Summary
Background
Establishment and maintenance of epithelial cell polarity is regulated in part by signaling from adhesion receptors. Loss of cell polarity is associated with multiple pathologies including the initiation and progression of various cancers. The β1-integrin adhesion receptor plays a role in the regulation of cell polarity; however, the identity of the signaling pathways that modulate β1-integrin function and connect it to the regulation of polarity pathways remains largely unknown.
Results
The present work identifies a role for Arg, a member of the Abl family non-receptor tyrosine kinases, in the regulation of adhesive signals and epithelial cell polarity. In a three-dimensional (3D) cell culture model, activation of Arg kinase leads to a striking inversion of apical-basal polarity. In contrast, loss of Arg function impairs the establishment of a polarized epithelial cyst structure. Activated Arg kinase disrupts β1-integrin signaling and localization and impairs Rac1-mediated laminin assembly. Disruption of β1-integrin function by active Arg results in altered distribution of selected polarity complex components mediated in part by Rap1 GTPase signaling. Whereas polarity inversion is partially rescued by a constitutively active Rap1, Rac1-dependent laminin assembly is not, indicating Rap1 and Rac1 signal independently during epithelial polarity.
Conclusions
These findings suggest that modulation of Arg kinase function may contribute not only to normal epithelial polarity regulation, but also may promote pathologies associated with loss of cell polarity.
Introduction
The establishment and maintenance of tissue architecture requires crosstalk from cell surface receptors to polarity regulatory complexes [1]. Disruption of this crosstalk has been associated with diverse pathological conditions, including cancer. The initiation and progression of many cancers are often linked to the disruption of tissue architecture and loss of cell polarity [2, 3]. Integrin signaling modulates cell polarity and has been shown to suppress or promote tumorigenesis. The β1-integrin is required for mammary gland development and regulation of polarized epithelial structures [4] and targeted disruption of β1-integrin in mice revealed that this integrin plays a critical role at distinct stages of tumor progression depending on the tumor model employed [5, 6]. Using three-dimensional (3D) culture models, a direct role for β1-integrin in the regulation of epithelial cell polarity has been demonstrated as blocking β1-integrin function in this system caused inversion of apical polarity [7]. The effects of β1-integrin-dependent polarity regulation required Rac1-mediated control of laminin assembly at the basal membrane [8]. However, the pathways that connect β1-integrin to the regulation of polarity complexes and the identity of upstream signaling pathways that modulate β1-integrin function remain largely unknown.
Epithelial cell polarity is dependent on distinct polarity complexes known as the PAR (Par3/Par6/aPKC), Crumbs (Crumbs/PATJ/PALS) and Scribble (Lgl/Dlg/Scrib) complexes. The PAR and the Crumbs protein complexes promote apical-membrane identity and establishment of the apical-basal border, respectively, while the Scribble complex directs basolateral membrane identity [2]. Aberrant activation of growth factor receptor tyrosine kinases (RTKs) disrupts cell polarity by targeting polarity protein complexes [3] as in the case of Erb2 hyperactivation which disrupts cell polarity by causing the dissociation of Par3 from the Par6/aPKC polarity complex [9].
The Abl non-receptor tyrosine kinases, Abl and Arg, are activated downstream of RTKs and adhesion molecules, and regulate cell migration, morphogenesis, proliferation and survival [10, 11]. We showed that Abl kinases regulate cadherin-mediated intercellular adhesion in part through modulation of the Rac1 and RhoA GTPases [12]. Abl kinases are also activated downstream of integrin engagement and regulate integrin function in neural and immune cells [13, 14]. Abl kinases were first identified as oncoproteins in leukemias associated with the production of fusion proteins with constitutive tyrosine kinase activity [10]. Recently, enhanced expression and activation of Abl and Arg has been reported in solid tumors including colorectal, breast, lung cancer and renal medullary carcinoma [15–19]. We showed that Abl kinases are activated by chemokines in breast cancer cells and are required for cancer cell invasion [20]. While our previous studies suggested that activated Abl kinases play a role at later stages of tumor progression, a role for Abl kinases in the disruption of cell polarity linked to tumor initiation has not yet been examined. Studies in Drosophila have implicated D-Abl in the regulation of epithelial cell polarity [21–23]. Loss of D-Abl disrupts cell migration and cell shape changes during dorsal closure and ventral furrow formation [22, 23]. However, whether mammalian Abl and Arg kinases play a role in epithelial cell polarity is unknown. In this report we uncover a role for the Abl family kinase member Arg in the regulation of β1-integrin signaling and apical-basal polarity using 3D epithelial cell cultures.
Results
Arg kinase regulates epithelial cell polarity
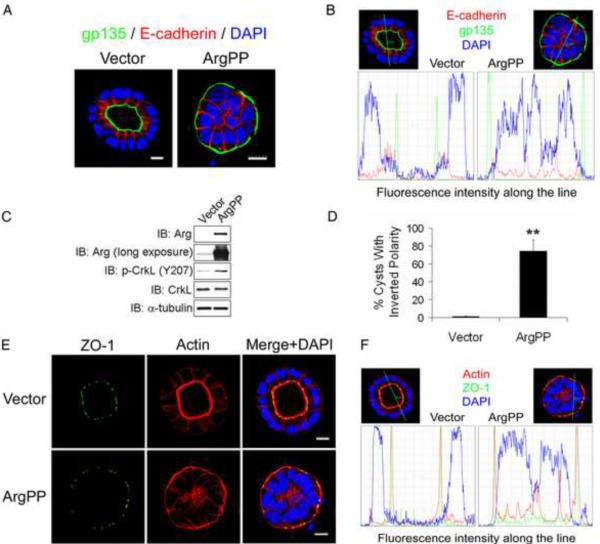
To investigate the consequences of Arg kinase activation on epithelial cell polarity, we employed a 3D cell culture system in which Madin-Darby canine kidney type II (MDCKII) cells form polarized cysts when grown in collagen matrix. MDCKII cells express both Arg and Abl kinases (Figure 1C, S1B). In contrast to control cells, overexpression of a constitutively-active Arg mutant (ArgPP) in MDCKII cells produced cysts without lumen and a striking inverted polarity phenotype characterized by the localization of the apical marker gp135 to the outer surface of the cyst (Figure 1A–D). Activation of the Arg kinase was confirmed by hyper-phosphorylation of CrkL, an Abl/Arg substrate, on tyrosine 207 (Figure 1C). Whereas control cysts displayed strong actin accumulation at the apical membrane and localization of the tight junction marker ZO-1 on the inner membrane surrounding the lumen, ArgPP-expressing cysts were characterized by inverted membrane distribution of both actin and ZO-1 (Figure 1E–F). Expression of an active mutant of the Abl kinase (AblPP) also produced cysts with inverted apical polarity (Figure S1A–C). Active ArgPP generated higher percentages of cysts with inverted polarity (~70%) (Figure 1D) than AblPP (~25%) (Figure S1C), and thus we focused primarily on the role of Arg in the regulation of cyst polarity.
Figure 1.
Active Arg inverts epithelial cyst polarity.
(A) MDCKII cells expressing either empty vector or constitutively-active Arg (ArgPP) were grown in collagen gels for 6 days. The gels were fixed and stained for the apical polarity marker gp135 and the adherens junction marker E-cadherin, and visualized by confocal microscopy. Scale bars, 10μm.
(B) Lines were drawn across representative cells in (A) and the fluorescence intensity distribution of the indicated markers along the lines are shown.
(C) Western blots show Arg expression and activity by detection of p-CrkL levels.
(D) Quantification of the percentage of cysts with inverted polarity at day 6 from 3 independent experiments; over 200 cysts from each group were analyzed by two-tailed unpaired Student's t-test; **, p<0.01. Error bars represent mean ± SD. (E, F) MDCKII cysts expressing either vector or ArgPP were stained for ZO-1 and actin and visualized by confocal microscopy. Fluorescence intensity distribution along the indicated lines are shown. Scale bars, 10μm.
Inhibition of β1-integrin signaling by activated Arg kinase
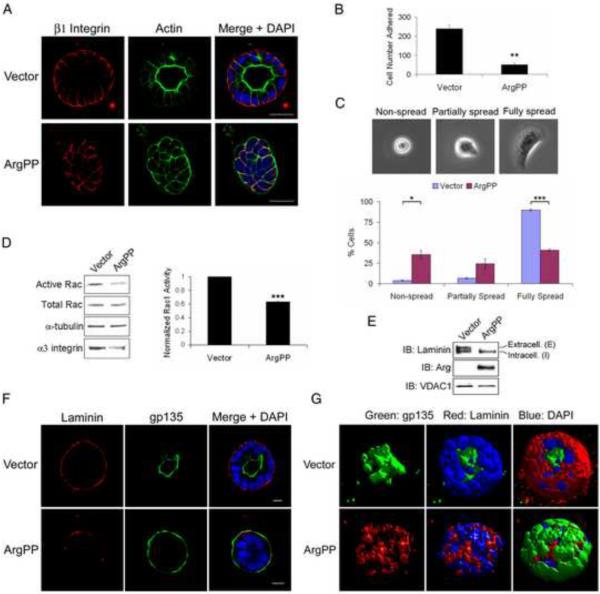
The phenotype induced by activated Arg is reminiscent of the inverted polarity induced by loss of β1-integrin function in MDCKII cysts [7]. Thus, we first examined whether active ArgPP altered β1-integrin function. While β1-integrin localized to the basolateral membrane in control cysts, ArgPP disrupted the localization of β1-integrin at the collagen-interacting outer membrane, though its localization at cell-cell junctions remained intact (Figures 2A and S2A). The inverted polarity phenotype in ArgPP-expressing cysts was detected as early as days 2 and 3 in 3D-culture, and was independent of cell numbers as both control and ArgPP-expressing cysts displayed similar cell numbers at this stage (Figure S2B–D). Similarly, the selective loss of β1-integrin at the cyst outer membrane occurred at the earliest stages of polarity establishment (Figure S2B–D). These data suggest that ligand-induced β1-integrin signaling might be impaired, a possibility which is supported by the observation that β1-integrin-mediated cell adhesion and spreading on collagen were dramatically inhibited by ArgPP (Figure 2B–C).
Figure 2.
Active Arg inhibits β1-integrin signaling.
(A) MDCKII cysts expressing either vector or ArgPP at day 6 were stained for β1-integrin and actin, and visualized by confocal microscopy. Scale bars, 25μm.
(B) MDCKII cells expressing vector or ArgPP were plated on collagen for 8 h and adherent cells were quantified. Data were analyzed by two-tailed unpaired Student's t-test; **, p<0.005. Error bars represent mean ± SEM. Vector, n=718; ArgPP, n=150.
(C) Cells quantified in (B) were classified into three morphological groups (top). The percentage of cells in each group was analyzed by two-tailed unpaired Student's t-test (bottom); *, p<0.03; ***, p<0.001. Error bars represent mean ± SEM.
(D) Cell lysates from MCDKII cells expressing either vector or ArgPP embedded in collagen for 6 to 12 h were subjected to active Rac1-GTP assay. Active Rac1 levels were detected by western blot (left panel) and analyzed by two-tailed unpaired Student's t-test (right panel); ***, p<0.001. Error bar represents mean ± SD. Lysates were also blotted for α3 integrin and tubulin (loading control).
(E) 4-day MDCKII cysts expressing either vector or ArgPP were isolated from collagen gels. Expression of cyst-associated laminin was detected by western blotting. VDAC1 was used as a loading control.
(F, G) MDCKII cysts expressing vector or ArgPP were stained for gp135 and laminin, and analyzed by confocal microscopy (F). Z-stack pictures were reconstructed to 3D models using the software “Volocity” (G). Scale bars, 10μm.
Similar to the phenotype induced by loss of β1-integrin, loss of Rac1 function causes inversion of apical polarity [8]. Rac1 is activated downstream of β1-integrin and promotes basal laminin assembly [8]. Activation of the laminin receptor α3β1 integrin on the basal membrane relays positional information promoting cyst polarity orientation [24]. Expression of ArgPP produced a 40% reduction of active Rac1 levels without affecting the levels of total Rac1 protein (Figure 2D). ArgPP-expressing cysts also exhibited decreased levels of α3 integrin (Figure 2D, left panel). A similar decrease in α3 integrin was reported in cysts treated with a β1-integrin function-blocking antibody or expressing dominant negative RacN17 mutant [7, 8]. The α3β1 integrin is required for basal laminin assembly [25]. Concomitant with decreased Rac1 activation and α3 integrin levels, laminin assembly was dramatically impaired in ArgPP-expressing cysts (Figure 2F–G). Consistent with published data [8] analysis of cyst-associated laminin protein in control cysts yields a doublet with an upper band that corresponds to extracellular laminin and a lower band for intracellular laminin (Figure 2E). ArgPP expression markedly decreased the levels of extracellular laminin without changing the levels of intracellular laminin (Figure 2E). Taken together, these data suggest that expression of active Arg impairs β1-integrin signaling, leading to decreased Rac1 activation and impaired laminin assembly. A role for Arg in the regulation of β1-integrin function during polarity establishment is consistent with its basolateral distribution in epithelial cysts (Figure S2E), a localization that is similar to that of β1-integrin (Figures 2A and S2A–D).
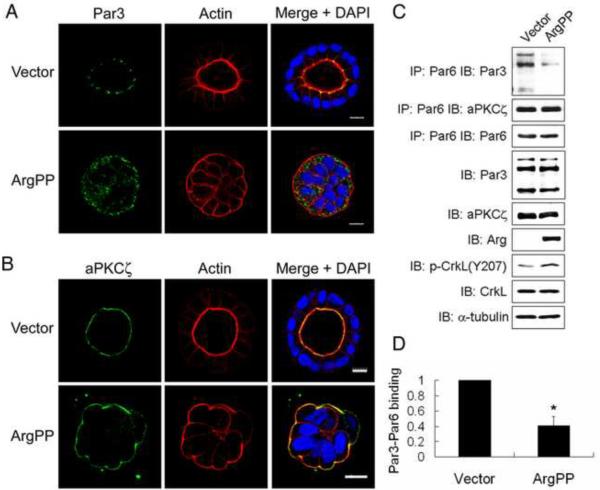
Active Arg or inhibition of β1-integrin disrupts polarity protein complexes in epithelial cysts
Epithelial cell polarity is dependent on the activity of distinct polarity complexes including the PAR, Scribble and Crumbs complexes [26]. Expression of ArgPP induced abnormal cytosolic localization of Par3, a component of the Par3/Par6/aPKC complex, which normally localizes to tight junctions in control cysts (Figure 3A and S3A). In contrast, aPKC and Par6 remained at the inverted apical membrane (Figures 3B and S3B–c). These findings suggested that ArgPP might promote dissociation of Par3 from the complex. Indeed, expression of ArgPP disrupted the interaction of Par3 with Par6/aPKC as assessed by co-immunoprecipiation of endogenous Par proteins (Figure 3C–D).
Figure 3.
Disruption of the Par complex by Arg activation.
(A, B) MDCKII cysts expressing either vector or ArgPP were stained for Par3 (A) or aPKC (B), and visualized by confocal microscopy. Scale bars, 10μm.
(C) Lysates of cells expressing vector or ArgPP were incubated with anti-Par6 antibody, and co-immunoprecipitates were blotted for Par3 and aPKCζ.
(D) Quantification of Par3 co-immunoprecipitated with Par6 by ImageJ and analyzed by two-tailed unpaired Student's t-test. *, p<0.04. Error bar represents mean ± SEM.
Active Arg did not affect the cytosolic and lateral membrane localization of mammalian Lgl (mLgl), a component of the Scribble complex (Figure S3D), but induced aberrant localization of Dlg which was found at the inverted apical membrane in cysts expressing ArgPP, rather than at the basolateral membrane (Figure S3F). To examine whether loss of β1-integrin function phenocopied the effects of ArgPP expression on the distribution of polarity proteins, cysts were treated with the β1-integrin function blocking antibody AIIB2 [7, 27]. While control IgG had no effect on cyst polarity, treatment with AIIB2 has been shown to induce inversion of apical polarity [7]. We found that AIIB2 treatment promoted the cytosolic localization of Par3 (Figure S3E) but had no effect on the localization of aPKCξ at the inverted apical membrane (data not shown). The effects of AIIB2 treatment phenocopied those induced by ArgPP. Similar to active Arg, blocking β1-integrin function induced Dlg mislocalization at the inverted apical membrane (Figure S3F-G). These findings suggest that the abnormal localization of polarity proteins in ArgPP-expressing cysts are likely to be a consequence of impaired β1-integrin signaling.
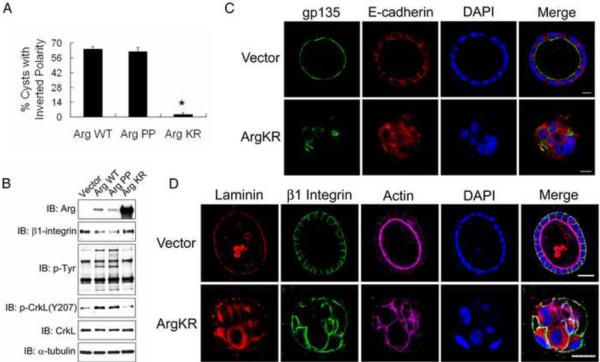
Loss of Arg function impairs epithelial polarity establishment
To determine whether the Arg-induced polarity inversion required Arg kinase activity, we overexpressed wild-type (WT), active (PP) or kinase-inactive (KR) forms of Arg in MDCKII cells. We consistently found that expression of ArgKR was higher than ArgWT and ArgPP, and that overexpression of ArgWT activated its kinase activity (Figure 4B). Both ArgWT and ArgPP but not ArgKR promoted inversion of cyst polarity (Figure 4A–C). Loss of Arg kinase activity in cysts expressing ArgKR or pharmacological inhibition with STI571 resulted in impaired polarity establishment characterized by fragmented localization of the apical marker gp135 and disruption of E-cadherin-positive adherens junctions in 3D cysts (Figures 4C and S4A–B). The loss of cell-cell junctions in the absence of functional Arg and Abl kinases in 3D cysts is consistent with our previous finding in 2D culture showing that Abl kinases are required for adherens junction formation and maintenance [12]. Cysts treated with STI571 or expressing ArgKR had decreased cyst radiuses compared to control cysts (Figure S4C–D), which may be due in part to cyst structure collapse induced by loss of polarity. The assembly of laminin was impaired in cysts expressing ArgKR despite the presence of β1-integrin at the cyst-collagen-interacting outer membrane (Figure 4D), suggesting that loss of Arg function interferes with the β1-integrin-mediated signaling leading to laminin assembly. The presence of β1-integrin at the cyst-collagen interface in cysts lacking functional Arg kinase is in contrast to the disruption of β1-integrin accumulation at the collagen-interacting outer membrane in cysts expressing activated ArgPP. Thus, loss of Arg activity produces cysts without obvious polarity, a phenotype that is distinct from the inverted polarity induced by active Arg.
Figure 4.
Loss of Arg function impairs establishment of epithelial cyst polarity while Arg activation inverts cyst polarity.
(A, B) MDCKII cells overexpressing either Arg wild-type (WT), constitutively active (PP) or kinase dead (KR) mutants were grown in collagen gels for 6 days. The percentage of cysts with inverted polarity was analyzed by two-tailed unpaired Student's t-test (A); *, p<0.02. ArgWT, n=332; ArgPP, n=195; ArgKR, n=446. Protein expression was analyzed by western blotting (B).
(C, D) MDCKII cells expressing either vector or ArgKR were embedded in collagen gels for 8~9 days. Cysts were stained for the indicated markers, followed by confocal microscopy. Scale bars, 10μm (C) and 25μm (D).
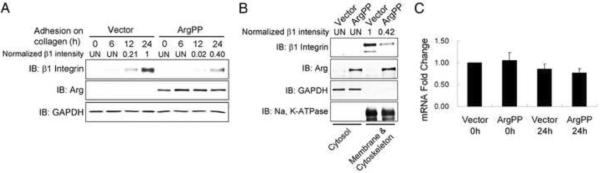
Active Arg suppresses collagen-induced β1-integrin expression
We showed that Abl/Arg kinases regulate cell surface levels of the epidermal growth factor receptor and MT1-MMP [20, 28]. Thus, we hypothesized that active Arg might affect β1-integrin function in part by modulating its surface expression. Flow cytometric analysis of non-adherent cells showed no statistically significant differences in the percentage of β1-integrin positive cells or average intensity of surface β1-integrin levels between control and ArgPP-expressing cells (Figure S5A–B). In contrast, β1-integrin levels were dramatically increased in control cells in response to adhesion to collagen, and this increase was significantly suppressed by ArgPP (Figure 5A). Cellular protein fractionation demonstrated that the upregulated β1-integrin in adherent cells was associated with the membrane/cytoskeleton fraction and this increase was impaired in ArgPP-expressing cells (Figure 5B). The β1-integrin mRNA levels were similar in control and ArgPP-expressing cells before and after adhesion to collagen (Figure 5C), suggesting post-transcriptional regulation of β1-integrin protein by active Arg in adherent cells. Loss of Arg function did not affect collagen-induced β1-integrin protein upregulation in adherent cells as expression of ArgKR kinase-inactive mutant or Abl-Arg knockdown did not affect β1-integrin levels upon collagen engagement (Figures 4B and S4E).
Figure 5.
Active Arg suppresses collagen-induced β1-integrin protein levels.
(A) MDCKII cells were plated on collagen-coated plates for the indicated times and β1-integrin protein levels were analyzed by western blotting and quantified with ImageJ. UN, undetectable.
(B) Cells plated on collagen for 24 hours were lysed, subjected to protein fractionation, and the lysates from the cytosolic and membrane/cytoskeleton fractions were analyzed by western blotting with the indicated antibodies. Relative β1-integrin levels were quantified with ImageJ. UN, undetectable.
(C) Quantification revealed no statistically significant difference in β1-integrin mRNA levels by real-time RT-PCR in cells expressing either vector or ArgPP before and after plating on collagen for 24 h. Data were analyzed by two-tailed unpaired Student's t-test. p>0.1. Error bars represent mean ± SD.
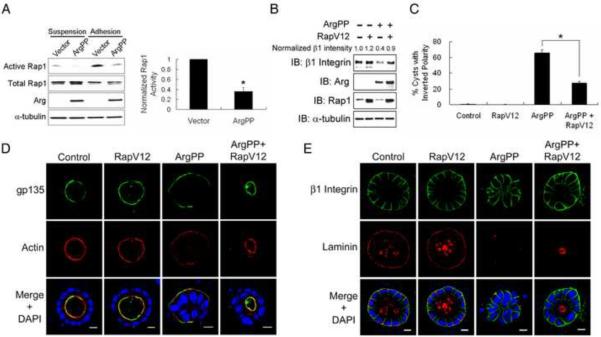
Regulation of β1-integrin levels and cyst polarity by active Arg is mediated by Rap1
The Rap1 GTPase is a critical regulator of β1-integrin in lymphocytes [29, 30], and Abl kinases are required for T cell receptor-mediated Rap1 activation and regulation of integrin affinity [31]. Recently, Rap1 has been implicated in post-transcriptional regulation of β1-integrin in epithelial cells grown under 2D culture conditions [32]. These findings prompted us to examine whether active Arg impaired β1-integrin function by altering Rap1 signaling. Adhesion of control MDCKII cells to collagen markedly increased the levels of active Rap1, which was significantly inhibited in cells expressing active ArgPP (Figure 6A). In contrast, loss of Abl/Arg function led to increased Rap1 activity upon collagen engagement (Figure S6A–B). To determine whether decreased Rap1 activation contributes in part to the regulation of β1-integrin levels and inversion of polarity in cells expressing active Arg, we expressed an active mutant of Rap1 (RapV12). RapV12 expression was sufficient to rescue the decrease in β1-integrin protein levels in ArgPP-expressing cells (Figure 6B) and partially rescued the inverted polarity phenotype induced by active Arg (Figure 6C–D). Further, RapV12 restored the basal membrane localization of β1-integrin in ArgPP-expressing cysts (Figure 6E). Notably, the loss of laminin assembly in ArgPP-expressing cysts was not restored by active Rap1 (Figure 6E), which is consistent with regulation of laminin assembly by Rac1 [7, 8]. These findings suggest that Arg may regulate Rac1-mediated laminin assembly independently of Rap1-dependent modulation of β1-integrin levels during polarity establishment. In support of this conclusion, we found RapV12 could rescue the polarity inversion but not defective laminin assembly in cysts expressing dominant negative RacN17 (Figure S6F–H). Moreover, low-level expression of dominant negative RapN17 in MDCKII cells promoted polarity inversion in about 20% of cysts, which further supports a role for Rap1 in the regulation of epithelial cyst polarity (Figure S6C–E).
Figure 6.
Regulation of β1-integrin and epithelial cyst polarity by active Arg are mediated by Rap1.
(A) Active Arg inhibits Rap1 activation upon β1-integrin engagement. Control or ArgPP-expressing cells were left suspended or allowed to adhere to collagen for 30min. Rap1-GTP pull down assays were carried out and levels of active Rap1 were detected by western blotting (left panel), quantified with ImageJ and analyzed by two-tailed unpaired Student's t-test (right panel); *, p<0.02. Error bar represents mean ± SEM.
(B, C) Active Rap1 (RapV12) partially rescues ArgPP-induced polarity inversion. MDCKII cells expressing the indicated proteins were plated on collagen-coated plates for 18h. Protein expression was analyzed by western blotting. Relative β1-integrin levels were quantified with ImageJ. Percentages of Cysts with inverted polarity were quantified and data were analyzed by two-tailed unpaired Student's t-test; *, p<0.04. Error bars represent mean ± SEM. Control, n=550; RapV12, n=600; ArgPP, n=197; ArgPP+RapV12, n=454.
(D, E) Active Rap1 rescues polarity inversion but not laminin assembly in ArgPP-expressing cysts. MDCKII cells expressing the indicated proteins were grown in collagen for 7 days, followed by confocal imaging for the indicated markers. Scale bars, 10μm.
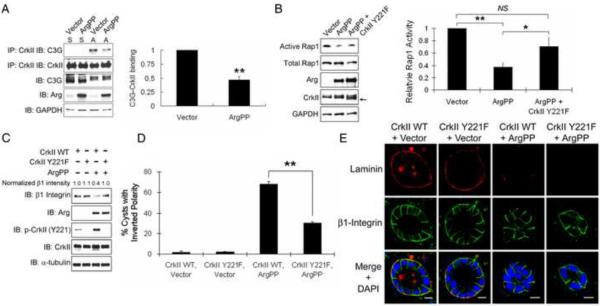
Active Arg-dependent regulation of Rap1-integrin signaling and cyst polarity is mediated by the CrkII adaptor
We next sought to define the mechanism that links active Arg to Rap1 in the modulation of cyst polarity. A potential target for Arg in the regulation of Rap1 activity is C3G, a Rap1 guanine nucleotide exchange factor (GEF). C3G is regulated by the formation of a protein complex with the Crk family of adaptor proteins, which are binding partners and substrates of the Abl/Arg kinases [33]. Tyrosine phosphorylation of CrkII Y221 disrupts the CrkII-C3G complex and inhibits Rap1 activation [34]. We found that engagement of β1-integrin by adhesion to collagen induced the formation of the CrkII-C3G complex, which was impaired in the presence of active Arg (Figure 7A). This finding suggested that active Arg may inhibit Rap1 activation by suppressing CrkII-C3G complex formation. To assess whether CrkII plays a role in ArgPP-induced Rap1 inhibition and polarity inversion, we employed a phospho-deficient mutant of CrkII lacking Y221, the Abl/Arg phosphorylation site. Expression of CrkII Y221F mutant but not wild-type (WT) CrkII significantly rescued the levels of active Rap1 in ArgPP-expressing cells upon collagen adhesion (Figures 7B and S7A–B). Further, we found that the CrkII Y221F mutant, but not CrkII WT, rescued β1-integrin protein levels and localization to the basal membrane in ArgPP-expressing cysts (Figures 7C–E). Moreover, ArgPP-induced polarity inversion could be partially rescued by CrkII Y221F, but not CrkII WT, to a similar extent as that induced by RapV12 (Figures 7D–E and S7C). However, like RapV12, expression of CrkII Y221F failed to rescue laminin assembly in ArgPP-expressing cysts (Figure 7E).These data suggest that CrkII and Rap1 function in the same pathway to regulate β1-integrin protein levels and localization, which is independent of laminin assembly.
Figure 7.
ArgPP-dependent regulation of Rap1 and β1-integrin signaling is mediated by CrkII.
(A) Active Arg inhibits CrkII-C3G complex formation in response to β1-integrin engagement. Control and ArgPP-expressing MDCKII cells were either left in suspension or allowed to adhere to collagen-coated plates for 25min. The C3G-CrkII interaction was examined by co-immunoprecipitation and western blotting with the indicated antibodies (left panel). S: suspension; A: adhesion. Levels of C3G bound to CrkII in adherent cells were quantified with ImageJ and analyzed by two-tailed unpaired Student's t-test (right panel); **, p<0.01. Error bar represents mean ± SD.
(B) CrkII Y221F mutant partially rescues Rap1 activity in ArgPP-expressing cells. MDCKII cells expressing the indicated proteins were plated on collagen for 10 h followed by Rap1-GTP pull-down assays and active Rap1 was detected by western blotting (left panel). The arrow marks the migration of the exogenous CrkII Y221F mutant protein. Active Rap1 levels were quantified with ImageJ and analyzed with two-tailed unpaired Student's t-test (right panel); *, p<0.05; **, p<0.01; NS, not statistically significant. Error bars represent mean ± SEM.
(C, D) CrkII Y221F partially rescues ArgPP-induced polarity inversion. MDCKII cells expressing the indicated proteins were plated on collagen for 18h. Protein expression was examined by western blotting and β1-integrin protein levels were quantified by ImageJ. The percentages of cysts with inverted polarity in each experimental group were quantified and analyzed by two-tailed unpaired Student's t-test; **, p<0.01. Error bars represent mean ± SEM. CrkII WT + Vector, n=528; CrkII WT + ArgPP, n=433; CrkII Y221F + Vector, n=548; CrkII Y221F + ArgPP, n=214.
(E) CrkII Y221F does not rescue laminin assembly in ArgPP-expressing cysts. Scale bars, 10μm.
Discussion
The present work has identified a previously unappreciated role for the Arg kinase in the regulation of apical-basal epithelial polarity through at least two distinct pathways: Rac1-mediated laminin assembly and CrkII-C3G-Rap1-dependent modulation of β1-integrin levels and localization in response to collagen engagement (Figure S7D). Expression of constitutively active Arg produces acinar structures with inverted polarity characterized by the apical membrane facing the collagen matrix rather than the central lumen. We show that the inverted polarity phenotype induced by active Arg kinase is linked to impaired β1-integrin function, and that Rap1 activation downstream of CrkII/C3G complex is required for β1-integrin expression and function, but not for laminin assembly, a process known to be Rac1-dependent [8]. Thus, the Rac1-laminin pathway functions independently from the Rap1-β1-integrin pathway in the regulation of epithelial cyst polarity.
Previous studies have shown that Abl kinases can promote or attenuate Rap1 activation dependent on the cellular context. Endogenous Abl kinase activity is required for Rap1 activation and increased integrin affinity in response to engagement of the T cell receptor (TCR) [31]. However, others reported that constitutively active Abl decreased Rap1 activation by phosphorylation of the CrkII adaptor and disruption of the CrkII/C3G complex leading to decreased β1-integrin affinity without altering β1-integrin levels [34]. These contrasting findings raise the possibility that transient activation of the endogenous Abl kinases may be required for Rap1 activation in response to specific stimuli, and that elevated and prolonged Abl/Arg kinase activity may negatively modulate Rap1 activity through CrkII phosphorylation and disruption of the CrkII/C3G complex. We propose that enhanced activation of Arg and/or Abl downstream of multiple growth factors, chemokines and oncogenic signals in epithelial cysts may disrupt the normal turnover of Rap1 activity required for β1-integrin regulation and maintenance of epithelial polarity (Figure S7D).
Our previous work demonstrated that Abl kinases modulate Rac1 activity by functioning both upstream and downstream of Rac1 [12, 35]. Rac1 activation has been linked to the formation of a CrkII-Cas-Dock180 complex, and phosphorylation of Y221 on CrkII disrupts this complex, leading to Rac inhibition [36]. Expression of CrkII Y221F did not rescue Rac1-mediated laminin assembly in ArgPP-expressing cysts. These findings suggest that active Arg might regulate Rac1 activation through pathways independent of CrkII phosphorylation in epithelial cysts.
We found that Arg kinase is functionally linked to β1-integrin signaling in the regulation of epithelial polarity. The role of β1-integrin in cancer is complex, as this integrin has been reported to be required for tumor progression [5, 6], but it has also been shown to have anti-neoplastic functions and suppress cancer progression [37]. The anti- and pro-neoplastic effects of the β1-integrins are likely to be dependent on the cellular context and the tumor stage. Targeted disruption of β1-integrin in transgenic mouse models of breast cancer has shown that this integrin is required for mammary tumorigenesis. In transgenic mice expressing polyoma middle T antigen in the mammary gland, loss of β1-integrin inhibits both tumor initiation and maintenance [5]. In contrast, while β1-integrin is dispensable for tumor initiation in transgenic mice expressing activated ErbB2 in the mammary gland, it is required for tumor metastasis [6]. Dynamic regulation of Arg kinase activity may play a role at distinct stages of tumor initiation and progression downstream of diverse oncogenic signals leading to altered β1-integrin function.
We found that both inhibition of β1-integrin function or expression of active Arg promoted the dissociation of Par3 from the Par6/aPKC complex and induced aberrant localization of Dlg with apical membrane components. Recently it was reported that the Par polarity complex together with Cdc42 regulate vesicular transport to the apical surface as well as orientation of cell division during MDCK epithelial polarization and lumen formation [38, 39]. A role for Abl kinases in the modulation of membrane-trafficking during epithelial polarization has yet to be explored. We have previously shown that Abl kinases are required for lysosomal trafficking and regulate cell surface levels of receptor tyrosine kinases and metalloproteinases [20, 28, 40]. It remains to be determined whether Abl and Arg affect membrane trafficking events through modulation of polarity protein complexes. The present work has revealed that gain- and loss-of-function of the Arg kinase results in distinct phenotypic abnormalities in epithelial cyst polarity, suggesting that Arg activity may be required for spatial and temporal regulation of polarity pathways during epithelial morphogenesis and organization in normal and pathological conditions.
Supplementary Material
Acknowledgements
We thank Drs. Sam Johnson and Yasheng Gao, Duke University Light Microscopy Core Facility, for advice; Dr. Mike Cook for FACS-based cell sorting; Elizabeth Chislock, Emileigh Greuber and Drs. Colleen Ring, Pameeka Smith-Pearson and Tso-Pang Yao for helpful comments. We thank Dr. Lawrence Quilliam (Indiana University) and Dr. Jing Jin Gu (Duke University) for Rap1 constructs, and Dr. Patrick Brennwald (University of North Carolina at Chapel Hill) for anti-mLgl antibodies. These studies were supported by a DOD Breast Cancer pre-doctoral grant W81XWH-06-1-0399 to R.L., and NIH grants CA070940 and HL084102 to A.M.P. The authors declare no financial conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online.
References
- 1.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Vasioukhin V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. Journal of cell science. 2008;121:1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 3.Feigin ME, Muthuswamy SK. Polarity proteins regulate mammalian cell-cell junctions and cancer pathogenesis. Curr Opin Cell Biol. 2009;21:694–700. doi: 10.1016/j.ceb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. The EMBO journal. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Huck L, Pontier SM, Zuo DM, Muller WJ. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proceedings of the National Academy of Sciences of the United States of America. 107:15559–15564. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 9.Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, Muthuswamy SK. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- 10.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 11.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. Journal of cell science. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui L, Chen C, Xu T, Zhang J, Shang X, Luo J, Chen L, Ba X, Zeng X. c-Abl kinase is required for beta 2 integrin-mediated neutrophil adhesion. J Immunol. 2009;182:3233–3242. doi: 10.4049/jimmunol.0802621. [DOI] [PubMed] [Google Scholar]
- 15.Chen WS, Kung HJ, Yang WK, Lin W. Comparative tyrosine-kinase profiles in colorectal cancers: enhanced arg expression in carcinoma as compared with adenoma and normal mucosa. Int J Cancer. 1999;83:579–584. doi: 10.1002/(sici)1097-0215(19991126)83:5<579::aid-ijc1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 17.Podtcheko A, Ohtsuru A, Tsuda S, Namba H, Saenko V, Nakashima M, Mitsutake N, Kanda S, Kurebayashi J, Yamashita S. The selective tyrosine kinase inhibitor, STI571, inhibits growth of anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88:1889–1896. doi: 10.1210/jc.2002-021230. [DOI] [PubMed] [Google Scholar]
- 18.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Simpson L, He X, Pins M, Huang X, Campbell SC, Yang XJ, Perlman EJ, Bergan RC. Renal medullary carcinoma and ABL gene amplification. The Journal of urology. 2005;173:1883–1888. doi: 10.1097/01.ju.0000158448.56888.09. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem. 285:40201–40211. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum B, Perrimon N. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat Cell Biol. 2001;3,:883–890. doi: 10.1038/ncb1001-883. [DOI] [PubMed] [Google Scholar]
- 22.Grevengoed EE, Loureiro JJ, Jesse TL, Peifer M. Abelson kinase regulates epithelial morphogenesis in Drosophila. The Journal of cell biology. 2001;155:1185–1198. doi: 10.1083/jcb.200105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- 24.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. Journal of cell science. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. The Journal of cell biology. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- 27.Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 2008;9:923–929. doi: 10.1038/embor.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanos B, Pendergast AM. Abl tyrosine kinase regulates endocytosis of the epidermal growth factor receptor. J Biol Chem. 2006;281:32714–32723. doi: 10.1074/jbc.M603126200. [DOI] [PubMed] [Google Scholar]
- 29.Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. The Journal of cell biology. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. The Journal of cell biology. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, Billadeau DD. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. The Journal of cell biology. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 33.Feller SM. Crk family adaptors-signalling complex formation and biological roles. Oncogene. 2001;20:6348–6371. doi: 10.1038/sj.onc.1204779. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. The Journal of cell biology. 2008;183:711–723. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zandy NL, Pendergast AM. Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases. Cell Cycle. 2008;7:444–448. doi: 10.4161/cc.7.4.5452. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 38.Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. The Journal of cell biology. 189:661–669. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yogalingam G, Pendergast AM. Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components. J Biol Chem. 2008;283:35941–35953. doi: 10.1074/jbc.M804543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.