Abstract
Secreted reporters which are detected in the blood/serum/urine have shown to be simple and useful tools for ex vivo real-time monitoring of in vivo biological processes. Here we explore the three most commonly used secreted blood reporters in experimental animals: secreted alkaline phosphatase, soluble peptides derived from human carcinoembryonic antigen and human chorionic gonadotropin, and Gaussia luciferase. We also comment on other recently discovered secreted reporters and their potential use as blood reporters for multiplexing applications.
Secreted blood reporter proteins are valuable tools for rapid and sensitive detection, quantification, and non-invasive monitoring of biological phenomena in pre-clinical animal experiments (Hughes et al., 2009; Pham et al., 2009; Wurdinger et al., 2008). Traditional enzyme-based reporter systems using cytosolic markers are sensitive, but require either a tissue lysis step which prevents their use for long-term monitoring, or frequent systemic anesthesia and substrate injection for detection of photon absorption. By contrast, the levels of secreted reporters can be evaluated repeatedly to generate multiple sets of data over time using conditioned medium from the same viable cell population or small amount of blood, serum, or urine without sacrificing the animal. This property allowed time-course study of embryo development, viral dissemination, gene transfer, tumor growth and response to therapy, as well as the fate of genetically engineered cells in animal models (Maelandsmo et al., 2005; Msaouel et al., 2009; Tannous, 2009).
Initial attempts were made using growth hormone as a secreted reporter in rats and humans. The restricted expression pattern of this hormone permits its use in mammalians (Kingston et al., 1986). However, the rate of its secretion varies significantly among tissue types and under different experimental conditions. Also, growth hormone is not “neutral”, i.e. it may interfere with metabolism, development, and other cellular signaling pathways (Larsen et al., 1986). These weaknesses make this protein an unreliable reporter. The first engineered secreted reporter was from human placental alkaline phosphatase, initially derived by Berger et al (Berger et al., 1988) and used for quantification experiments in cell culture. Bettan et al. then applied this reporter for ex vivo analysis in a mouse model (Bettan et al., 1999). To avoid immune response, at least two forms of mouse specific alkaline phosphatase were later produced (Maelandsmo et al., 2005; Wang et al., 2001). More recently, virus-encoded soluble marker peptides have been exploited to monitor viral therapy. Edmonston vaccine strain of measles virus (MV-Edm) was engineered to express a soluble peptide derived from the human carcinoembryonic antigen (hCEA) or human chorionic gonadotropin (hCG) (Liu et al., 2007; Peng et al., 2002b; Pham et al., 2009). Infection of this oncolytic virus was easily detected by measuring the concentration of these polypeptides in tissue culture supernatant or in body fluids (Msaouel et al., 2009; Peng et al., 2002a).
Light emission from bioluminescence provides a rapid and sensitive method to detect gene expression in a quantitative manner. The Photinus pyralis firefly luciferase (Fluc) (de Wet et al., 1987) and the Renilla reniformis luciferase (Rluc) (Lorenz et al., 1991) have been mostly studied. The typical luciferase reaction occurs intracellularly and therefore a lysis step is required for detection in cultured cells. Several attempts have been made to engineer secreted luciferases in mammalian cells (Kim et al., 2005; Liu et al., 1997). More recently, naturally secreted luciferases from marine copepods such as Gaussia princeps and marine ostracod Vargula hilgendorfii have been cloned and optimized for mammalian gene expression (Tannous et al., 2005; Thompson et al., 1989). Here we address the three most popular and promising secreted blood reporters, the secreted alkaline phosphatase, soluble peptide markers, as well as the Gaussia luciferase, and their use for non-invasive ex vivo monitoring of in vivo biological processes. Further, we comment on other recently discovered secreted proteins and their potential use as blood reporters for multiplexing applications in experimental animal models and potential applications in humans.
Secreted Alkaline Phosphatase
The secreted alkaline phosphatase (SEAP) is the most commonly used secreted reporter for monitoring in vivo processes. Alkaline phosphatases are normally membrane-bound, thus not secreted. By introducing a termination codon at sequence encoding the membrane anchoring domain, the 513-amino acid cell-surface form was converted into a truncated (489-amino acid), fully active protein (Berger et al., 1988). This recombinant reporter gene can be constitutively expressed and efficiently released from transfected cells. Changes in SEAP levels detected in conditioned medium of Chinese hamster ovary cells are directly proportional to changes in intracellular SEAP mRNA and cell number (Bettan et al., 1999; Cullen and Malim, 1992). Since then, SEAP has been widely used as a secreted serum reporter for accurate long-term monitoring of gene expression in a variety of gene transfer studies, from direct injection of plasmid DNA into muscles of adult mice (Abruzzese et al., 1999) to electroporating into large animals (e.g pigs) (Brown et al., 2008), and delivering the plasmid DNA in the form of bioreducible multilayered polyelectrolyte films in subcutaneous model in rats (Blacklock et al., 2009). A single intrathecal injection of naked plasmid DNA in rats yields a sensitive detection of SEAP in serum for up to 4 months (Hughes et al., 2009). More recently, SEAP has been applied in screening mini-peptide-coding sequences to increase gene transfer activity (Cutrera et al., 2011). Further, SEAP expression in serum is a useful indicator in early and long-term measurement of tumor growth and response to therapeutics. In different human cancer xenograft models (Bao et al., 2000; Chaudhuri et al., 2003; Nilsson et al., 2002), SEAP was detectable in mouse plasma as soon as one day after injection, long before evident tumor was present. In all these studies, changes in SEAP levels reflected changes in tumor volume and cell numbers.
SEAP has also been used in detection of endoplasmic reticulum (ER) stress which is implicated in a number of diseases such as diabetes and neurodegeneration (Zhao and Ackerman, 2006). In transgenic mice constitutively producing SEAP, in vivo induction of systemic ER stress using intraperitoneally administration of thapsigargin caused rapid down-regulation of serum SEAP levels (Hiramatsu et al., 2006). SEAP serum assay has been used in measuring promoter and transcription factors activation and inhibition. Its activity detected in serum and protein extract of pancreas in transgenic mice could represent the activity of pancreatic and duodenal homeobox gene-1 promoter (Shiraiwa et al., 2007). Real-time monitoring of the nuclear factor kappa B (NFκB) activation, a transcription factor that plays a major role in many human disorders, including immune diseases and cancer, was achieved first using SEAP (Meng et al., 2005). In addition, SEAP has been extended to clinic use and was recently used to evaluate systemic and cervical antibody levels after vaccination. In 50 women vaccinated with HPV16/18 AS04-adjuvanted vaccine, serum SEAP levels correlated with antibody levels measured using ELISA (Kemp et al., 2008). In addition to the measurement of SEAP in blood, it is a useful reporter for in situ histo-enzymatic revelation to correlate the level of its secretion and the number of transduced cells (Shimajiri et al., 2011).
Human SEAP are immunogenic in rodents, which are the most commonly used laboratory animals. Circulating human SEAP levels is transient and decreases rapidly after reaching a maximum level in animal models, since it elicits cytotoxic T lymphocyte or neutralizing antibody responses that can suppress its own expression. To improve SEAP stability in transgenic mice, the mouse SEAP (mSEAP) has been developed by modification of the murine embryonic alkaline phosphatase (Wang et al., 2001) or isolation the cDNA using mouse plancental RNA as template (Maelandsmo et al., 2005). After intravenous administration in mice, both forms of mSEAP continued to express at high levels for >1 month, and no antibodies to mSEAP were detected. This species-specific form of SEAP has high potential in preclinical transplantation studies. When mSEAP-expressing muscle precursor cells were injected into the muscle of dystrophin-deficient mdx mice, there was a good correlation between the level of circulating mSEAP, the number of mSEAP-positive fibers, and the expression of dystrophin in regenerating fibers (Gerard et al., 2009).
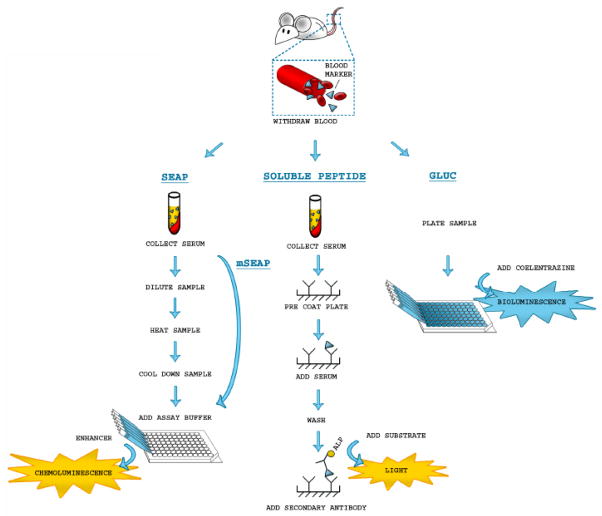
The expression level of SEAP is relatively low thus a relatively large amount of blood (up to 100 μl in some cases) is required for the assay. The high molecular weight of SEAP (64 kDa) significantly lessens its release into blood and almost completely blocks its excretion into urine (Hiramatsu et al., 2005). Most blood samples contain naturally occurring serum alkaline phosphatases which can interfere with the SEAP assay. Also, some treatments may cause liver damage and increase the presence of circulating liver-derived alkaline phosphatases in the experimental animals leading to non-specific SEAP activity. However, the heat stability of SEAP and resistance to phosphatase inhibitors (such as L-homoarginine) permit the sample to be pre-treated by either incubation at 65 °C or with this inhibitor to eliminate endogenous alkaline phosphatase activity (Fig. 1a). The requirement for sample processing and this inactivation step limits throughput and can be especially problematic for screening assays (Bettan et al., 1999). Since native mSEAP is sensitive to L-homoarginine and heat labile, high background activity cannot be inactivated by these strategies. Also, most mSEAP remains cell-associated due to glycan phosphatidylinositol linkage to the cell surface. A mutant (H451E) of mSEAP has been reported to enhance secretion and heat stability (Christou and Parks, 2011).
Figure 1.
Experimental set up for the secreted Alkaline phosphatase, soluble peptides and Gaussia luciferase assays in body fluids
Soluble marker peptides
Many viruses have shown oncolytic activity in preclinical tumor models. A major challenge in cancer virotherapy studies is the lack of a convenient method to monitor the in vivo spread and elimination of the virus, as well as to measure the profile of viral gene expression and kinetics over time (Msaouel et al., 2009). One approach to this problem is to introduce into the virus a second secreted marker which can be accurately and non-invasively detected over time. Measurement of the amount of these inert peptide markers in serum ex vivo could therefore provide important feedback on in vivo kinetics of viral infection and spread (Fig. 1b)(Peng et al., 2002a; Ricci et al., 2008). Important characteristics for choosing a soluble peptide includes lack of biological activity, constant circulation half-life, minimal immunogenicity, and the existence of a validated assay that allows its detection in blood/serum (Msaouel et al., 2009).
Edmonston vaccine strain of measles virus (MV-Edm) has been effectively engineered to express different soluble marker peptides, including the ones derived from the human carcinoembryonic antigen (hCEA) and β subunit of human chorionic gonadotropin (βhCG) (Peng et al., 2002b; Pham et al., 2009). The replication-competent virus was engineered to express the soluble extracellular N-terminal domain of hCEA or βhCG as an additional transcription unit before the viral N gene. Viral replication and gene expression following infection of the target cell results in encoded marker peptide production and secretion (Peng et al., 2006; Ricci et al., 2008). When expressoion cassettes for both hCEA and βhCG as well as the reporter gene human sodium iodide symporter (hNIS; a membrane ion channel detected non-invasively by radioisotopic single photon emission computed tomography imaging) were inserted into the same recombinant adenoviral vector backbone, strong correlation between hNIS image intensity and serum level of both soluble markers was noted (Liu et al., 2008; Liu et al., 2007). In athymic rats, blood and urine levels of βhCG were highly concordant with serum levels of hCEA at all time points. More recently, these soluble peptides have been successfully used in Lewis inbred rats to monitor transplanted heart (Liu et al., 2007; Pham et al., 2009).
Iankov et al. used the same concept and engineered an oncolytic measles virus to express a human light immunoglobulin chain reporter gene for the treatment of multiple myeloma. The vector-encoding λ protein would recombine with myeloma IgG-κ immunoglobulin, which is only expressed in tumor cells. In human myeloma xenograft mouse models, the level of specific chimeric IgG-κ/λ correlated with response to the therapy. This strategy allows discrimination between tumor and normal cells using secreted blood markers (Iankov et al., 2009).
Real-time monitoring of the profile and kinetics of viral gene expression could facilitate tailoring of individualized treatment protocol to generate a specific dose size intervals between repeat treatment cycles both at the pre-clinical and clinical levels (Msaouel et al., 2009). In a phase-1 clinical trial of an intraperitoneal administration of an oncolytic measles virus strain in recurrent ovarian cancer patients, a dose-dependent CEA elevation was observed in peritoneal fluid and serum without any development of anti-CEA antibodies (Galanis et al., 2010). Soluble marker peptides were used in two additional trials in patients with recurrent glioblastoma multiforme and multiple myeloma (Galanis et al., 2010; Msaouel et al., 2009).
Gaussia luciferase
Our laboratory has established a novel naturally secreted reporter from the marine copepod Gaussia princeps (Gluc) (Tannous et al., 2005). This 185 amino acid monomeric luciferase is the smallest luciferase cloned (19.9 kDa). The Gluc gene possesses a secretory signal consisting of 16 amino acids and therefore it is naturally secreted in an active form upon expression in mammalian cells. Gluc does not require any cofactors for activity (e.g., ATP) and catalyzes the oxidation of the substrate coelenterazine in a reaction that leads to emission of blue light (480 nm). The levels of Gluc in the conditioned medium are linear with respect to cell number, growth and proliferation (Badr et al., 2007; Wurdinger et al., 2008). Compared to Fluc or Rluc, human codon-optimized Gluc generates over 1000-fold higher bioluminescent signal intensity when expressed in mammalian cells. Gluc also has a much shorter assay time and increased sensitivity and linear range over other secreted blood reporters (Fig. 1c, Table 1) (Tannous, 2009).
Table 1.
Application of Secreted Blood Reporters
| Secreted reporters | Strategy | Applications | References |
|---|---|---|---|
| Secreted Alkaline Phosphatase | Constitutive expression driven by promoters | Monitor gene expression in gene transfer studies Monitor tumor growth and response to therapy |
Brown et al. 2008 Hughes et al. 2009 Cutrera et. al 2011 Nilsson et al. 2002 Chaudhuri et al. 2003 |
| Expression under the control of transcription factors/gene promoters | Monitor transcription factors, signaling pathways, such as NFκB, ER stress |
Meng et al. 2005 Hiramatsu et al. 2006 |
|
| Expression using modified murine embryonic alkaline phosphatase | Long-term expression of SEAP in mice |
Wang et al. 2001 Gerard et al. 2009 |
|
| Expression using alkaline phosphatase isolated from mouse plancental RNA | Mælandsmo et al. 2005 | ||
| Soluble Peptide Markers | Measles virus to express human carcinoembryonic antigen | Non-invasively monitor virus expression and kinetics |
Peng et al. 2002a Pham et al. 2009 Rocci et al. 2008 Liu et al. 2008 |
| Measles virus to express human chorionic gonadotropin β subunit | |||
| Measles virus to express human sodium iodide symporter | Use the image intensity of a membrane protein as control | ||
| Measles virus to express human light immunoglobulin chain | Discrimination between tumor and normal cells | Iankov et al. 2009 | |
| Gaussia Luciferase | Constitutive expression driven by promoters | Monitor cell growth and response to therapy, Viral infection/replication, Viability of circulation cells |
Wurdinger et al. 2008 Tannous et al. 2009 Chung et al. 2009 Marquardt et al. 2011 |
| Expression under the control of transcription factors/gene promoters | Monitor transcription factors, signaling pathways, ER stress etc. |
Badr et al. 2007 Yang and Richmond 2009 Badr et al. 2010a Badr et al. 2011 |
|
| Fusing to microRNA target site at the 3′ UTR | Monitor expression and activity of miRNAs | Kim et al. 2009 | |
| Fusing a short peptide recognized and cleaved by caspase-3 | Real-time in vivo monitoring of apoptosis | Niers et al. 2011 | |
| Isolation Gluc variant that catalyzes stable light emission | High-throughput screening | Maguire et al. 2009 |
As a secreted blood reporter, Gluc has been extensively used to monitor different biological processes including tumor growth and response to therapy, viral infection and replication, as well as viability of circulating cells (Wurdinger et al., 2008). In one application, Gluc blood assay was used to monitor MDA-MB-231 BR human breast cancer metastasis and treatment response in experimental animal models (Chung et al., 2009). Good correlation between the primary tumor volume and Gluc concentrations in blood and urine was observed. More importantly, Gluc blood assay revealed early detection of tumor growth and metastasis in mice and in large animal models (e.g. sheep) for breast, airway and lung cancer which were not attainable by typical in vivo imaging techniques (Griesenbach et al., 2011). When Gluc was constructed into a piggyBac transposon, gene expression in mice could be detected up to 80 days after transfection, suggesting that this transposon is useful for organ-selective somatic integration and sustained gene expression in mammals, and could potentially contribute to basic genetic studies and gene therapies (Nakanishi et al., 2010).
Gluc offers a tool for evaluating transcriptional regulation associated with signaling pathways which are dysregulated in many human disorders including inflammation and cancer. A reporter system was generated by cloning Gluc under the control of tandem repeats of NFκB responsive elements. This reporter demonstrated to be a highly sensitive for non-invasive continuous monitoring of the kinetics of NFκB activation and inhibition over time using blood or urine samples in mice (Badr et al., 2010a; Badr et al., 2009; Yang and Richmond, 2009). Recently, An apoptosis blood assay was generated by fusing GFP to the N-terminus of Gluc (including its signal sequence) separated by a short peptide consisting of aspartic acid, glutamic acid, valine, and aspartic acid (DEVD). This peptide is recognized and cleaved by caspase-3, whose activation reflects both the intrinsic and extrinsic apoptosis pathways. Under normal conditions, this fusion protein resides in the cytoplasm in an inactive form. During apoptosis, DEVD is cleaved, freeing Gluc which can now enters the secretory pathway, release from cells, and be detected in the conditioned medium in culture or blood of animals ex vivo. This reporter has been proved to be useful in real-time monitoring of apoptosis both in subcutaneous and metastatic tumor models (Niers et al., 2011).
Gluc was also shown to be a sensitive marker for viral infection, replication, and reactivation. In one study, a mouse cytomegalovirus reporter that expresses Gluc under control of a strong major immediate early promoter was constructed. Gluc blood assay revealed virus reactivation 3 days after latent infection, preceding the detection of infectious virus by approximately 4 days (Marquardt et al., 2011). In another study, the efficiency of lentivirus infection and replication in tumors was monitored using the Gluc blood assay (Badr et al., 2010a; Wurdinger et al., 2008).
A major challenge in the cell therapy field is the ability to monitor the fate of implanted cells in vivo. By engineering neural precursor cells to express Gluc, viability, growth and proliferation of these cells could be monitored in real-time. This assay has the unique advantage in that the Gluc activity in blood reports from all viable circulating cells and not only from subpopulation of cells localized at a single site, typically imaged with other techniques.
microRNAs (miRNA) comprise a large group of endogenous non-coding short sequence RNA that can block mRNA translation or negatively regulate mRNA stability and thereby play a central role in the regulation of gene expression (Cortez and Calin, 2009). miRNAs are known to be extremely stable. Recently, endogenous naturally expressed miRNA circulating in blood has been tested as diagnostic markers in both fresh and archived serum or plasma (Reid et al., 2010). At the pre-clinical level, several recent studies utilized Gluc to monitor in vivo miRNA biogenesis by cloning seed sequences of a particular miRNA to the 3′-untranslated region of Gluc under the control of a constitutively active promoter. The Gluc blood assay allowed continuous detection of in vivo miR-122, miR142, or miR-34a activities (Kim et al., 2009).
One limitation for the Gluc assay in general is the rapid decay of its bioluminescence reaction, thereby requiring a luminometer with a built-in injector for assaying its activity. Our laboratory has isolated a Gluc variant (GlucM43I) that catalyzes a stable light emission output (half-life of bioluminescence reaction is >10 min versus approximately 3min for wild type) in the presence of a detergent, triton X-100 using a screen of a mutant library created by DNA shuffling and error-prone PCR (Maguire et al., 2009). This Gluc variant proved to be a useful reporter for high-throughput screening applications where sensitivity and stable light emission are desired, and could potentially replace the wild-type Gluc for blood assay allowing semi-throughput screening/validation of novel therapeutics in vivo (Badr et al., 2010b; Marquardt et al., 2011).
Trends in development of novel secreted reporters
An ideal reporter for ex vivo monitoring of in vivo biological processes should encode a protein that is secreted from various tissues into the bloodstream where its endogenous level is minimal and distinguishable, be expressed in a stable manner over time in immunocompetent animals, and be easy to detect with rapid, specific and sensitive assays. The secreted protein should not be retained in different tissues through heparin-binding or matrix attachment region or through its interaction with its naturally occurring receptor (Baumgartner et al., 1998). Another important factor in the development of a blood reporter is the potential inactivation of these non-endogeneous proteins through interaction with serum proteins such as albumin, limiting their detection ex vivo (Hiramatsu et al., 2005).
Secreted reporters are advantageous when can be detected in urine, since this specimen can be sampled more easily, frequently, and noninvasively in large animals. However, the bottleneck for a protein to pass into urine is the glomerular basement membrane in the kidney. Filtration rates of individual macromolecules are dependent on their molecular size. Proteins with a molecular mass more than 60 kDa can be rarely passed through. In contrast, a protein with a molecular mass smaller than 20 kDa often has a good chance to be filtered into the urinary space (Hiramatsu et al., 2005; Salgado et al., 2010).
Another field that can benefit from repeated data acquisition from live cells is toxicology. A secreted reporter allows non-invasive monitoring of the toxic effect of a given compound or treatment strategy over a prolonged exposure time in animals by withdrawing a few microliters of blood or urine and assaying for their activity. It also allows monitoring of potential drug interaction, dependent on the time as well as the order in which the drug is administered (Haugwitz et al., 2008; Tannous, 2009).
Various bioluminescent marine organisms exists with naturally occurring secreted luciferases. Among them, Vargula luciferase (Vluc) was the first to be cloned from the small marine ostracod crustacean Vargula (formerly Cypridine) hilgendorfii (Thompson et al., 1989). In the presence of the substrate vargulin, Vluc emits a blue light (emission maxima at 462nm). However, the inavailability of the vargulin substrate limited its use (Thompson et al., 1995). Another naturally secreted luciferase was recently cloned from the marine copepod Metridia longa (Mluc). The cDNA of Mluc is 897 bp and encodes a 219-amino acid polypeptide with a M.W. of 23.9 kDa (Takenaka et al., 2008). Mluc has a more “glow-like” bioluminescence characteristic (Haugwitz et al., 2008). However, Mluc was reported to be rapidly inactivated by rat serum, fetal bovine serum, or human serum. Albumin was identified as the potential factor for the inhibition of its activity (Hiramatsu et al., 2005). This problem led to the limited use of Mluc as a blood reporter. In addition, several attempts were made to create a secreted Rluc variant by tagging it with a secretion signal peptide sequence. Liu et al., fused the 5′ end of the Rluc gene in frame with a short DNA sequence encoding the signal peptide of the human interleukin-2 (IL-2) protein. When expressed in mammalian cells, this construct encodes a protein product which can be secreted as a functional Rluc enzyme (Liu et al., 1997). Unfortunately, the specific activity of the secreted form of Rluc was only 7% of the cytosolic Rluc in mammalian cells. This may be in part due to cysteine residues in Rluc sequence which are oxidized in the secretory pathway environment (Liu et al., 2000).
Simultaneous monitoring of multiple biological processes in the same experimental animal facilitates understanding of disease development and expedites findings of novel therapeutics and translation into the clinic. The discovery of new secreted reporters which either utilizes different substrates, emits at different regions of the spectrum, or detects using specific assays allows their potential use for multiplex applications. For instance, different secreted reporters/soluble peptides can be used to report for different molecular event such as activation of different promoters, viral transduction, gene expression (etc.) simultaneously. In order for secreted reporters be suited for multiplexing, the detection of their signals must be distinguishable from each other, and their chemical reactions must be compatible or separable. Recently, a multiplex blood assay was developed using Gluc and SEAP secreted reporters to monitor the kinetics of NFκB activation during tumor development (Badr et al., 2009). Since Gluc and Vluc utilizes different substrates, they can potentially multiplexed together along with SEAP, giving that Vluc is a suited blood reporter. Many luciferases which are expressed intracellularly and emit different colored light (green and red-emitting luciferases from click beetle (Caysa et al., 2009; Takeuchi et al., 2010) and the American or Italian firefly (Branchini et al., 2007)) have been cloned. Secreted variants of these lucifrases could be engineered and multiplexed with Gluc for multicolor applications using spectral unmixing (Gammon et al., 2006; Michelini et al., 2008).
Secreted blood reporters have proven to be sensitive tools for real-time ex vivo monitoring of in vivo biological processes. Since secreted reporters are first processed in the secretory pathway (i.e. ER and Golgi) before released from the cells, conditions which interfere with this pathway such as protein folding and ER stress could affect protein secretion leading to false data interpretation (Hiramatsu et al., 2006). Careful consideration of the secretory pathway is a must when applying blood assays in different disease models.
Table 2.
Comparison of Secreted Blood Reporter
| SEAP | Soluble peptide | Gluc | |
|---|---|---|---|
| Time to complete the assay | hours | hours | minutes |
| Sensitivity | ng/ml | ng/ml | 1,000-fold more than SEAP |
| Dynamic range to cell number | <3 orders of magnitudes | >4 orders of magnitudes | >5 orders of magnitudes |
| Assay in the whole blood | No, requires sample processing | No, requires sample processing | Yes |
| Half-life | 3 h leading to accumulation | hours | 20 minute |
| Detected in urine | No | Yes, also in peritoneal fluid | Yes, cleared by kidneys |
| Localized with in vivo imaging | In situ histology | No | Yes, non-invasive imaging |
| Immunogenicity | Need species-specific | No | Yes |
| Tested in clinic | No | Yes | No |
| Cost | $$ | $$$ | $ |
Acknowledgments
Dr. Tannous is supported by grants from the National Institutes of Health, the National Cancer Institute (4R00CA126839) and the National Institute of Neurological Disorders and Stroke (NS045776 and 1R01NS064983).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abruzzese RV, Godin D, Burcin M, Mehta V, French M, Li Y, O’Malley BW, Nordstrom JL. Ligand-dependent regulation of plasmid-based transgene expression in vivo. Hum Gene Ther. 1999;10:1499–1507. doi: 10.1089/10430349950017833. [DOI] [PubMed] [Google Scholar]
- Badr CE, Hewett JW, Breakefield XO, Tannous BA. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS One. 2007;2:e571. doi: 10.1371/journal.pone.0000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr CE, Niers JM, Morse D, Koelen JA, Vandertop P, Noske D, Wurdinger T, Zalloua PA, Tannous BA. Suicidal gene therapy in an NF-kappaB-controlled tumor environment as monitored by a secreted blood reporter. Gene Ther. 2010a doi: 10.1038/gt.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr CE, Niers JM, Tjon-Kon-Fat LA, Noske DP, Wurdinger T, Tannous BA. Real-time monitoring of nuclear factor kappaB activity in cultured cells and in animal models. Mol Imaging. 2009;8:278–290. [PMC free article] [PubMed] [Google Scholar]
- Badr CE, Wurdinger T, Tannous BA. Functional Drug Screening Assay Reveals Potential Glioma Therapeutics. Assay Drug Dev Technol. 2010b doi: 10.1089/adt.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao R, Selvakumaran M, Hamilton TC. Use of a surrogate marker (human secreted alkaline phosphatase) to monitor in vivo tumor growth and anticancer drug efficacy in ovarian cancer xenografts. Gynecol Oncol. 2000;78:373–379. doi: 10.1006/gyno.2000.5925. [DOI] [PubMed] [Google Scholar]
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Bettan M, Darteil R, Scherman D. Secreted human placental alkaline phosphatase as a reporter gene for in vivo gene transfer. Anal Biochem. 1999;271:187–189. doi: 10.1006/abio.1999.4144. [DOI] [PubMed] [Google Scholar]
- Blacklock J, You YZ, Zhou QH, Mao G, Oupicky D. Gene delivery in vitro and in vivo from bioreducible multilayered polyelectrolyte films of plasmid DNA. Biomaterials. 2009;30:939–950. doi: 10.1016/j.biomaterials.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchini BR, Ablamsky DM, Murtiashaw MH, Uzasci L, Fraga H, Southworth TL. Thermostable red and green light-producing firefly luciferase mutants for bioluminescent reporter applications. Anal Biochem. 2007;361:253–262. doi: 10.1016/j.ab.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Brown PA, Khan AS, Draghia-Akli R. Delivery of DNA into skeletal muscle in large animals. Methods Mol Biol. 2008;423:215–224. doi: 10.1007/978-1-59745-194-9_15. [DOI] [PubMed] [Google Scholar]
- Caysa H, Jacob R, Muther N, Branchini B, Messerle M, Soling A. A redshifted codon-optimized firefly luciferase is a sensitive reporter for bioluminescence imaging. Photochem Photobiol Sci. 2009;8:52–56. doi: 10.1039/b814566k. [DOI] [PubMed] [Google Scholar]
- Chaudhuri TR, Cao Z, Krasnykh VN, Stargel AV, Belousova N, Partridge EE, Zinn KR. Blood-based screening and light based imaging for the early detection and monitoring of ovarian cancer xenografts. Technol Cancer Res Treat. 2003;2:171–180. doi: 10.1177/153303460300200214. [DOI] [PubMed] [Google Scholar]
- Christou C, Parks RJ. Rational design of murine secreted alkaline phosphatase for enhanced performance as a reporter gene in mouse gene therapy preclinical studies. Hum Gene Ther. 2011;22:499–506. doi: 10.1089/hum.2010.171. [DOI] [PubMed] [Google Scholar]
- Chung E, Yamashita H, Au P, Tannous BA, Fukumura D, Jain RK. Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLoS One. 2009;4:e8316. doi: 10.1371/journal.pone.0008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- Cullen BR, Malim MH. Secreted placental alkaline phosphatase as a eukaryotic reporter gene. Methods Enzymol. 1992;216:362–368. doi: 10.1016/0076-6879(92)16033-g. [DOI] [PubMed] [Google Scholar]
- Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a Linear Peptide for Improving Tumor Targeting of Gene Products and Treatment of Distal Tumors by IL-12 Gene Therapy. Mol Ther. 2011 doi: 10.1038/mt.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ, Jr, Aderca I, Zollman PJ, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon ST, Leevy WM, Gross S, Gokel GW, Piwnica-Worms D. Spectral unmixing of multicolored bioluminescence emitted from heterogeneous biological sources. Anal Chem. 2006;78:1520–1527. doi: 10.1021/ac051999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard X, Vignaud L, Charles S, Pinset C, Scherman D, Kichler A, Israeli D. Real-time monitoring of cell transplantation in mouse dystrophic muscles by a secreted alkaline phosphatase reporter gene. Gene Ther. 2009;16:815–819. doi: 10.1038/gt.2009.28. [DOI] [PubMed] [Google Scholar]
- Griesenbach U, Vicente CC, Roberts MJ, Meng C, Soussi S, Xenariou S, Tennant P, Baker A, Baker E, Gordon C, et al. Secreted Gaussia luciferase as a sensitive reporter gene for in vivo and ex vivo studies of airway gene transfer. Biomaterials. 2011;32:2614–2624. doi: 10.1016/j.biomaterials.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Haugwitz M, Nourzaie O, Garachtchenko T, Hu L, Gandlur S, Olsen C, Farmer A, Chaga G, Sagawa H. Multiplexing bioluminescent and fluorescent reporters to monitor live cells. Curr Chem Genomics. 2008;1:11–19. doi: 10.2174/1875397300801010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu N, Kasai A, Hayakawa K, Yao J, Kitamura M. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res. 2006;34:e93. doi: 10.1093/nar/gkl515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu N, Kasai A, Meng Y, Hayakawa K, Yao J, Kitamura M. Alkaline phosphatase vs luciferase as secreted reporter molecules in vivo. Anal Biochem. 2005;339:249–256. doi: 10.1016/j.ab.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Hughes TS, Langer SJ, Johnson KW, Chavez RA, Watkins LR, Milligan ED, Leinwand LA. Intrathecal injection of naked plasmid DNA provides long-term expression of secreted proteins. Mol Ther. 2009;17:88–94. doi: 10.1038/mt.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther. 2009;17:1395–1403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, Rodriguez AC, Porras C, Herrero R, Hildesheim A, Pinto LA. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26:3608–3616. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Chung JK, Hwang do W, Lee DS, Kim S. In vivo imaging of miR-221 biogenesis in papillary thyroid carcinoma. Mol Imaging Biol. 2009;11:71–78. doi: 10.1007/s11307-008-0188-6. [DOI] [PubMed] [Google Scholar]
- Kim SB, Ozawa T, Umezawa Y. Genetically encoded stress indicator for noninvasively imaging endogenous corticosterone in living mice. Anal Chem. 2005;77:6588–6593. doi: 10.1021/ac0510078. [DOI] [PubMed] [Google Scholar]
- Kingston RE, Cowie A, Morimoto RI, Gwinn KA. Binding of polyomavirus large T antigen to the human hsp70 promoter is not required for trans activation. Mol Cell Biol. 1986;6:3180–3190. doi: 10.1128/mcb.6.9.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PR, Harney JW, Moore DD. Sequences required for cell-type specific thyroid hormone regulation of rat growth hormone promoter activity. J Biol Chem. 1986;261:14373–14376. [PubMed] [Google Scholar]
- Liu C, Erlichman C, McDonald CJ, Ingle JN, Zollman P, Iankov I, Russell SJ, Galanis E. Heat shock protein inhibitors increase the efficacy of measles virotherapy. Gene Ther. 2008;15:1024–1034. doi: 10.1038/gt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M, Carlson B, Decker PA, Wu W, James CD, et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. 2007;13:7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- Liu J, O’Kane DJ, Escher A. Secretion of functional Renilla reniformis luciferase by mammalian cells. Gene. 1997;203:141–148. doi: 10.1016/s0378-1119(97)00505-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Szalay AA, Escher A. Visualizing and quantifying protein secretion using a Renilla luciferase-GFP fusion protein. Luminescence. 2000;15:45–49. doi: 10.1002/(SICI)1522-7243(200001/02)15:1<45::AID-BIO553>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lorenz WW, McCann RO, Longiaru M, Cormier MJ. Isolation and expression of a cDNA encoding Renilla reniformis luciferase. Proc Natl Acad Sci U S A. 1991;88:4438–4442. doi: 10.1073/pnas.88.10.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelandsmo GM, Ross PJ, Pavliv M, Meulenbroek RA, Evelegh C, Muruve DA, Graham FL, Parks RJ. Use of a murine secreted alkaline phosphatase as a non-immunogenic reporter gene in mice. J Gene Med. 2005;7:307–315. doi: 10.1002/jgm.666. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Deliolanis NC, Pike L, Niers JM, Tjon-Kon-Fat LA, Sena-Esteves M, Tannous BA. Gaussia luciferase variant for high-throughput functional screening applications. Anal Chem. 2009;81:7102–7106. doi: 10.1021/ac901234r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt A, Halle S, Seckert CK, Lemmermann NA, Veres TZ, Braun A, Maus UA, Forster R, Reddehase MJ, Messerle M, Busche A. Single cell detection of latent cytomegalovirus reactivation in host tissue. J Gen Virol. 2011 doi: 10.1099/vir.0.029827-0. [DOI] [PubMed] [Google Scholar]
- Meng Y, Kasai A, Hiramatsu N, Hayakawa K, Takeda M, Shimizu F, Kawachi H, Yao J, Kitamura M. Real-time monitoring of mesangial cell-macrophage cross-talk using SEAP in vitro and ex vivo. Kidney Int. 2005;68:886–893. doi: 10.1111/j.1523-1755.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- Michelini E, Cevenini L, Mezzanotte L, Ablamsky D, Southworth T, Branchini B, Roda A. Spectral-resolved gene technology for multiplexed bioluminescence and high-content screening. Anal Chem. 2008;80:260–267. doi: 10.1021/ac7016579. [DOI] [PubMed] [Google Scholar]
- Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Higuchi Y, Kawakami S, Yamashita F, Hashida M. piggyBac transposon-mediated long-term gene expression in mice. Mol Ther. 2010;18:707–714. doi: 10.1038/mt.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niers JM, Kerami M, Pike L, Lewandrowski G, Tannous BA. Multimodal In Vivo Imaging and Blood Monitoring of Intrinsic and Extrinsic Apoptosis. Mol Ther. 2011 doi: 10.1038/mt.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Westfall SD, McDonald C, Lison T, Sadler-Riggleman I, Skinner MK. An in vivo mouse reporter gene (human secreted alkaline phosphatase) model to monitor ovarian tumor growth and response to therapeutics. Cancer Chemother Pharmacol. 2002;49:93–100. doi: 10.1007/s00280-001-0396-0. [DOI] [PubMed] [Google Scholar]
- Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002a;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Peng KW, Hadac EM, Anderson BD, Myers R, Harvey M, Greiner SM, Soeffker D, Federspiel MJ, Russell SJ. Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 2006;13:732–738. doi: 10.1038/sj.cgt.7700948. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002b;62:4656–4662. [PubMed] [Google Scholar]
- Pham L, Nakamura T, Gabriela Rosales A, Carlson SK, Bailey KR, Peng KW, Russell SJ. Concordant activity of transgene expression cassettes inserted into E1, E3 and E4 cloning sites in the adenovirus genome. J Gene Med. 2009;11:197–206. doi: 10.1002/jgm.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol Hematol. 2010 doi: 10.1016/j.critrevonc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Ricci D, Mennander AA, Pham LD, Rao VP, Miyagi N, Byrne GW, Russell SJ, McGregor CG. Non-invasive radioiodine imaging for accurate quantitation of NIS reporter gene expression in transplanted hearts. Eur J Cardiothorac Surg. 2008;33:32–39. doi: 10.1016/j.ejcts.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado JV, Neves FA, Bastos MG, Franca AK, Brito DJ, Santos EM, Salgado Filho N. Monitoring renal function: measured and estimated glomerular filtration rates - a review. Braz J Med Biol Res. 2010;43:528–536. doi: 10.1590/s0100-879x2010007500040. [DOI] [PubMed] [Google Scholar]
- Shimajiri Y, Kosaka Y, Scheel DW, Lynn FC, Kishimoto N, Wang J, Zhao S, German MS. A mouse model for monitoring islet cell genesis and developing therapies for diabetes. Dis Model Mech. 2011;4:268–276. doi: 10.1242/dmm.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa T, Kaneto H, Miyatsuka T, Kato K, Yamamoto K, Kawashima A, Kajimoto Y, Matsuoka TA, Matsuhisa M, Yamasaki Y, Fujitani Y. Establishment of a non-invasive mouse reporter model for monitoring in vivo pdx-1 promoter activity. Biochem Biophys Res Commun. 2007;361:739–744. doi: 10.1016/j.bbrc.2007.07.101. [DOI] [PubMed] [Google Scholar]
- Takenaka Y, Masuda H, Yamaguchi A, Nishikawa S, Shigeri Y, Yoshida Y, Mizuno H. Two forms of secreted and thermostable luciferases from the marine copepod crustacean, Metridia pacifica. Gene. 2008;425:28–35. doi: 10.1016/j.gene.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nagaoka Y, Yamada T, Takakura H, Ozawa T. Ratiometric bioluminescence indicators for monitoring cyclic adenosine 3′,5′-monophosphate in live cells based on luciferase-fragment complementation. Anal Chem. 2010;82:9306–9313. doi: 10.1021/ac102692u. [DOI] [PubMed] [Google Scholar]
- Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Thompson EM, Adenot P, Tsuji FI, Renard JP. Real time imaging of transcriptional activity in live mouse preimplantation embryos using a secreted luciferase. Proc Natl Acad Sci U S A. 1995;92:1317–1321. doi: 10.1073/pnas.92.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EM, Nagata S, Tsuji FI. Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii. Proc Natl Acad Sci U S A. 1989;86:6567–6571. doi: 10.1073/pnas.86.17.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Orsini C, Casanova D, Millan JL, Mahfoudi A, Thuillier V. MUSEAP, a novel reporter gene for the study of long-term gene expression in immunocompetent mice. Gene. 2001;279:99–108. doi: 10.1016/s0378-1119(01)00754-5. [DOI] [PubMed] [Google Scholar]
- Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, Tannous BA. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5:171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Richmond AJ. Monitoring NF-kappaB mediated chemokine transcription in tumorigenesis. Methods Enzymol. 2009;460:347–355. doi: 10.1016/S0076-6879(09)05217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]