Abstract
Left ventricular mass (LVM) is a highly heritable trait1 and an independent risk factor for all-cause mortality2. To date, genome-wide association studies (GWASs) have not identified the genetic factors underlying LVM variation3 and the regulatory mechanisms for blood pressure (BP)-independent cardiac hypertrophy remain poorly understood4,5. Unbiased systems-genetics approaches in the rat6,7 now provide a powerful complementary tool to GWAS and we applied integrative genomics to dissect a highly replicated, BP-independent LVM locus on rat chromosome 3p. We identified endonuclease G (Endog), previously implicated in apoptosis8 but not hypertrophy, as the gene at the locus and demonstrated loss-of-function mutation in Endog associated with increased LVM and impaired cardiac function. Inhibition of Endog in cultured cardiomyocytes resulted in an increase in cell size and hypertrophic biomarkers in the absence of pro-hypertrophic stimulation. Genome-wide network analysis unexpectedly inferred ENDOG in fundamental mitochondrial processes unrelated to apoptosis. We showed direct regulation of ENDOG by ERRα and PGC1α, master regulators of mitochondrial and cardiac function9,10,11, interaction of ENDOG with the mitochondrial genome and ENDOG-mediated regulation of mitochondrial mass. At baseline, Endog deleted mouse heart had depleted mitochondria, mitochondrial dysfunction and elevated reactive oxygen species (ROS), which was associated with enlarged and steatotic cardiomyocytes. Our studies establish further the link between mitochondrial dysfunction, ROS and heart disease and demonstrate a new role for Endog in maladaptive cardiac hypertrophy.
Elevated left ventricular mass (LVM) is a clinically important trait that independently predicts the risk of heart failure, sudden death and all-cause mortality2. Although LVM is a heritable complex trait1, large genome wide association studies (GWASs) have not identified new LVM genes3. Blood pressure (BP)-dependent regulation of LVM, which is perhaps surprisingly limited7, has been studied extensively in model systems and acts through well-characterised and overlapping signalling modules12. In contrast, the pathways underlying BP-independent cardiac hypertrophy, commonly seen in obesity and type 2 diabetes and related to mitochondrial dysfunction and liptoxicity4,5, remain largely unknown. Here, we took advantage of the recent step-changes in integrative systems-genetics approaches in the rat6,7 to dissect a BP-independent cardiac mass quantitative trait locus (QTL) and identified the causative gene and underlying mechanism.
The rat is unique for the study of cardiac mass with over 75 QTLs identified for this trait (rat genome database; http://rgd.mcw.edu/). Rat chromosome 3p (0-25Mbp) contains a highly replicated and BP-independent QTL for cardiac mass, which was mapped in crosses of the Spontaneously Hypertensive Rat (SHR) or SHR Stoke Prone (SHRSP) to Wistar Kyoto (WKY) or Salt Sensitive (SS)13,14. To dissect genetically this locus, we generated an F2 intercross from SHR and Brown Norway (BN) strains and replicated further the LVM QTL (LOD=4.2) (Fig. 1a). We confirmed the BP-independent QTL effect in a congenic strain (SHR.BN-(3L)) that had lower LVM and smaller cardiomyocytes than the SHR (Fig. 1b,c) and refined the QTL region (6.4Mbp-11.2Mpb) using a second congenic strain (SHR.BN-(3S)) (Supplementary Fig. 1). In the F2 cross, in the SHR.BN-(3L) strain and in previous experimental crosses13,14, the SHR allele at the locus was associated with increased cardiac mass and this effect was BP-independent (Fig. 1a and d). Functional assessment in vivo revealed that the SHR.BN-(3L) strain had better cardiac performance at baseline and following stimulation, as compared to the SHR (Supplementary Fig. 1). These data demonstrate that an SHR allele at the cardiac mass QTL on rat chromosome 3p increases LVM and adversely affects cardiac function.
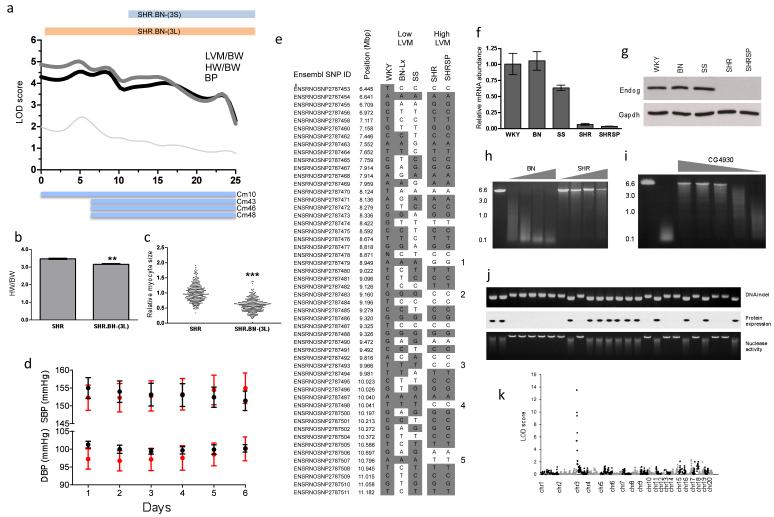
Figure 1. Positional cloning of Endog as the gene underlying the rat chromosome 3p cardiac mass quantitative trait locus (QTL).
a, Mapping of heart weight (HW) and left ventricular mass (LVM) corrected for body weight (BW) to chromosome 3p in the Brown Norway (BN) x Spontaneously Hypertensive (SHR) F2 population. The telomeric limits of the congenic strains (SHR.BN-(3L) and SHR.BN-(3S)) and the previously mapped cardiac mass (CM) QTLs13,14 are shown; x-axis, physical position in Mbp. b, HW indexed to BW in the SHR (n=4) and the SHR.BN-(3L) congenic strains (n=5). c, Relative cardiomyocyte cross-sectional area in SHR and SHR.BN-(3L) congenic strains. d, In vivo telemetric systolic- and diastolic-blood pressure (SBP and DBP) measurements in the SHR (red circles) and SHR.BN-(3L) (black circles) (n=8 per genotype). e, Haplotype analysis of the refined QTL region. SNPs are depicted with reference to WKY/NCrl alleles (grey, identical; white, dissimilar) with numbers (1-5) denoting the polymorphic regions between strains with either high or low HW. QPCR of Endog mRNA expression (f) and immunoblot of Endog protein expression (g) in strains with low or high CM at the chromosome 3p locus. h, Nuclease activity in BN and SHR heart extracts over a range of cardiac protein extract amounts (grey wedge) (Supplementary Methods). i, Reversal of nuclease activity in cardiac lysates by a drosophila-derived inhibitor of Endog18 (range 1500nM-1.5nM, grey wedge). j, Association of the Endog indel with loss of Endog protein expression and diminished nuclease activity in the recombinant inbred (RI) strains. Upper, middle and lower panels display the DNA indel, protein expression and nuclease activity, respectively. k, Linkage mapping of nuclease activity in RI strains using a quantitative fluorescence-based assay (Supplementary Methods). All data are represented as mean+s.e.m. *, P<0.05, **, P<0.01, ***, P<0.001.
We used the new genotypes generated in our F2 cross and those from previous experiments13,14 to refine the QTL region, and identified five distinct loci (spanning 750kbp in total) that co-segregated with the haplotypes associated with LVM variation (Fig. 1e). Endonuclease G (Endog), which we had previously shown to be cis-regulated in the heart (P=3×10−6)7, was the only gene at the loci to be differentially regulated with consistent direction of effect in the SHR and SHRSP heart as compared to the WKY heart (Supplementary Table 1). Endog is a nuclear-encoded, mitochondrial-localised nuclease with a proposed but disputed function in apoptosis8,15,16,17 and no known effect on cardiac mass or function. We observed reduced expression of Endog transcript and lack of Endog protein in all strains with elevated cardiac mass (Fig. 1f and g). Sequencing of Endog revealed promoter and coding sequence variation and we identified an SHR-specific, frameshift-causing insertion in Endog exon one that was associated with increased heart weight and LVM (Supplementary Fig. 2). There was marked reduction in cardiac nuclease activity, which was Endog-dependent18, in SHR heart as compared to BN heart (Fig. 1h and i). In recombinant inbred strains derived from the SHR and BN6,7 we confirmed the direct relationship between the SHR insertion and the lack of nuclease activity (Fig. 1j) and mapped Endog-dependent nuclease activity to a single locus that encodes Endog (Fig. 1k) . These data identify Endog as the candidate gene at the QTL and infer Endog loss-of-function as the mechanism for increased cardiac mass and impaired heart function.
We performed immunoblotting across rat and mouse tissues and determined that Endog was most highly expressed in the heart, localised to cardiomyocytes (Fig. 2) and co-localised with mitochondria (Supplementary Fig. 3). Using short hairpin RNA (shRNA) knockdown of Endog (shEndog)19 we tested the effect of Endog loss-of-function in cardiomyocytes and observed an increase in hypertrophic biomarkers and cell size in the absence of pro-hypertrophic stimulation (Fig. 2). Conventional BP-dependent hypertrophic signalling pathways12 were not activated in shEndog treated cells but we established activation of AMPK (Supplementary Fig. 4), which can induce cardiac hypertrophy20. We also observed increased reactive oxygen species (ROS), an additional pro-hypertrophic stimulus21 that acts through multiple downstream effectors (Supplementary Fig. 4). These data show that Endog loss-of-function directly induces cardiac myocyte hypertrophy in vitro that is associated with the activation of two pro-hypertrophic pathways both of which have previously been linked with mitochondrial dysfunction20,21,22.
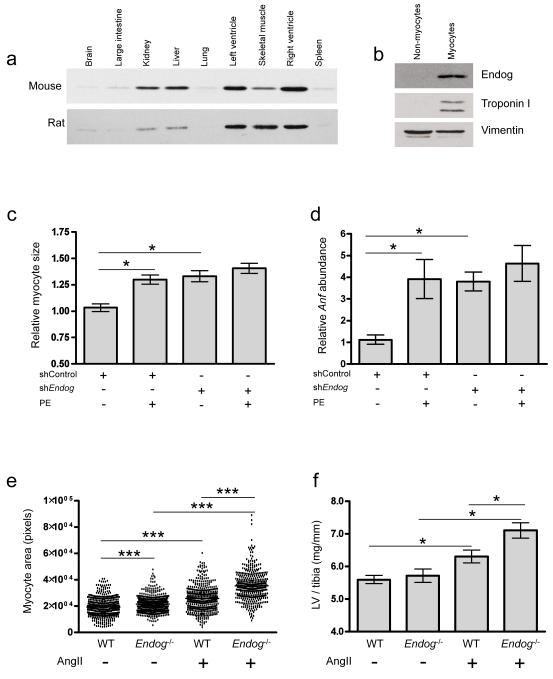
Figure 2. Endog regulates cardiac hypertrophy.
a, Immunoblot of Endog expression in mouse and rat tissues (Endog: ~30 kDa). b, Immunoblot of Endog expression in myocyte and non-myocyte populations isolated from neonatal rat heart. c, Cardiomyocyte size (n≥100 cells, n=3 independent experiments) treated with shRNA against Endog (shEndog) or control shRNA (shControl) in the presence or absence of the hypertrophic stimulant phenylephrine (PE, 100μM, 24 h). d, Expression of the hypertrophic biomarker Anf in shEndog and shControl treated cells. e, Cardiomyocyte size (Supplementary Fig. 5) in Endog-/- and wildtype (WT) mice at baseline and following angiotensin II-induced cardiac hypertrophy. f, LVM/tibial length in Endog-/- and WT mice at baseline and following AngII stimulation. Data are represented as mean+s.e.m. *, P<0.05, **, P<0.01, ***, P<0.001.
We then examined the effects of Endog loss-of-function in vivo in the Endog deleted mouse (Endog-/-)17 that exhibits no detectable variation in apoptotic phenotypes, an observation that was confirmed in an independent Endog deleted strain16. As compared to controls, Endog-/- mice had larger cardiomyocytes at baseline (Fig. 2) in the absence of stimulation, in keeping with our observations in the SHR.BN-(3L) rat (Fig. 1) and in vitro (Fig. 2). Following angiotensin II stimulation of hypertrophy, which is largely ROS-dependent21, we observed an increase in cardiomyocyte size, hypertrophic biomarkers and LVM in Endog-/- mice (Fig. 2 and Supplementary Fig 5). Endog-/- mice had BPs equivalent to control mice at baseline (P=0.49) and following angiotensin II stimulation (P=0.51). Our combined in vitro and in vivo data confirm a role for Endog in cardiomyocyte hypertrophy and identify ROS as a conserved pro-hypertrophic stimulus in both systems.
Endog was proposed8 but subsequently disputed16,17 as important for apoptotic cell death and it was unclear how Endog loss-of-function was associated with cardiac hypertrophy and dysfunction. To infer ENDOG function in the human heart, we carried out genome-wide coexpression network analysis23 in a large human cardiac expression dataset (n=210) (Supplementary Methods). ENDOG was identified in a network that was highly enriched for mitochondrial genes (P=1.8×10−58) and oxidative metabolism processes (P=4.7×10−38) (Fig. 3) (Supplementary Tables 2 and 3). Taken together, the high levels of Endog expression in metabolically active organs (Fig. 2) and in brown fat (Supplementary Fig. 6), the unique co-expression of ENDOG with oxidative metabolism genes and the link with AMPK signalling and ROS production pointed to an unappreciated effect of Endog in physiological mitochondrial processes.
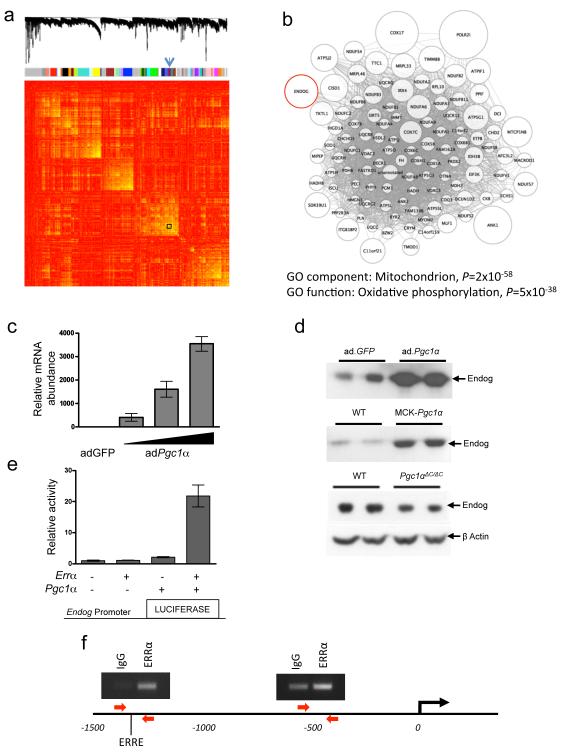
Figure 3. ENDOG is co-expressed with a mitochondrial-specific gene network and regulated by Pgc1α and ERRα.
a, Genes (8,490 from 210 datasets) are clustered and plotted based on the dissimilarity metric between their expression profiles (Supplementary Methods). From top to bottom: low-hanging branches in the dendrogram represent groups of genes (modules) that have a high similarity metric. Modules are shown beneath the dendrogram and are colour coded. The arrow indicates the module (also boxed) containing ENDOG. In the heat-map of the correlations between expression profiles, high and low similarities are coloured yellow and red, respectively. b, Weighted gene co-expression network analysis (WGCNA23) for the module containing ENDOG, providing functional annotation by cellular localization by Gene Ontology classification (Supplementary Tables 2 and 3). Nodes represent genes and edges represent significant co-expression between genes. The node size is proportional to the relative degree of interconnectivity of each gene within the module. c, QPCR analysis of Endog expression in cultured cardiomyocytes following infection with adenovirus (ad) expressing GFP (ad.GFP) or Pgc1α (ad.Pgc1α). d, Immunoblot of Endog expression in ad.Pgc1α-infected cardiomyocytes (top panel), skeletal muscle of wild-type (WT) mice and transgenic mice expressing Pgc1α under the control of muscle creatine kinase (MCK-Pgc1α) (middle panel), and in hearts of WT and cardiac-specific Pgc1α deleted mice (Pgc1αΔC/ΔC) (bottom panel). e, Endog promoter activity in HEK293 cells infected with ad.Pgc1α and and/or ad.Errα. f, ERRα chromatin immunoprecipitation (ChIP) and PCR of two regions of the ENDOG promoter. Red arrows denote primers and ERRE specifies the location of a consensus ERR response element (1304 bases upstream). The experiment was repeated three times with similar results and PCR products quantified by QPCR.
Peroxisome proliferator activated receptor gamma coactivator 1 alpha (Pgc1α) is widely recognised as a master regulator of mitochondrial function24, and activates many target genes component of the ENDOG-associated network (Fig. 3) through interaction with estrogen-related receptor alpha (Errα)9. Therefore, we tested whether Pgc1α also regulated Endog and observed robust Pgc1α-induced Endog transcript and Endog protein expression in cardiomyocytes in vitro (Fig. 3). We confirmed the effects of Pgc1α variation on Endog protein expression in vivo using mice over-expressing Pgc1α under the control of muscle creatine kinase (MCK-Pgc1α) and in cardiac-specific Pgc1α deleted mice (Pgc1αΔC/ΔC) (Fig. 3, Supplementary Methods). Luciferase studies revealed strong activation of the Endog promoter by Pgc1α and Errα together (Fig. 3e) and we confirmed direct binding of ESRRα to the ENDOG promoter by chromatin immuno-precipitation and PCR (ChIP-PCR) in a region containing an ERRα response element (P<0.001) (Fig. 3f). These data show that Endog is a direct target of ESRRα and Pgc1α, master regulators of mitochondrial and heart function, further inferring a role for Endog in mitochondrial and cardiac biology.
It was apparent that the effects of Endog loss-of-function on cardiac hypertrophy might be mediated through perturbation of mitochondrial physiology, which we examined. Electron microscopy revealed no gross morphological changes of mitochondria but we observed lipid-like droplets associated with mitochondria from Endog-/- mice that were more numerous and larger than those seen in control mice. Molecular studies revealed marked elevation of triglyceride levels in the Endog-/- mouse heart that was manifest as cardiomyocyte steatosis (Fig. 4 and Supplementary Fig. 7) but not associated with variation in expression levels of fatty acid metabolism or mitochondrial biogenesis genes (Supplementary Fig. 8 and 9). As compared to wildtype littermates, Endog-/- mice had impaired mitochondrial respiration and increased ROS production (Fig. 4).
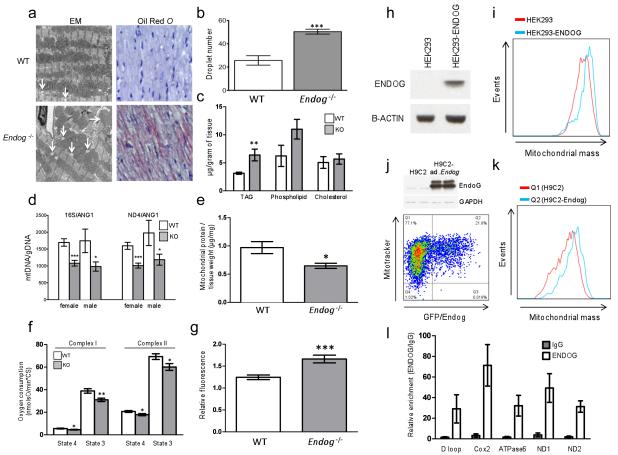
Figure 4. Endog regulates mitochondrial function and cardiac lipid metabolism.
a, Transmission electron micrographs and oil red O stained micrographs (high resolution, Supplementary Fig. 7) of left ventricular sections from WT and Endog-/- mice. b, Quantification of the number of mitochondrial-associated droplets in WT and Endog-/- mice. c, Quantification of cardiac triglyceride (TAG), phospholipid and cholesterol content in WT and Endog-/- mice (n=5). d, Ratio of mitochondrial DNA (mtDNA) to genomic DNA (gDNA) in hearts of WT and Endog-/- mice. e, Quantification of mitochondrial protein content in WT and Endog-/- mice (n=5). f, State 3 and state 4 oxygen consumption in the presence of complex I or complex II substrates in isolated cardiac mitochondria from WT (n=6) and Endog-/- (n=5) mice. g, Relative fluorescence-based measurement of ROS production by mitochondria isolated from WT (n=6) and Endog-/- (n=5) mice. h-k, Representative flow cytometry analysis of mitochondrial mass in HEK293 and H9C2 cells over-expressing ENDOG or Endog, respectively (n=4). h, Stable expression of ENDOG in HEK293 cells (HEK293-ENDOG). i, Flow cytometry analysis of HEK293 and HEK293-ENDOG cells stained with mitotracker. j, Adenovirus (ad)-mediated expression of GFP and Endog in myocytes and flow cytometry analysis of ad.Endog infected cells (Q2) and uninfected control cells (Q1). k, Number of events plotted against mitochondrial mass in ad.Endog infected (Q2) and control (Q1) H9C2 cells. l, QPCR of mtDNA-protein complexes following ChIP of mitochondrial chromatin using anti-ENDOG antibody or IgG. All data are represented as mean+s.e.m. *, P<0.05, **, P<0.01, ***, P<0.001.
To assess for mitochondrial depletion we examined mitochondrial DNA (mtDNA)/genomic DNA and mitochondrial protein/tissue weight ratios, which were both diminished in the Endog-/- mouse heart (Fig. 4) in the absence of mtDNA structural variation (Supplementary Fig. 10). This was an intriguing finding given the previously proposed roles for Endog in mtDNA synthesis, processing of poly-cistronic mtRNA and mitochondrial biogenesis25,26 that were subsequently discarded based primarily on experiments in Endog deleted mice16,17. We re-examined a role of ENDOG in mitochondrial biogenesis and demonstrated an increase in mitochondrial mass with chronic ENDOG expression in HEK cells (P<0.01)and acute Endog over-expression in a cardiomyocyte-derived cell line (P<0.001) (Fig. 4k-k in the absence of an effect on apoptotic or necrotic cell death (Supplementary Fig. 11). A role for ENDOG in mtDNA biology25,26 was supported further by ChIP-PCR experiments that showed direct binding of ENDOG throughout the mtDNA molecule (Fig. 4n) as previously demonstrated for mitochondrial transcription factor A (TFAM)27, which is a critical determinant of mtDNA synthesis and repair that when deleted causes eccentric cardiac hypertrophy and heart failure28.
Mitochondria are essential for oxidative metabolism and mitochondrial dysfunction/depletion in the heart causes maladaptive cardiac hypertrophy and cardiac dysfunction associated with increased ROS and lipotoxicity4,5,28,29. Here we identified Endog loss-of-function as a primary determinant of maladaptive cardiac hypertrophy that was associated with mitochondrial dysfunction/depletion and marked cardiomyocyte steatosis. Although the mechanism underlying cardiac hypertrophy due to impaired mitochondrial function is not limited to a single pathway we demonstrated a conserved increase in ROS, an established hypertrophic stimulus21,22, in Endog loss-of-function models. Our studies resolve some of the uncertainty as to the non-apoptotic function of Endog15,16,17 and reveal it’s importance in mitochondrial biology, which has intriguing parallels with the dual roles of apoptosis-inducing-factor30. We propose that ENDOG, which we show binds to mtDNA, modulates mtDNA synthesis, maintenance and/or transcription, in keeping with previous hypotheses25,26. Therapeutic targeting the Pgc1α/Errα axis has been proposed to improve mitochondrial function in cardiac failure11 and our studies suggest that regulation of Endog is an important component of this process. We conclude that Endog is a novel determinant of maladaptive cardiac hypertrophy with previously unappreciated mitochondrial functions.
Methods Summary
Linkage mapping was carried out using microsatellite genotypes in the BNxSHR F2 population. Ex vivo heart weight analysis was performed in the congenic strains, which were characterised using in vivo BP telemetry. Comparative haplotype analysis was performed using SNP data (Rat Genome Database; http://rgd.mcw.edu/) for all strains used in the QTL mapping studies. Microarray-based expression analysis was conducted as previously described6,7. Cell size and hypertrophy biomarker expression were measured in cardiomyocytes following lentivirus-mediated Endog knockdown. Heart weight, hypertrophic biomarker expression and cardiomyocyte cell size were measured in Endog-/- mice at baseline and following angiotensin II-induced hypertrophy. Triglyceride abundance, mitochondrial mass and respiratory activity were measured in Endog-/- mice as described in the Supplementary material. Weighted gene co-expression network analysis (WGCNA)23 was applied to the largest publicly available human heart transcriptome dataset. Regulation of Endog by Pgc1α was investigated in Ad.Pgc1α-infected neonatal cardiomyocytes, MCK-Pgc1 skeletal muscle and Pgc1 αΔC/ΔC heart samples. ERRα association with the ENDOG promoter and ENDOG-mtDNA interaction were determined using ChIP. Histological analysis and electron microscopy of Endog-/- hearts was carried out to study mitochondrial structure and abundance as well as lipid deposition. MtDNA and gDNA copy number were assessed by QPCR. Mitochondrial abundance was studied in cells by flow cytometry. Full methods are provided in Supplementary Methods.
Supplementary Material
Acknowledgements
We acknowledge funding from the Medical Research Council (MRC) UK, the National Institute for Health Research (NIHR) UK, the Royal Brompton and Harefield Cardiovascular Biomedical Research Unit, the Imperial College Healthcare Biomedical Research Centre, the British Heart Foundation, the Fondation Leducq, the Wellcome Trust, 301/08/0166 from the Grant Agency of the Czech Republic and 1M0520 from the Ministry of Education of the Czech Republic, PTQ-08-03-07880, SAF2008-02271, SAF2008-03067 and SAF2010-19125 from the Ministerio de Ciencia e Innovacion (MICINN, Spain), 2009-SGR-346 from the Agència de Gestió d’Ajuts Universitaris i Recerca (AGAUR, Spain), PS09/02034, PS09/01602 and PS09/01591 from Fondo de Investigaciones (FIS, Spain). The European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. HEALTH-F4-2010-241504 (EURATRANS). The German National Genome Research Network (NGFN-Plus) Heart Failure. We thank Dr Michael R. Lieber (University of Southern California) for providing the Endog deleted mice and Prof E. Wahle (Institute of Biochemistry and Biotechnology, Halle) for providing the CG4930 expression plasmid. We thank the National BioResource Project-Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing rat strains.
Footnotes
Author contributions C.M-R, J.Y., X-M.S., A.S., J.Z., A.B., R.B., D.H., H.L., G.C.R., R.M. and E.G-A. performed the lab-based experiments. R.A, P.M., M.M., V.Z., F.P, M.C., M.R-M. and F.K. performed the physiology experiments. N.H., H.J., L.E.F., P.J.R.B. and T.S., provided gene expression and physiology data. J.W, L.B. and E.P. performed genetic mapping and network studies. X.C., J.X.C., Z.A., M.P. and D.C-D. supervised data analysis and contributed to the experimental design. S.A.C and D.S. planned the experiments. S.A.C. wrote the manuscript with input and discussion from all co-authors.
References
- 1.Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: the Framingham Heart Study. Hypertension. 1997;30:1025–1028. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- 2.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA. 2009;302:168–178. doi: 10.1001/jama.2009.978-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 5.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–443. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- 6.Heinig M, et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petretto E, et al. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat Genet. 2008;40:546–552. doi: 10.1038/ng.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 9.Dufour CR, et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 11.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 13.Inomata H, et al. Identification of quantitative trait loci for cardiac hypertrophy in two different strains of the spontaneously hypertensive rat. Hypertens Res. 2005;28:273–281. doi: 10.1291/hypres.28.273. [DOI] [PubMed] [Google Scholar]
- 14.Siegel AK, et al. Genetic loci contribute to the progression of vascular and cardiac hypertrophy in salt-sensitive spontaneous hypertension. Arterioscler Thromb Vasc Biol. 2003;23:1211–1217. doi: 10.1161/01.ATV.0000079509.20542.C9. [DOI] [PubMed] [Google Scholar]
- 15.Buttner S, et al. Endonuclease G regulates budding yeast life and death. Mol Cell. 2007;25:233–246. doi: 10.1016/j.molcel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 16.David KK, Sasaki M, Yu SW, Dawson TM, Dawson VL. EndoG is dispensable in embryogenesis and apoptosis. Cell Death Differ. 2006;13:1147–1155. doi: 10.1038/sj.cdd.4401787. [DOI] [PubMed] [Google Scholar]
- 17.Irvine RA, et al. Generation and characterization of endonuclease G null mice. Mol Cell Biol. 2005;25:294–302. doi: 10.1128/MCB.25.1.294-302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temme C, et al. The Drosophila melanogaster Gene cg4930 Encodes a High Affinity Inhibitor for Endonuclease G. J Biol Chem. 2009;284:8337–8348. doi: 10.1074/jbc.M808319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahi N, et al. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA proces sing of differentiated cardiomyocytes. J Biol Chem. 2006;281:22943–22952. doi: 10.1074/jbc.M601025200. [DOI] [PubMed] [Google Scholar]
- 20.Arad M, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai DF, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1128. Article17. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. discussion 63-69. [PubMed] [Google Scholar]
- 25.Cote J, Ruiz-Carrillo A. Primers for mitochondrial DNA replication generated by endonuclease G. Science. 1993;261:765–769. doi: 10.1126/science.7688144. [DOI] [PubMed] [Google Scholar]
- 26.Tiranti V, et al. Chromosomal localization of mitochondrial transcription factor A (TCF6), single-stranded DNA-binding protein (SSBP), and endonuclease G (ENDOG), three human housekeeping genes involved in mitochondrial biogenesis. Genomics. 1995;25:559–564. doi: 10.1016/0888-7543(95)80058-t. [DOI] [PubMed] [Google Scholar]
- 27.Rothfuss O, et al. Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum Mol Genet. 2009;18:3832–3850. doi: 10.1093/hmg/ddp327. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet. 1999;21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 29.Lewis W, et al. Decreased mtDNA, oxidative stress, cardiomyopathy, and death from transgenic cardiac targeted human mutant polymerase gamma. Lab Invest. 2007;87:326–335. doi: 10.1038/labinvest.3700523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahsen N, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.