Abstract
HIV-infected patients have metabolic abnormalities that put them at increased risk of cardiovascular disease (CVD), including abnormalities associated with HIV infection itself, antiretroviral treatment, restoration to health, and body composition changes. The 2 major components of dyslipidemia in HIV-infected patients are hypertriglyceridemia and reduction in high-density lipoprotein (HDL) cholesterol (with likely altered function of HDL cholesterol); these abnormalities contribute to increased atherosclerotic risk. Adverse effects of antiretroviral drugs on lipids are not class specific but rather are associated with particular drugs. Thus, practitioners need to be cognizant of the risks of metabolic abnormalities posed by individual drugs. HIV infection increases CVD risk independent of the effects of traditional risk factors. The relative risk of CVD in HIV-infected patients has decreased in recent years with increasing use of lipid-lowering therapy. However, use of lipid-lowering therapy is complicated by numerous potential drug interactions with antiretroviral drugs that practitioners need to consider when prescribing lipid-lowering therapy. This article summarizes a presentation made by Carl Grunfeld, MD, PhD, at the International AIDS Society–USA continuing medical education program in Los Angeles in March 2010. The original presentation is available as a Webcast at www.iasusa.org.
HIV-infected patients have metabolic abnormalities that make them susceptible to atherosclerosis. These abnormalities are multifactorial and include the effects of HIV infection itself, toxicities of specific antiretroviral therapies, restoration to health, and body composition changes, as well as the traditional factors that increase atherosclerotic risk in the non–HIV-infected population (eg, factors related to genes, diet, alcohol use, obesity, and inactivity).
Assessment of Cardiovascular Disease Risk in HIV Infection
Several studies have examined whether and to what degree HIV infection is a risk factor for cardiovascular disease (CVD), independent of the effects of traditional risk factors. Registry and other database studies have found increased rates of CVD in patients with HIV (Triant et al, J Clin Endocrinol Metab, 2007; Klein et al, JAIDS, 2002) that may be restricted to younger patients (Currier et al, JAIDS, 2003). However, such studies are limited in that they cannot fully adjust for traditional CVD risk factors such as smoking and lipid levels. For example, lipid levels are usually controlled for in such studies using a diagnosis of “dyslipidemia” rather than actual lipid level measurements.
The D:A:D (Data Collection on Adverse Events of Anti-HIV Drugs) study suggested a rate of CVD in HIV infection that was higher than that predicted using the Framingham risk equation, but risk factor data in the study population were incomplete (Law et al, HIV Med, 2006). The D:A:D study also found that an association of antiretroviral therapy with risk of myocardial infarction (MI) was attenuated after adjusting for traditional risk factors (D:A:D Study Group, N Engl J Med, 2007).
Several studies that controlled for traditional CVD risk factors compared carotid intima media thickness (IMT; a predictor of CVD events) in HIV-infected patients with that in control subjects. Of the studies, 5 did not observe increased IMT in HIV infection (Kaplan et al, AIDS, 2008; Currier et al, AIDS, 2007; Johnsen et al, J Clin Endocrinol Metab, 2006; Currier et al, AIDS, 2005; Depairon et al, AIDS, 2001), and 2 did find increased IMT relative to controls (Lorenz et al, Atherosclerosis, 2008; Hsue et al, Circulation, 2004).
The FRAM (Fat Redistribution and Metabolic Change in HIV Infection) investigative group measured both the common carotid artery and the internal carotid artery bulb region in 433 HIV-infected patients and 5749 control subjects. They found a statistically significant increase in the mean internal carotid IMT of HIV-infected patients versus the control subjects (1.17 mm vs 1.06 mm; P < .0001). The difference between groups was still highly statistically significant after adjustment for demographics (0.19 mm; P < .0001) and after adjustment for both demographics and traditional CVD risk factors (smoking, diabetes, lipid levels, blood pressure) (0.15 mm; P = .0001).
HIV infection was also associated with a smaller but statistically significant increase in common carotid IMT (0.033 mm vs uninfected control subjects; P < .01). The finding that the HIV effect was stronger in the internal than the common carotid artery led the researchers to analyze the previously published papers. The 5 studies that did not find increased IMT in HIV infection had examined only the common carotid artery. The 2 reports that found an increase in HIV infection had examined both the bulb and the internal regions. Thus, the data from the larger FRAM study explain the discrepancy and make the case that HIV infection is an independent risk factor for atherosclerosis, as measured by the surrogate of IMT.
The effect of HIV infection on internal carotid IMT was similar to the effects associated with male sex (0.13 mm vs female sex), current smoker status (0.17 mm vs nonsmoker status), and diabetes (0.12 mm vs no diabetes; all P < .0001). The effect of HIV infection on common carotid IMT was of a magnitude similar to the increase associated with male sex (0.054 mm; P < .0001), current smoker status (0.020 mm; P < .001), and diabetes (0.026 mm; P < .0001). The study also showed that the effect of HIV infection in increasing risk of atherosclerosis, as measured by IMT, may be stronger in women than in men, similar to the relatively increased risk of CVD posed by diabetes in women versus men. Thus, these findings suggest that HIV infection is associated with increased risk of atherosclerosis similar to the level of risk associated with diabetes, smoking, and male sex after adjustment for traditional risk factors.
Metabolic Abnormalities and Dyslipidemia in HIV Infection
How does the finding of increased risk of atherosclerosis in HIV infection enter into treatment considerations? To answer this question first requires consideration of the factors contributing to dyslipidemia in HIV disease.
Initial studies showed that lipid changes in HIV-infected patients include an early decrease in high-density lipoprotein cholesterol (HDL-C) and a somewhat later decrease in low-density lipoprotein cholesterol (LDL-C), followed by increases in levels of triglycerides (TG) and very low-density lipoprotein cholesterol (VLDL-C) in later-stage disease (Grunfeld et al, J Clin Endocrinol Metab, 1992). The loss of the antiatherosclerotic effect of HDL-C likely increases risk of atherosclerosis more than the reduction in LDL-C reduces the risk, and the later-stage increase in VLDL-C firmly tips the scale toward a proatherogenic effect. The effect is further amplified by the fact that HDL-C does not function optimally in the setting of infection or inflammation. Recent studies have shown statistically significant correlations between increasing plasma HIV RNA level and decreasing levels of HDL-C and LDL-C and increasing levels of TG and VLDL-C, as well as a statistically significant relationship between decreasing CD4+ cell count and decreasing levels of HDL-C (El-Sadr et al, HIV Med, 2005).
The lipodystrophy associated with HIV disease is expressed as lipoatrophy that affects the lower body (especially the legs) more than the upper body (where effects are lowest in the upper trunk), rather than as a disorder of central versus peripheral fat redistribution. HIV lipoatrophy is associated with the use of stavudine. Although visceral obesity does occur in HIV disease, stavudine plays no role in it and the major predictors are age, restoration to health through antiretroviral treatment, and inactivity.
Increased visceral adipose tissue is associated with increased TG levels in HIV-infected patients and control subjects (both men and women), with TG levels higher in HIV-infected patients than in control subjects with similar amounts of visceral adipose tissue. Perhaps less well known is that increased leg subcutaneous adipose tissue is associated with reduced TG levels (Currier et al, JAIDS, 2008; Wohl et al, JAIDS, 2008). Hence, patients with HIV-related lipoatrophy who have strikingly lower amounts of leg adipose tissue are at particular risk of elevated TG levels.
HIV infection itself appears to be associated with elevated TG levels. An early study showed that TG levels remained stable in patients receiving placebo but decreased in patients receiving zidovudine monotherapy (in association with reduced alpha-interferon levels) (Mildvan et al, Lancet, 1992). The addition of a protease inhibitor (PI) to nucleoside analogue reverse transcriptase inhibitor (nRTI) treatment, however, leads to a substantial increase in TG levels (Mulligan et al, JAIDS, 2000)–a counterintuitive effect given that a reduction in HIV replication appears to lower TG levels.
Observations such as these led to a prevailing opinion that PIs were responsible for numerous metabolic abnormalities. For example, in the same study that documented a TG increase with the addition of a PI to nRTI treatment, LDL-C level was also observed to increase, appearing to pose increased CVD risk. A point that seemed to escape notice in this and other studies was that LDL-C levels in HIV-infected patients were low initially. After starting PIs, patients’ mean LDL-C levels increased to 112 mg/dL (Mulligan et al, JAIDS, 2000), a level that would be well accepted by many physicians for their dyslipidemic patients.
It is difficult to determine whether metabolic changes observed in patients receiving PIs are the direct effect of the drugs or secondary to a reactivated immune system, restoration to health, or body composition changes. Therefore, investigators examined the effects of short-term PI administration on HIV-seronegative volunteers, studying their metabolism and body composition before and after treatment. No change was observed in body composition, indicating that fat accumulation or loss during PI treatment does not account for metabolic alterations.
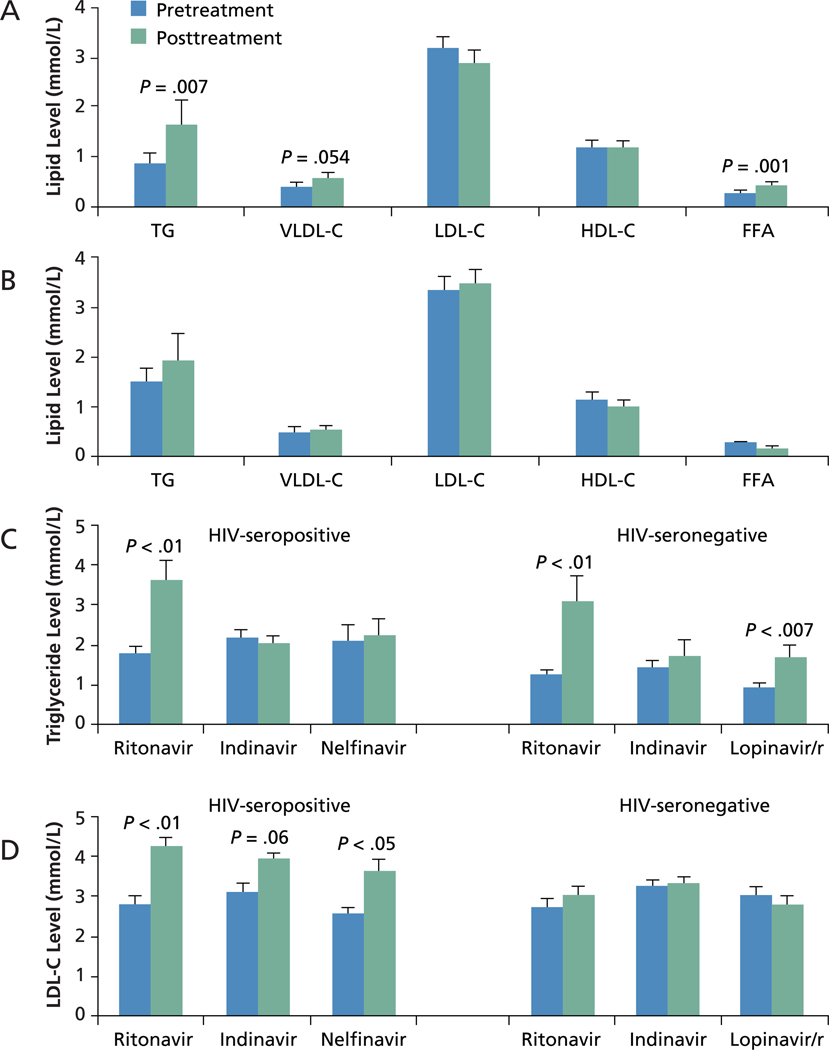
In 4-week studies of HIV-seronegative subjects, ritonavir-boosted (/r) lopinavir use was associated with statistically significant increases in levels of TG, VLDL-C, and free fatty acids but not of LDL-C (Lee et al, AIDS, 2004). Ritonavir use alone was statistically significantly associated with increased levels of TG (Purnell et al, AIDS, 2000). In contrast, another study showed no change in TG or lipoprotein profiles associated with indinavir use (Noor et al, AIDS, 2001) (Figure 1). Studies including HIV-infected patients showed a statistically significant increase in TG levels associated with use of ritonavir but not with use of indinavir or nelfinavir (Periard et al, Circulation, 1999). These results indicate that lipid alterations are not a class effect and that ritonavir is associated with a marked effect in raising TG levels.
Figure 1.
Effects of protease inhibitors on lipid measures. A, Effects of ritonavir-boosted (/r) lopinavir and B, indinavir on levels of triglycerides (TG), very low-density lipoprotein cholesterol (VLDL-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and free fatty acids (FFA) in HIV-seronegative subjects. Based on data for lopinavir/r from Lee et al, AIDS, 2004, and for indinavir from Noor et al, AIDS, 2001. C, Effects on TG levels of ritonavir, indinavir, and nelfinavir in HIV-seropositive patients and of ritonavir, indinavir, and lopinavir/r in HIV-seronegative subjects. Based on data for HIV-seronegative patients from Periard et al, Circulation, 1999, and for HIV-seropositive subjects from Purnell et al, AIDS, 2000; Lee et al, AIDS, 2004; and Noor et al, AIDS, 2001. D, Effects on LDL-C levels of ritonavir, indinavir, and nelfinavir in HIV-seropositive patients and of ritonavir, indinavir, and lopinavir/r in HIV-seronegative subjects. Based on data for HIV-seronegative subjects from Periard et al, Circulation, 1999, and for HIV-seropositive subjects from Purnell et al, AIDS, 2000; Lee et al, AIDS, 2004; and Noor et al, AIDS, 2001
These studies also showed a statistically significant or nearly significant increase in LDL-C levels associated with ritonavir, nelfinavir, or indinavir administration in HIV-infected patients but not in HIV-seronegative control subjects. These data suggest that the increase in LDL-C is not a drug effect but rather likely represents restoration to health.
Further support for the concept that LDL-C changes observed during antiretroviral treatment frequently occur as part of restoration to health–and are not associated solely with PI administration–comes from findings indicating that treatment with non-nucleoside reverse transcriptase inhibitors (NNRTIs) is also associated with increases in LDL-C levels. A study reported in 2001 (van der Valk et al, AIDS, 2001) showed that treatment with the NNRTI nevirapine was associated with increases in LDL-C levels of approximately 20% (similar to the increase observed with indinavir). As is well recognized, nevirapine treatment was associated with an approximately 50% increase in HDL-C levels, with smaller increases observed associated with use of the nRTI lamivudine and the PI indinavir. The NNRTI efavirenz was also associated with increased levels of HDL-C, LDL-C, and TG. The fact that increased TG levels were observed with efavirenz treatment but not nevirapine treatment indicates that increased TG level is not a class effect of NNRTIs and underscores the need to determine which drugs are associated with which lipid effects.
The situation becomes even more complicated when it is appreciated that the nRTI base in a regimen affects the lipid profile. Study 903 (Gilead Sciences, Inc, Foster City, CA) showed statistically significantly greater increases in levels of TG, total cholesterol, and LDL-C associated with the regimen that included stavudine versus the increase associated with tenofovir when each drug was administered with lamivudine and efavirenz for 48 weeks. Although these findings were widely taken as evidence of stavudine toxicity, it is important to recognize that efavirenz, which is associated with increased levels of VLDL-C and TG, was included in both regimens. The findings thus could also indicate an off-target effect of tenofovir in improving non-HDL-C level, as is indeed suggested by other, recent data. Some of the effect of stavudine on TG level is likely to be specific to the drug, however. In one study, initial increases in TG levels were greater with the nRTI combination of didanosine plus stavudine than with abacavir plus lamivudine, whereas moderate increases were observed over the long term (2 – 3 years) with both regimens (Shlay et al, JAIDS, 2005).
In another long-term follow-up study, LDL-C level was initially increased in association with PI-, NNRTI-, and PI plus NNRTI-containing treatments and then drifted back toward baseline levels after 3 years to 4 years (Shlay et al, JAIDS, 2007). Taken together, these results suggest the initial increase in LDL-C levels occurs in association with restoration to health, irrespective of the drug class used. The decline thereafter may occur as the result of a return of some of the immune responses that act to decrease LDL-C levels in HIV infection, and it is likely that ongoing infection and inflammation cause continuing dysfunction of HDL-C.
Table 1 summarizes the effects on lipid levels associated with various anti-retroviral drugs. The most pronounced effect on TG level occurs with full-dose ritonavir; somewhat attenuated effects are associated with ritonavir-boosted PIs; and a neutral effect is noted with unboosted PIs. Among non-PI drugs, efavirenz and probably stavudine lead to TG elevation, whereas tenofovir may reduce the TG level. The HDL-C level is raised to the greatest extent in association with use of the NNRTIs nevirapine and efavirenz, with a smaller increase or neutral effect observed with some PI-based regimens and decreases observed in some studies of ritonavir-boosted PIs. LDL-C levels are increased in association with most PI-and NNRTI-based regimens, with the exception of atazanavir monotherapy, which appears to have a neutral effect. Tenofovir may lower LDL-C level.
Table 1.
Changes in Lipid Levels Associated With Antiretroviral Drugs
| Effects on Triglyceride Level | |
|---|---|
| ↑↑↑ | Full-dose ritonavir |
| ↑↑ | Ritonavir-boosted (/r) lopinavir, tipranavir/r, fosamprenavir/r |
| ↑↑ | Efavirenz |
| ↑ | Darunavir/r, some other ritonavir-boosted protease inhibitors (PIs), possibly stavudine |
| ↔ | Most non–ritonavir-boosted PIs, possibly raltegravir, maraviroc |
| ↓ | Likely tenofovir |
| Effects on High-Density Lipoprotein Cholesterol Level | |
| ↑↑↑ | Nevirapine |
| ↑↑ | Efavirenz |
| ↑ or ↔ | Some PI-based antiretroviral regimens, maraviroc |
| ↓ | Some ritonavir-boosted PIs (eg, lopinavir or darunavir) |
| Effects on Low-Density Lipoprotein Cholesterol Level | |
| ↑ | Most PI- and NNRTI-based regimens |
| ↔ | Non–ritonavir-boosted atazanavir, maraviroc |
| ↓ | Likely tenofovir |
Note: Arrows indicate effects as follows: ↑↑↑, very large increase; ↑↑, moderate increase; ↑, small increase; ↔, no change; ↓, small decrease. NNRTI indicates nonucleoside analogue reverse transcriptase inhibitor.
There are too few data on drugs in new antiretroviral classes for firm conclusions on their lipid effects. However, in one study the integrase strand transfer inhibitor (INSTI) raltegravir was associated with no increase in TG or LDL-C level and a smaller increase in HDL-C level than with efavirenz when each drug was used in combination with tenofovir and lamivudine (Markowitz et al, JAIDS, 2007). In addition, switching from lopinavir/r to raltegravir was associated with reductions in TG and non-HDL-C levels. The investigational INSTI elvitegravir requires ritonavir boosting for once-daily dosing, and its effects on lipids are not yet known. Also unknown are the effects the investigational drug cobicistat (GS-9350) may have on lipids. The CC chemokine receptor 5 entry inhibitor maraviroc has been reported to have no effect on levels of TC, LDL-C, HDL-C, or TG (Arribas, Enferm Infecc Microbiol Clin, 2008).
Considering this information, what steps should clinicians take to address the metabolic effects of antiretroviral therapy? First, it is imperative for clinicians to be thoroughly versed in drug interactions and the metabolic effects of various drugs. Reviews on the topic are useful, as is posting summary tables showing the effects of individual drugs. Some patients and their caregivers may be too quick to blame antiretroviral drugs for effects that may be more strongly associated with lifestyle factors (eg, obesity, physical inactivity, alcohol intake, and diet). Patients need to be informed that the effects of HIV disease outweigh the CVD risks associated with some antiretroviral drugs; therefore, the regimen to treat the HIV infection should be selected based on the virus, and appropriate treatment for dyslipidemia will be provided if necessary. Patient education also includes emphasis that lifestyle changes will likely be necessary as well.
Treatment of Dyslipidemia in HIV Infection
The rate of MI in HIV-infected patients has declined in recent years, as indicated by data from the Kaiser Permanente California integrated health system (Table 2). The relative rate of MI hospitalization in HIV-infected persons was more than 2-fold greater than that of controls, but the most recent data (2006 – 2008) show a 1.3-fold increased rate (Hurley et al, CROI, 2009). This reduction in risk is associated with increasing use of lipid-lowering treatment, mostly statin drugs and fibrate drugs. Since the period of 1996 to 1997, the proportions of HIV-infected patients in the cohort using lipid-lowering therapy has increased from 1.5% to 33.5% in PI-treated patients, 1.5% to 30.1% in PI-naive patients, and 1.0% to 6.4% in antiretroviral drug-naive patients.
Table 2.
Rates of Myocardial Infarction per 1000 Person-Years in HIV-Infected Versus HIV-Uninfected Persons, 1996 to 2008
| Years | Rate of Myocardial Infarction | |
|---|---|---|
| HIV-Infected | HIV-Uninfected | |
| 1996 – 1997 | 2.5 | 1.3 |
| 1998 – 1999 | 3.3 | 1.5 |
| 2000 – 2001 | 2.8 | 1.4 |
| 2002 – 2003 | 3.7 | 1.7 |
| 2004 – 2005 | 3.1 | 1.9 |
| 2006 – 2008a | 2.5 | 2.0 |
Data for 2008 were from January through June.
Based on data from Hurley et al, CROI, 2009.
The increased risk of CVD by HIV infection itself has yet to be reflected in conventional risk calculations. Nevertheless, the increased risk is a strong argument for more aggressive lipid-lowering treatment in dyslipidemic HIV-infected patients than would be suggested by use of conventional risk calculation. Appropriate use of lipid-lowering drugs in these patients requires knowledge of the drugs’ effects in HIV-infected persons and potential interactions with antiretroviral drugs.
Recent data from Kaiser Permanente California show that lipid-lowering therapy produces LDL-C reductions in HIV-infected patients similar to those in HIV-uninfected patients (Silverberg et al, Ann Intern Med, 2009). Reductions in LDL-C were 19.2% versus 19.9% (P = .38) with any lipid-lowering therapy and 25.6% versus 28.3% (P = .001) with any statin therapy. The problem with statin therapy in patients receiving antiretroviral therapy is not lack of efficacy in reducing LDL-C level, but drug interactions with PIs. The PIs inhibit cytochrome P450 (CYP) 3A4 isoenzymes, which metabolize some statins. Although simvastatin and lovastatin are popular because generic versions are available, blood levels of simvastatin (and likely lovastatin) increase 32-fold when used concomitantly with PIs (Fichtenbaum et al, AIDS, 2002). Thus, simvastatin or lovastatin should not be used by PI-treated patients. Atorvastatin activity increases 2-fold when used concomitantly with PIs (Fichtenbaum et al, AIDS, 2002); thus, the 80-mg dose should be avoided. Pravastatin levels decrease by 50% (Aberg et al, AIDS, 2006; Fichtenbaum et al, AIDS, 2002), and a case of rhabdomyolysis has been reported at high dose.
Some PIs increase rosuvastatin blood levels via a non-CYP3A4-related mechanism. Rosuvastatin maximum concentration was increased 4.7-fold by lopinavir/r (Kiser et al, JAIDS, 2008) and 6-fold by atazanavir/r (Busti et al, J Cardiovasc Pharmacol, 2008). Thus, high-dose rosuvastatin should be avoided by patients receiving these PIs. No change in rosuvastatin concentration was observed with fosamprenavir/r (Busti et al, J Cardiovasc Pharmacol, 2008). Darunavir increases levels of pravastatin (5-fold), rosuvastatin, and atorvastatin. Efavirenz is a mixed inducer and inhibitor of CYP3A4 isoenzymes. Efavirenz lowers simvastatin (and likely lovastatin) level by 60%, atorvastatin activity by 34%, and, paradoxically, pravastatin level by 40% (Gerber et al, JAIDS, 2005). Higher levels of such statin drugs are needed, but there is potential risk of rhabdomyolysis with use of higher doses.
With regard to other lipid-lowering therapies, ezetimibe is effective in reducing LDL-C levels in HIV-infected patients and is a useful addition for patients unable to reach LDL-C goals with statin treatment (its primary indication) (Bennet et al, Lipids Health Dis, 2007; Negredo et al, AIDS, 2006). Ezetimibe can also be used as a primary therapy (Coll et al, AIDS, 2006; Wohl et al, Clin Infect Dis, 2008). Using ezetimibe in combination with a statin drug may be safer than using a higher dose of the statin drugs. A trial including HIV-seronegative patients, using the change in mean IMT as the primary endpoint, did not show benefit of ezetimibe in reducing progression of atherosclerosis (Kastelein et al, N Engl J Med, 2008), but baseline IMT was not increased, so it was difficult to show any change. Trials using clinical events as endpoints for ezetemibe efficacy are still ongoing.
Use of bile acid-binding drugs is complicated in HIV-infected patients because such drugs raise the TG level and hypertriglyceridemia is the primary lipid abnormality in HIV-infected patients. Further, bile acid–binding drugs may affect antiretroviral drug absorption.
Triglyceride-lowering therapy appears less effective in HIV-infected patients than in HIV-uninfected patients. Data from Kaiser Permanente California show that HIV-infected patients start with higher TG levels than HIV-uninfected persons and achieve smaller percentage TG reductions with lipid-lowering therapy, as well as little improvement in HDL-C levels. Reductions in TG levels with any lipid-lowering therapy were 41.2% versus 52.1%, respectively (P < .001), and 44.2% versus 59.3%, respectively (P < .001), with the fibrate gemfibrozil (Silverberg et al, Ann Intern Med, 2009). Fibrate drugs do reduce TG level and seem safe used as monotherapy to reduce lipid levels in HIV-infected patients.
Fish oil also may not work as well in HIV-infected patients as in HIV-uninfected patients. Available data indicate average TG reductions of 14% in HIV-uninfected patients (Eslick et al, Int J Cardiol, 2009) and 20% in HIV-infected patients (Wohl et al, Clin Infect Dis, 2005). However, in these analyses, baseline TG levels were 216 mg/dL in HIV-uninfected patients and 461 mg/dL in HIV-infected patients. Fish oil treatment resulted in a 2% increase in LDL-C levels in HIV-uninfected patients versus a 16% increase in HIV-infected patients. It is likely that higher initial TG levels result in smaller percentage decreases in TG and greater increases in LDL-C levels with fish oil treatment.
In HIV-infected patients, niacin decreased TG level by 32% to 34%, non-HDL-C level by 9% to 19%, and apolipo-protein B-100 level by 9% but produced no change in LDL-C level and small increases of 3% to 15% in HDL-C level (Dubé et al, Antivir Ther, 2006; Gerber et al, Clin Infect Dis, 2004). However, niacin also induces substantial insulin resistance. Small increases in fasting glucose levels that improved over time have been observed, but 3% to 25% of patients have developed impaired glucose tolerance as indicated by results of oral glucose tolerance testing (Dubé et al, Antivir Ther, 2006; Gerber et al, Clin Infect Dis, 2004). Although niacin is potentially useful, patients receiving it need close monitoring for signs of diabetes.
More than one drug may be needed to treat dyslipidemia in HIV-infected patients, especially patients with hypertriglyceridemia. Because gemfibrozil inhibits glucuronidation of statins (Jacobson, Nat Rev Endocrinol, 2009; Davidson, Expert Opin Drug Saf, 2006), drug labeling restricts the concomitant use of gemfibrozil and statin drugs to lower doses of statin drugs (eg, rosuvastatin 10 mg). However, the most prudent course may be to avoid adding gemfibrozil to statin treatment altogether. Fenofibrate does not alter statin metabolism (Jacobson, Nat Rev Endocrinol, 2009; Davidson, Expert Opin Drug Saf, 2006) and is approved by the US Food and Drug Administration for use in combination with statin drugs. When recommending combined use of fenofibrate with a statin, practitioners should inform patients that the benefits of the combination likely outweigh the risks. As stated, ezetimibe enhances lipid-lowering effects but was not found to decrease IMT when combined with a statin; long-term outcome trials of ezetimibe are under way.
Conclusion
HIV infection and its therapies are associated with dyslipidemia (and diabetes) and therefore with the promotion of atherosclerosis. HIV infection is also associated with increased risk of CVD, even after adjusting for traditional risk factors. The degree of increased risk suggests that aggressive lipid-lowering therapy should be considered in HIV-infected patients with moderate CVD risk. Safely treating dyslipidemia in HIV-infected patients requires knowledge of HIV infection, antiretroviral therapy, and potential drug interactions between antiretroviral drugs and lipid-lowering drugs.
Acknowledgments
Financial Disclosure: Dr Grunfeld has received grants and research support awarded to the Northern California Institute for Research and Education from Theratechnologies Inc.
Footnotes
Presented by Dr Grunfeld in March 2010. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Grunfeld in August 2010.
Suggested Reading
- Aberg JA, Rosenkranz SL, Fichtenbaum CJ, et al. Pharmacokinetic interaction between nelfinavir and pravastatin in HIV-seronegative volunteers: ACTG Study A5108. AIDS. 2006;20:725–729. doi: 10.1097/01.aids.0000216373.53819.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas JR. Role of the new molecules in antiretroviral therapy. Position of raltegravir. Enferm Infecc Microbiol Clin. 2008;26 suppl 12:53–59. doi: 10.1016/s0213-005x(08)76574-1. [DOI] [PubMed] [Google Scholar]
- Bennett MT, Johns KW, Bondy GP. Ezetimibe is effective when added to maximally tolerated lipid lowering therapy in patients with HIV. Lipids Health Dis. 2007;6:15. doi: 10.1186/1476-511X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busti AJ, Bain AM, Hall RG, 2nd, et al. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J Cardiovasc Pharmacol. 2008;51:605–610. doi: 10.1097/FJC.0b013e31817b5b5a. [DOI] [PubMed] [Google Scholar]
- Coll B, Aragonés G, Parra S, Alonso-Villaverde C, Masana L. Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. AIDS. 2006;20:1675–1677. doi: 10.1097/01.aids.0000238418.43937.3b. [DOI] [PubMed] [Google Scholar]
- Currier J, Scherzer R, Bacchetti P, et al. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. JAIDS. 2008;48:35–43. doi: 10.1097/QAI.0b013e318164227f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- Currier JS, Kendall MA, Zackin R, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS. 2005;19:927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. JAIDS. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- Friis-Møller N, Reiss P, et al. D:A:D Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- Davidson MH. Statin/fibrate combination in patients with metabolic syndrome or diabetes: evaluating the risks of pharmacokinetic drug interactions. Expert Opin Drug Saf. 2006;5:145–156. doi: 10.1517/14740338.5.1.145. [DOI] [PubMed] [Google Scholar]
- Depairon M, Chessex S, Sudre P, et al. Premature atherosclerosis in HIV-infected individuals–focus on protease inhibitor therapy. AIDS. 2001;15:329–334. doi: 10.1097/00002030-200102160-00005. [DOI] [PubMed] [Google Scholar]
- Dubé MP, Wu JW, Aberg JA, et al. Safety and efficacy of extended-release niacin for the treatment of dyslipidaemia in patients with HIV infection: AIDS Clinical Trials Group Study A5148. Antivir Ther. 2006;11:1081–1089. [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6:114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol. 2009;136:4–16. doi: 10.1016/j.ijcard.2008.03.092. [DOI] [PubMed] [Google Scholar]
- Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: AIDS Clinical Trials Group (ACTG) study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- Gerber JG, Rosenkranz SL, Fichtenbaum CJ, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. JAIDS. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- Gerber MT, Mondy KE, Yarasheski KE, et al. Niacin in HIV-infected individuals with hyperlipidemia receiving potent antiretroviral therapy. Clin Infect Dis. 2004;39:419–425. doi: 10.1086/422144. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- Hurley L, Leyden W, Xu L, et al. Updated surveillance of cardiovascular event rates among HIV-infected and HIV-uninfected Californians, 1996 to 2008; 16th Conference on Retroviruses and Opportunistic Infections; February 8-11, 2009; Montreal, Canada. [Abstract 710.] [Google Scholar]
- Jacobson TA. Myopathy with statin-fibrate combination therapy: clinical considerations. Nat Rev Endocrinol. 2009;5:507–518. doi: 10.1038/nrendo.2009.151. [DOI] [PubMed] [Google Scholar]
- Johnsen S, Dolan SE, Fitch KV, et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4916–4924. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- Kiser JJ, Gerber JG, Predhomme JA, Wolfe P, Flynn DM, Hoody DW. Drug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. JAIDS. 2008;47:570–578. doi: 10.1097/QAI.0b013e318160a542. [DOI] [PubMed] [Google Scholar]
- Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? JAIDS. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- Law MG, Friis-Møller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006;7:218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- Lee GA, Seneviratne T, Noor MA, et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS. 2004;18:641–649. doi: 10.1097/00002030-200403050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MW, Stephan C, Harmjanz A, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. JAIDS. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- Mildvan D, Machado SG, Wilets I, Grossberg SE. Endogenous interferon and triglyceride concentrations to assess response to zidovudine in AIDS and advanced AIDS-related complex. Lancet. 1992;339:453–456. doi: 10.1016/0140-6736(92)91058-g. [DOI] [PubMed] [Google Scholar]
- Mulligan K, Grunfeld C, Tai VW, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. JAIDS. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- Negredo E, Moltó J, Puig J, et al. Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins. AIDS. 2006;20:2159–2164. doi: 10.1097/01.aids.0000247573.95880.db. [DOI] [PubMed] [Google Scholar]
- Noor MA, Lo JC, Mulligan K, et al. Metabolic effects of indinavir in healthy HIV-seronegative men. AIDS. 2001;15:F11–F18. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Zambon A, Knopp RH, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- Shlay JC, Bartsch G, Peng G, et al. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. JAIDS. 2007;44:506–517. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- Shlay JC, Visnegarwala F, Bartsch G, et al. Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. JAIDS. 2005;38:147–155. doi: 10.1097/01.qai.0000143599.64234.15. [DOI] [PubMed] [Google Scholar]
- Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150:301–313. doi: 10.7326/0003-4819-150-5-200903030-00006. [DOI] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an antiatherogenic lipid profile. AIDS. 2001;15:2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- Wohl D, Scherzer R, Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. JAIDS. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl DA, Tien HC, Busby M, et al. Randomized study of the safety and efficacy of fish oil (omega-3 fatty acid) supplementation with dietary and exercise counseling for the treatment of antiretroviral therapy-associated hypertriglyceridemia. Clin Infect Dis. 2005;41:1498–1504. doi: 10.1086/497273. [DOI] [PubMed] [Google Scholar]
- Wohl DA, Waters D, Simpson RJ, Jr, et al. Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2008;47:1105–1108. doi: 10.1086/592116. [DOI] [PMC free article] [PubMed] [Google Scholar]