Abstract
Tyrosine kinase inhibitor (TKI) therapy has revolutionized the therapy of CML. Thus, while in the near past allogeneic transplantation was the curative option for CML, imatinib, nilotinib, and dasatinib have pushed transplantation to the role of salvage therapy in CML. Still, TKI therapy still fails some patients, and so the clinical challenge is to integrate transplantation in a safe and sane manner. This manuscript reviews the data on the variables that influence outcome following transplantation, and discusses the variables to consider in determining which patients should receive transplantation and when.
Keywords: Chronic myeloid leukemia, stem cell transplant, imatinib
Introduction
CML is characterized by a natural history of a transition from chronic phase to blast crisis. The role of transplantation in CML (and hence, the role of transplanters) has had it's own unique natural history. In the early 1980s, transplantation was a revolutionary therapy; by the 1990s, the therapy became so refined that it was the standard of care, offering “cures” in most chronic phase patients. Transplanters thus enjoyed unusually high regard. With the advent of imatinib, an oral targeted therapy could alter the natural history of CML. Soon it was advocated that STI 571 (the original name for imatinib in early trials) stood for “stop transplantation immediately.” However, as experience with imatinib grew, issues of resistance in chronic phase emerged, and the treatment of advanced phase disease (accelerated and blast phases) proved to be inadequate with imaitinib (or later, other second generation tyrosine kinase inhibitors). Thus, transplantation still has a definite, though more limited, role in the treatment of CML.
Factors that determine outcome following transplantation in CML
Allogeneic stem cell transplantation is remarkably effective in CML, and outcomes have benefited from several factors such as better HLA typing, better infectious disease therapy, better treatment of graft versus host disease, (GVHD), etc. The outcome of allogeneic HCT in CML is influenced by many factors, most importantly the phase of disease, but also, the type of donor used, the nature of the stem cell product, and the age of the patient.
Phase of disease
As with all treatments of CML, outcomes following transplantation are superior for patients in chronic phase compared to advanced phase disease. The first large study on 167 CML patients transplanted through 1983 from matched siblings 1 demonstrated a strong phase-dependent survival. After 20 years of follow-up, 40% of patients transplanted in chronic phase survive. Contemporary data demonstrate a significant improvement in survival. Data from the International Bone Marrow Transplant Registry (IBMTR) between 1994 and 1999 show a probability of long-term (>5 year) survival of ∼70% patients transplanted in chronic phase within the first year from diagnosis, and ∼60% for 1391 patients transplanted more than 1 year from diagnosis (IBMTR, http://wwwibmtrorg2002). Contemporary results from selected single institutions continue to demonstrate the excellent outcomes with HCT. For example, the use of a preparative regimen of targeted busulfan (BU) plus CY in chronic phase patients yielded 3 year survival rates of 86%; nearly 90% of these survivors were in molecular remission. 2. These data are keeping with studies from the German consortium (Figure 1) and Hammersmith groups, where survival in chronic phase have shown similar excellent results, with 5 year disease free survivals of ∼90%. 3
Figure 1.
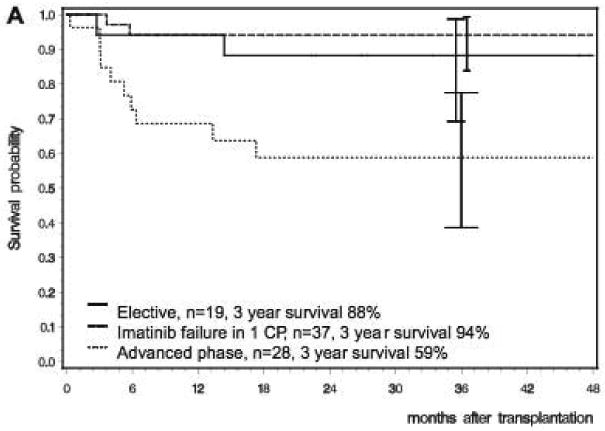
Overall survival of chronic phase CML patients receiving allogeneic transplants grouped by elective transplantation, those with imatinib failure, and advanced phase disease. The figure is courtesy of Sauselle and Hehlman (3).
Outcomes in advanced phase disease (accelerated phase and blast crisis) are far inferior to chronic phase 4,5. Outcomes in accelerated are approximately half of that of chronic phase, with survival and event-free survival of ∼50 and 40%, respectively 6. The outcome of transplantation for patients in blast crisis is very poor, both because of a high risk of disease recurrence and a high incidence of transplant-related deaths. Most studies have found 3-5 year survival for transplants in blast crisis to be only 10-20%1,7. Data from the FHCRC from 1995 to the present are shown in Figure 2, and show that while major advances have occurred in the treatment of chronic phase disease, no such gains have been seen in advanced phase disease (data courtesy of Dr. Ted Gooley, unpublished).
Figure 2.
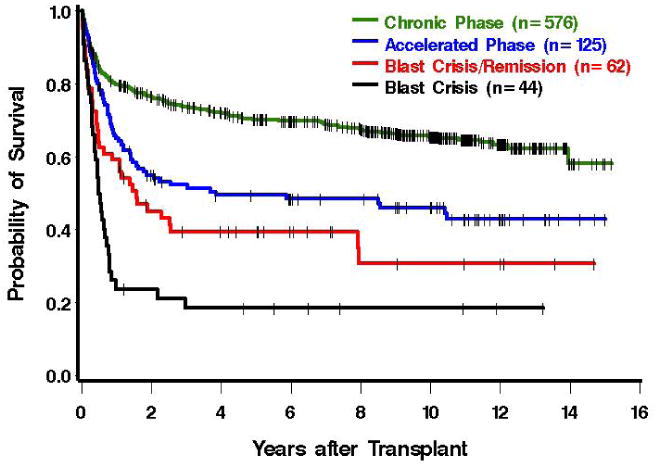
Overall survival of patients receiving allografts at the Fred Hutchinson Cancer Research Center from 1995 to the present. The data includes both matched related and unrelated donors. The figure is courtesy of Dr. Ted Gooley.
Preparative regimen
The majority of patients treated in the early 1980s received a preparative regimen of 120mg/kg cyclophosphamide (CY), followed by total body irraditation (TBI), generally 12Gy applied in fractionated doses over 6 consecutive days1. Later, similar results were shown using an all chemotherapy preparative regimen of 16mg/kg busulfan (BU) and 60mg/kg CY8. A randomized trial compared the BU-CY regimen with CY plus 12Gy TBI in myeloid malignancies showed no differences were found in survival, relapse, or event-free survival between the CY-TBI and BU-CY treatment groups 9.
The absorption and subsequent metabolism of oral BU varies dramatically among patients. The development of an assay to measure plasma concentration of BU showed a strong relationship of BU level with response and toxicity10. Subsequent trials in CML using targeted BU/CY in matched related transplants showed excellent results, with a 3-year survival of 86%, a relapse rate of 8% and non-relapse mortality rate of 14%2. Moreover, age no longer had no major impact on outcome (up to the study limit of 65 years of age).
Patient age
Initial studies of transplantation for CML demonstrated a strong age effect, with younger patients far better than older patients 1. As noted above, a variety of changes in donor selection, preparative regimens, and supportive care have largely dampened the effect of age and outcome11. However, age seems to be more clearly related to transplant outcome in the unrelated donor setting12.
Time from diagnosis to transplant
Early studies showed that an increased interval from diagnosis to transplant was associated with a worse transplant outcome, even for patients in chronic phase at the time of transplant. This finding was confirmed by others and is evident in the most recent IBMTR data13,14. Reasons for the effect of delay on outcome are not readily apparent, but a longer time to transplant has been associated with a higher relapse rate and an increase in non-relapse mortality. This may reflect both the fact that a longer delay to transplantation allows resistant clones to develop, as well as potentially affecting subsequent toxicity of the transplant if the due to pre-transplant therapy (discussed below).
Donor type
For cases without a matched-related donor, a majority of cases can find an unrelated matched donor from the world donor registries. Advances in HLA typing and GVHD therapy has made marked improvements in transplant outcomes. For many diseases, including CML, results with a fully matched unrelated donor are quite similar to that achieved with a matched related donor 12,15. In general, related transplants have less non-relapse mortality (from GVHD associated maladies) compared to unrelated transplants, while having more relapse (due to less graft versus leukemia effect).
The experience with identical twin (syngeneic) transplants is obviously limited, but instructive. The first four twin chronic phase CML transplants were performed in 1979, using dimethyl busulfan, cyclophosphamide, and a single 920cGy exposure of TBI16. All four recovered with Ph-negative normal hematopoiesis. A follow-up study of 22 twin transplants was performed17. After 20 years of follow-up 7/12 chronic phase patients are alive post-transplant, and five have maintained their initial post-transplant complete remission (unpublished, data courtesy of Drs. Alex Fefer and Ted Gooley). These data suggest that a high-dose preparative regimen can, in some cases, cure CML without an allogeneic graft-vs-leukemia effect.
Source of hematopoietic stem cells
Bone marrow served as the initial source of stem cells in allogeneic transplants. With the advent of peripheral blood mobilization and collection, two separate large randomized trials involving patients with a variety of hematologic malignancies demonstrated that use of G-CSF mobilized peripheral blood stem cells, compared to bone marrow, lead to faster myeloid and platelet recovery, no significant difference in acute or chronic GVHD and an overall survival advantage. In these studies there was a trend towards improved survival in CML patients with the use of peripheral blood18,19. Lastly, the results of a randomized study of chronic phase CML showed no statistically significant differences in outcome between the bone marrow and peripheral blood groups. Relapse rates were lower in the peripheral blood group, but chronic GVHD higher in cases received peripheral blood stem cells20.
The impact of prior therapy on transplant outcomes
One hypothesis for the effect of the delay to transplant and poorer outcome was the effect of prior therapy. IBMTR data suggested that exposure to low-dose BU led to a worse outcome with subsequent transplantation14. In addition, several reports suggested that exposure to interferon (IFN) might worsen the outcome of unrelated donor transplant, though the effect on matched sibling transplantation were unclear. In a recent report of 856 patients randomized to hydroxyurea, BU or IFN, 197 went on to transplant. Although there was no overall difference in transplant outcome according to the initial treatment, the 5-year survival from transplant was only 46% patients who received IFN within the last 90 days before transplant, as opposed to 71% for those who did not (p=0.0057)21.
Now that we are in the Age of Imatinib, should we be worried that imatinib prior to transplant may have a deleterious effect of transplant outcomes? Probably not. There have been several studies on the effect of prior imatinib and transplant outcomes. Early reports warned of an increase in regimen-related toxicity and mortality, especially from hepatic causes22. Larger studies have failed to show a deleterious effect of pre-transplant imatinib 23,24. A single institution study of 140 CML patients showed no difference in regimen-related mortality, survival or relapse in patients who received imatinib versus 200 historical controls25. Indeed, a IBMTR study of 409 patients who received imatinib therapy transplant compared to 900 cases that did not suggested that those chronic phase patients who received imatinib enjoyed a slightly better outcome following transplant, though this effect seemed to be limited in cases who moved to transplantation because they could not tolerate imatinib, rather than treatment failure 26. There was no positive or negative effect of imatinib in the outcome of advanced phase patients. So far there is no evidence that resistant patients with Abl mutations have a poorer outcome following transplantation.
Summary of myeloablative allogeneic transplant results
To generalize the status of modern transplantation results, one must merge data from single center (or country) data with that from international consortiums (Seattle, German, IBMTR). Single center data suffer from possible selection biases (perhaps from the type of patient who is economically solvent and sufficiently motivated to relocate), but may reflect best possible practice outcomes, due to experience. Multiple site studies may reflect “real world” experience, but may include data from centers without optimal experience, and may incorporate a wide range of practice patterns. Nonetheless, today one should expect survival to be greater than 80% for chronic phase patients, 40-50% for accelerated phase patients, and ∼20% for blast crisis patients. For patients in blast crisis placed back into remission, results are similar to accelerated phase (please note: while these patients are often referred to as “second chronic phase,” a better description is “blast crisis in remission.” This not only since they behave differently than chronic phase cases, but also because by gene expression patterns, these patients are clearly not chronic phase, but rather retain their blast crisis gene expression signature. 27
Pre-transplant variables can define a prognostic scoring system for transplantation in CML. The system devised by Gratwohl uses HLA matching, stage, age, sex of donor/recipient, and time from diagnosis to transplant28. The scoring system is effective in defining post-transplant outcomes following an ablative transplant, with survival in European bone marrow transplant (EBMT) data 70% for the best score to 20% for the worst score (Figure 3). This scoring was recently applied again to EBMT cases transplanted in three cohorts, 1980-90, 1991-99, and 2000-02 29. In all risk groups survival has improved over time, improved overall by over 50%, largely from halving of regimen-related mortality. However, as discussed below, given that transplantation is being reserved for cases of TKI failure and/or advanced phase disease, the value of a prognostic scoring system is largely for patient information rather than decision-making. Thus, in Table 1, we outline transplant groups in the neo-classic scheme of The Good, The Bad, and The Ugly (with apologies to Mr. Sergio Leone and Mr. Clint Eastwood).
Figure 3.
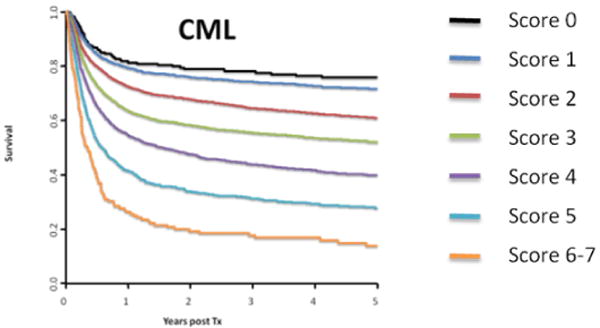
Overall survival based on the transplant score. The figure is courtesy of Dr. Alois Gratwohl, and is derived from the data presented in reference 29.
Table 1. Transplant risk groups in CML.
| Class | Phase | Comments |
|---|---|---|
| The Good | CML CP | Intolerant to TKI |
| Not able to obtain TKI therapy | ||
| The Bad | CML CP | Resistant with T315I mutation |
| Poor response to secondary TKI therapy | ||
| The Ugly | CML AP | Treat with TKI until a donor is found |
| CML BC |
Reduced Intensity Conditioning in CML
Reduced intensity conditioning (RIC) or non-myeloablative (NMA) transplants have been used to fight leukemia based on the immunological GVL effect. This has been a highly popular and successful strategy for many hematological malignancies. However, the use of RIC and NMA transplantation in CML has been relatively infrequent given the success of both TKI therapy and traditional ablative transplantation.
Initial published results on CML patients who underwent a NMA regimen of Flu/TBI or 2 Gy TBI in 24 patients with CML-chronic phase or accelerated phase (AP)30 showed a 100-day non-relapse mortality (NRM) rate of 4%, while the 2-year estimates of survival were 70% for patients in chronic phase and 56% for patients beyond first CP. However, the results using URD were not terribly encouraging because of a high graft rejection rate of nearly fifty percent. This likely occurred since the CML cases had little therapy before transplantation, and thus after a non-ablative regimen, still had an adequate enough host immune systems to mount a rejection in the setting of an unrelated donor. Another report of a younger cohort of 24 CP CML patients (median age 35 years) using a RIC regimen of Flu, 180 mg/m2, oral Bu, 8 mg/kg, and ATG, 20-40 mg/kg was subsequently published31. The 100-day NRM was 0% and there were only three late deaths, all from complications of GVHD. The estimated 5-year OS and RFS was 85%. In addition, at a median of 42 months from HCT, all patients had a complete molecular response by RT-PCR testing. Lastly, the EBMT group reported results on 186 patients with CML (median age 50 years) who received RIC32. Patients with all stages of CML were included, with various RIC regimens, donor types, and stem cell products used. The 100-day NRM was 6%, the 2-year NRM was 23%, and the 3-year OS and RFS was 58% and 37%, respectively. Over 60% of patients enjoyed a complete cytogenetic remission, while 40% attained a complete molecular response by PCR testing. In sum, these studies suggest that RIC/NMA transplantation is a promising approach for CML patients, and one can imagine a combined approach lower intensity transplantation followed by TKI prophylaxis.
Using TKI to treat or prevent relapse following transplant
Relapse is the main cause of treatment failure after an allogeneic transplant. The pace of disease progression after post-transplant relapse is variable. If relapse is defined at a molecular (PCR) level, some patients may never progress, or at least may not progress for a very long time 33,34. Similarly, patients whose only evidence of relapse is detection of low levels of Ph-positive metaphases may remain stable for many years, and even patients with clinical relapse may not progress rapidly.
An increasing number of potential interventions are available to the patient who has relapsed after allogeneic transplantation for CML. Treatment with IFN can produce both clinical and cytogenetic remissions in patients who have relapsed after transplantation 35. Results with IFN appear better if treatment is initiated at the time of cytogenetic relapse instead of waiting until hematologic relapse, and in some cases IFN induced molecular remissions. Nonetheless, early treatment of relapse in CML has been supplanted by imatinib.
Imatinib appears highly active as post-transplant therapy for relapse 36,37 Not surprisingly, response to imatinib maps to the stage of disease. Complete hematological response was seen in >90% of chronic phase cases, >50% of accelerated phase, and >20% of blast crisis cases. A complete cytogenetic remission was accomplished in >40% of cases, higher in chronic phase than advanced phase disease. In addition, imatinb appears to be effective in the prophylactic setting in order to prevent relapse in high-risk Ph+ disease38. Twenty-two patients (15 with Ph+ ALL, 7 with CML) were given imatinib at a median of 28 days post-engraftment. In all but one case patients tolerated imatinb at the targeted dose (400 mg/d for adults, 260 mg/m2/d for children), and 19 patients completed the planned year of treatment post-transplant. At a median follow-up of ∼1.4 years, 5/7 of the CML patients and 12/15 of the Ph+ ALL cases were in a molecular remission. Thus, the tactic of early tyrosine kinase inhibitor therapy, coupled with molecular monitoring, may prevent relapse and improve outcomes for advanced cases of CML and Ph+ ALL.
Rationale for Transplantation
As outlined in other chapters in this book, TKIs have revolutionized the therapy of CML. To summarize the salient points of TKI therapy:
Primary therapy for chronic phase is highly effective, and can produce a CCyR is ∼80% of cases. For these patients, survival at 7+ years is nearly 90%. However, approximately 20-30% of cases will fail primary therapy, either from intolerance, relapse, or progression to advanced phase disease.
For patients receiving secondary therapy for resistant disease, approximately 50% will achieve a CCyR. The survival for these patients is ∼80% at 3 years. Those who do not achieve and maintain a CCyR often relapse with new mutations.
Patients with AP or BC can achieve a CCyR with TKI therapy, but this does not appear to be associated with long-term PFS.
Patients who have a T315I mutation do not respond to currently available TKI therapy. As natural selection would dictate, selection of this mutation increases as patients become resistant to more and more TKIs.
Efforts have been made to identify clinical or laboratory variables that will predict outcome after the initiation of secondary TKI therapy. At present, early cytogenetic response to a secondary TKI seems to do well. Thus, patients who are placed on a secondary TKI, but who fail to have any cytogeneic response after 3 months of therapy are very unlikely to ever achieve a CCyR. Likewise, failure to achieve at least a minor cytogenetic response by 6 months, or a MCyR by 12 months of secondary therapy, bodes poorly for sustained disease control 39. A scoring system based on the best cytogenetic score on imatinib therapy, Sokal score, and a history of neutropenia during imatinib therapy has been proposed by the Hammersmith group at the ASH 2008 meeting (Milojkovic, Blood 112, 331a, 2008). Patients with a low score (few adverse features) had a >90% chance of achieving a CCyR on as secondary agent, compared to <20% for cases with a high score (several adverse features).
Who and when do we transplant?
Given the above summary of the TKI treatment experience, and the contemporary results of transplant, can we develop general “rules of engagement” for who and when we should offer transplantation? While this strategy is evolving, we can ask and and answer some basic questions that can clarify our treatment course.
Should we ever offer transplantation as a first option?
Given the great results of front-line imatinib in newly diagnosed chronic phase CML, the situations were transplantation should be offered first seem few and far between. There have been a few attempts to compare upfront transplantation with non-transplant therapy40. One trial enrolled 621 patients with chronic phase CML. Of this set, only 354 patients were eligible for transplantation and “biologically randomized” based on the availability of a related donor. Of the 123 patients who received a transplant, the 10-year estimate of survival was 53 percent (surprisingly low given other data from this group3. Those 219 patients without a related donor were treated with IFN until imatinib became available later in the trial. Imatinib was then offered to patients with a poor response to IFN. The 10-year estimate of survival in this group was 52 percent. The survival curves of these two groups show that the transplant group suffered a higher early mortality, with a flattening of the survival curves thereafter. The non-transplant group had a better early outcome, but the survival curves continuing to drop over the history of the trial. The cross-over of the curves (favoring transplantation) came at eight years. There are several issues that make this study difficult to interpret in the 2010s, however. The “non-transplant” group contained patients who underwent an URD transplant; the survival in the URD transplant group was better than all other groups (69%). Moreover, both the transplant group and the IFN/Imatinib group had outcomes worse than anticipated. As one cannot imagine a randomized study occurring with a up-front TKI versus transplant, it seems prudent to unreservedly recommend imatinib as initial therapy of chronic phase CML. However, in health systems where a TKI cannot be obtained (due to financial limitations or other bureaucratic/infrastructure restrictions), then transplant would be a good option for initial therapy of chronic phase disease 41.
Very few CML cases present in advanced phase disease. Given the relatively poor response to TKI in these cases, transplantation is the option of choice, though it would be prudent to treat the patient with a TKI until a donor is secured.
When should transplantation be used in resistance?
There is increasing evidence that resistance may be forever. Less than 50% of cases of IM failure achieve a CCyR with secondary TKI therapy 42-44. Moreover, patients who do fail secondary therapy often have a new mutation45-47. Thus, secondary TKI therapy may be an unfortunate example of the Darwinian selection of fit clones that survive each successive therapeutic attack. Moreover, molecular data has shown than the biological states of resistance and progression have a large overlap27,48,49. Given these facts, it is reasonable to prepare patients for transplantation at the time of first line failure. This means a consultation at a transplant center, and lining up a donor, either sibling or unrelated.
It is certainly reasonable, and sensible, to start a secondary TKI at the time of resistance, for two reasons-first, in case the patient is one of the fortunate who obtain a CCyR on secondary therapy; secondly, a donor search can take months (especially an unrelated donor), so therapy is needed to blunt the progression of the disease. For the bulk of the patients receiving secondary TKI, patients should be monitored closely, and taken to transplant if there is not optimal cytogenetic response by 3, 6, or 12 months 39
If a patient has relapsed on IM with a T315I mutation, then it seems reasonable to proceed to transplant, given the low response rate to secondary therapy. Likewise, those who have a mutation within the P loop region, which seem in many studies to be associated with an increased risk of progression to advance phase disease, transplant may seem a reasonable option. For the rare patient who is intolerant to all TKI, transplant may be reasonable, though one worries that if a patient cannot tolerated TKIs, whether or not he/she will do well on the plethora of medications needed for a successful transplant.
Conclusion
In CML clinicians are blessed with several effective modalities of therapy, and relatively easy monitoring techniques that serve as good surrogate outcome measures. The trick is obviously using the right therapy at the right time. For patients with chronic phase disease, TKI should be the first choice; careful monitoring will discover those patients needing secondary therapy. The choice of therapy can be made based on the presence of an Abl mutation (especially what type), and the early response of secondary therapy, if applied. For patients with advanced phase disease, transplantation is the option of choice, though therapy with a TKI while the transplant is being arranged is recommended.
Table 2. HLA typing and timing of transplantation.
| Phase | At diagnosis | Transplant during TKI | Comments |
|---|---|---|---|
| CP | Consider HLA typing if high risk Sokal | If IM failure first line, then failure of 2nd generation TKI If IM failure first line, and T315I mutation present |
Consider transplant if patients young and have HLA matched sibling |
| AP, BC | HLA typing for all | Transplant all patients | Give TKI until HLA matched sib or URD found, then transplant |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED, Clift RA, Fefer A, et al. Marrow transplantation for the treatment of chronic myelogenous leukemia. Ann Intern Med. 1986;104:155–163. doi: 10.7326/0003-4819-104-2-155. [DOI] [PubMed] [Google Scholar]
- 2.Radich JP, Gooley T, Bensinger W, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102:31–35. doi: 10.1182/blood-2002-08-2619. [DOI] [PubMed] [Google Scholar]
- 3.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115:1880–1885. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 4.Devergie A, Reiffers J, Vernant JP, et al. Long-term follow-up after bone marrow transplantation for chronic myelogenous leukemia: factors associated with relapse. Bone Marrow Transplant. 1990;5:379–386. [PubMed] [Google Scholar]
- 5.Martin PJ, Clift RA, Fisher LD, et al. HLA-identical marrow transplantation during accelerated-phase chronic myelogenous leukemia: analysis of survival and remission duration. Blood. 1988;72:1978–1984. [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for patients in accelerated phase of chronic myeloid leukemia. Blood. 1994;84:4368–4373. [PubMed] [Google Scholar]
- 7.Goldman JM, Gale RP, Horowitz MM, et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann Intern Med. 1988;108:806–814. doi: 10.7326/0003-4819-108-6-806. [DOI] [PubMed] [Google Scholar]
- 8.Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70:1382–1388. [PubMed] [Google Scholar]
- 9.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–2043. [PubMed] [Google Scholar]
- 10.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060. [PubMed] [Google Scholar]
- 11.Clift RA, Appelbaum FR, Thomas ED. Treatment of chronic myeloid leukemia by marrow transplantation. Blood. 1993;82:1954–1956. [PubMed] [Google Scholar]
- 12.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 13.Enright H, Daniels K, Arthur DC, et al. Related donor marrow transplant for chronic myeloid leukemia: patient characteristics predictive of outcome. Bone Marrow Transplant. 1996;17:537–542. [PubMed] [Google Scholar]
- 14.Goldman JM, Szydlo R, Horowitz MM, et al. Choice of pretransplant treatment and timing of transplants for chronic myelogenous leukemia in chronic phase. Blood. 1993;82:2235–2238. [PubMed] [Google Scholar]
- 15.Davies SM, DeFor TE, McGlave PB, et al. Equivalent outcomes in patients with chronic myelogenous leukemia after early transplantation of phenotypically matched bone marrow from related or unrelated donors. Am J Med. 2001;110:339–346. doi: 10.1016/s0002-9343(01)00629-5. [DOI] [PubMed] [Google Scholar]
- 16.Fefer A, Cheever MA, Thomas ED, et al. Disappearance of Ph1-positive cells in four patients with chronic granulocytic leukemia after chemotherapy, irradiation and marrow transplantation from an identical twin. N Engl J Med. 1979;300:333–337. doi: 10.1056/NEJM197902153000702. [DOI] [PubMed] [Google Scholar]
- 17.Fefer A, Cheever MA, Greenberg PD, et al. Treatment of chronic granulocytic leukemia with chemoradiotherapy and transplantation of marrow from identical twins. N Engl J Med. 1982;306:63–68. doi: 10.1056/NEJM198201143060202. [DOI] [PubMed] [Google Scholar]
- 18.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 19.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–1531. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 20.Oehler VG, Radich JP, Storer B, et al. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005;11:85–92. doi: 10.1016/j.bbmt.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Hehlmann R, Hochhaus A, Kolb HJ, et al. Interferon-alpha before allogeneic bone marrow transplantation in chronic myelogenous leukemia does not affect outcome adversely, provided it is discontinued at least 90 days before the procedure. Blood. 1999;94:3668–3677. [PubMed] [Google Scholar]
- 22.Shimoni A, Kroger N, Zander AR, et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia. 2003;17:290–297. doi: 10.1038/sj.leu.2402808. [DOI] [PubMed] [Google Scholar]
- 23.Deininger M, Schleuning M, Greinix H, et al. The effect of prior exposure to imatinib on transplant-related mortality. Haematologica. 2006;91:452–459. [PubMed] [Google Scholar]
- 24.Zaucha JM, Prejzner W, Giebel S, et al. Imatinib therapy prior to myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36:417–424. doi: 10.1038/sj.bmt.1705087. [DOI] [PubMed] [Google Scholar]
- 25.Oehler VG, Gooley T, Snyder DS, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood. 2007;109:1782–1789. doi: 10.1182/blood-2006-06-031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Kukreja M, Wang T, et al. Impact of prior imatinib mesylate on the outcome of hematopoietic cell transplantation for chronic myeloid leukemia. Blood. 2008;112:3500–3507. doi: 10.1182/blood-2008-02-141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–1092. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 29.Gratwohl A, Brand R, Apperley J, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91:513–521. [PubMed] [Google Scholar]
- 30.Kerbauy FR, Storb R, Hegenbart U, et al. Hematopoietic cell transplantation from HLA-identical sibling donors after low-dose radiation-based conditioning for treatment of CML. Leukemia. 2005;19:990–997. doi: 10.1038/sj.leu.2403730. [DOI] [PubMed] [Google Scholar]
- 31.Or R, Shapira MY, Resnick I, et al. Nonmyeloablative allogeneic stem cell transplantation for the treatment of chronic myeloid leukemia in first chronic phase. Blood. 2003;101:441–445. doi: 10.1182/blood-2002-02-0535. [DOI] [PubMed] [Google Scholar]
- 32.Crawley C, Szydlo R, Lalancette M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;106:2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 33.Radich J, Gehly G, Lee A, et al. Detection of bcr-abl transcripts in Philadelphia chromosome-positive acute lymphoblastic leukemia after marrow transplantation. Blood. 1997;89:2602–2609. [PubMed] [Google Scholar]
- 34.Radich JP, Gooley T, Bryant E, et al. The significance of bcr-abl molecular detection in chronic myeloid leukemia patients “late,” 18 months or more after transplantation. Blood. 2001;98:1701–1707. doi: 10.1182/blood.v98.6.1701. [DOI] [PubMed] [Google Scholar]
- 35.Higano CS, Chielens D, Raskind W, et al. Use of alpha-2a-interferon to treat cytogenetic relapse of chronic myeloid leukemia after marrow transplantation. Blood. 1997;90:2549–2554. [PubMed] [Google Scholar]
- 36.Kantarjian HM, Talpaz M, O'Brien S, et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha: follow-up results. Clinical Cancer Research. 2002;8:2177–2187. [PubMed] [Google Scholar]
- 37.Olavarria E, Craddock C, Dazzi F, et al. Imatinib mesylate (STI571) in the treatment of relapse of chronic myeloid leukemia after allogeneic stem cell transplantation. Blood. 2002;99:3861–3862. doi: 10.1182/blood.v99.10.3861. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–2793. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam CS, Kantarjian H, Garcia-Manero G, et al. Failure to achieve a major cytogenetic response by 12 months defines inadequate response in patients receiving nilotinib or dasatinib as second or subsequent line therapy for chronic myeloid leukemia. Blood. 2008;112:516–518. doi: 10.1182/blood-2008-02-141580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hehlmann R, Berger U, Pfirrmann M, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109:4686–4692. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 41.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. Jama. 303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 43.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 44.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid zleukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 45.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27:4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second-or third-line tyrosine kinase inhibitors. Blood. 2009;114:2168–2171. doi: 10.1182/blood-2009-01-197186. [DOI] [PubMed] [Google Scholar]
- 48.McWeeney SK, Pemberton LC, Loriaux MM, et al. A gene expression signature of CD34+ cells to predict major cytogenetic response in chronic-phase chronic myeloid leukemia patients treated with imatinib. Blood. 115:315–325. doi: 10.1182/blood-2009-03-210732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oehler VG, Yeung KY, Choi YE, Bumgarner RE, Raftery AE, Radich JP. The derivation of diagnostic markers of chronic myeloid leukemia progression from microarray data. Blood. 2009;114:3292–3298. doi: 10.1182/blood-2009-03-212969. [DOI] [PMC free article] [PubMed] [Google Scholar]


