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 44.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 45.Duncan SR, Paradis IL, Yousem SA, et al. Sequelae of cytomegalovirus pulmonary infections in lung allograft recipients. The American review of respiratory disease. 1992;146:1419–1425. doi: 10.1164/ajrccm/146.6.1419. [DOI] [PubMed] [Google Scholar]
- 46.Gao LH, Zheng SS. Cytomegalovirus and chronic allograft rejection in liver transplantation. World J Gastroenterol. 2004;10:1857–1861. doi: 10.3748/wjg.v10.i13.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostareli E, Hadzidimitriou A, Stavroyianni N, et al. Molecular evidence for EBV and CMV persistence in a subset of patients with chronic lymphocytic leukemia expressing stereotyped IGHV4-34 B-cell receptors. Leukemia. 2009;23:919–924. doi: 10.1038/leu.2008.379. [DOI] [PubMed] [Google Scholar]
- 48.Duncan SR, Cook DJ. Survival of ganciclovir-treated heart transplant recipients with cytomegalovirus pneumonitis. Transplantation. 1991;52:910–913. [PubMed] [Google Scholar]
- 49.Krensky AM, Weiss A, Crabtree G, Davis MM, Parham P. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med. 1990;322:510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 50.Ely LK, Burrows SR, Purcell AW, Rossjohn J, McCluskey J. T-cells behaving badly: structural insights into alloreactivity and autoimmunity. Curr Opin Immunol. 2008;20:575–580. doi: 10.1016/j.coi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Duncan SR, Valentine V, Roglic M, et al. T cell receptor biases and clonal proliferations among lung transplant recipients with obliterative bronchiolitis. J Clin Invest. 1996;97:2642–2650. doi: 10.1172/JCI118714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duncan SR, Leonard C, Theodore J, et al. Oligoclonal CD4(+) T cell expansions in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2002;165:1439–1444. doi: 10.1164/rccm.2107009. [DOI] [PubMed] [Google Scholar]
- 53.Kuo E, Bharat A, Dharmarajan S, et al. Animal models for bronchiolitis obliterans syndrome following human lung transplantation. Immunologic research. 2005;33:69–81. doi: 10.1385/IR:33:1:069. [DOI] [PubMed] [Google Scholar]
- 54.George MP, Zhu X, Stoner MW, Myerburg M, Duncan SR. A human-murine chimeric model of lung transplantation. Proc Am Thorac Soc. 2006:A538. (abstract) [Google Scholar]
- 55.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 57.Studer SM, George MP, Zhu X, et al. CD28 down-regulation on CD4 T cells is a marker for graft dysfunction in lung transplant recipients. Am J Respir Crit Care Med. 2008;178:765–773. doi: 10.1164/rccm.200701-013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends in molecular medicine. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Gadgil AS, Zhu Z, Stoner MW, Duncan SR. Allograft tolerance conferred by adoptive transfer of splenic dendritic cells. Proc Am Thorac Soc. 2005:A859. (abstract) [Google Scholar]
- 60.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 61.White DA, Wong PW, Downey R. The utility of open lung biopsy in patients with hematologic malignancies. Am J Respir Crit Care Med. 2000;161:723–729. doi: 10.1164/ajrccm.161.3.9904016. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki T, Saijo Y, Ebina M, et al. Bilateral pneumothoraces with multiple bullae in a patient with asymptomatic bronchiolitis obliterans 10 years after bone marrow transplantation. Bone Marrow Transplant. 1999;23:829–831. doi: 10.1038/sj.bmt.1701660. [DOI] [PubMed] [Google Scholar]
- 63.Chadwick C, Marven SM, Vora AJ. Autologous blood pleurodesis for pneumothorax complicating graft-versus-host disease-related bronchiolitis obliterans. Bone Marrow Transplant. 2004;33:451–453. doi: 10.1038/sj.bmt.1704370. [DOI] [PubMed] [Google Scholar]
- 64.Kumar S, Tefferi A. Spontaneous pneumomediastinum and subcutaneous emphysema complicating bronchiolitis obliterans after allogeneic bone marrow transplantation--case report and review of literature. Ann Hematol. 2001;80:430–435. doi: 10.1007/s002770100301. [DOI] [PubMed] [Google Scholar]
- 65.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 66.Yokoi T, Hirabayashi N, Ito M, et al. Broncho-bronchiolitis obliterans as a complication of bone marrow transplantation: a clinicopathological study of eight autopsy cases. Nagoya BMT Group. Virchows Arch. 1997;431:275–282. doi: 10.1007/s004280050099. [DOI] [PubMed] [Google Scholar]
- 67.Diez Herranz A. RV/TLC% ratio: alternative criteria of normality. Eur Respir J. 1995;8:1812–1813. doi: 10.1183/09031936.95.08101812. [DOI] [PubMed] [Google Scholar]
- 68.Guttadauria M, Ellman H, Emmanuel G, Kaplan D, Diamond H. Pulmonary function in scleroderma. Arthritis Rheum. 1977;20:1071–1079. doi: 10.1002/art.1780200506. [DOI] [PubMed] [Google Scholar]
- 69.Gunn ML, Godwin JD, Kanne JP, Flowers ME, Chien JW. High-resolution CT findings of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. J Thorac Imaging. 2008;23:244–250. doi: 10.1097/RTI.0b013e3181809df0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark JG. The challenge of bone marrow transplantation. Mayo Clin Proc. 1990;65:111–114. doi: 10.1016/s0025-6196(12)62115-6. [DOI] [PubMed] [Google Scholar]
- 72.Chan CK, Hyland RH, Hutcheon MA, et al. Small-airways disease in recipients of allogeneic bone marrow transplants. An analysis of 11 cases and a review of the literature. Medicine (Baltimore) 1987;66:327–340. doi: 10.1097/00005792-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Hollmen M, Tikkanen JM, Nykanen AI, Koskinen PK, Lemstrom KB. Tacrolimus treatment effectively inhibits progression of obliterative airway disease even at later stages of disease development. J Heart Lung Transplant. 2008;27:856–864. doi: 10.1016/j.healun.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 74.Snell GI, Esmore DS, Williams TJ. Cytolytic therapy for the bronchiolitis obliterans syndrome complicating lung transplantation. Chest. 1996;109:874–878. doi: 10.1378/chest.109.4.874. [DOI] [PubMed] [Google Scholar]
- 75.Fullmer JJ, Fan LL, Dishop MK, Rodgers C, Krance R. Successful treatment of bronchiolitis obliterans in a bone marrow transplant patient with tumor necrosis factor-alpha blockade. Pediatrics. 2005;116:767–770. doi: 10.1542/peds.2005-0806. [DOI] [PubMed] [Google Scholar]
- 76.Ratjen F, Rjabko O, Kremens B. High-dose corticosteroid therapy for bronchiolitis obliterans after bone marrow transplantation in children. Bone Marrow Transplant. 2005;36:135–138. doi: 10.1038/sj.bmt.1705026. [DOI] [PubMed] [Google Scholar]
- 77.Duncan CN, Buonanno MR, Barry EV, et al. Bronchiolitis obliterans following pediatric allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:971–975. doi: 10.1038/bmt.2008.19. [DOI] [PubMed] [Google Scholar]
- 78.Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J. 2005;25:490–493. doi: 10.1183/09031936.05.00020804. [DOI] [PubMed] [Google Scholar]
- 79.Bashoura L, Gupta S, Jain A, et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:63–67. doi: 10.1038/sj.bmt.1705877. [DOI] [PubMed] [Google Scholar]
- 80.Smith EP, Sniecinski I, Dagis AC, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs.-host disease. Biol Blood Marrow Transplant. 1998;4:27–37. doi: 10.1016/s1083-8791(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 81.Besnier DP, Chabannes D, Mahe B, et al. Treatment of graft-versus-host disease by extracorporeal photochemotherapy: a pilot study. Transplantation. 1997;64:49–54. doi: 10.1097/00007890-199707150-00010. [DOI] [PubMed] [Google Scholar]
- 82.Ilhan O, Arat M, Arslan O, et al. Extracorporeal photoimmunotherapy for the treatment of steroid refractory progressive chronic graft-versus-host disease. Transfus Apher Sci. 2004;30:185–187. doi: 10.1016/j.transci.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Majhail NS, Schiffer CA, Weisdorf DJ. Improvement of pulmonary function with imatinib mesylate in bronchiolitis obliterans following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:789–791. doi: 10.1016/j.bbmt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Johnson BA, Iacono AT, Zeevi A, McCurry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med. 2003;167:1271–1278. doi: 10.1164/rccm.200205-410OC. [DOI] [PubMed] [Google Scholar]
- 85.Or R, Gesundheit B, Resnick I, et al. Sparing effect by montelukast treatment for chronic graft versus host disease: a pilot study. Transplantation. 2007;83:577–581. doi: 10.1097/01.tp.0000255575.03795.df. [DOI] [PubMed] [Google Scholar]
- 86.Islam SA, Thomas SY, Hess C, et al. The leukotriene B4 lipid chemoattractant receptor BLT1 defines antigen-primed T cells in humans. Blood. 2006;107:444–453. doi: 10.1182/blood-2005-06-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilborn J, Bailie M, Coffey M, et al. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97:1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medoff BD, Seung E, Wain JC, et al. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202:97–110. doi: 10.1084/jem.20042481. [DOI] [PMC free article] [PubMed] [Google Scholar]