Abstract
DJ-1 (PARK7) is a neuroprotective protein that protects cells from oxidative stress. Accordingly, loss-of-function DJ-1 mutations have been linked with a familial form of early onset Parkinson disease. Mechanisms by which DJ-1 level could be enriched in the CNS are poorly understood. Recently we have discovered anti-inflammatory activity of sodium benzoate (NaB), a metabolite of cinnamon and a widely-used food additive. Here we delineate that NaB is also capable of increasing the level of DJ-1 in primary mouse and human astrocytes and human neurons highlighting another novel neuroprotective effect of this compound. Reversal of DJ-1-inducing effect of NaB by mevalonate, farnesyl phosphate, but not cholesterol and ubiquinone, suggests that depletion of intermediates, but not end products, of the mevalonate pathway is involved in the induction of DJ-1 by NaB. Accordingly, either an inhibitor of p21ras farnesyl protein transferase (FPTI) or a dominant-negative mutant of p21ras alone was also able to increase the expression of DJ-1 in astrocytes suggesting an involvement of p21ras in DJ-1 expression. However, an inhibitor of geranyl geranyl transferase (GGTI) and a dominant-negative mutant of p21rac had no effect on the expression of DJ-1, indicating the specificity of the effect. Similarly lipopolysaccharide (LPS), an activator of small G proteins, also inhibited the expression of DJ-1, and NaB and FPTI, but not GGTI, abrogated LPS-mediated inhibition. Together, these results suggest that NaB upregulates DJ-1 via modulation of mevalonate metabolites and that p21ras, but not p21rac, is involved in the regulation of DJ-1.
Keywords: Astrocytes, DJ-1, PARK, Neurons, Sodium benzoate, p21ras, p21rac
Introduction
Parkinson disease (PD) is a common age associated neurodegenerative disorder of the central nervous system (Dauer and Przedborski 2003) that was first described in an essay entitled “An essay of the Shaking Palsy” by James Parkinson in 1817. Clinically PD is characterized by resting tremor, bradykinesia, rigidity and postural instability (Olanow and Tatton 1999; Dauer and Przedborski 2003). The pathological hallmarks of PD includes progressive degeneration of the dopaminergic neurons associated with the presence of intracytoplasmic inclusions (Lewy bodies) and gliosis in the substantia nigra pars compacta (SNpc) (Dauer and Przedborski 2003). Causes for the disease are not well known however environmental, genetic, and immunological factors have been associated with the onset of this disease (Hardy et al. 2006). The discovery of various genes linked to familial forms of PD provides important clues in understanding the pathogenesis of the disease and confirms the role of genetics in the disease development. Various genes implicated in PD are α-synuclein (Polymeropoulos et al. 1997), parkin (Kitada et al. 1998), DJ-1 (Bonifati et al. 2003), PTEN-induced kinase 1 (PINK-1) (Valente et al. 2004), HtrA2/Omi gene (Strauss et al. 2005) and leucine-rich repeat kinase 2 (LRRK2) (Paisan-Ruiz et al. 2004).
Oxidative stress plays an important role in the pathogenesis of PD and among all these PD-associated genes, DJ-1 is the most important one in providing antioxidant defense. In one hand, by facilitating the transcription of MnSOD, DJ-1 helps a damaged cell to scavenge superoxide (Zhong and Xu 2008). On the other, by mediating the upregulation of glutamate cysteine ligase, the rate limiting enzyme of glutathione (GSH) biosynthesis, it enriches the level of master antioxidant GSH (Zhou and Freed 2005). However, mechanisms by which DJ-1 expression could be upregulated is poorly understood and such steps may be vital for understanding the disease progression as well as designing therapeutic approaches for PD.
Cinnamon contains three major compounds (cinnamaldehyde, cinnamyl acetate and cinnamyl alcohol), which are converted into cinnamic acid by oxidation and hydrolysis, respectively. In the liver, this cinnamic acid is β-oxidized to benzoate that exists as sodium salt (sodium benzoate; NaB) or benzoyl-CoA. It has been reported that minor amount of NaB is also excreted in the urine of humans (Kubota and Ishizaki 1991). NaB is also of medical importance because it is a component of Ucephan, a Food and Drug Administration (FDA)-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia such as urea cycle disorder (Leonard and Morris 2002; Scaglia et al. 2004). It is also widely used as a preservative in broad range of foods and cosmetic products (Nair 2001). It is nontoxic and can be administered as a solution in drinking water. It has been reported that 2% solution of NaB in drinking water is safe for lifelong treatment in mice without any noticeable side effects (Toth 1984). Recently, we have demonstrated that NaB is useful in protecting mice from relapsing-remitting experimental allergic encephalomyelitis (Brahmachari et al. 2009) and that NaB is also capable of inhibiting the expression of various proinflammatory molecules from activated glial cells (Brahmachari et al. 2009).
Here we provide the first evidence that NaB is capable of increasing the expression of DJ-1 in astrocytes and neurons. Interestingly, NaB executes the induction of DJ-1 via involving intermediates, but not end product(s), of the mevalonate pathway. Furthermore, we demonstrate that p21ras, but not p21rac, is involved in the expression of DJ-1. Our findings raise a possibility that NaB, a component of a prescribed drug for human urea cycle disorder and a widely used food preservative, may be used to upregulate DJ-1 in PD and other neurodegenerative disorders.
Materials and methods
Reagents
Sodium benzoate (NaB), sodium formate (NaFO), lipopolysaccharide (LPS) (Escherichia coli), mevalonate, farnesylpyrophosphate, cholesterol, and ubiquinone were purchased from Sigma (St. Louis, MO). Inhibitors of farnesyl protein transferase and geranylgeranyl transferase were purchased from EMD Chemicals (Gibbstown, NJ). Fetal bovine serum (FBS), Hank’s balanced salt solution (HBSS), trypsin and DMEM/F-12 were from Mediatech (Manassas, VA). Real-time primer for DJ-1 was purchased from Applied Biosystems. Antibodies against DJ-1 and GFAP were obtained from Abcam (Cambridge, MA) and DAKO (Carpinteria, CA), respectively. Dominant-negative mutants of p21ras and p21rac were kindly provided by Prof. John R. Raymond of the Medical University of South Carolina.
Isolation of primary mouse astroglia
Astroglia were isolated from mixed glial cultures as described by us (Brahmachari and Pahan 2007; Saha and Pahan 2007) according to the procedure of Giulian and Baker (Giulian and Baker 1986). Briefly, on day 9, the mixed glial cultures were washed three times with Dulbecco’s modified Eagle’s medium/F-12 and subjected to shaking at 240 rpm for 2 h at 37°C on a rotary shaker to remove microglia. After 2 days, the shaking was repeated for 24 for the removal of oligodendroglia and to ensure the complete removal of all nonastroglial cells. The attached cells were seeded onto new plates for further studies.
Isolation of primary human astroglia
Primary human astroglia were prepared as described by us in many studies (Jana and Pahan 2010; Saha et al. 2007). All of the experimental protocols were reviewed and approved by the Institutional Review Board of the Rush University Medical Center. Briefly, 11- to 17-week-old fetal brains obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA, USA) were dissociated by trituration and trypsinization. On 9th day, these mixed glial cultures were placed on a rotary shaker at 240 rpm at 37°C for 2 h to remove loosely attached microglia. Then on 11th day, flasks were shaken again at 190 rpm at 37°C for 18 to remove oligodendroglia. The attached cells remaining were primarily astrocytes. These cells were trypsinized and subcultured in complete media at 37°C with 5% CO2 in air to yield more viable and healthy cells. By immunofluorescence assay, these cultures homogeneously expressed GFAP, a marker for astrocytes (Jana et al. 2007).
Isolation of primary human neurons
Human primary neurons were prepared as described by us earlier (Jana and Pahan 2010; Jana et al. 2007). All of the experimental protocols were reviewed and approved by the Institutional Review Board of the Rush University Medical Center. Briefly, 11–17-week-old fetal brains obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA) were dissociated by trituration and trypsinization (0.25% trypsin in PBS at 37°C for 15 min). The trypsin was inactivated with 10% heat-inactivated fetal bovine serum (Mediatech). The dissociated cells were pelleted by centrifugation and the cell pellet was washed once with PBS and once with Neurobasal medium containing 2% B27 and 1% antibiotic-antimycotic mixture (Sigma). In the first step, neurons were enriched by allowing the cells (3 × 106/ml) to adhere to poly-D-lysine-coated plates or coverslips for 5 min. Nonadherent cells were removed, and adherent cells (mostly neurons) were further treated with 10 µM Ara-C to prevent the proliferation of dividing cells. After 10 days of Ara-C treatment, the cells were used for this study. More than 98% of this preparation was positive for microtubule-associated protein-2 (MAP-2), a marker for neurons.
Expression of Δp21ras and Δp21rac in astrocytes
In the dominant-negative form of p21ras (Δp21ras), the Ser residue at position 17 has been changed to Asn (S17N mutant). This mutant binds preferentially to GDP and acts as the dominant inhibitor of p21ras function presumably by blocking access to exchange factors (Garnovskaya et al. 1996). Similarly, Δp21rac (S17N rac mutant) having a preference for GDP to GTP also prevents the function of p21rac (Qiu et al. 1995). For transient transfection, primary astrocytes plated at 60–70% confluency in 12-well plates were transfected with 0.5 µg of either p21ras or p21rac using Lipofectamine Plus following manufacturer’s protocol as described by us earlier, (Liu et al. 2002; Pahan et al. 2002; Saha et al. 2006).
Immunoblot analysis for DJ-1
Immunoblot analysis for DJ-1 was carried out as described earlier (Saha et al. 2006, 2007). Briefly, cells homogenates were electrophoresed, proteins were transferred onto a nitrocellulose membrane, and the DJ-1 band was visualized with Odyssey infrared scanner after immunolabeling with primary antibodies followed by infra-red fluorophore-tagged secondary antibody (Molecular Probes).
Semi-quantitative RT-PCR analysis
To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out described earlier (Ghosh et al. 2007, 2009), using a RT-PCR kit from Clontech. Briefly, 1 µg of total RNA was reverse transcribed using oligo(dT) as primer and MMLV reverse transcriptase (Clontech). The resulting cDNA appropriately-diluted, and diluted cDNA was amplified. Amplified products were electrophoresed on a 1.8% agarose gels and visualized by ethidium bromide staining.
| DJ-1: | Sense: 5′- CCCCGTGCAGTGTAGCCGTG -3′ Antisense: 5′- CAGGCCGTCCTTCTCCACGC-3′ |
| α-synuclein: | Sense: 5′- GGTGTGGCAGAGGCAGCTGG-3′ Antisense: 5′- CCCCTCCTCACCCTTGCCCA-3′ |
| Parkin: | Sense: 5′- GCCCAGGGCCCATCTTGCTG -3′ Antisense: 5′- GCCAGCTACACACGGCAGGG-3′ |
| PINK-1: | Sense: 5′- GTTGCTGGAGGGCGTGGACC -3′ Antisense: 5′- CGAGGTGGGCACTGCCTTGG-3′ |
| LRRK2: | Sense: 5′- ACCAGAACAGTTTGCATGAGA-3′ Antisense: 5′- AGCCCAGACACTGAATTTCTTG-3′ |
| HtrA2: | Sense: 5′- GCCAGGGACCTGGGACTCCC-3′ Antisense: 5′- TGTCTCTGATCCGCGCCGGA-3′ |
| GAPDH: | Sense: 5′-GGTGAAGGTCGGTGTGAACG3′ Antisense: 5′-TTGGCTCCACCCTTCAAGTG-3′ |
Real-time PCR analysis
It was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems) as described earlier (Ghosh et al. 2007, 2009). The mRNA expressions of respective genes were normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
Immunofluorescence analysis
It was performed as described earlier (Saha et al. 2006; Jana and Pahan 2007). Briefly, cover slips containing 100–200 cells/mm2 were fixed with 4% paraformaldehyde followed by treatment with cold ethanol and two rinses in phosphate-buffered saline (PBS). Samples were blocked with 3% bovine serum albumin (BSA) in PBS-Tween-20 (PBST) for 30 min and incubated in PBST containing 1% BSA and mouse anti-DJ-1 (1:200) or rabbit anti-GFAP (1:1000). After three washes in PBST (15 min each), slides were further incubated with Cy2 (Jackson ImmunoResearch Laboratories, Inc.). For negative controls, a set of culture slides was incubated under similar conditions without the primary antibodies. The samples were mounted and observed under a Bio-Rad MRC1024ES confocal laser-scanning microscope.
Results
NaB upregulates DJ-1 expression in primary mouse astrocytes
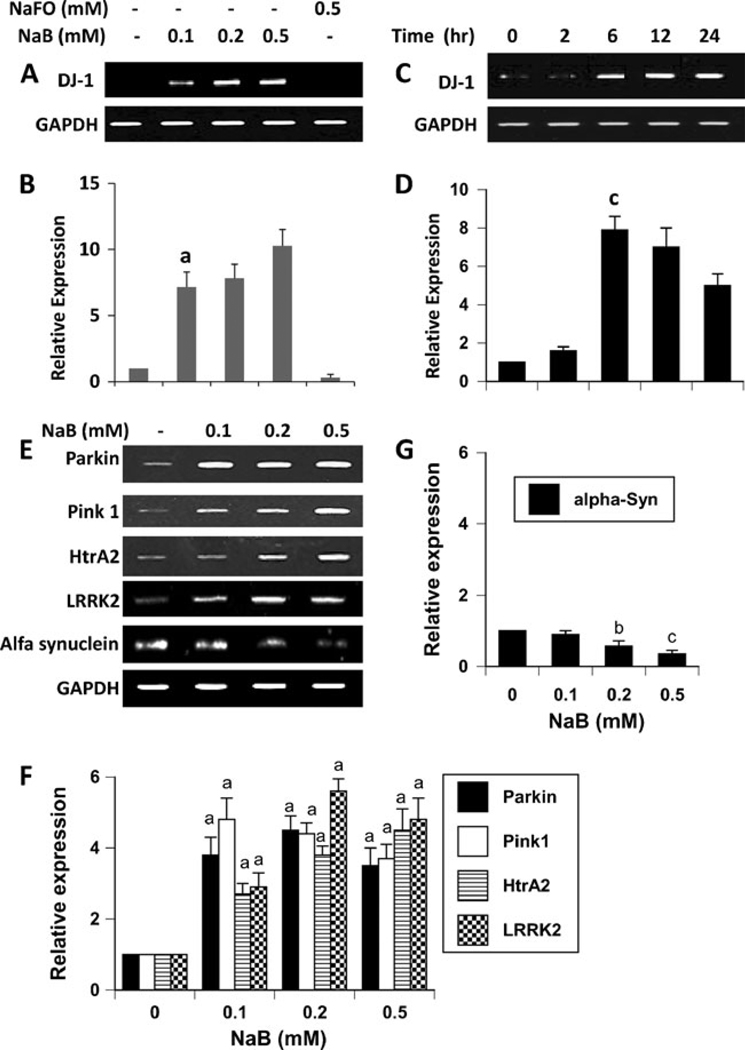
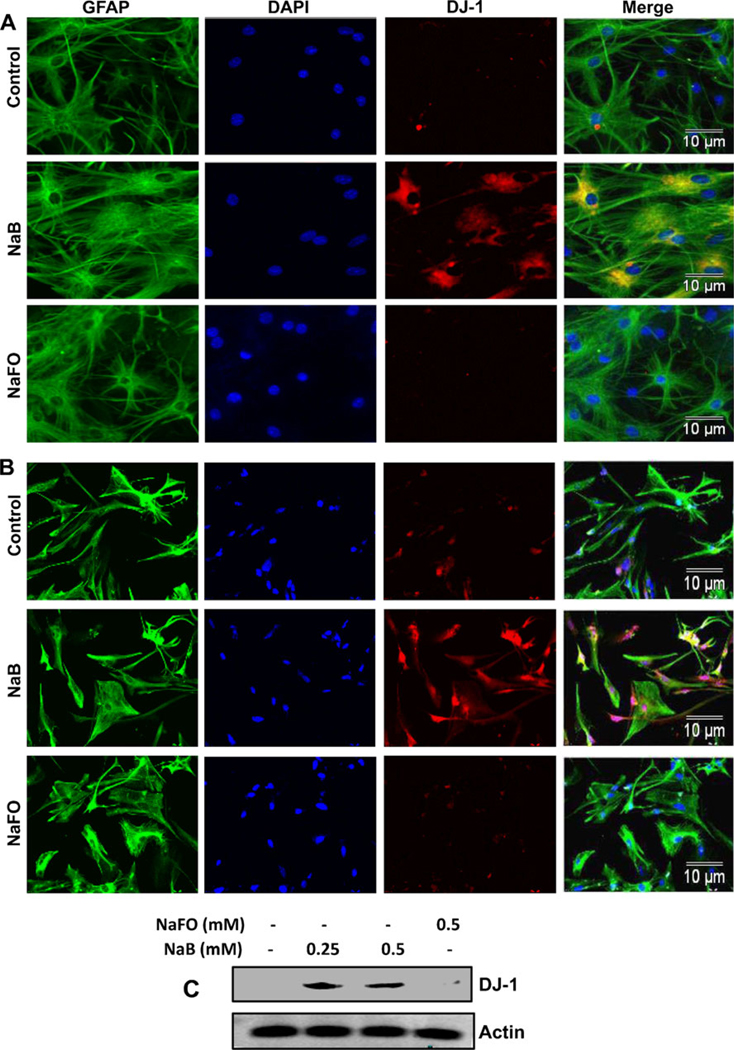
DJ-1 is a neuroprotective protein having implications in neurodegenerative disorders like Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Bonifati et al. 2003; Baulac et al. 2009). Astrocytes, the major glial cell type in the CNS, are known to harbor many neuroprotective arsenals including DJ-1 and support neurons. Because cinnamon has been being used as a natural supplement for normal human health for centuries, we investigated the effect of NaB, a major metabolite of cinnamon, on the expression of DJ-1 in astrocytes. Therefore, primary astrocytes were incubated with different doses of NaB and sodium formate (NaFO), the negative control for NaB, for 6 h followed by mRNA analysis. Although at lower concentrations (25 and 50 µM), NaB was not effective in inducing the mRNA expression of DJ-1 (data not shown), at higher concentrations, NaB markedly increased the mRNA expression of DJ-1 in astrocytes as evident from semi-quantitative RT-PCR (Fig. 1a) and real-time PCR (Fig. 1b). On the other hand, NaFO had no effect on DJ-1 mRNA expression (Fig. 1a and b). Time course study shows that 1 h or 2 h of treatment was not enough for this molecule to exhibit DJ-1-inducing effect (Fig. 1c and d). However, marked increase in DJ-1 mRNA expression was observed when astrocytes were stimulated for 6 h or longer (Fig. 1c and d). Induction of DJ-1 protein by NaB was monitored by immunofluorescence and western blot. Consistent to the upregulation of DJ-1 mRNA, NaB increased the protein level of DJ-1 in mouse astrocytes (Fig. 2a). However, NaFO remained unable to do that. Next we investigated if NaB could increase the level of DJ-1 in human astrocytes. As appeared from Fig. 2b, NaB, but not NaFO, also increased the level of DJ-1 protein in primary human astrocytes. Similarly, western blot analysis also demonstrates the increase in DJ-1 protein in human astrocytes by NaB, but not NaFO (Fig. 2c). On the other hand, the level of actin did not change after treatment with either NaB or NaFO (Fig. 2c) suggesting the specificity of the effect.
Fig. 1.
Effect of NaB on the expression of DJ-1 and other PARK genes in primary mouse astrocytes. Cells were treated with different concentrations of NaB under serum-free condition. Sodium formate (NaFO) was used as a negative control. After 6 h of treatment, the expression of DJ-1 was analyzed in cells by semi-quantitative RT-PCR (a) and real-time PCR (b). Next, cells were treated with 0.5 mM NaB under serum-free conditions for different time periods and DJ-1 expression was analyzed by semi-quantitative RT-PCR (c) and real-time PCR (d). Results are mean ± SD of three different experiments. a,bp < 0.001 vs. control. e) Cells were treated with different concentrations of NaB and after 6 h of treatment, the expression of Parkin, Pink1, HtrA2, LRRK2, and α-synuclein was analyzed by semi-quantitative RT-PCR. The relative expression of Parkin, Pink1, HtrA2, and LRRK2 (e) and α-syn (f) was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech). Results represent mean ± SD of three separate experiments. a,c,p < 0.001 vs. control; bp < 0.05 vs. control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase
Fig. 2.
Effect of NaB on the expression of DJ-1 protein in primary astrocytes. Primary mouse astrocytes (a) and human astrocytes (b) were treated with 500 µM of NaB or NaFO under serum-free condition. After 24 h of treatment, levels of GFAP and DJ-1 were monitored by double-label immunofluorescence. c) Primary human astrocytes were treated with 500 µM of NaB or NaFO under serum-free condition. After 24 h of treatment, levels of DJ-1 and actin were monitored by Western blot analysis. Actin was used as a housekeeping protein. Results represent three independent analyses
Does NaB upregulate other PARK genes?
In addition to DJ-1, five other PARK genes have been delineated: α-synuclein (PARK1), leucine-rich repeat kinase 2 (LRRK2, also known as PARK8), Parkin (PARK2), PTEN-induced kinase 1 (PINK1, also known as PARK6), and Omi/HtrA2 (PARK13). Because NaB upregulated the expression of DJ-1, we investigated the effect of NaB on other PARK genes. As evident from Fig. 1e and f, NaB increased the mRNA expression of Parkin, PINK1, HtrA2, and LRRK2. While the mRNA expression of PINK1 was maximum at 100 µM NaB, Parkin and LRRK2 showed maximum expression at 200 µM (Fig. 1e and f). On the other hand, HtrA2 always showed optimum expression at 500 µM NaB (Fig. 1e and f). However, in contrast to the upregulation of DJ-1, Parkin, PINK1, LRRK2, and HtrA2, NaB dose-dependently decreased the mRNA expression of α-synuclein (Fig. 1e and g). These effects were specific as NaFO had no effect on the expression of any of these genes (data not shown). Taken together, while NaB upregulated the expression of DJ-1, Parkin, PINK1, LRRK2, and HtrA2, this drug down-regulated the expression of α-synuclein.
Does NaB upregulate DJ-1 in neurons?
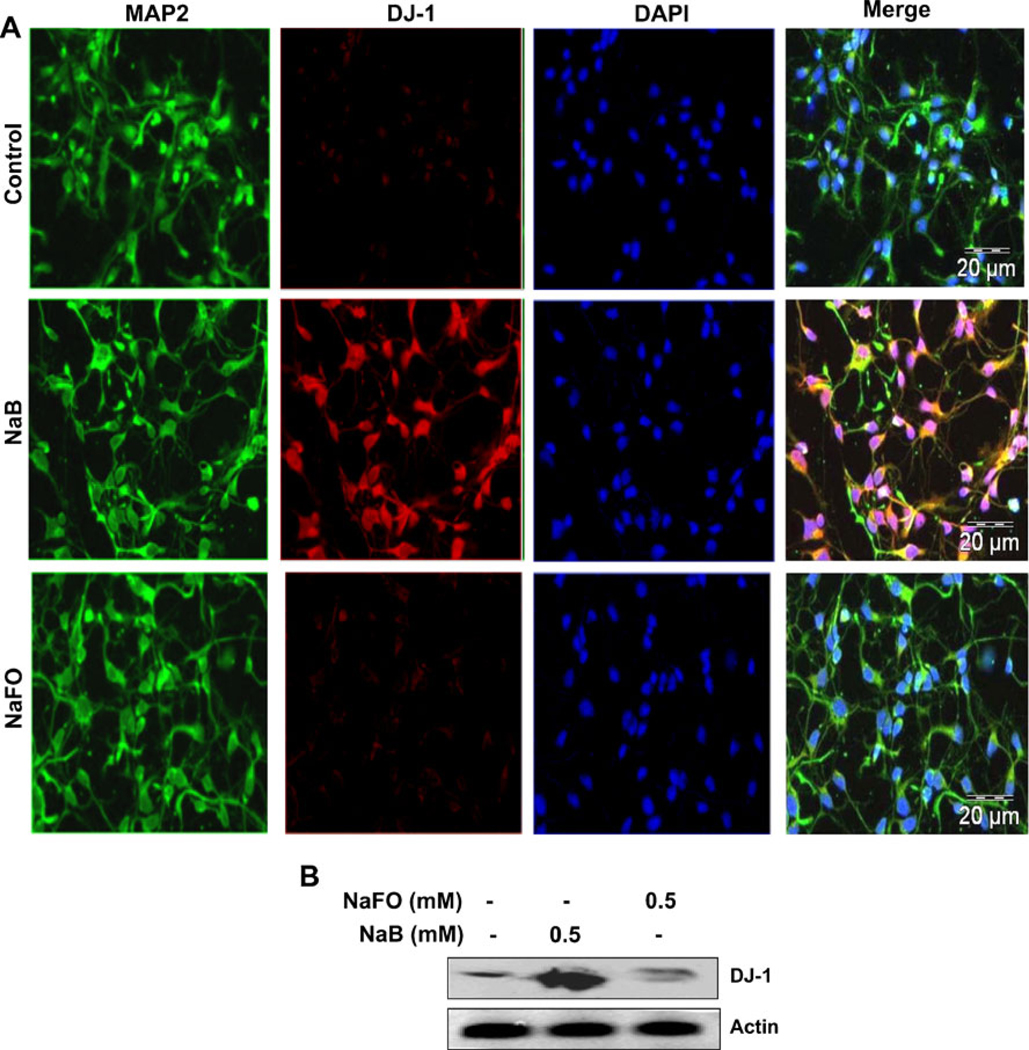
Similar to astrocytes, neurons have been also shown to express DJ-1 (Bandopadhyay et al. 2004). Therefore, we examined whether NaB was capable of inducing DJ-1 in neurons. Primary human neurons were treated with NaB and NaFO for 24 h followed by double-label immunofluorescence analysis. Similar to astrocytes, NaB also markedly increased the level of DJ-1 in primary neurons (Fig. 3a). This increase was specific as NaFO could not upregulate DJ-1 in neurons (Fig. 3a) and both NaB and NaFO had no effect on MAP-2 (Fig. 3a). Next we performed immunoblot analysis in SH-SY5Y cells. As evident from Fig. 3b, NaB, but not NaFO, markedly increased the expression of DJ-1 protein in SH-SY5Y neuronal cells. On the other hand, both NaB and NaFO had no such effect on actin. These results suggest NaB can also upregulate DJ-1 in neurons.
Fig. 3.
Effect of NaB on the expression of DJ-1 protein in primary human neurons and SH-SY5Y neuronal cells. a Primary human neurons were treated with 500 µM of NaB or NaFO under serum-free condition. After 24 h of treatment, levels of MAP2 and DJ-1 were monitored by double-label immunofluorescence. b SH-SY5Y neuronal cells were treated with 500 µM of NaB or NaFO under serum-free condition. After 24 h of treatment, levels of DJ-1 and actin were monitored by Western blot analysis. Results represent three independent analyses
Intermediates of the mevalonate pathway negate the inducing effect of NaB on the expression of DJ-1 in mouse primary astrocytes
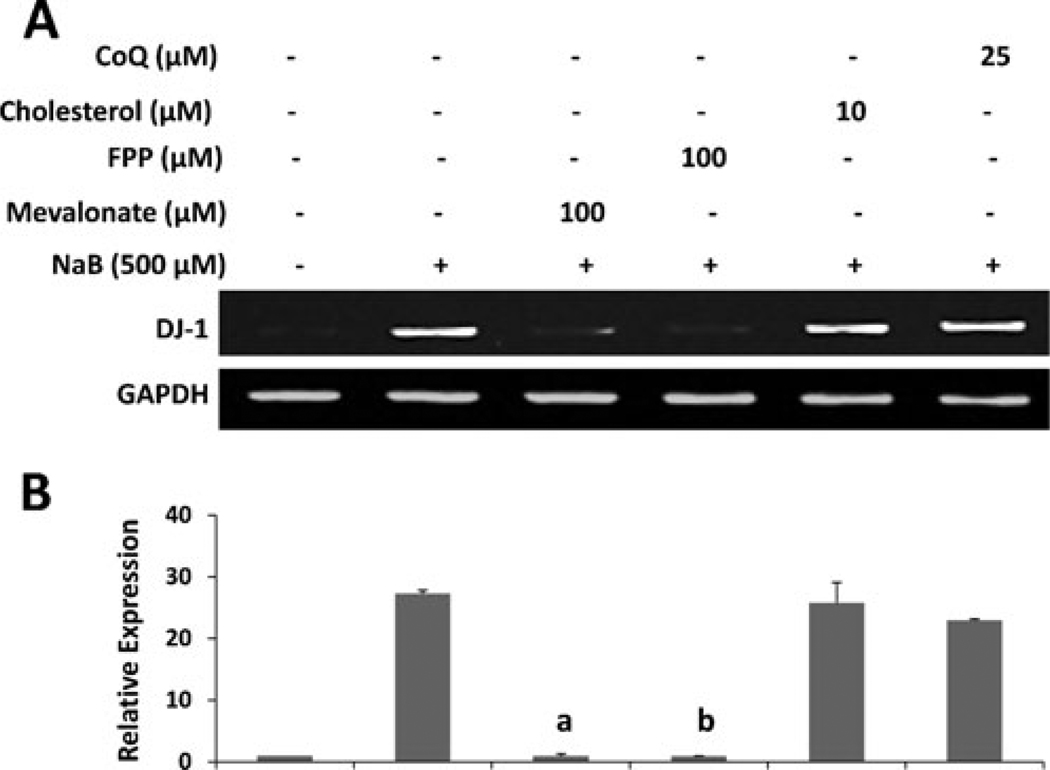
Next we tried to delineate mechanisms by which NaB upregulated DJ-1. Our time-course study shows that at least 6 h of incubation was required for NaB to upregulate DJ-1 (Fig. 1c and d). This suggests that metabolite (s) sensitive to NaB may be involved in the upregulation of DJ-1. Recently we have demonstrated that NaB exhibits anti-inflammatory efficacy in activated glial cells via modulating the mevalonate pathway (Brahmachari et al. 2009). Although NaB treatment inhibits the level of cholesterol, the end product of the mevalonate pathway, intermediates, but not the end product, was involved in NaB-mediated inhibition of iNOS in microglia (Brahmachari et al. 2009). Therefore, we investigated the role of various metabolites of the mevalonate pathway in NaB-mediated upregulation of DJ-1. As evident from semi-quantitative RT-PCR (Fig. 4a) and quantitative real-time PCR (Fig. 4b), both mevalonate and farnesyl pyrophosphate abrogated the DJ-1-inducing effect of NaB in primary astrocytes. On the other hand, cholesterol (the end product of the mevalonate pathway) and coenzyme Q (an unrelated lipid molecule) had no effect on NaB-mediated increase in DJ-1 mRNA (Fig. 4). These results suggest that depletion of intermediary products rather than end products of the mevalonate pathway is responsible for the observed DJ-1-inducing effect of NaB.
Fig. 4.
Mevalonate pathway intermediates negate the DJ-1-upregulating effect of NaB in primary mouse astrocytes. Cells were treated with NaB in the presence or absence of various intermediates of the mevalonate pathway namely mevalonate, farnesyl pyrophosphate, cholesterol, and coenzyme Q (CoQ) under serum free condition. After 6 h of treatment, the expression of DJ-1 was analyzed in cells by semi-quantitative RT-PCR (a) and real-time PCR (b). Results are mean ± SD of three different experiments. ap < 0.001 vs. control;, bp < 0.001 vs. NaB
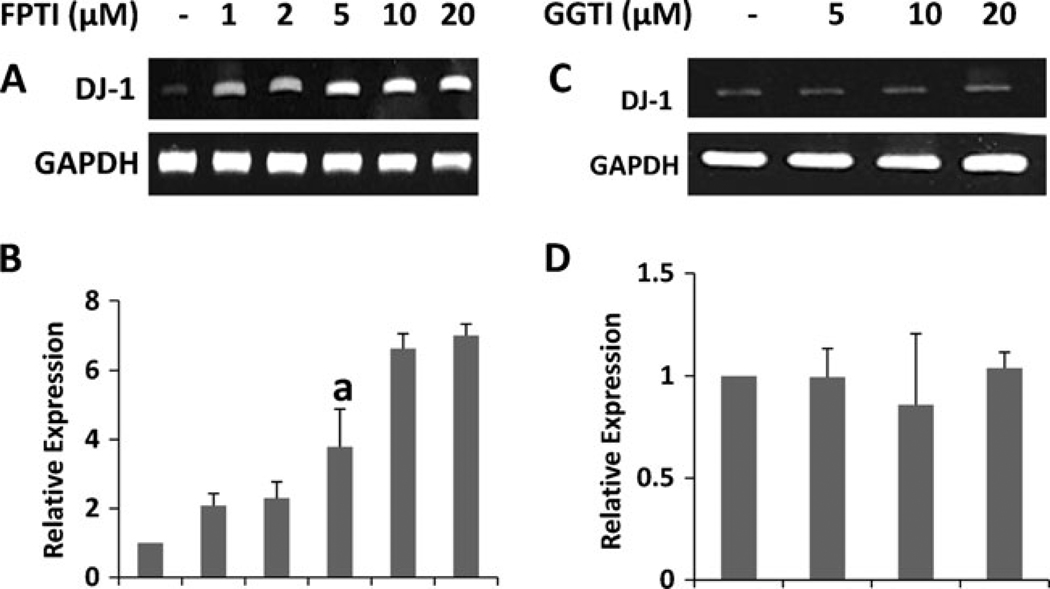
Farnesyl Protein Transferase Inhibitor (FPTI), but not Geranylgeranyl Transferase Inhibitor (GGTI), increases the expression of DJ-1 m RNA in primary mouse astrocytes
The reversal of NaB-mediated upregulation of DJ-1 by the intermediates of mevalonate pathway like mevalonate and farnesyl pyrophosphate, but not by the end product of the same pathway, suggest a possible involvement of the isoprenylation process in the regulation of DJ-1 gene. While farnesylation is a necessary step for the activation of p21ras, geranylgeranylation is required for the activation of p21rac (Qiu et al. 1995; Garnovskaya et al. 1996). Therefore, we examined the effect of FPTI, an inhibitor of p21ras farnesyl protein transferase, and GGTI, an inhibitor of p21rac geranylgeranyl transferase, on the expression of DJ-1 in astrocytes. It is clearly evident from semi-quantitative RT-PCR (Fig. 5a) and real-time PCR (Fig. 5b) that FPTI alone dose-dependently upregulated the expression of DJ-1 mRNA. Interestingly, GGTI had no effect on the mRNA expression of DJ-1 (Fig. 5c and d). These results suggest that the expression of DJ-1 may be regulated by p21ras, but not p21rac.
Fig. 5.
Farnesyl protein transferase inhibitor (FPTI), but not geranylgeranyl transferase inhibitor (GGTI), increases the expression of DJ-1 in primary mouse astrocytes. Cells were treated with increasing concentration of FPTI (A&B) and GGTI (C&D) under serum-free condition. After 6 h of treatment, the mRNA expression of DJ-1 was analyzed by semi-quantitative RT-PCR (A&C) and real-time PCR (B&D). Results are mean ± SD of three different experiments. ap < 0.01 vs. control
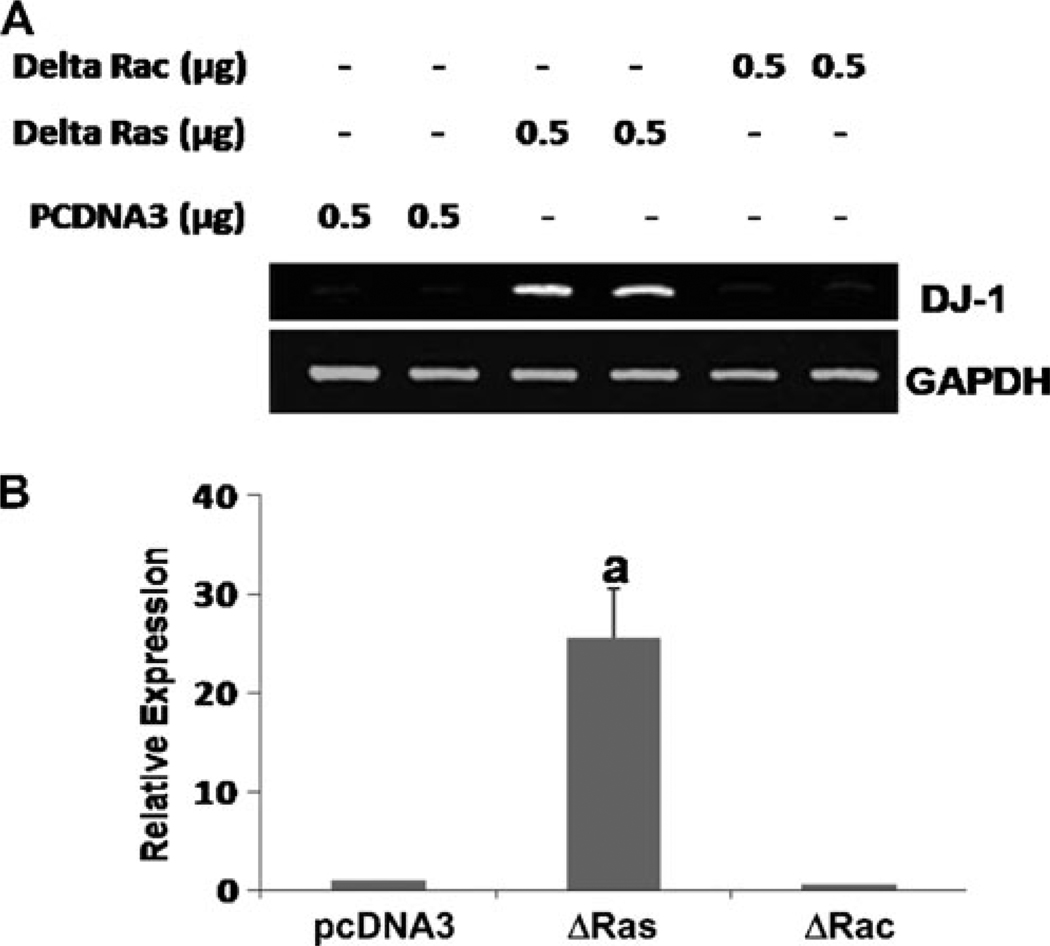
Dominant-negative mutant of p21ras, but not p21rac, increases the expression of DJ-1 in primary mouse astrocytes
To further confirm the role of p21ras and p21rac in the expression of DJ-1, primary astrocytes were transfected with dominant-negative mutant of p21ras (Δp21ras) and p21rac (Δp21rac). For comparison, cells were also transfected with an empty vector (pcDNA3). Consistent to the induction of DJ-1 by FPTI, Δp21ras alone markedly induced the mRNA expression of DJ-1 in astrocytes suggesting that inhibition of p21ras alone is sufficient for the induction of DJ-1 (Fig. 6a and b). Again, the empty vector and Δp21rac had no effect on the mRNA expression of DJ-1 (Fig. 6a and b) highlighting the specificity of the effect. These results confirm that DJ-1 expression is regulated by p21ras, but not p21rac.
Fig. 6.
Dominant-negativemutant of p21ras, but not p21rac, increases the expression of DJ-1 in primary mouse astrocytes. Cells were transfected with pcDNA3 (empty vector), Δp21ras and Δp21rac for 24 h. After 18 h of transfection, cells were incubated under serum-free condition for 6 h followed analysis of DJ-1 mRNA expression by semi-quantitative RT-PCR (a) and real-time PCR (b). Results are mean ± SD of three different experiments. ap < 0.0001 vs. empty vector
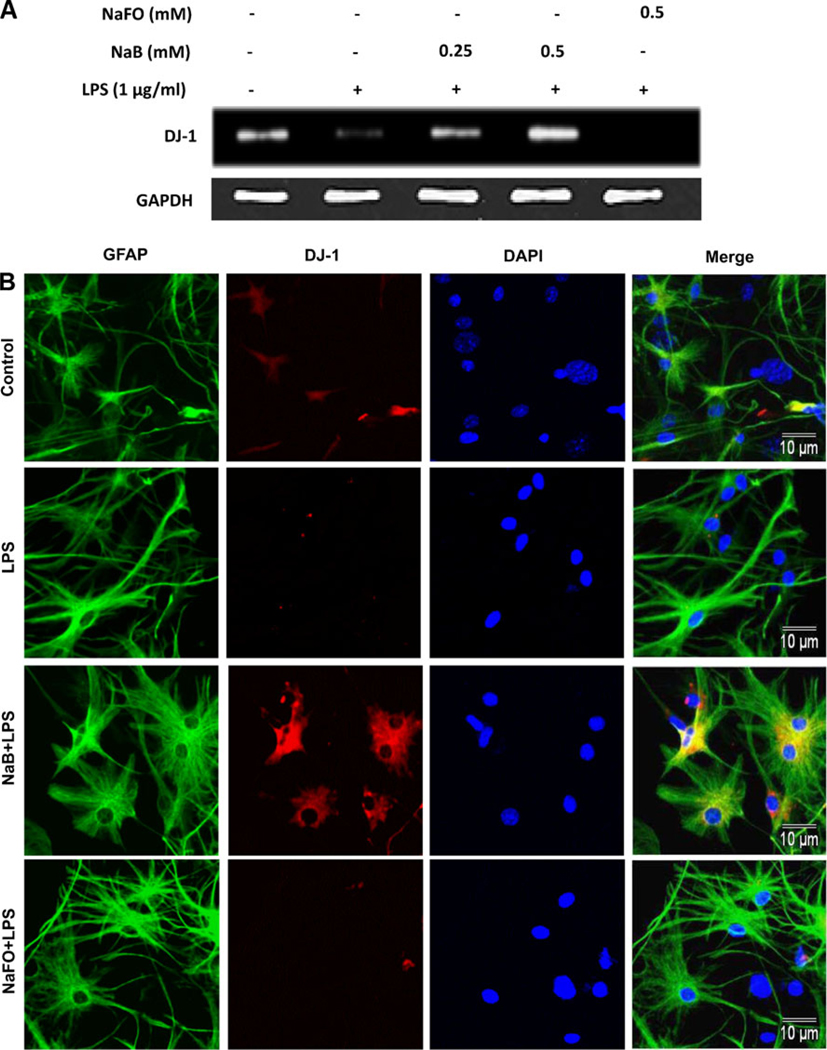
Reversal of LPS-mediated down-regulation of DJ-1 by NaB in primary mouse astrocytes
Results presented above suggest that NaB is capable of increasing the level of DJ-1 in normal astrocytes. However, astrocytes undergo activation and it has been reported that level of DJ-1 goes down in the CNS of patients with neurodegenerative disorders (Bonifati et al. et al. 2003). Therefore, we investigated the effect of NaB on the expression of DJ-1 in activated astrocytes. As evident from semi-quantitative RT-PCR (Fig. 7a), real-time PCR (Fig. 7b) and immunofluorescence (Fig. 7c) analyses, bacterial LPS suppressed the expression of DJ-1 mRNA and protein. However, NaB pretreatment markedly abrogated LPS-mediated inhibition of DJ-1 in astrocytes (Fig. 7). This effect was specific as NaFO could not abrogate LPS-mediated suppression of DJ-1 in astrocytes. These results suggest that the level of DJ-1 goes down in activated astrocytes and that NaB is also capable of blocking LPS-mediated down-regulation of DJ-1.
Fig. 7.
Effect of NaB on LPS mediated decrease in DJ-1 expression in primary mouse astrocytes. Cells pretreated with NaB or NaFO for 6 h were stimulated with 1 µg/ml of LPS under serum-free conditions. After 6 h of stimulation, the mRNA expression of DJ-1 was monitored by semi-quantitative RT-PCR (a) and real-time PCR (b). Results are mean ± SD of three different experiments. ap < 0.05 vs. control; bp < 0.05 vs. LPS; cp < 0.01 vs. LPS. After 24 h of stimulation, the protein level of DJ-1 was monitored by immunofluorescence analysis (c)
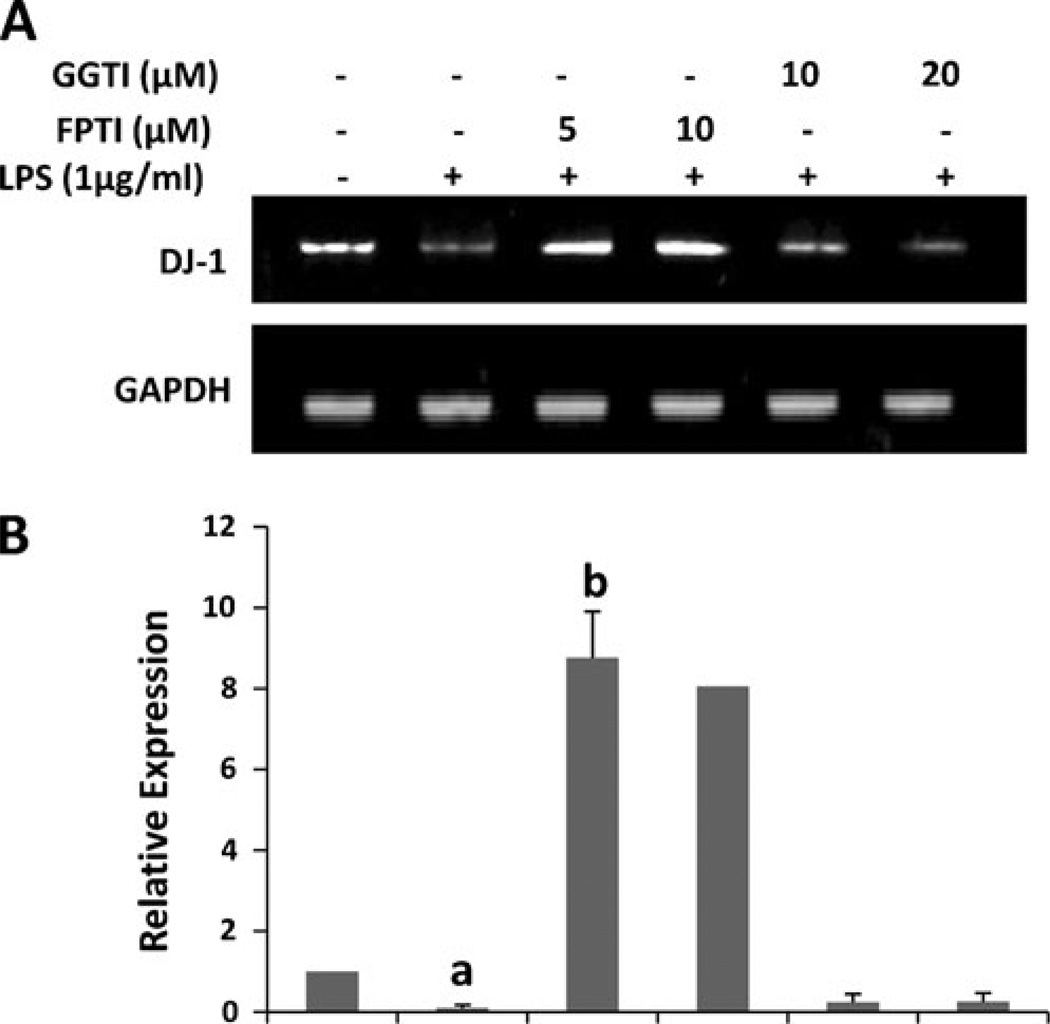
Does LPS down-regulate DJ-1 in primary mouse astrocytes via activation of p21ras?
Results presented above show that NaB upregulates the level of DJ-1 via modulation of mevalonate metabolites and that p21ras, but not p21rac, is involved in the regulation of DJ-1. We have recently shown that NaB inhibits the activation of small G proteins in LPS-stimulated microglial cells (Brahmachari et al. 2009). Because LPS is a known activator of small G proteins like p21ras and p21rac (Pahan et al. 2000), we investigated if LPS required p21ras for suppressing DJ-1. As shown above, LPS down-regulated the mRNA expression of DJ-1 (Fig. 8a). However, FPTI markedly blocked the suppressive effect of LPS and induced the expression of DJ-1 in activated astrocytes (Fig. 8a and b). Again, these effects were specific as GGTI had no effect on LPS-mediated suppression of DJ-1. These results suggest that LPS inhibits the expression of DJ-1 via p21ras, but not p21rac.
Fig. 8.
FPTI, but not GGTI, abrogates LPS-mediated inhibition of DJ-1 in primary mouse astrocytes. Cells pretreated with different concentrations of FPTI and GGTI for 30 min were stimulated with LPS for 6 h under serum-free condition followed by monitoring the mRNA expression of DJ-1 by semi-quantitative RTPCR (a) and real-time PCR (b). Results are mean ± SD of three different experiments. ap < 0.01 vs. control, bp < 0.001 vs. LPS
Discussion
Upregulation of DJ-1 has been suggested to be beneficial for the damaged nigrostriatum in PD. DJ-1 is a key molecule in the cell survival process by positively regulating the synthesis of reduced glutathione (Zhou and Freed 2005), facilitating Nrf2-dependent detoxification pathways (MacKeigan et al. 2003; Clements et al. 2006) and inducing Akt activity through the inhibition of phosphatase and tensin homolog (PTEN) (Aleyasin et al. 2011; Kim et al. 2005), thus suggesting an overall beneficial role of DJ-1 in PD. It can act as peroxiredoxin-like peroxidase in vivo, where it can protect against oxidative stress in mitochondria (Lesage and Brice 2009). Most importantly, DJ-1 has been shown to transcriptionally upregulate the human tyrosine hydroxylase gene by inhibiting sumoylation of pyrimidine tract-binding protein-associated splicing factor (Zhong et al. 2006). Accordingly, while genetic inactivation of DJ-1 is associated to early onset PD (Bonifati et al. 2003), down-regulation of DJ-1 is found in brains of patients with sporadic forms of PD (Bossers et al. 2009). Therefore, identification of compounds capable of increasing the level of DJ-1 and characterization of associated intracellular pathways required to transduce the signal from the cell surface to the nucleus for the induction of DJ-1 are active areas of investigation.
Cinnamon, the brown bark of cinnamon tree, is a commonly used spice and flavoring material for desert, candies, chocolate etc. It has a long history as a medicine as well. Medieval physicians used cinnamon in medicines to treat a variety of disorders including arthritis, coughing, hoarseness, sore throats, etc. In addition to containing manganese, dietary fiber, iron, and calcium, cinnamon contains three major compounds - cinnamaldehyde, cinnamyl acetate and cinnamyl alcohol. After intake, these three active compounds are converted into cinnamic acid by oxidation and hydrolysis, respectively. Then cinnamic acid is β-oxidized to benzoate in the liver. This benzoate exists as sodium salt (NaB) or benzoyl-CoA. This NaB is a widely-used food preservative due to its anti-microbial properties. Earlier, our lab has demonstrated that NaB inhibits the expression of proinflammatory molecules in activated glial cells (Brahmachari et al. 2009) and protects mice from experimental allergic encephalomyelitis via modification of T cells at various stages (Brahmachari and Pahan 2007). Here we demonstrate for the first time that NaB is capable of upregulating neuroprotective DJ-1 in astrocytes and neurons. These results are specific since similar to DJ-1, NaB also upregulated other protective PARK molecules like Parkin, PINK1, LRRK2, and HtrA2. In contrast, α-syn is proinflammatory and neurotoxic (Dawson et al. 2011). Accordingly, NaB suppressed the expression of α-syn in astrocytes. DJ-1 is a key regulator of GSH synthesis (Zhou and Freed 2005) and astrocytes play an important role in the GSH metabolism of the brain since of different cell types, only astrocyte is capable of releasing substantial amount of GSH and supplying GSH and GSH precursors to neurons (Dringen and Hirrlinger 2003). Accordingly, it has been shown that DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone (Mullett and Hinkle 2009). Because DJ-1 has been implicated in the pathogenesis of PD, upregulation of DJ-1 in astrocytes and neurons by NaB opens up a potentially important option whereby cinnamon metabolite NaB may ameliorate nigrostriatal injury in PD.
The intracellular signaling events required for the transcription of DJ-1 are poorly understood and mechanisms by which NaB could upregulate DJ-1 were not known. Recently we have demonstrated that NaB is capable of reducing the level of cholesterol in vivo in mice at a level comparable to pravastatin (Brahmachari et al. 2009) suggesting that this drug attenuates the cholesterol biosynthesis pathway. Here we show that mevalonate and farnesyl pyrophosphate, but not cholesterol, reverse the DJ-1-inducing activity of NaB in astroglia. These results suggest that intermediates, but not the end product of the cholesterol biosynthesis pathway, play an important role in the upregulation of DJ-1 by NaB. Intermediates of the cholesterol biosynthesis pathway are key regulators of isoprenylation of small G proteins like p21ras and p21rac (Boguski and McCormick 1993). For example, while farnesyl pyrophosphate is involved in the farnesylation and activation of p21ras, geranylgeranyl pyrophosphate is required for geranylgeranylation of p21rac. It is interesting to note that p21ras, but not p21rac, is involved in the regulation of DJ-1. Our conclusion is based on the following observations. First, FPTI, capable of inhibiting farnesylation of p21ras, alone increased the level of DJ-1 in astrocytes. In contrast, GGTI that inhibits the geranylger-anylation of p21rac, was unable to upregulate DJ-1. Second, a dominant-negative mutant of p21ras, but not p21rac, enhanced the expression of DJ-1. Third, LPS is a prototype activator of small G proteins like p21ras and p21rac in various cell types including glial cells. Accordingly, LPS decreased the expression of DJ-1. Again, reversal of LPS-mediated inhibition of DJ-1 in astrocytes by FPTI, but not GGTI, indicates a role of p21ras, but not p21rac in the down-regulation of DJ-1 in activated astrocytes.
The p21ras proto-oncogene proteins, a family of GTP-binding proteins, function by binding to the cytoplasmic surface of the plasma membrane. This membrane localization of p21ras involves prenylation of cysteine in CAAX motif present at the C-terminus, proteolytic removal of AAX tripeptide, and then carboxymethylation of C-terminal cysteine (Hancock et al. 1991). The activation of p21ras by receptor tyrosine kinase occurs through conversion of the GDP-bound inactive form to the GTP-bound active form by Sos and Grb2 and then transduction of signal to down-stream effector molecules. The GTP-bound form is converted to the inactive form by the intrinsic GTPase activity, which is accelerated by GTPase-activating proteins (Boguski and McCormick 1993). NaB attenuates the farnesylation of p21ras and thereby inhibits the signal transmission to the downstream signaling molecules. One such downstream candidate is Raf-1 (serine-threonine kinase). The p21ras interacts directly with Raf-1 and is believed to function by positioning Raf-1 at the plasma membrane in the vicinity of its activator, and tyrosine phosphorylation of Raf-1 seems to be essential for p21ras-induced activation of Raf-1 (Chung and Kondo 2011; Avruch et al. 1994; Garnovskaya et al. 1996). Raf-1, in turn, phosphorylates and activates MEKs and ERKs (members of mitogen-activated protein kinase cascade). Therefore, the observed induction of DJ-1 by NaB and FTI may be due to decrease and/or lack of signal transmission from receptor tyrosine kinase to Raf/mitogen-activated protein kinase cascade via p21ras. At present, we are investigating such possibilities.
At present, no effective interdictive therapy is available for PD. Administration of a DA agonist or levodopa has been the standard treatment for PD symptoms but does not affect the disease course. Moreover, these treatments are associated with side effects and often times unsatisfactory outcomes. There are several advantages of NaB over other proposed anti-neurodegenerative therapies. First, NaB is fairly nontoxic. Cinnamon, which has been widely used as flavoring material and spice throughout the world for centuries, is metabolized to NaB. NaB is excreted through the urine, if in excess. NaB is an FDA-approved drug against Urea cycle disorders in children. Second, NaB can be taken orally, the least painful route. Recently, we have demonstrated that NaB treatment of mice with relapsing-remitting EAE, an animal model of MS, via drinking water suppresses the disease process of EAE (Brahmachari and Pahan 2007). Third, NaB is very economical compared to other existing anti-neurodegenerative therapies. Fourth, although here we have not tested, NaB being a lipophilic molecule is most likely able to diffuse through the BBB. For example, glycine toxicity is a problem in urea cycle disorders. After treatment of patients with urea cycle disorders, NaB is known to combine with glycine to produce hippurate, a compound that is readily excreted in the urine. Simultaneous serum and CSF sampling in those patients showed comparable levels of NaB and hippurate in the CSF (Nair 2001; Leonard and Morris 2002; Scaglia et al. 2004) suggesting that NaB is capable of crossing the BBB.
In summary, we have demonstrated that NaB (a metabolite of cinnamon, commonly-used food additive and a FDA-approved drug for Urea cycle disorders) increases the level of DJ-1 by modulating the mevalonate pathway and that p21ras, but not p21rac, is involved in the upregulation of DJ-1. These results highlight an undiscovered property of NaB and indicate that this drug may be used for therapeutic intervention in PD as primary or adjunct therapy.
Acknowledgements
This study was supported by grants from NIH (NS64564, NS71479 and AT6681).
References
- Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW. Park DS DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A. 2011;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J, Zhang XF, Kyriakis JM. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Baulac S, Lu H, Strahle J, Yang T, Goldberg MS, Shen J, Schlossmacher MG, Lemere CA, Lu Q, Xia W. Increased DJ-1 expression under oxidative stress and in Alzheimer’s disease brains. Mol Neurodegener. 2009;4:12. doi: 10.1186/1750-1326-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bossers K, Meerhoff G, Balesar R, van Dongen JW, Kruse CG, Swaab DF, Verhaagen J. Analysis of gene expression in Parkinson’s disease: possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathol. 2009;19:91–107. doi: 10.1111/j.1750-3639.2008.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Kondo M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol Res. 2011;49:248–268. doi: 10.1007/s12026-010-8187-5. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2011;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Garnovskaya MN, van Biesen T, Hawe B, Casanas Ramos S, Lefkowitz RJ, Raymond JR. Ras-dependent activation of fibroblast mitogen-activated protein kinase by 5-HT1A receptor via a G protein beta gamma-subunit-initiated pathway. Biochemistry. 1996;35:13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2009;29:13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Marshall CJ. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B) EMBO J. 1991;10:641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson’s disease and parkinsonism. Ann Neurol. 2006;60:389–398. doi: 10.1002/ana.21022. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer’s disease. J Neurosci. 2010;30:12676–12689. doi: 10.1523/JNEUROSCI.1243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Jana A, Pal U, Pahan K. A simplified method for isolating highly purified neurons, oligodendrocytes, astrocytes, and microglia from the same human fetal brain tissue. Neurochem Res. 2007;32:2015–2022. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW. DJ-1, a novel regulator of the tumor suppressor PTEN. Canc Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kubota K, Ishizaki T. Dose-dependent pharmacokinetics of benzoic acid following oral administration of sodium benzoate to humans. Eur J Clin Pharmacol. 1991;41:363–368. doi: 10.1007/BF00314969. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Morris AA. Urea cycle disorders. Semin Neonatol. 2002;7:27–35. doi: 10.1053/siny.2001.0085. [DOI] [PubMed] [Google Scholar]
- Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, Pahan K. Human immunodeficiency virus type 1 (HIV-1) that induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–39319. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKeigan JP, Clements CM, Lich JD, Pope RM, Hod Y, Ting JP. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha. Canc Res. 2003;63:6928–6934. [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol. 2001;20(Suppl 3):23–50. doi: 10.1080/10915810152630729. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Pahan K, Liu X, McKinney MJ, Wood C, Sheikh FG, Raymond JR. Expression of a dominant-negative mutant of p21(ras) inhibits induction of nitric oxide synthase and activation of nuclear factor-kappaB in primary astrocytes. J Neurochem. 2000;74:2288–2295. doi: 10.1046/j.1471-4159.2000.0742288.x. [DOI] [PubMed] [Google Scholar]
- Pahan K, Jana M, Liu X, Taylor BS, Wood C, Fischer SM. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J Biol Chem. 2002;277:45984–45991. doi: 10.1074/jbc.M200250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Differential regulation of Mn-superoxide dismutase in neurons and astroglia by HIV-1 gp120: implications for HIV-associated dementia. Free Radic Biol Med. 2007;42:1866–1878. doi: 10.1016/j.freeradbiomed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia F, Carter S, O’Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81(Suppl 1):S79–S85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Toth B. Lack of tumorigenicity of sodium benzoate in mice. Fundam Appl Toxicol. 1984;4:494–496. doi: 10.1016/0272-0590(84)90208-2. [DOI] [PubMed] [Google Scholar]
- Valente EM, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Zhong N, Xu J. Synergistic activation of the human MnSOD promoter by DJ-1 and PGC-1alpha: regulation by SUMOylation and oxidation. Hum Mol Genet. 2008;17:3357–3367. doi: 10.1093/hmg/ddn230. [DOI] [PubMed] [Google Scholar]
- Zhong N, Kim CY, Rizzu P, Geula C, Porter DR, Pothos EN, Squitieri F, Heutink P, Xu J. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem. 2006;281:20940–20948. doi: 10.1074/jbc.M601935200. [DOI] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]