Abstract
Thermolysin and other secreted broad-specificity proteases, such as subtilisin or alpha-lytic protease, are produced as pre-pro-proteins that stay at least partially unfolded while in the cytosol. After secretion, the pro-proteases fold to their active conformations in a process that includes the autolytic removal of the pro-peptide. We review the life cycle of the thermolysin-like protease from Bacillus stearothermophilus in light of the calcium dependent stability and instability of the N-terminal domain. The protease binds calcium ions in the regions that are involved in the autolytic maturation process. It is generally assumed that the calcium ions contribute to the extreme stability of the protease, but experimental evidence for TLP-ste indicates that at least one of the calcium ions plays a regulatory role. We hypothesize that this calcium ion plays an important role as a switch that modulates the protease between stable and unstable states as appropriate to the biological need.
Keywords: calcium and instability, folding, protease, thermolysin like proteases, unfolding
Introduction to TLPs
Bacteria of the genus Bacillus are known to secrete proteins with a wide variety of functions. Many of these proteins are involved in cell wall biogenesis and integrity, but extracellular proteins have been detected with roles in processes as diverse as amino acid metabolism, mobility, sporulation, and nutrient scavenging. An important class of secreted proteins are the enzymes involved in detoxification and metabolism such as DNAses, RNAses, lipases, and a series of proteases. Some secreted enzymes are dangerous for the producer cell. Therefore mechanisms have evolved to ensure that such enzymes only become active after secretion from the cell. The best-known examples of such enzymes in the bacilli are secreted broad-specificity proteases such as the alkaline proteases often referred to as subtilisins and the metallo-proteases or ‘neutral proteases’ referred to in this review as thermolysin-like proteases (TLPs).
The secreted proteases are produced as pre- pro-proteins. The pre-peptide is cleaved-off during Sec-controlled secretion, and the active protease emerges outside the cell after folding of the pro- protein and autolytic removal of the pro-peptide. The autolytic processing step and the role of the pro-peptide have been described in considerable detail for several proteases, including TLPs.1–12 The pro-peptide acts as an intra-molecular chaperone, helping the protease to obtain its correct structure. It is cleaved-off through intra-molecular proteolysis upon completion of the folding process.
Thermolysin was among the first enzymes to have its crystal structure determined.13 The thermolysin structure was the first structure of a thermostable enzyme. The T50 of thermolysin, i.e. the temperature at which it loses 50% of its activity under standard conditions after 30 minutes of incubation, is 86.9°C.14 Unexpectedly at that time, but confirmed by many subsequent crystallographic studies on natural and engineered thermostable enzymes, the structure of thermolysin did not show obvious features that could be linked to its stability. It was concluded that “the enhanced stability of thermostable proteins relative to thermolabile ones cannot be attributed to a common determinant, such as metal ions or hydrophobic stabilization, but in a given instance may be due to rather subtle differences in hydrophobic character, metal binding, hydrogen binding, ionic interactions, or a combination of all of these.”15 Many subsequent studies have supported this idea. Today we indeed know that the stability of a protein can never be attributed to one single factor and there are many alternative ways to reach extreme protein stability.16–18 Calcium ions have long been known to contribute to the stability of thermolysin and are also important for TLP stability,19,20 albeit to different extents as was shown by Veltman et al.21,22
TLP structure
Structurally, TLPs consist of two domains (Fig. 1) that are connected by a central helix containing several catalytically crucial residues. It has been shown that substrate-binding involves a hinge-bending motion between the two domains.23,24 The C-terminal domain is rich in alpha-helices and the final 62 residues form a four-helix bundle that displays considerable stability on its own.25 The N-terminal domain contains a series of β-strands that cradle one long helix. The N-terminal 13 residues form the middle β-strand of a three-stranded β-sheet (Fig. 2).
Figure 1.
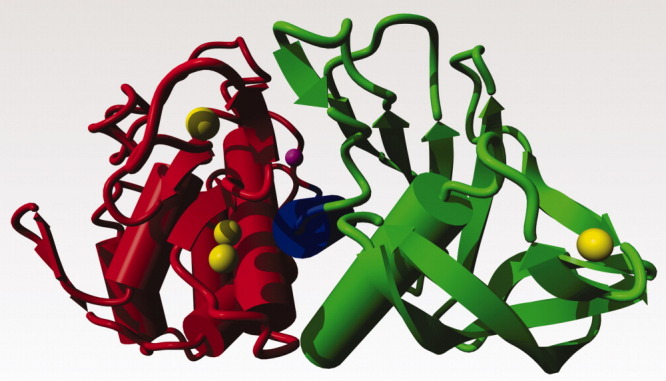
TLPs have a two domain structure. The N-terminal domain that typically comprises residues 1-135 is shown in green. The central helix connecting the two domains is shown in blue. This helix holds several active site residues. The C-terminal domain is in red. The active site Zn is shown in magenta, and the calcium ions are in yellow. Ca3 is at the far right; Ca4 at the top left; Ca1 is located just above and behind Ca2. This picture is based on a homology model of the TLP from B. stearothermophilus that shares 85% sequence identity with thermolysin.
Figure 2.
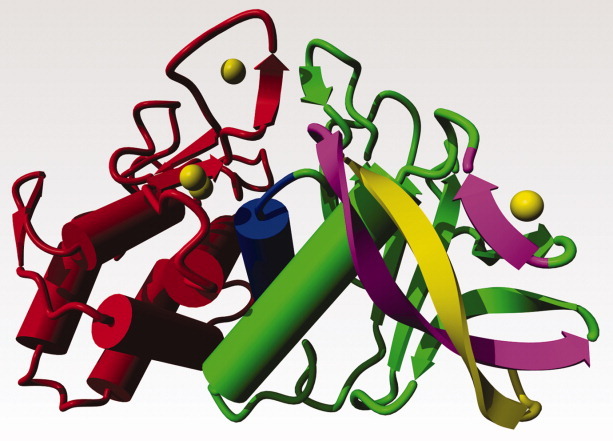
As Figure 1, but slightly rotated to better show the N-terminal β-strands. The N-terminal β-strand of 13 amino acids, shown in yellow, is sandwiched between the β-strands 14-26 and 60-63 that are shown in purple. The structural integrity of the β-strand 60-63 depends on the presence of calcium ion Ca3 (at the top right). Note that Gln61 in the strand 60-63 has a double role. Its side chain interacts with Phe63, while its backbone carbonyl is one of the Ca3 ligands (see also Figure 3).
TLP Stability
TLPs have been the subject of many site-directed mutagenesis studies aimed at manipulating their thermal stability, in particular the TLP from Bacillus stearothermophilus (TLP-ste; T50 = 73.4°C) and the TLP from Bacillus subtilis (TLP-sub; T50 = 58.6°C; see De Kreij et al.26 for comparative stability data). TLP-ste is a close relative of thermolysin; the two proteins share 85% sequence identity and have all important residues in common (including all residues involved in catalysis and ion-binding). Thermal inactivation of TLPs is a consequence of inter-molecular proteolysis and the rate-limiting step is the local unfolding that precedes this process.17,27,28 In this review, we refer to inter-molecular proteolysis as cannibalysis to distinguish this process from the autolytic step during protease maturation. It is important to note that the first order rate constant of thermal inactivation does not reflect the rate of the actual proteolytic cleavage nor of a bi-molecular association needed for cannibalysis to occur. Instead, it reflects the rate of an unfolding process that makes the protein proteolytically susceptible. All data suggest that the rate-limiting unfolding processes have a local character. While stability data on the mesophilic TLP-sub have not revealed a specific stability determining area,29–31 studies on TLP-ste have shown that stability is governed by local unfolding processes in the N-terminal domain,14,28,32–34 involving the three β-strands and Ca3 (Figs. 2, 3).
Figure 3.

Left. The area around Ca3 in thermolysin. The small yellow sphere is Ca3, the yellow lines connect the seven ligands for this calcium (2 Oδ atoms of Asp57, 1 Oδ atom of Asp59, the backbone carbonyl of Gln61, and 3 water molecules. The three water molecules are not shown).
Calciums
Thermally stable TLPs such as thermolysin and TLP-ste bind four calcium ions numbered Ca1–Ca4 (Fig. 1), whereas less stable TLPs seem to bind only two (Ca1 and Ca2). Ca1 and Ca2 bind in a double calcium site close to the interface between the two domains. Ca4 binds near the Ca1-2 site. All ligands for these three calciums are located in the C-terminal domain. Ca3 binding to the N-terminal domain is known to be an important stability determinant of TLPs. The calcium affinity of the four sites in thermolysin has been studied in early work by Dahlquist, Voordouw, Roche and co-workers.19,20 On the basis of structural inspections it seems safe to assume that the TLPs cannot be folded without calciums bound to the double calcium binding site and most experimental studies on calcium binding concluded that Ca1 binds with the highest affinity. The ranking of the other three affinities is not straightforward as has been discussed extensively.22,35 Veltman et al.22 were able to show that the dependency of the thermal stability of TLP-ste on calcium in the 0.1–10 mM concentration range involves the Ca3 site (Figs. 3, 4).
Figure 4.
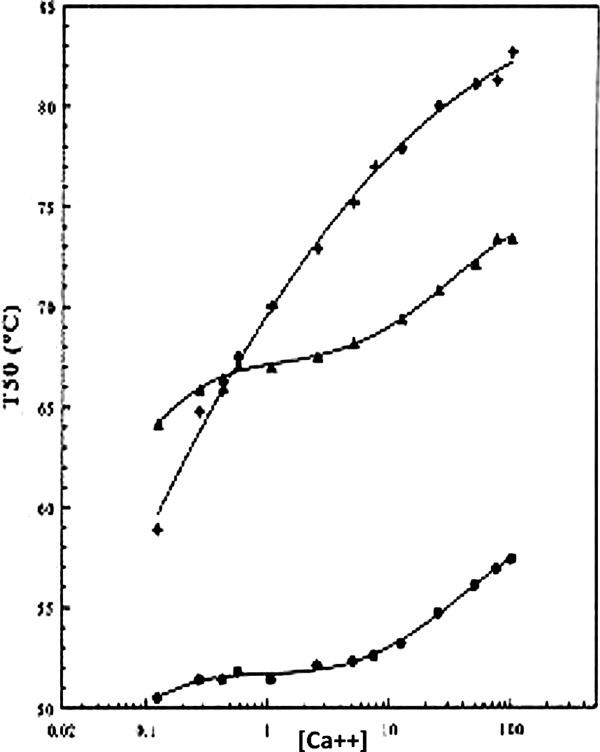
Stability of TLP-ste as a function of the Ca2+ concentration. Top curve: wild-type; middle curve Asp57Ala mutant; bottom curve Asp59Ala mutant. TLP-ste variants with mutations in the Ca3 site show reduced stability and an almost abolished calcium-dependency of this stability in the 0.1 – 10 mM concentration range. Asp 57 is buried in the molecule. If no Ca3 is bound, as at low [Ca2+] or in the case of the Asp59Ala mutant, the buried Asp57 residue will be very detrimental to stability. In the Asp57Ala mutant, also, relatively little calcium is bound, but in this case the remaining aspartic acid, Asp59, is located at the surface, limiting the detrimental effect of the D57A mutation relative to D59A. (Figure copied with permission from O.R. Veltman, dissertation; 1997; see also Veltman et al.22).
Figure 3 shows a detailed picture of the Ca3 site. Ca3 is liganded by seven atoms, three of which are water oxygens. It is important to note that integrity of the Ca3 site is crucial for the integrity of the β-strand 60–63 that in the mature, folded TLP-ste forms a β-sheet with the two N-terminal β-strands (Fig. 2). Most residues that are known from comparisons of naturally occurring enzymes32 and from site-directed mutagenesis experiments to be crucial for TLP-ste stability are in the vicinity of Ca3.
Figure 4 shows that the protease is highly unstable at low Ca2+ concentrations, indicating that local unfolding of the region around Ca3 and subsequent cannibalistic degradation are promoted as the Ca3 site becomes less populated. The lowest Ca2+ concentration that can be achieved in these stability assays is ∼100 μM. The Ca2+ concentration in the cytosol is a factor of 1000 lower.36
The Hypothesis
We hypothesize that in order to carry out its full range of biological functions the stability of TLP-ste is variable. Under some circumstances it is stable and fully folded, whereas it can also be partially unfolded and susceptible to cannibalism. We also propose that this change in stability is regulated by calcium ion binding. Other secreted broad specificity proteases such as subtilisins and alpha-lytic proteases are likely to need similar regulatory mechanisms and it may be noted that the regulation of the stability and instability of such enzymes often involves calcium binding.
The 5 Steps in the Life of TLP-ste
In this section, we summarize the life of TLP-ste starting at the ribosome. Five steps are identified which will be described briefly followed by a more detailed discussion. In Step 1 TLP-ste is synthesized as a pre-pro-protein. While in the cytosol, the pre-pro-protein remains sufficiently unfolded not to be active and to be secretion competent. All healthy living cells, eukaryotes and prokaryotes alike, have an intracellular Ca2+ concentration that is on the order of 100 nM.36,37 This low Ca2+ concentration plays a series of roles in signalling38 but for the purpose of this study, it is important to note that it will contribute to keeping TLP-ste in an at least partially unfolded state because one or more of the calcium sites is not sufficiently occupied. In Step 2, the pre-pro-protein is secreted via the Sec-system and the pre-peptide is removed. In Step 3, outside the cell, the pro-protein folds and the pro-peptide is autolytically cleaved off and degraded.7 The relatively high Ca2+ concentration outside the cell is an essential aspect of the folding and maturation of the protease. In Step 4, the protease does its work. This normally will be defence or food scavenging. At some point the activity of the protease is, for a series of reasons that we can at best speculate on, no longer needed and in Step 5 the enzyme is degraded. The same Ca3-containing region that is needed to stabilize the β-sheet involving the N-terminal β-strand (Fig. 2) is now the weak link, permitting digestion of the protease, either cannibalistic, or perhaps by members from other protease families.
Step 1: The partially unfolded cytosolic phase
Bacteria have developed several ways to accomplish the complex process of protein secretion.39 The challenge for a bacterium is to coordinate translation, transport to the secretion machinery, translocation through the membrane, and folding. The most ubiquitous export system, used for TLPs, is the so-called Sec-system.39–41 Studies on protein secretion of mainly the mesophile B. subtilis have been instrumental for generating insight into the functioning of the Sec machinery in bacilli. In the current model for Sec-driven secretion in bacilli, summarized by Harwood and Cranenburgh,41 a nascent TLP would start its life as a pre-pro-protein that remains at least partially unfolded. The so-called signal recognition particle (SRP) recognizes the N-terminal signal sequence (the ‘pre’ part). The SRP directs the protein to the translocase complex in the cell membrane while its chaperone function keeps the nascent protein in a ‘secretion competent’, partially unfolded state.
There are several good reasons for the Bacillus to keep the pre-pro-protein in the secretion competent and at least partially unfolded state. Obviously, it would be energetically inefficient to fold the protease in the cytosol, to unfold it again during translocation, and to refold it again outside the cell. In the particular case of broad specificity proteases such as subtilisins or TLPs, there is the additional problem that these proteases potentially can digest many cytosolic proteins. The bacilli combine several mechanisms to keep TLPs inactive: first, both the secretion machinery and the low intracellular calcium concentration will promote (partial) unfolding. Second, in the unlikely case that a pre-pro-protein occasionally would populate an active conformation, the pro- protein would act as an inhibitor.10,42,43
Step 2: Translocation
The protein is translocated through the membrane at the expense of ATP and the pre-peptide is cleaved off by a Type I signal peptidase. Once outside, the still unfolded pro-protein must fold rapidly and correctly. Failure to do so may damage the producer cell (e.g. by aggregate formation in the cell wall) or may lead to the protein being degraded by quality-control proteases. The pro-part plays two roles in this process: it facilitates folding by acting as an intra-molecular chaperone and it inhibits protease activity of the folded pro-enzyme.10
Step 3: Folding, maturation, and stability
Once at the trans-side of the membrane and without folding restraints conferred by the secretion machinery, the protein folds in an environment that is rich in bivalent metal ions (including Ca2+), possibly assisted by foldases such as peptidyl prolyl cis-trans isomerases and in some cases also disulphide isomerases (most TLPs, though, neither contain cis-peptide bonds nor cysteine bridges and thus seem not to rely on these enzymes). Many secreted Bacillus proteins are metallo-proteins and there is experimental data supporting the idea that bivalent cations such as Ca2+ act as folding catalysts.41,44 Looking at what is known about calcium binding in TLP-ste and thermolysin and considering intra- and extra-cellular calcium concentrations, it can only be concluded that calcium ions indeed play a crucial role in the maturation process of TLP-ste. The calcium titrations by Veltman et al.22 (see also Fig. 4) show that the intracellular calcium concentration of a few hundred nanomolar is so low that in the cytosol not all calcium sites can be occupied in TLP-ste, which would contribute to keeping it at least partly unfolded and thus inactive. Once outside the cell, i.e. in or near the Gram-positive cell wall, the calcium concentration typically is in the millimolar range due to the ability of the B. subtilis cell wall to concentrate calcium ions present in the surrounding medium.44,45 Consequently, all calcium binding sites will become occupied, leading to structural stabilization and protease activation.
Pro-enzymes secreted and folded in this manner will not become active before the pro-peptide is cleaved off and removed. This process has been studied in varying detail for different secreted proteases. It is well established that in most cases, including TLP-ste, the necessary cleavage is intra-molecular,6,7,46 but in trans processing is possible.47 Intra-molecular processing seems to be the default and biologically most relevant situation. Once separated from the mature protease, the pro-parts have low stability48,49 and are readily degraded by the mature protease or by other, exogenous proteases.7,50,51
This folding and maturation mechanism has important implications for the conformational flexibility and stability of the N-terminus of the mature proteins. During autolytic maturation, a stretch of amino acids comprising the pro-peptide/N-terminus boundary needs to insert productively into the catalytic centre, a process that obviously requires conformational flexibility. Structural studies on the isolated pro-domain from alpha lytic protease showed a disordered C-terminus that became ordered in the complex of the protease with its pro- domain.50 During maturation, the N-termini of the mature proteases need to relocate from a position in the catalytic centre to their final position in the mature enzyme. Structural studies on the intact or processed precursor complexes of subtilisin,9,52 alpha-lytic protease,50 a TLP with a long, ‘thermolysin-like’ pro sequence,12 and a TLP with a short pro-sequence,11 have shown that these relocations are considerable.
Figure 5 shows the result of a short (unrealistic) molecular dynamics simulation on the thermolysin structure. In this simulation, a strong force was applied manually (interactively) to pull the N-terminal amino acid into the active site pocket (such that Val-1 occupies the S1′ pocket). Obviously, this simulation is highly unrealistic, but it shows that the N-terminal residue can be located in the active site without the need for any large rearrangements other than the location of the N-terminal 13 amino acids. Recent structural, mutagenesis and modelling studies of the autolytic processing of a TLP from Pseudoalteromonas led to similar conclusions.12 Interestingly, this study showed that the reorganization of the N-terminal beta-strand (amounting to a displacement of 33 Å for the N-terminal amino acid) takes place prior to release of the pro-part, i.e. in a complex of the pro-part and the mature enzyme.
Figure 5.
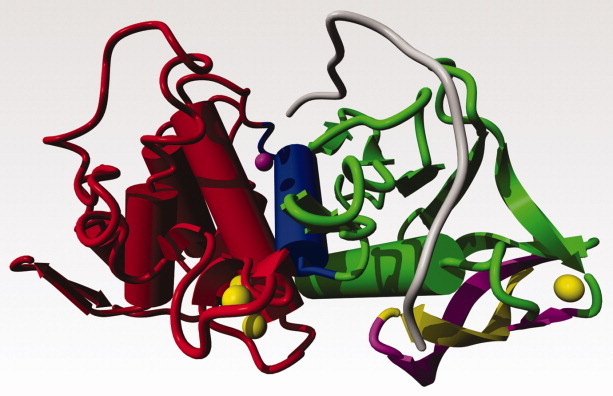
Thermolysin shown in the same colouring scheme as figure 2. The gray loop shows a possible conformation of the residues 1–13 that has the N-terminal amino acid in the active site of the protease. The gray loop was interactively brought into the conformation shown by pulling the N-terminal residue along a wide curve towards the active site Zn while continuously energy minimizing the structure with the YASARA software.
The N-termini of all the aforementioned proteases have extended structures and tend to be a part of β-sheets. Mature, fully folded TLPs have an N-terminal β-hairpin that is detailed in Figures 1, 2, and 5. Figure 6 shows the result of a molecular dynamics simulation on subtilisin E, performed similar to the one described above for thermolysin. In subtilisin, only 10 N-terminal residues need to be relocated to place the N-terminal residue in front of the active site serine. Also in this case, relocation of the N-terminus takes place prior to release of the pro-part, as observed in the crystal structure of a complex of the pro-part and the mature enzyme.52
Figure 6.
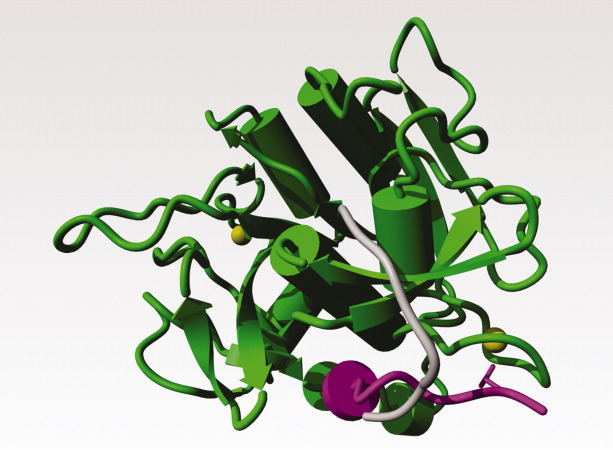
Subtilisin E from Bacillus subtilis; PDB file 1scj52. The two calcium ions are shown as yellow balls. Residues 1–10 in the mature protease are shown in magenta. Residues 1–10 after the energy minimisation-supported manual operation that brought the N-terminal residue close to the serine in the active site cleft are shown in gray. The side chain of Gln-2, which interacts strongly with one of the calcium ions, is shown as a magenta stick-model.
It seems crucial for both proteases that relocation of the intrinsically flexible N-terminus is accompanied by the establishment of new stabilizing interactions to fix the N-terminus in its correct position and to confer sufficient stability to the now fully folded and active protease. In thermolysin and in subtilisin E, calcium is crucially involved in stabilizing the N-terminal residues in the mature protease. The structural aspects like packing, location, etc. of the calciums in thermolysin and subtilisin are different, but their functional roles seem highly similar. In both cases, it has been shown that the occupancy of this N-terminal calcium-binding site has a large effect on stability.22,53
One highly successful, and at the time rather surprising stabilizing mutation in TLP-ste concerns the introduction of a phenylalanine at the surface-located position 63. This phenylalanine is naturally present in thermolysin (T50 = 86.9°C versus T50 = 73.4°C for TLP-ste) and stabilizes the N-terminal β-sheet through interactions with the aliphatic parts of the side chains of residues 9, 11, 17 and 61 (see Fig. 7).32,54 The introduction of this phenylalanine in TLP-ste adds stabilizing interactions between the three strands in the N-terminal β-sheet and causes a 7° increase in T50.
Figure 7.
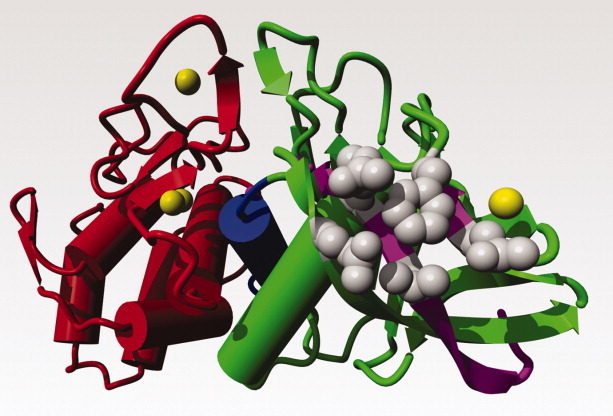
Hydrophobic packing of Phe-63 at the surface. TLP-ste ribbon coloured similar as in Figure 1. Side chains in gray. Phe-63 bridges, Gln-61 (and thus indirectly also Ca3), Val-9, Gln-17, and Arg-11 (shown clockwise around Phe-63).
The relocation of the N-termini needs to happen in an otherwise pre-formed mature enzyme. Thus, it would seem that those parts of the mature enzyme that interact with the N-terminal residues must be among the conformational least restricted and potentially least stable parts of the protein. Interestingly, mutational studies on TLP-ste have clearly shown that the N-terminal domain is considerably less stable than the C-terminal domain. Thus, the instability of the N-terminal domain, which is crucial during maturation of the enzyme, is to some extent maintained in the fully mature protein. Further details are provided in the description of Step 5.
Step 4: The protease is active
Once folded and without its pro-domain, the protease acts in the extracellular space, its primary functions being to digest food and to detoxify the environment. Broad-specificity proteases including TLP-ste are prone to cannibalistic proteolysis. This process is reduced by the presence of abundant substrate, whereas it is promoted by factors that destabilize the protein such as high temperature or the removal of calcium ions. As the secreted proteases deplete their substrates, the degree of cannibalysis will increase, meaning that the proteases will be removed when they are no longer needed. The resulting peptides may potentially be taken up by the bacterium and re-used. It is also possible that protease turnover is accomplished by other secreted broad-specificity proteases (e.g. subtilisin-like) in the environment Most importantly, as described in detail below (Step 5), the proteolytic degradation of an enzyme, such as TLP-ste, be it by cannibalistic or by the action of another type of protease, requires that the sites of proteolytic attack are sufficiently accessible. This requires some degree of local instability.
Step 5. TLP-ste degradation; N-terminal instability and unfolding
Extensive protein engineering studies of TLP-ste have shown that the N-terminal domain, and in particular the region comprising the N-terminal β-sheet and the Ca3 site are a “weak link” in mature TLP-ste.14,27,34 While mutations in the C-terminal domain generally have stability effects on the order of maximally 1° (often close to zero24,33,55), larger mutational effects were obtained when mutating in or around the proposed ‘weak link’.14,28,54–57 Although it has not been possible yet to map the first, kinetically critical cannibalistic site in TLP-ste, the studies summarized in Figure 8 show that the N-terminal region of the protein, including the N-terminal three stranded β-sheet and the Ca3 binding site, governs the partial unfolding processes that lead to cannibalistic TLP-ste inactivation. The validity of this model for local instability and unfolding was further illustrated by Vriend et al.34 who showed that mutations in the C-terminal part of TLP-ste, which had minimal effects on stability in the wild-type background, had notable effects in extremely stable engineered variants with optimized N-terminal regions. Thus, as the N-terminal ‘weak link’ becomes stabilized, other parts of the protein become relatively vulnerable and their unfolding starts contributing to the overall thermal inactivation process.
Figure 8.
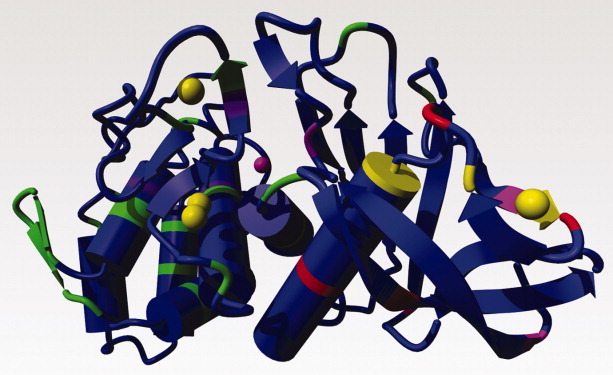
TLP-ste shown as a ribbon, coloured by mutational effect of stabilizing mutations. The relation between colour and mutational effect is: blue = not measured; green = marginal effect; purple = ΔT50 up to 2°; magenta = ΔT50 ∼ 3°; red = ΔT50 ∼ 4°; yellow = ΔT50 > 5°. Calciums shown as yellow balls; zinc as a magenta ball.
The many mutational studies on TLP-ste also showed that binding of Ca3 is merely one of many ways to stabilize the N-terminal region. Using a variety of stabilization strategies, highly stable variants of TLP-ste could be engineered.14,56 One particularly successful example was the introduction of a designed disulfide bridge (through mutations G8C & N60C) that covalently linked the N-terminal β-strand to one of the other strands in the N-terminal three-strand β-sheet.28,58 Other mutations in the region were also successful for stabilization of TLP-ste (e.g. positions 4, 56, 58, 65, and 69).14,57 Several of these stabilizing mutations were discovered when replacing residues in TLP-ste with the corresponding residue in the more stable thermolysin.14,55
In light of the life cycle of TLP-ste, the relative instability of the N-terminal region and the tendency of this region to be (partially) unfolded can be seen to permit a dual biological function: unfolding of this region is not only critical during maturation (Step 3) but also plays a critical role in cannibalistic turnover (Step 5). Residues such as those at positions 4, 56, 58, 63, and 69, whose mutation to the corresponding residue in thermolysin leads to improved TLP-ste stability, may in fact be crucial to ensure appropriate INstability of TLP-ste. Turnover requires that a certain degree of N-terminal instability is maintained at the temperatures at which the producing organism thrives. A TLP-ste variant containing the stabilizing mutations A4T, T56A, G58A, T63F, and A69P has a T50 of 93.3°C14 and will not show detectable cannibalistic turnover at e.g. the T50 of the wild-type enzyme (73.4°C).
The instability of the N-terminal domain, also in the mature protein, places the role of calcium binding to the Ca3 site in another, rather unusual light. The binding affinity of the Ca3 site and the importance of this site for local (un)folding and (in)stability seem to provide an ideal regulatory tool for TLP-ste. Intra-cellular, there is little if any calcium binding to the Ca3 site and this contributes to the protein remaining unfolded. In the calcium rich environment of the Gram-positive cell wall,44 calcium concentrations are such that all binding sites are occupied and the protease can fold to a stable, active conformation after the pro-part has been cleaved off. Outside the cell and further away from the calcium rich cell wall, the turnover process will be largely prevented as long as there is sufficient substrate present, i.e. as long as the protease is needed. The presence of substrate is likely to contribute to protease stability in two ways: cannibalism will be reduced because of substrate competition, while substrate binding additionally may stabilize the protease against unfolding, simply by the law of mass action While protein engineering has shown that stabilization of the critical N-terminal region may be obtained through a variety of mechanisms, nature has selected calcium-binding to the Ca3 site to be the primary tool. Calcium-induced stability can, of course, be regulated post-translationally, whereas stability caused by incorporation of certain amino acids cannot.
A closer look at the N-terminal part of TLP-ste
The sequence of the N-terminal beta-hairpin of TLP-ste is rich in β-branched residues that are known to favor β-strand formation. On the other hand, it is rich in glycines which are known to allow for flexibility and mobility and are not good candidates for formation of regular secondary structures. The β-turn in the middle of the β-hairpin is formed by the residues Val and Leu, relatively uncommon in β-turns. In summary the β-hairpin sequence seems to suffer from schizophrenia; half of the residues want to be in β-strands, and the other half preferably not. The sequence is shown in Figure 9.
Figure 9.

The sequence of the N-terminal β-hairpin of TLP-ste. β-branched residues are shown in red. Glycines, known to be uncommon in helices and β-strands, are shown in blue. Residues mentioned in the text are labelled with their residue number. The Val and Leu that, surprisingly, form the β-turn in this β-hairpin are labelled in brackets.
The first 10 amino acids, VAGASTVGVG, are mostly small. From the sequence, one would expect this fragment to have an extended flexible conformation, and the lack of bulky aromatic side chains suggests that this stretch will not easily be lured from its productive folding path (from the active site to its final location in the mature protease) by accidental interactions.
Figure 5 shows that the stretch of amino acids up to the β-turn made by Val-Leu at 13-14 has the appropriate length to reach the active site zinc with the N-terminal Val-1 in the S1′ pocket, permitting the pro-peptide to be cleaved off. The sequence shown in Fig. 9 shows that the N-terminal beta-strand is well suited to display the large flexibility that is needed to make the necessary conformational change happen.
Concluding Remarks
In this review, we have combined information from extensive stability engineering of TLP-ste, from the secretion and processing of broad-specificity pre-pro-proteases, and from bioinformatics to argue (1) that enzymes such as TLP-ste need to be partially unfolded during several stages of their life cycle, (2) that the N-terminal region is a logical key to this process due to its role during precursor processing, and (3) that bound calcium ions may provide an effective mechanism to regulate the necessary (in)stability of the N-terminal domain. The emerging key hypothesis is that calcium binding sites such as the Ca3 site in TLP-ste should not only be viewed as sites of stabilization but as a mechanism for regulating protein stability.
We do not wish to suggest that the mechanism for TLP-ste stability regulation suggested in this article is universal. Even within the TLP family we find TLPs that do not use this mechanism. However, the fact that there seem to be similar mechanisms in all thermostable TLPs and in subtilisins suggests that calcium ions have a broad role in folding and unfolding of these proteases. The fact that nature often uses the calcium ion concentration as a regulation or signalling mechanism strongly suggests that in many cases calcium ions are not only responsible for protein stability but can also contribute to protein instability.
Acknowledgments
Over the years, the authors have enjoyed stimulating discussion with Gerard Venema, Rob Veltman, Florence Hardy, Rob van der Zee, Johanna Mansfeld, Renate Ulbrich-Hofmann, Arno de Kreij, Bauke Dijkstra, Jan Drenth, Sigrid Gåseidnes, Bertus van den Burg, Herman Berendsen, Chris Sander, Arthur Lesk, Anna Tramontano, colleagues at the EMBL in Heidelberg, the CMBI at the Radboud University Nijmegen Medical Centre, the Groningen University, and the Norwegian University of Life Sciences at Ås, Norway. Over the years, the authors have enjoyed financial support from NBIC's BioRange and BioAssist programs, and the EU EMBRACE and BioSapiens projects.
References
- 1.Zhu XL, Ohta Y, Jordan F, Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989;339:483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]
- 2.Karlin S, Zhu ZY. Classification of mononuclear zinc metal sites in protein structures. Proc Natl Acad Sci USA. 1997;94:14231–14236. doi: 10.1073/pnas.94.26.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silen JL, Agard DA. The alpha-lytic protease pro-region does not require a physical linkage to activate the protease domain in vivo. Nature. 1989;341:462–464. doi: 10.1038/341462a0. [DOI] [PubMed] [Google Scholar]
- 4.Strausberg S, Alexander P, Wang L, Schwarz F, Bryan P. Catalysis of a protein folding reaction: thermodynamic and kinetic analysis of subtilisin BPN' interactions with its propeptide fragment. Biochemistry. 1993;32:8112–8119. doi: 10.1021/bi00083a009. [DOI] [PubMed] [Google Scholar]
- 5.Eder J, Fersht AR. Pro-sequence-assisted protein folding. Mol Microbiol. 1995;16:609–614. doi: 10.1111/j.1365-2958.1995.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 6.Marie-Claire C, Roques BP, Beaumont A. Intramolecular processing of prothermolysin. J Biol Chem. 1998;273:5697–5701. doi: 10.1074/jbc.273.10.5697. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham EL, Jaswal SS, Sohl JL, Agard DA. Kinetic stability as a mechanism for protease longevity. Proc Natl Acad Sci USA. 1999;96:11008–11014. doi: 10.1073/pnas.96.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaswal SS, Sohl JL, Davis JH, Agard DA. Energetic landscape of alpha-lytic protease optimizes longevity through kinetic stability. Nature. 2002;415:343–346. doi: 10.1038/415343a. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Saito K, Chon H, Matsumura H, Koga Y, Takano K, Kanaya S. Crystal structure of unautoprocessed precursor of subtilisin from a hyperthermophilic archaeon: evidence for Ca2+-induced folding. J Biol Chem. 2007;282:8246–8255. doi: 10.1074/jbc.M610137200. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Inouye M. The intramolecular chaperone-mediated protein folding. Curr Opin Struct Biol. 2008;18:765–770. doi: 10.1016/j.sbi.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Demidyuk IV, Gromova TY, Polyakov KM, Melik-Adamyan WR, Kuranova IP, Kostrov SV. Crystal structure of the protealysin precursor: insights into propeptide function. J Biol Chem. 2010;285:2003–2013. doi: 10.1074/jbc.M109.015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Wang J, Yu DQ, Bian F, Xie BB, Chen XL, Zhou BC, Lai LH, Wang ZX, Wu JW, Zhang YZ. Structural basis for the autoprocessing of zinc metalloproteases in the thermolysin family. Proc Natl Acad Sci USA. 2010;107:17569–17574. doi: 10.1073/pnas.1005681107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews BW, Jansonius JN, Colman PM, Schoenborn BP, Dupourque D. Three-dimensional structure of thermolysin. Nature New Biol. 1972;238:37–41. doi: 10.1038/newbio238037a0. [DOI] [PubMed] [Google Scholar]
- 14.Eijsink VGH, Veltman OR, Aukema W, Vriend G, Venema G. Structural determinants of the stability of thermolysin-like proteinases. Nat Struct Biol. 1995;2:374–379. doi: 10.1038/nsb0595-374. [DOI] [PubMed] [Google Scholar]
- 15.Matthews BW, Weaver LH, Kester WR. The conformation of thermolysin. J Biol Chem. 1974;249:8030–8044. [PubMed] [Google Scholar]
- 16.Van den Burg B, Eijsink VGH. Selection of mutations for increased protein stability. Curr Opin Biotechnol. 2002;13:333–337. doi: 10.1016/s0958-1669(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 17.Eijsink VGH, Bjørk A, Gåseidnes S, Sirevåg R, Synstad B, Van den Burg B, Vriend G. Rational engineering of enzyme stability. J Biotechnol. 2004;113:105–120. doi: 10.1016/j.jbiotec.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Eijsink VGH, Gåseidnes S, Borchert TV, Van den Burg B. Directed evolution of enzyme stability. Biomol Eng. 2005;22:21–30. doi: 10.1016/j.bioeng.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Dahlquist FW, Long JW, Bigbee WL. Role of calcium in the thermal stability of thermolysin. Biochemistry. 1976;15:1103–1111. doi: 10.1021/bi00650a024. [DOI] [PubMed] [Google Scholar]
- 20.Roche RS, Voordouw G. The structural and functional roles of metal ions in thermolysin. CRC Crit Rev Biochem. 1978;5:1–23. doi: 10.3109/10409237809177138. [DOI] [PubMed] [Google Scholar]
- 21.Veltman OR, Vriend G, Van den Burg B, Hardy F, Venema G, Eijsink VGH. Engineering thermolysin-like proteases whose stability is largely independent of calcium. FEBS Lett. 1997;405:241–244. doi: 10.1016/s0014-5793(97)00193-2. [DOI] [PubMed] [Google Scholar]
- 22.Veltman OR, Vriend G, Berendsen HJ, Van den Burg B, Venema G, Eijsink VGH. A single calcium binding site is crucial for the calcium-dependent thermal stability of thermolysin-like proteases. Biochemistry. 1998;37:5312–5319. doi: 10.1021/bi9725879. [DOI] [PubMed] [Google Scholar]
- 23.Holland DR, Tronrud DE, Pley HW, Flaherty KM, Stark W, Jansonius JN, McKay DB, Matthews BW. Structural comparison suggests that thermolysin and related neutral proteases undergo hinge-bending motion during catalysis. Biochemistry. 1992;31:11310–11316. doi: 10.1021/bi00161a008. [DOI] [PubMed] [Google Scholar]
- 24.Veltman OR, Eijsink VGH, Vriend G, de Kreij A, Venema G, Van den Burg B. Probing catalytic hinge bending motions in thermolysin-like proteases by glycine --> alanine mutations. Biochemistry. 1998;37:5305–5311. doi: 10.1021/bi972374j. [DOI] [PubMed] [Google Scholar]
- 25.Conejero-Lara F, De Filippis V, Fontana A, Mateo PL. The thermodynamics of the unfolding of an isolated protein subdomain. The 255-316 C-terminal fragment of thermolysin. FEBS Lett. 1994;344:154–156. doi: 10.1016/0014-5793(94)00358-0. [DOI] [PubMed] [Google Scholar]
- 26.De Kreij A, Venema G, Van den Burg B. Substrate specificity in the highly heterogeneous M4 peptidase family is determined by a small subset of amino acids. J Biol Chem. 2000;275:31115–31120. doi: 10.1074/jbc.M003889200. [DOI] [PubMed] [Google Scholar]
- 27.Vriend G, Eijsink VGH. Prediction and analysis of structure, stability and unfolding of thermolysin-like proteases. J Comput Aided Mol Des. 1993;7:367–396. doi: 10.1007/BF02337558. [DOI] [PubMed] [Google Scholar]
- 28.Mansfeld J, Vriend G, Dijkstra BW, Veltman OR, Van den Burg B, Venema G, Ulbrich-Hofmann R, Eijsink VGH. Extreme stabilization of a thermolysin-like protease by an engineered disulfide bond. J Biol Chem. 1997;272:11152–11156. doi: 10.1074/jbc.272.17.11152. [DOI] [PubMed] [Google Scholar]
- 29.Eijsink VGH, Vriend G, Van den Burg B, Van der Zee JR, Venema G. Increasing the thermostability of a neutral protease by replacing positively charged amino acids in the N-terminal turn of alpha-helices. Protein Eng. 1992;5:165–170. doi: 10.1093/protein/5.2.165. [DOI] [PubMed] [Google Scholar]
- 30.Frigerio F, Margarit I, Nogarotto R, de Filippis V, Grandi G. Cumulative stabilizing effects of hydrophobic interactions on the surface of the neutral protease from Bacillus subtilis. Protein Eng. 1996;9:439–445. doi: 10.1093/protein/9.5.439. [DOI] [PubMed] [Google Scholar]
- 31.Eijsink VGH, Vriend G, Van Den Burg B, Venema G, Stulp BK. Contribution of the C-terminal amino acid to the stability of Bacillus subtilis neutral protease. Protein Eng. 1990;4:99–104. doi: 10.1093/protein/4.1.99. [DOI] [PubMed] [Google Scholar]
- 32.Van den Burg B, Enequist HG, Van der Haar ME, Eijsink VGH, Stulp BK, Venema G. A highly thermostable neutral protease from Bacillus caldolyticus: cloning and expression of the gene in Bacillus subtilis and characterization of the gene product. J Bacteriol. 1991;173:4107–4115. doi: 10.1128/jb.173.13.4107-4115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eijsink VGH, Dijkstra BW, Vriend G, van der Zee JR, Veltman OR, Van der Vinne B, Van den Burg B, Kempe S, Venema G. The effect of cavity-filling mutations on the thermostability of Bacillus stearothermophilus neutral protease. Protein Eng. 1992;5:421–426. doi: 10.1093/protein/5.5.421. [DOI] [PubMed] [Google Scholar]
- 34.Vriend G, Berendsen HJ, Van den Burg B, Venema G, Eijsink VGH. Early steps in the unfolding of thermolysin-like proteases. J Biol Chem. 1998;273:35074–35077. doi: 10.1074/jbc.273.52.35074. [DOI] [PubMed] [Google Scholar]
- 35.Weaver LH, Kester WR, Ten Eyck LF, Matthews BW. Zuber H. Symposium on Enzymes and Proteins from Thermophilic Microorganisms, Zurich. 1976. The structure and stability of thermolysin; pp. 31–39. Experientia Supplement 36. [DOI] [PubMed] [Google Scholar]
- 36.Herbaud M, Guiseppi A, Denizot F, Haiech J, Kilhoffer M. Calcium signalling in Bacillus subtilis. Biochim Biophys Acta. 1998;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 37.Lodish HF. Molecular cell biology. New York: Scientific American Books; 1999. [Google Scholar]
- 38.Clapham DE. Calcium signalling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol. 2007;5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 40.Tjalsma H, Antelmann H, Jongbloed JDH, Braun PG, Darmon E, Dorenbos R, Dubois J-YF, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, Van Dijl JM. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol Mol Biol Rev. 2004;68:207–233. doi: 10.1128/MMBR.68.2.207-233.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harwood CR, Cranenburgh R. Bacillus protein secretion: an unfolding story. Trends Microbiol. 2008;16:73–79. doi: 10.1016/j.tim.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Baker D, Silen JL, Agard DA. Protease pro region required for folding is a potent inhibitor of the mature enzyme. Proteins. 1992;12:339–344. doi: 10.1002/prot.340120406. [DOI] [PubMed] [Google Scholar]
- 43.O'Donohue MJ, Beaumont A. The roles of the prosequence of thermolysin in enzyme inhibition and folding in vitro. J Biol Chem. 1996;271:26477–26481. doi: 10.1074/jbc.271.43.26477. [DOI] [PubMed] [Google Scholar]
- 44.Petit-Glatron MF, Grajcar L, Munz A, Chambert R. The contribution of the cell wall to a transmembrane calcium gradient could play a key role in Bacillus subtilis protein secretion. Mol Microbiol. 1993;9:1097–1106. doi: 10.1111/j.1365-2958.1993.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 45.Beveridge TJ, Murray RG. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141:876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yabuta Y, Takagi H, Inouye M, Shinde U. Folding pathway mediated by an intramolecular chaperone: propeptide release modulates activation precision of pro-subtilisin. J Biol Chem. 2001;276:44427–44434. doi: 10.1074/jbc.M107573200. [DOI] [PubMed] [Google Scholar]
- 47.Power SD, Adams RM, Wells JA. Secretion and autoproteolytic maturation of subtilisin. Proc Natl Acad Sci USA. 1986;83:3096–3100. doi: 10.1073/pnas.83.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson DE, Peters RJ, Wilk B, Agard DA. Alpha-lytic protease precursor: characterization of a structured folding intermediate. Biochemistry. 1999;38:4728–4735. doi: 10.1021/bi982165e. [DOI] [PubMed] [Google Scholar]
- 49.Falzon L, Patel S, Chen Y, Inouye M. Autotomic behavior of the propeptide in propeptide-mediated folding of prosubtilisin E. J Mol Biol. 2007;366:494–503. doi: 10.1016/j.jmb.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Sauter NK, Mau T, Rader SD, Agard DA. Structure of alpha-lytic protease complexed with its pro region. Nat Struct Biol. 1998;5:945–950. doi: 10.1038/2919. [DOI] [PubMed] [Google Scholar]
- 51.Yabuta Y, Subbian E, Takagi H, Shinde U, Inouye M. Folding pathway mediated by an intramolecular chaperone: dissecting conformational changes coincident with autoprocessing and the role of Ca(2+) in subtilisin maturation. J Biochem. 2002;131:31–37. doi: 10.1093/oxfordjournals.jbchem.a003074. [DOI] [PubMed] [Google Scholar]
- 52.Jain SC, Shinde U, Li Y, Inouye M, Berman HM. The crystal structure of an autoprocessed Ser221Cys-subtilisin E-propeptide complex at 2.0 A resolution. J Mol Biol. 1998;284:137–144. doi: 10.1006/jmbi.1998.2161. [DOI] [PubMed] [Google Scholar]
- 53.Alexander PA, Ruan B, Bryan PN. Cation-dependent stability of subtilisin. Biochemistry. 2001;40:10634–10639. doi: 10.1021/bi010797m. [DOI] [PubMed] [Google Scholar]
- 54.Van den Burg B, Dijkstra BW, Vriend G, Van der Vinne B, Venema G, Eijsink VGH. Protein stabilization by hydrophobic interactions at the surface. Eur J Biochem. 1994;220:981–985. doi: 10.1111/j.1432-1033.1994.tb18702.x. [DOI] [PubMed] [Google Scholar]
- 55.Veltman OR, Vriend G, Middelhoven PJ, Van den Burg B, Venema G, Eijsink VGH. Analysis of structural determinants of the stability of thermolysin-like proteases by molecular modelling and site-directed mutagenesis. Protein Eng. 1996;9:1181–1189. doi: 10.1093/protein/9.12.1181. [DOI] [PubMed] [Google Scholar]
- 56.Van den Burg B, Vriend G, Veltman OR, Venema G, Eijsink VGH. Engineering an enzyme to resist boiling. Proc Natl Acad Sci USA. 1998;95:2056–2060. doi: 10.1073/pnas.95.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veltman OR, Vriend G, Hardy F, Mansfeld J, van den Burg B, Venema G, Eijsink VGH. Mutational analysis of a surface area that is critical for the thermal stability of thermolysin-like proteases. Eur J Biochem. 1997;248:433–440. doi: 10.1111/j.1432-1033.1997.00433.x. [DOI] [PubMed] [Google Scholar]
- 58.Dürrschmidt P, Mansfeld J, Ulbrich-Hofmann R. An engineered disulfide bridge mimics the effect of calcium to protect neutral protease against local unfolding. FEBS J. 2005;272:1523–1534. doi: 10.1111/j.1742-4658.2005.04593.x. [DOI] [PubMed] [Google Scholar]


