Abstract
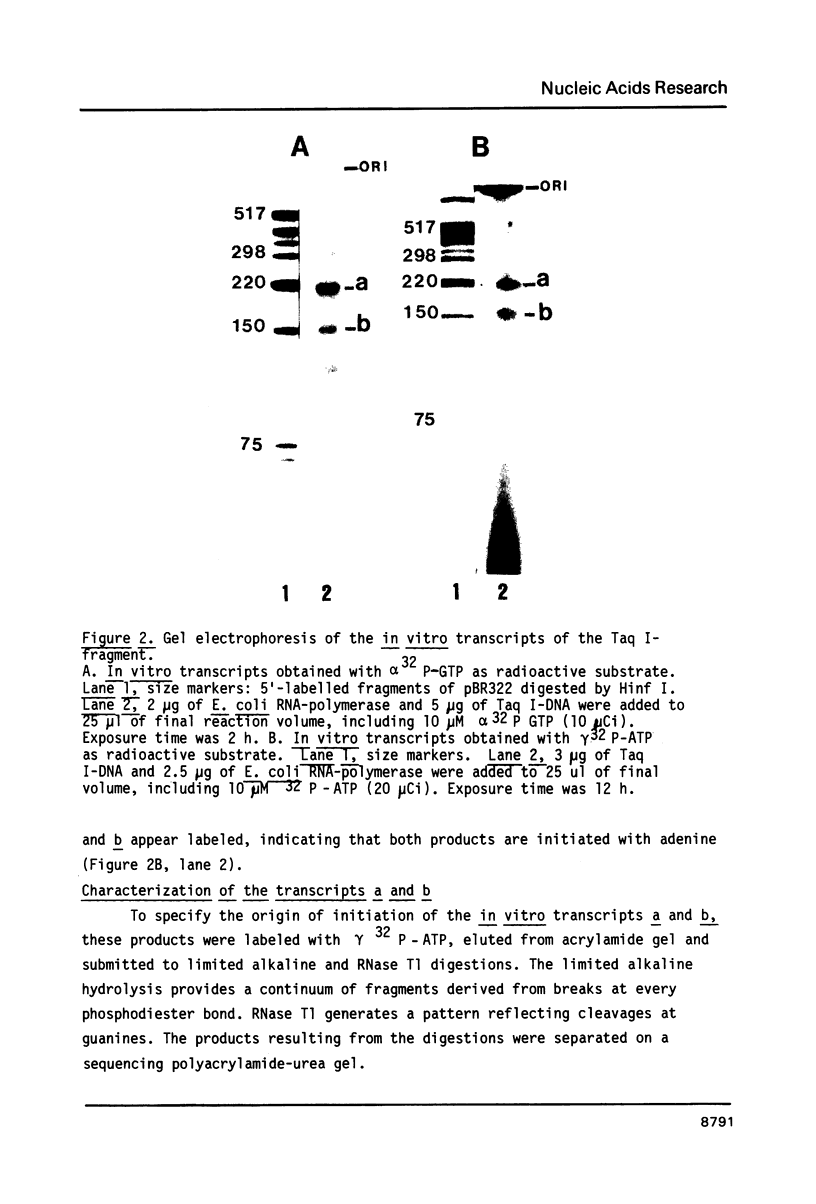
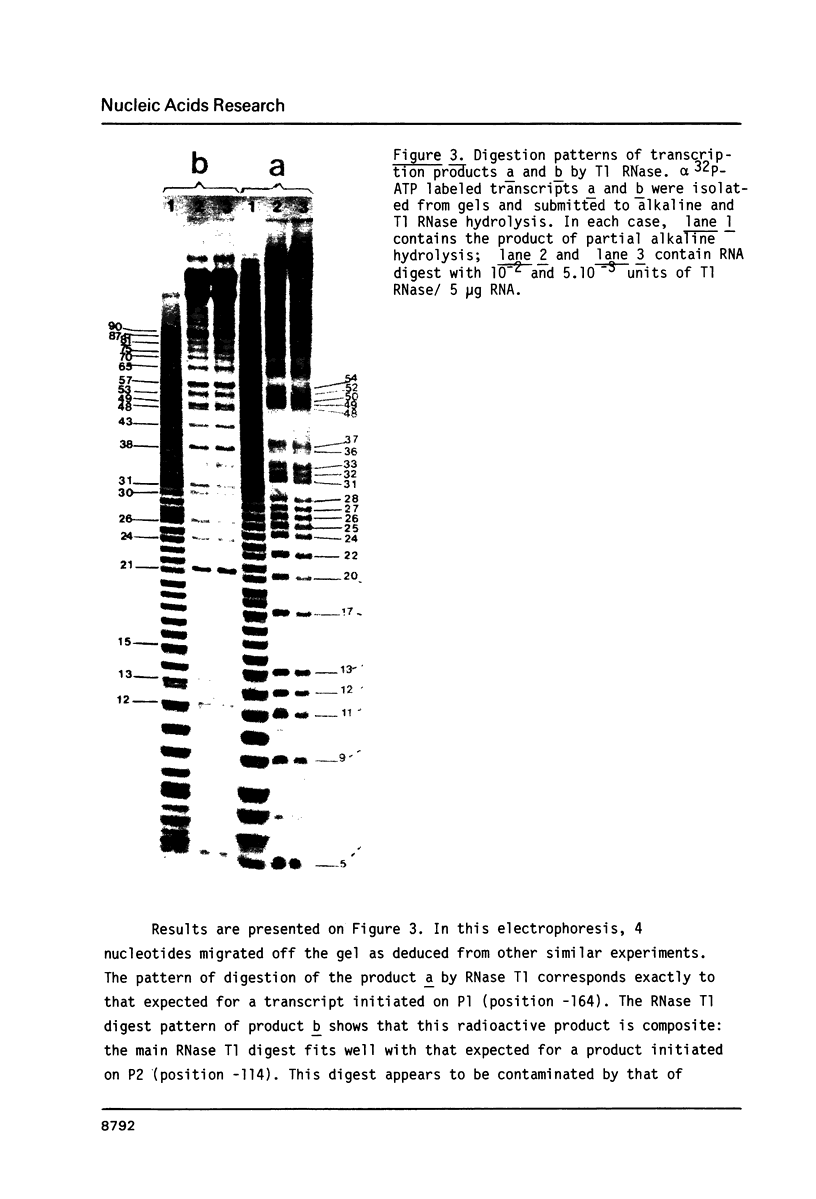
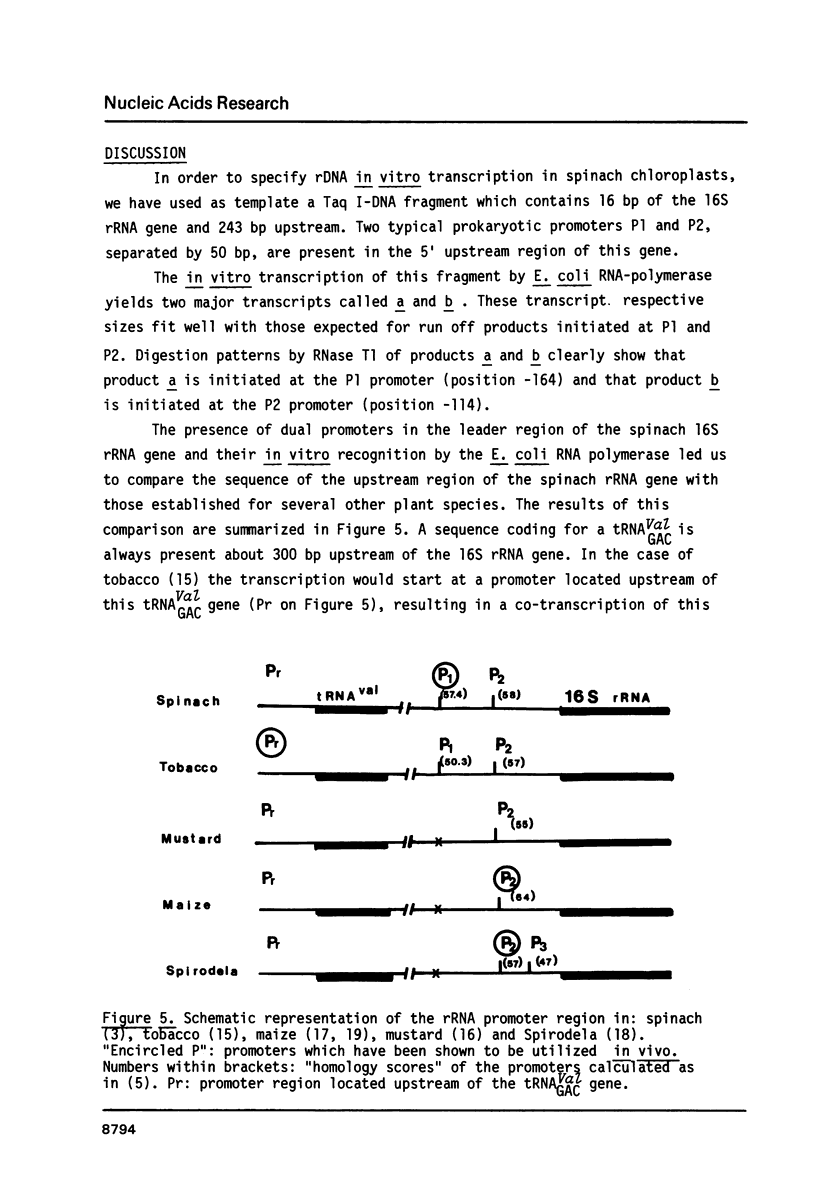
Two potential prokaryotic promoters, P1 and P2, are characterized 164 and 114 bp upstream of the spinach chloroplast 16S rRNA gene. The strengths of these promoters, calculated according to an homology score established for E. coli RNA-polymerase, are identical. Experiments performed with a Taq I-DNA fragment, containing 16 bp of the 16S rDNA and 243 bp upstream of the gene, give evidence that in vitro, E. coli RNA-polymerase starts transcription at these two promoters. These results are based on both the size of the transcripts and their nucleotide sequences. A possible regulation by differential control of these dual promoters is suggested. S1 mapping with RNAs extracted either from green or from etiolated spinach plants, indicates that, at these two steps of plastid development, transcription in vivo starts at P1. Surprisingly only P2 appears to be conserved in the homologous sequences reported for maize, mustard and Spirodela.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briat J. F., Dron M., Loiseaux S., Mache R. Structure and transcription of the spinach chloroplast rDNA leader region. Nucleic Acids Res. 1982 Nov 11;10(21):6865–6878. doi: 10.1093/nar/10.21.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Keus R. J., Dekker A. F., van Roon M. A., Groot G. S. The nucleotide sequences of the regions flanking the genes coding for 23S, 16S and 4.5S ribosomal RNA on chloroplast DNA from Spirodela oligorhiza. Nucleic Acids Res. 1983 Sep 24;11(18):6465–6474. doi: 10.1093/nar/11.18.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- Lerbs S., Bräutigam E., Parthier B. Polypeptides of DNA-dependent RNA polymerase of spinach chloroplasts: characterization by antibody-linked polymerase assay and determination of sites of synthesis. EMBO J. 1985 Jul;4(7):1661–1666. doi: 10.1002/j.1460-2075.1985.tb03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Langridge U. Structure of the chloroplast gene for the precursor of the Mr 32,000 photosystem II protein from mustard (Sinapis alba L.). Nucleic Acids Res. 1984 Jan 25;12(2):945–958. doi: 10.1093/nar/12.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. A modified pBR322 vector with improved properties for the cloning, recovery, and sequencing of blunt-ended DNA fragments. Gene. 1982 Feb;17(2):189–196. doi: 10.1016/0378-1119(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Sarmientos P., Sylvester J. E., Contente S., Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivo in multicopy plasmids. Cell. 1983 Apr;32(4):1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- Schnepf E. Types of plastids: their development and interconversions. Results Probl Cell Differ. 1980;10:1–27. doi: 10.1007/978-3-540-38255-3_1. [DOI] [PubMed] [Google Scholar]
- Schwarz Z., Kössel H., Schwarz E., Bogorad L. A gene coding for tRNA is located near 5' terminus of 16S rRNA gene in Zea mays chloroplast genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4748–4752. doi: 10.1073/pnas.78.8.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter G., Gozdzicka-Jozefiak A., Kössel H. Identification of an rRNA operon promoter from Zea mays chloroplasts which excludes the proximal tRNAValGAC from the primary transcript. EMBO J. 1985 Mar;4(3):599–604. doi: 10.1002/j.1460-2075.1985.tb03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist C., Björn L. O., Virgin H. I. Factors in chloroplast differentiation. Results Probl Cell Differ. 1980;10:201–224. doi: 10.1007/978-3-540-38255-3_7. [DOI] [PubMed] [Google Scholar]
- Tohdoh N., Shinozaki K., Sugiura M. Sequence of a putative promoter region for the rRNA genes of tobacco chloroplast DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5399–5406. doi: 10.1093/nar/9.20.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. Nucleotide sequence analysis of DNA. I. Partial sequence of the cohesive ends of bacteriophage lambda and 186 DNA. J Mol Biol. 1970 Aug;51(3):501–521. doi: 10.1016/0022-2836(70)90004-5. [DOI] [PubMed] [Google Scholar]






