Abstract
Multiple sclerosis (MS) is a debilitating disease of the central nervous system (CNS) that has been extensively studied using the animal model experimental autoimmune encephalomyelitis (EAE). It is believed that CD4+ T lymphocytes play an important role in the pathogenesis of this disease by mediating the demyelination of neuronal axons via secretion of proinflammatory cytokines resulting in the clinical manifestations. Although a great deal of information has been gained in the last several decades about the cells involved in the inflammatory and disease mediating process, important questions have remained unanswered. It has long been held that initial neuroantigen presentation and T cell activation events occur in the immune periphery and then translocate to the CNS. However, an increasing body of evidence suggests that antigen (Ag) presentation might initiate within the CNS itself. Importantly, it has remained unresolved which antigen presenting cells (APCs) in the CNS are the first to acquire and present neuroantigens during EAE/MS to T cells, and what the conditions are under which this takes place, ie, whether this occurs in the healthy CNS or only during inflammatory conditions and what the related cytokine microenvironment is comprised of. In particular, the central role of interferon-γ as a primary mediator of CNS pathology during EAE has been challenged by the emergence of Th17 cells producing interleukin-17. This review describes our current understanding of potential APCs in the CNS and the contribution of these and other CNS-resident cells to disease pathology. Additionally, we discuss the question of where Ag presentation is initiated and under what conditions neuroantigens are made available to APCs with special emphasis on which cytokines may be important in this process.
Introduction
Understanding multiple sclerosis
Multiple sclerosis (MS) is the most common idiopathic inflammatory demyelinating disease of the central nervous system (CNS). MS is estimated to affect more than 2.1 million individuals worldwide, predominantly young adults in North America and Europe (Hauser and Oksenberg 2006). The disease is characterized by immune-mediated axonal damage producing demyelinating lesions in the brain and spinal cord that significantly disrupt normal neuronal signaling (Carswell 1838; Charcot 1868). The progression of demyelination/axonal damage and impairment of autonomic, cognitive, and motor functions results in a wide range of clinical symptoms such as muscle weakness, difficulties in speech and motor coordination, partial to complete paralysis, pain, and vision loss (Compston and Coles 2008). The disease course is highly variable and unpredictable (Barkhof and others 1992; Miller and others 1993; Filippi and others 1998). MS is believed to be predominantly mediated by autoreactive T lymphocytes that are initially primed in peripheral lymphatic tissues and subsequently infiltrate the CNS (Pettinelli and McFarlin 1981). Upon the arrival of T cells in the CNS, they encounter antigen presenting cells (APCs), which provide the final signals for their activation. Current treatment is primarily focused on anti-inflammatory and immunomodulatory therapy, which has been somewhat successful in treating the acute episodes of MS. Unfortunately, no treatment has been developed that inhibits long-term progression to disability or cures the disease entirely, primarily because there are still major gaps in our understanding of the disease etiology and pathophysiology.
Experimental autoimmune encephalomyelitis (EAE) is a rodent model developed around 50 years ago (Kabat 1947) that shares many similarities with the acute inflammatory episodes of MS both clinically and histopathologically. EAE has been instrumental in elucidating potential mechanisms driving events that occur in MS and its use has fostered the development of currently available treatment strategies. EAE can be induced in susceptible mouse strains either by active immunization with myelin antigen (Ag) combined with a strong adjuvant or by passive immunization using adoptive transfer of T cells already primed against myelin Ag (Becher and others 2000b; Sospedra and Martin 2005).
T lymphocyte effector function in MS and EAE
Autoreactive T lymphocytes are widely accepted as the primary mediators of pathogenesis in MS and EAE. Therefore, much research is focused on elucidating the life cycle of T cells and the factors that direct their differentiation into any of several potential effector phenotypes as defined by the production of certain classes of cytokines that affect their effector function and pathogenic potential, such as Th1, Th2, and Th17 cytokines.
It is known that T cells begin their life as common lymphoid progenitors in the bone marrow, and then travel to the thymus where they mature through a process of positive and negative selection to become either cytotoxic (CD8+) or helper (CD4+) cells. Most T cells are eliminated during thymic development due to lack of stringent affinity for self-major histocompatibility complex (MHC) molecules or overt autoreactivity (positive and negative selection, respectively). T cells that do survive the thymic selection process should possess T cell receptors (TCRs) that recognize foreign but not self-Ags presented by the host's MHC class I or class II molecules. The TCRs of CD8+ T cells are activated by interaction with an MHC class I molecule. Every nucleated cell in the body constitutively expresses MHC I and presents self-peptides. If any cell is invaded by a foreign pathogen, it begins to present foreign peptide with its MHC I molecules, and CD8+ cytotoxic T cells mediate its destruction.
CD4+ T cells only recognize Ag presented by MHC class II molecules, which are present on a select population of cells called professional APCs. CD4+ T cells can potentially differentiate into one of several phenotypes with different effector functions, and therefore studies to understand the complex myriad of molecular mechanisms underlying effector differentiation of CD4+ T cells are ongoing. The process of the TCR-MHC I interaction between CD8+ T cells and somatic cells is relatively straightforward and different cytokine profiles have been reported (Cerwenka and others 1998); however, the significance of cytokine differentiation for these cells is not as well understood.
A critical factor in determining the development and polarization of CD4+ T helper cells into different effector subsets is the particular microenvironment created by the presence of cytokines, which are known to dramatically affect the behavior of nearby cells bearing the appropriate receptors. Initially, research defined 2 distinct subsets of CD4+ T helper cells based on their differential cytokine secretions and effector functions as Th1 and Th2 (Mosmann and others 1986). Th1 cells were found to predominantly release interferon (IFN)-γ and promote macrophage activities, whereas Th2 cells were associated with interleukin (IL)-4 secretion and the promotion of B cell functions. Th2 cells have been described as protective and Th1 cells as pathogenic in EAE/MS based on evidence in animal models and studies showing IFN-γ in the lesions of MS patients (Traugott and Lebon 1988; Liblau and others 1995; Heeger and others 2000). This was reinforced when treatment with recombinant IFN-γ was shown to cause relapses in patients (Panitch and others 1987). Consistent with this idea, the adoptive transfer of myelin-specific CD4+ Th1 cells causes EAE in mice. CD4+ cells can be differentiated into Th1 or Th2 cells in vitro and to some extent in vivo by adding or antagonizing cytokines (Seder and Paul 1994; Constant and Bottomly 1997). IL-12 is a potent differentiation factor toward the Th1 phenotype and has also been shown to be crucial for the development of EAE in mice (Leonard and others 1995; Segal and Shevach 1996; Segal and others 1998). However, subsequently it was discovered that Ifng−/− and Ifngr−/− mice are actually hypersusceptible to EAE (Ferber and others 1996) as are Il12−/− mice (Becher and others 2002), indicating that IL-12-driven, IFN-γ-producing Th1 cells may not be the only factor determining disease outcome. A remarkable development in our understanding of autoimmune pathology occurred when it was discovered that the p35 subunit knocked out in Il12−/− mice was not specific to IL-12 (Oppmann and others 2000) but was shared with the cytokine IL-23, which promotes the cellular secretion of IL-17. Thus, it was found that IL23−/− mice were resistant to EAE (Cua 2003) and IL-23-driven, IL-17-producing cells (Th17) have become a major research focus in the context of autoimmune disease pathogenesis. Since these discoveries there have been additional reports of other potential T cell effector subsets based on the secretion of signature cytokines such as Th9 (IL-9) or Th22 (IL-22) or even regulatory T cells, which are less well defined. However, it may be an oversimplification to define T cell subsets in this manner as it is possible that T cells, including Th17 cells, may not terminally differentiate into a particular cytokine phenotype but rather retain enough developmental plasticity to change effector function when the situation calls for it (Hirota and others 2011). Therefore, it is imperative that further research is directed to identifying the cells that are responsible for activating naïve T cells into any particular phenotype to develop a successful treatment strategy for blocking disease progression entirely.
Ag presentation to T cells in autoimmune inflammation
Ag presentation is a critical event involved in the generation of protective T cell responses against infectious agents or tumor cells, and of pathogenic, autoreactive T cell responses against self-Ags (Murphy 2008). Ag presentation requires interaction between the TCR and the processed antigenic peptides bound to MHC molecules on the surface of APCs. Studies in both EAE and MS have implicated oligodendrocytes, the myelinating cells of the CNS, and neuronal axons as the main targets of the inflammatory response associated with disease pathology. However, it is still unresolved which cells are the most potent APCs and whether, where, and why the initiation of autoimmune disease pathology begins in the CNS.
In EAE, the immune response is initiated in the secondary lymphoid tissues and the CNS becomes a target of that response, indicating that this scenario may also be similar in the human disease. It has also been proposed that an immune response can be initiated within the CNS and that infiltration of immune cells is merely a response to processes that occur in the CNS (Becher and others 2000b; Bailey and others 2007). This possible course of pathology has been demonstrated by the Theiler's murine encephalitis virus (TMEV) model of MS. In this model, persistent infection by a member of the nonenveloped Picornaviridae family (Brahic and Roussarie 2009) results in immune system-mediated CNS demyelination similar to that in MS (Vanderlugt and Miller 1996; Miller 1997). TMEV is only neurotropic in murine models, so it likely does not fully represent the disease course in humans; however, the idea that a viral infection can initiate an immune response against the CNS poses intriguing questions. If autoimmunity does not require peripheral APCs to present autoantigens to naïve T cells, then which cells are acting as APCs in the CNS and under what conditions did these APCs acquire the Ag? These questions will be the primary focus of this review.
Potential APCs in MS and EAE
In general, for APCs to activate T cells within the CNS, multiple signals must be engaged. The first is an Ag-specific signal provided by the engagement of the TCR on the surface of CD4+ T cells with an MHC-II molecule on the surface of the APC (Janeway and Golstein 1992). Evidence for this was shown in the EAE model where activated T cells were reactivated within the subarachnoid space on encounter with cognate Ag during early stages of the disease (Kivisäkk and others 2009). The second is an Ag nonspecific co-stimulatory signal that involves the interaction of different T-cell surface receptors with their respective ligands on APCs (Lenschow and others 1996; Lanzavecchia 1997) If a T cell interacts with an APC that is presenting Ag through MHC class II but does not express the appropriate co-stimulatory molecules, then the autoreactive T cell will actually be deactivated through apoptosis or anergy (Kishimoto and Sprent 1999). Important co-stimulatory molecules on the APC belong to the B7 family of molecules, predominantly CD80/B7.1 and CD86/B7.2 and their associated receptors on lymphocytes (Salomon and others 2001; Greenwald and others 2002) which regulate T cell activity for either stimulation (CD28) or inhibition (CTLA), respectively (Karandikar and others 1996; Sansom 2000). Activated T cells also transiently express CD154 (CD40L, gp39), which is the natural ligand for CD40. CD40 is a member of the tumor necrosis factor-R (TNF-R) superfamily and is expressed by dendritic cell (DC), B cells, macrophages, and endothelial cells (Banchereau and others 1994; Foy and others 1996). CD40/CD154 interactions have been shown to be crucial for the development of humoral as well as cell-mediated immune responses. Transgenic animals with defective CD40/CD154 interactions do not develop EAE, and CD154 antagonist can inhibit ongoing EAE induced by adoptive transfer as well as active immunization (Gerritse and others 1996; Howard 1999).
APCs also secrete cytokines such as IL-12, IL-4, and IL-23, which are potent differentiation factors into the Th1, Th2, or Th17 phenotypes, respectively, of CD4+ T helper cell populations and therefore APCs regulate and direct the T cell response. Cytokine production by APCs is tightly regulated (Trembleau and others 1995; Ma and others 1996). For example, TNF-α has been reported to be required to drive IL-12 production (Flesch and Kaufmann 1995). Therefore, for a cell to be a successful APC it must produce pathogenic cytokines in addition to possessing MHC-loaded Ag and co-stimulatory factors at the right time and the right place.
Microglia
Substantial evidence points to microglia as the potential primary candidate for immune activation in the CNS by a tissue-resident cell population. It is well accepted that microglia do not arise from a neuroectodermal progenitor but from a hematopoietic precursor that colonize the CNS during development. In the first few postnatal weeks these monocytes develop an increasingly complex morphology as they differentiate to form microglia. Microglia are believed to play an important role in CNS remodeling during the developmental process, but their function in the adult CNS is not fully understood. Unfortunately, one of the complicating factors in the efforts to describe the behavior of microglia in the naïve brain, or under inflammatory conditions, is the lack of consensus regarding cell-type-specific markers that can be used to distinguish this CNS-resident population from infiltrating myeloid-derived cells arriving from the periphery. As a result, one of the major questions in the field today is determining which population of cells is responsible for the Ag presenting mechanism driving the pathogenesis behind MS and EAE. Almolda and others recently showed that along the different phases of EAE both MHC class I and II molecules co-localized with 2 different markers of microglial cells, IBA1 (Ito and others 1998) and TL (Acarin and others 1994; Almolda and others 2010). F4/80 is one marker that has been used to identify tissue-resident macrophages such as microglia (Austyn and Gordon 1981) although the differential staining of CD11b+/CD45hi (circulating macrophages) and CD11b+CD45lo (microglia) has proven to be useful for determining cellular origin as well. The pattern recognition receptor CD14 has also been suggested to serve as a de novo activation marker for human microglia (Becher and Antel 1996; Becher and others 1996).
Despite these uncertainties, within the CNS parenchyma, the microglia appear to be the most capable to initiate and sustain a T cell-mediated immune response (Carson and others 1998; Fischer and Reichmann 2001). Along these lines, among the tissue-resident cell populations in the CNS, only microglia appear to have the necessary machinery to process and present Ag. Several studies have shown microglia as the only CNS-resident cells that possess mature immunoproteasome expression, a specialized multicatalytic protease complex believed to be involved in the generation of peptides presented as Ag in MHC molecules (Stohwasser and others 2000), which appears to be upregulated in the presence of lipopolysaccharide (LPS) or IFN-γ. In humans, both parenchymal microglia and perivascular microglia/macrophages have been shown to constitutively express MHC II within the CNS in normal nonpathologic conditions (Gehrmann and others 1993; Bo and others 1994; Ulvestad 1994; Williams and others 1994b; Becher and Antel 1996). Under virtually all inflammatory conditions, parenchymal microglial cells rapidly upregulate MHC class II expression, suggesting that they participate in the Ag presentation process that occurs during the inflammatory process. Microglia express MHC class II in different EAE models in both rats (Craggs and Webster 1985; Matsumoto and Fujiwara 1986; McCombe and others 1992, 1994) and mice (Lindsey and Steinman 1993; Pope and others 1998; Juedes and Ruddle 2001; Ponomarev and others 2005).
CNS perivascular macrophages have been demonstrated to be competent APCs by both in vitro and in vivo studies. In the rat, perivascular macrophages were found to support significantly greater proliferation of myelin basic protein (MBP)-reactive CD4+ T cells compared to the capacity of parenchymal microglia isolated from the same animals (Ford and others 1995). Additionally and in contrast to resident parenchymal microglia, perivascular cells are frequently repopulated by bone marrow-derived monocytes. Perivascular APCs are important for the homing of MBP-reactive T cells into the CNS (Hickey and Kimura 1988). Depletion of macrophages using toxin-loaded liposome treatment inhibits the onset of EAE (Tran and others 1998) and results in depletion of perivascular macrophages and accumulation of inflammatory T cells in the perivascular areas but not infiltration into the parenchyma. Thus, it is conceivable that parenchymal microglia initiate the inflammatory cascade, and perivascular microglia/macrophages perpetuate it. However, at this time most of the available data support the idea that initiation of T cell reactivation occurs in the choroid plexus or meninges.
Several co-stimulatory molecules are found on microglia as well (Miller 1995; Hurwitz and others 1997; Karandikar and others 1998; Perrin and others 1999; Chang and others 2000b, 2003; Girvin and others 2000). Human microglia in situ in MS lesions express B7.1 (Williams and others 1994a; Windhagen and others 1995). Using microglia isolated from the noninflamed human CNS, it was later shown that the pattern of B7.1-2 expression is similar to that seen in professional Ag-presenting DCs, in that B7.2 is constitutively expressed by human microglia and B7.1 can be induced upon their activation (Becher and Antel 1996). Microglial cells in MS lesions have also been shown to express CD40 (Gerritse and others 1996).
Microglia are one of the most important cells in the CNS cytokine network. Microglia are known to produce IL-1, IL-5, IL-6, TNF-α, and transforming growth factor-β (TGF-β) and respond to several other cytokines such as granulocyte macrophage colony-stimulating factor (GM-CSF), IL-4, TGF-β, and IFN-γ (Sawada and others 1995). Human (Windhagen and others 1995; Becher and others 1996) and mouse (Aloisi and others 1998) microglia are potent producers of IL-12 and can therefore act as regulators of Th1 differentiation. Early response inflammatory mediators produced by microglia include proinflammatory cytokines (IL-1B, TNF-α, and IL-6) that can permeabilize the blood-brain barrier (BBB) as well as chemokines (Mip-1α, IP-10, etc.) a complex family of molecules that are involved in the regulation of immune cell migration (Pan and others 1997; Utans-Schneitz and others 1998). Microglia are a potent source of TNF-α, and Becher and others (2000a) showed that TNF-α levels increase rapidly after CD40 engagement and only hours later IL-12 is produced, providing further evidence that cytokines are tightly regulated in microglia as they are in professional APCs. Additionally, TGF-β and IL-2 can also be produced by microglia and together these cytokines are believed to drive Th cells into a regulatory phenotype. The production of IL-23 by microglia is especially intriguing, as it has recently been shown that microglia also express its receptor, IL-23R, implicating that it may function in an autocrine manner to enhance the IFN-γ-induced signal transducer and activator of transcription 1 phosphorylation and subsequent cytokine production (Sonobe and others 2008), particularly of IL-17. IL-17 induction by microglia could mean that they are capable of orchestrating a stronger immune response than previously appreciated and contributing significantly to autoimmune disease such as EAE/MS (Kawanokuchi and others 2008).
Taken together, these studies indicate that microglia have the potential to participate in Ag presentation and T cell activation in the CNS and therefore may be crucial for the development of EAE and MS. However, it remains to be determined if microglia do indeed play this role as so far it is unknown under what conditions, if any, microglia process and present myelin Ags in situ in the CNS or at what point in the disease process this may occur. Furthermore, the factors that regulate microglia activation and effector function need better refinement. For example, how do CD4+ T cell-derived pro- and anti-inflammatory cytokines regulate microglia, both perivascular and parenchymal? Lastly, it is unclear if microglia can activate naïve T cells in situ, as has been suggested for DCs. The vast majority of studies related to this topic have predominantly focused on the status of microglia at the peak of acute inflammatory conditions, which has provided a great deal of useful information. However, to further pinpoint mechanisms of disease initiation or progression, it would be beneficial to have a complete picture of microglial activity over the entire disease course.
Dendritic cells
DCs are considered the archetypical professional APCs because they continuously capture and present Ag to T cells, but very little is known about their role in the CNS. Like microglia, DCs begin their life as hematopoietic bone marrow progenitor cells. However, rather than extravasating into tissues and becoming morphologically distinct tissue-resident cells, DCs continue to circulate in the periphery, constantly sampling their surrounding environment for pathogens such as bacteria or viruses. Once they have come into contact with presentable Ag, they mature and migrate to the spleen or lymph nodes where they activate T cells.
Despite that DCs are not CNS-resident cells, they have been found in the lesions of MS patients (Greter and others 2005; Serafini and others 2006; Lande and others 2008), indicating that they do play a role in disease pathology. Therefore, an important question is where peripheral DCs encounter the CNS-specific Ag.
The exact lineage divergence of DCs from monocytes is still unclear, and the use of antibody recognition of cell-type-specific surface markers has demonstrated that diverse heterogeneity exists among hematopoietic-derived progenitors. It has been proposed that 2 major subsets of conventional myeloid-derived DCs (mDCs) can be defined based on the differential expression of the C-type lectins CD205 (DEC205) and DCIR2 (33D1) as well as the glycoprotein CD8 (Iyoda and others 2002; Shortman and Liu 2002; Dudziak and others 2007). A recently described class of plasmacytoid DCs (pDCs) that are morphologically similar to plasma cells but share many characteristics with mDCs appear to arise from a common progenitor of monocytes and classical DCs now referred to as the macrophage and DC precursor (McKenna and others 2005). A complex characteristic surface phenotype (Lin- cKithi CD115+ Flt3+) was used to distinguish cells from this lineage in contrast to the common DC precursor lineage (Lin- cKitlo CD115+ Flt3+) (Naik and others 2007; Onai and others 2007), further emphasizing that we have a long way to go before we fully understand the complete differentiation pathway of hematopoietic stem cells produced in the bone marrow into functional APCs.
As a result, most studies describing the specific activities of microglia/macrophages and/or DCs need to be evaluated in the broader context of overlapping phenotypes and functions. Recent studies have reported that a specific population of DCs infiltrates the CNS in EAE rats (Matyszak and Perry 1996; Serafini and others 2000) and plays a central role in Ag presentation in murine EAE models (Greter and others 2005; Bailey and others 2007), opening the possibility that DCs can also be involved in the mechanism of Ag presentation in this acute model. In one study, mice with MHC II expressed only on DCs developed EAE similar in onset and severity to wild-type mice, indicating that DCs have the capability to present myelin Ag to pathogenic T cells even in the absence of Ag presentation by other functional APCs (Greter and others 2005). However, it has also been suggested that microglial cells are a population of tissue-resident immature DCs (Carson and others 1998; Almolda 2011) that, when activated under particular circumstances, may acquire a mature DC phenotype (Fischer and Reichmann 2001; Santambrogio and others 2001). Recent studies have shown that a subpopulation of microglial cells constitutively express CD11c (Bulloch and others 2008), an integrin frequently used as a cell-specific marker of DCs. Additionally, CD1, an immature DC marker (Serafini and others 2006), is also expressed by a set of CD11b+ or TL+ cells that morphologically appear to be an early monocyte/macrophage/microglia population (Almolda and others 2010). Although pDCs are widely viewed as capable of providing only immunomodulatory support, recent evidence suggests that they may express MHC II and secrete pro-inflammatory cytokines (Villadangos and Young 2008). More recently, mDCs have been shown to be capable of perpetuating CNS targeted autoimmunity when Ags are readily available, but other APCs are required to efficiently initiate pathogenic cognate CD4+ T cell responses (Wu and others 2011).
As professional APCs, DCs are capable of communicating with other cells via direct contact through ligand–receptor interactions as well as from a distance through cytokine signaling, primarily to induce T cell differentiation and expansion. DCs secrete proinflammatory cytokines to activate T cells into a particular effector phenotype and also possess cytokine receptors in order to respond to stimulation from their environment. Immature DCs respond to IFN-γ strongly and quickly change their gene expression to upregulate transcription of genes related to migration and Ag presentation. Once a DC becomes activated, it upregulates the chemokine receptor CCR7 upon activation, which induces them to travel through the bloodstream to the spleen or through the lymphatic system to a lymph node where they present Ag to T cells. Activation also results in upregulation of costimulatory molecules and proinflammatory cytokines such as IL-12, which directs the Th1 response. After their migration, DCs would have to infiltrate the CNS to present myelin-Ag to primed, autoreactive T cells for them to gain full effector function. It was previously believed that T cells infiltrate the CNS parenchyma via meningeal vessels; however, recent evidence has pointed to an alternative path of entry via vessels of the choroid plexus and it is possible that DCs follow this route as well.
Potential Sites of T Cell–APC Encounters and Neuroantigen Presentation
The CNS is comprised of the brain and spinal cord, which are encased in bone and a 3-layer membrane referred to as dura mater, arachnoid mater, and pia mater from outside to inside (Ransohoff and others 2003), which are bathed and nourished by cerebrospinal fluid (CSF). The BBB is an efficient physiological checkpoint for regulation of CNS biochemical homeostasis and filtering out of blood-borne infections from entering the CNS, but in doing so, it also cordons off peripheral immune surveillance. This was historically looked upon as a benefit, as a robust immune attack on nonreplicating viral-infected neurons might cause significantly greater damage than the infection itself, a fatal risk that such a vital organ system cannot afford to take. Highly regulated Ag presentation to infiltrating T cells along with the lack of a conventional lymphatic drainage system in the CNS, in addition to constitutive neurotrophin and TGF-β secretion, have all been proposed as key factors responsible for maintaining this immunological quiescence (Dorries 2001). However, there is evidence that in healthy individuals activated memory T cells enter the CSF directly from the systemic circulation and monitor the subarachnoid space retaining the capacity to initiate local immune reactions (Kivisakk and others 2003). Therefore, immune surveillance from the point of view of T cells and myeloid cells is potentially involved in disease initiation.
As pointed out earlier, auto-aggressive CD4+ T cells infiltrating the CNS most likely received their primary stimulation in the immune periphery. Upon entry to the CNS, it is necessary for their full activation that they re-encounter an MHC class II-bearing APC carrying their target Ag. This Ag presentation could conceivably be conducted by peripheral macrophages, resident microglia or DCs, or other cells with similar Ag presenting properties such as B cells. Answering this question is complicated by a lack of definite cell-specific markers for CNS intrinsic or extrinsic APC populations although some markers have been suggested, including recently the chemokine receptors CCR2 and CX3CR1 to differentiate inflammatory macrophages from resident microglia (Geissmann and others 2003; Saederup and others 2010). A fundamental question that has thus far not been addressed is where and under what conditions APCs acquire neuroantigens for MHC II-restricted presentation to CD4+ T cells. Therefore, answering this question may provide clues to solving the puzzle of the role and importance of specific APCs in the context of demyelinating diseases such as MS.
Possible anatomic locations for the activation of T cells responsive to myelin Ags include both peripheral lymphoid organs, such as the spleen or deep cervical lymph nodes, where myelin debris might accumulate (Hochwald and others 1988; Yamada and others 1991; Ling and others 2003; Karman and others 2004) and the CNS, where myelin epitopes might be processed and presented by the resident microglia, infiltrating macrophages (Hickey and Kimura 1988; Katz-Levy 2000; Mack and others 2003) and/or DCs (Serafini and others 2000; Fischer and Reichmann 2001). It has also been shown that naïve T cells might be able to gain access to the inflamed CNS, potentially bypassing the need for activation in peripheral lymphoid sites (Krakowski and Owens 2000; Greter 2005; McMahon 2005).
Although research predominantly focuses on white matter locations, MS patients also develop gray matter lesions and cortical demyelination plays an important role in the pathology of MS (Brownell and Hughes 1962; Lumsden 1970; Kidd and others 1999; Peterson and others 2001; Bo and others 2003; Kutzelnigg and Lassmann 2005). Microglia are stationed throughout the normal adult brain, although substantially more are located in gray matter than white. Because current research indicates that gray matter is affected first in MS and EAE, it is possible that microglia in this region are the first to initiate the Ag presentation signal in the disease context. There is more evidence of vascular changes than glial involvement in gray matter lesions when compared with white matter lesions, suggesting that one possible mechanism during injury or infection consists of microglia presenting Ag in the gray matter to incoming peripheral macrophages, which then can travel to cervical lymph nodes to recruit lymphocytes and professional APCs to intensify the immune response and enhance cleanup of the CNS. Thus, while research using models established so far indicates that initiation of T cell reactivation more often occurs in the choroid plexus or meninges, it may be useful to consider alternative locations.
Contribution of CNS-Resident Cells to Immune-Mediated Demyelination
Disease symptoms of MS arise from the demyelination of neuronal axons; therefore, this is likely to be the primary location of neuroantigen release. To fully understand the mechanisms underlying this event, it is important to examine the potential contribution of CNS-resident cells inherently involved in the synaptic microenvironment affected by immune-mediated demyelination (Table 1).
Table 1.
Characteristics of Cells Potentially Contributing to Disease Pathogenesis in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis
| Cell type | Characteristic features | Potential contribution to MS/EAE |
|---|---|---|
| Parenchymal microglia | MHC class II, PRRs, co-stimulatory molecules, immunoproteasome machinery, pro-inflammatory cytokines | APC, disease initiation |
| Peripheral microglia/macrophages | MHC class II, PRRs, co-stimulatory molecules, immunoproteasome machinery, pro-inflammatory cytokines | APC, disease initiation or progression |
| Myeloid-derived DCs | MHC class II, PRRs, co-stimulatory molecules, immunoproteasome machinery, pro-inflammatory cytokines | APC, disease progression |
| Plasmacytoid DCs | MHC II (inducible), PRRs, pro-inflammatory cytokines | APC, immunoregulation |
| neurons | PRRs, pro-inflammatory cytokines | Immunoregulation |
| Astrocytes | MHC class II (inducible), PRRs (TLR3 only), pro-inflammatory cytokines | Immunoregulation |
| Vascular endothelial cells | MHC class II (inducible), co-stimulatory molecules (inducible) | Immunoregulation |
DC, dendritic cell; MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; TLR3, toll-like receptor 3; APC, antigen presenting cell; MHC, major histocompatibility complex; PRR, pattern recognition receptor.
Neurons
Neuronal axons of all vertebrates are myelinated (Bullock and others 1984) to allow for rapid impulse propagation. In MS the demyelination caused by inflammatory lesions impairs neuronal signaling and communication in the CNS. Although neurons are thought of as passive bystanders during viral infection or traumatic injury, recent evidence suggests that extracellular insult causes neurons to express immune factors characteristic of lymphoid tissues and therefore potentially contribute to immunoregulation in the CNS (Chakraborty and others 2010). Furthermore, damaged neurons release many factors into the surrounding microenvironment that can have an effect on CNS-resident or infiltrating cells. For example, damaged or overactive neurons release nucleotides such as ATP (Wang and others 2004) or UDP (Koizumi and others 2007) and microglia express several receptors whose nucleotide binding activity can trigger a variety of cellular responses both in vitro and in vivo (Honda and others 2001; Suzuki and others 2004; Davalos and others 2005; Haynes and others 2006; Inoue 2006; Koizumi and others 2007; Ohsawa and others 2007). P2Y6 receptors on microglia have been associated with phagocytic activities that are upregulated in vivo after neuronal damage (Koizumi and others 2007). Excessive glutamate levels are associated with neurodegeneration (Farber and others 2005) and although free glutamate directly leads to neuronal death it may also serve as an activation signal for microglia (Pocock and Kettenmann 2007), who express a variety of glutamate receptors (Biber and others 1999; Hagino and others 2004; Taylor and others 2005). Matrix metalloproteinases (MMPs) are proteolytic enzymes that may be released from neurons undergoing apoptosis and recombinant MMP-3 causes microglial cultures to release the cytokines TNF-α, IL-6, IL-1β, and the IL-1 receptor antagonist IL-1Rα (Kim and others 2005, 2007). Damaged neurons have even been shown to release chemokines (Erichsen and others 2003; Dijkstra and others 2004; Limatola and others 2005), which suggests that upon insult or injury neurons use both short distance and long distance communication to call for help.
All neuronal signaling methods mentioned result in microglial activation and less likely affect CNS homing T cells directly. Thus, substantial evidence indirectly implicate microglia as the main immunological mediator in the CNS, although thus far no one has been able to directly demonstrate the cascade of events that occur in complex neuroinflammatory conditions such as MS, which is necessary to advance successful therapeutic modalities. Importantly, neurons themselves are not capable of Ag presentation via MHC class II molecules and must therefore signal via another cell in order for CD4+ T cells to become activated and mediate pathology. Whether this particular role is played by microglia remains to be determined.
Oligodendrocytes
Oligodendrocytes are the myelinating cells of the CNS. The myelin sheaths are protein- and lipid-rich structures that stabilize, protect, and electrochemically insulate axons. They are an extension of oligodendrocytes and both the cell body and the sheaths are main targets of infiltrating immune cells in EAE and MS along with neurons. Oligodendrocytes myelinate several axons simultaneously (Nave 2010); therefore, it is important to understand what effects immune-mediated demyelination has on the entire microenvironment of a lesion area including the cell body as well as the sheaths that it produces. From animal studies it has been shown that myelin is formed by oligodendrocytes that are derived from morphologically complex oligodendrocyte precursor cells (OPCs) that can be distinguished by their expression of the proteoglycan protein NG2. However, the life cycle of OPCs is still not well understood (Richardson and others 2006; Trotter and others 2010), and although remyelination can be observed as a result of OPC activation under noninflammatory conditions, this population of cells appears to be relatively quiescent in MS due to a blockade of oligodendrocyte differentiation and maturation processes (Scolding and others 1998; Wolswijk 1998; Chang and others 2000a, 2002; Kuhlmann and others 2008) perhaps due to a block in ERK2 signaling (Fyffe-Maricich and others 2011), an avenue that is also currently being explored in our laboratory. Mature oligodendrocytes generate myelin by enveloping axons with their own cell membrane in a spiral shape, which eventually becomes a multilayered sheath covering a long segment of axon (Nave 2010). In the CNS, compact myelin has been shown by electron microscopy to have a periodic ultrastructure which requires the expression of structural proteins such as MBP, myelin oligodendrocyte glycoprotein (MOG), and proteolipid protein. The functions of most myelin-associated proteins, which have only recently been identified by proteomics (Jahn and others 2009), are not well understood. It is clear that that they can be presented as Ags and that autoreactive T cells specific to these and other less well-studied Ags play a key role in driving demyelinating pathogenesis. Although oligodendrocytes produce and contain myelin, they cannot present it as an Ag to pathogenic CD4+ T cells because they do not constitutively express MHC class II (Wong and others 1984) and cannot be induced to in vitro either. Nevertheless, they likely play a significant role in the release of self-Ags for the capture and presentation by neighboring microglia.
Although oligodendrocytes are not believed to secrete cytokines to initiate an immune response, they do have the capability to respond to cytokines in their microenvironment. For example, both IL-1β and IL-1β receptor are constitutively expressed in the CNS by oligodendrocytes and IL-1β inhibits OPC proliferation and enhances the survival of mature MBP+ oligodendrocytes (Vela and others 2002). IL-6R is present on oligodendrocytes and binding of IL-6 supports oligodendrocyte differentiation and survival (Pizzi and others 2004, Valerio and others 2002). Of particular interest, IFN-γ protects against myelin damage in a cuprizone-induced demyelination animal model (Gao and others 2000), whereas ectopic overexpression of IFN-γ in the retina protects against neurotropic viral infections but leads to CNS demyelination (Geiger and others 1994), although the exact mechanism of this differential protection remains undetermined.
Vascular endothelial cells
Cerebral microvascular endothelial cells have a unique anatomical location between circulating T cells and the extra-vascular sites of Ag exposure. Endothelial cells, unlike perivascular microglia, do not constitutively express MHC class II molecules in vivo or in vitro; however, they can be induced to do so (Jemison and others 1993; Prat and others 2000). Furthermore, murine brain endothelial cells are reported to process and present MBP and can induce the proliferation of sensitized T cells (McCarron and others 1986). The inducibility of MHC II molecules on brain endothelium by IFN-γ has been linked to the activation of the MHC-II transactivator (CIITA) and seems to correlate with EAE susceptibility (Nikcevich and others 1997). In EAE, expression of MHC II molecules on endothelial cells increases before immune cell infiltration of the brain (Sobel and others 1984), suggesting that this may be a significant event during brain inflammation. Human and mouse endothelial cells express B7 molecules in culture when stimulated with IFN-γ (Prat and others 2000). However, two different labs both failed to induce T cell proliferation with brain endothelial cells in two different models of Ag presentation (Risau and others 1990; Prat and others 2000). It is well established that T cells invade tissues by extravasating through vascular endothelial cells via integrin receptors such as VCAM-1; thus, it is this interaction that is targeted by the MS drug natalizumab. Endothelial cells can also secrete CSFs and could therefore potentially participate in the activation or proliferation activities of CNS-resident microglia or perivascular macrophages and possibly even extravasating immune cells. Therefore, vascular endothelial cells can contribute to the cytokine microenvironment, but it remains unclear if they can activate T cells in the CNS.
Astrocytes
Astrocytes are the link between the BBB and the CNS, providing structural support for neurons and isolating synapses into microenvironments and aiding in the smooth transmission of neural messages (Barker and Ullian 2010). It is well accepted that they have a role in regulating CNS immunity (Nair and others 2008); however, their contribution and capacity to present Ag and activate T cells are still controversial. Astrocytes can contribute significantly to the cytokine crosstalk in the CNS because they can produce many of the same cytokines as microglia and DCs. Astrocytes also secrete the cytokine leukemia inhibitory factor that promotes the myelinating activity of oligodendrocytes when they detect extracellular ATP (Ishibashi and others 2006) and have been shown to exhibit phagocytic activity (Sellers and others 2009) in an injured brain. Human and murine astrocytes only express 1 toll-like receptor (TLR) constitutively, TLR3, which detects double-stranded RNA (dsRNA) common to viruses (Farina and others 2005; Jack and others 2005; Scumpia and others 2005), and its upregulation can be triggered by IFN-γ, a T-cell derived cytokine, or IL-1β, which is produced by microglia (Farina and others 2005). Poly I:C stimulation, which synthetically mimics dsRNA, induces upregulation of TLR2-4 in vitro as well as several growth and neuroprotective factors (Jack and others 2005; Scumpia and others 2005; Bsibsi and others 2006; Rivieccio and others 2006). Even though astroglia do not express detectable amounts of MHC II under normal conditions, they can be induced to do so by stimulation with IFN-γ (Fontana and others 1984; Fierz and others 1985; Takiguchi and Frelinger 1986), although this appears to favor induction of T cell anergy rather than effector activation (Matsumoto and others 1992; Ulvestad 1994; Williams and others 1995), which is consistent with a lack of co-stimulatory molecules (Satoh and others 1995). Astrocytes have also been shown to express B7 upon stimulation with IFN-γ in mice and can therefore present Ag to naïve T cells under certain conditions (Tan and others 1998), although this implies that an already activated T cell is giving the signal to the astrocytes and that another cell type must be responsible for initiating the cascade. Thus, it has remained unclear to what extent astrocytes can process and present protein Ags via MHC II (Kort and others 2006).
Potential Conditions of Neuroantigen Release
An important factor to consider in understanding the disease course of immune-mediated demyelination is the conditions in which CNS Ags are made available to APCs. Cells with compromised membrane integrity release their contents into the environment causing extracellular debris, making it possible that neuroantigen release could arise from several potential scenarios such as a proinflammatory cytokine environment created by infection with neurotropic pathogens, synaptic remodeling via neuronal apoptosis, or even normal oligodendrocyte/myelin turnover.
Over the past decade, extensive studies on members of the membrane-bound TLRs and cytosolic retinoic acid-inducible gene-like receptors have characterized these proteins to play a central role in specific sensing of foreign pathogens for initiating immune responses in the host cells. CD14 is the primary receptor for LPS, a cell-wall component of gram-negative bacteria, and once complexed together they bind to TLR4, a mechanism that has also been shown to be involved in the recognition of apoptotic cells, and therefore to stimulate the phagocytic machinery of mononuclear phagocytes (Devitt and others 1998). The flipping of phosphatidylserine from inner to outer leaflet of the cell membrane is one signal that has been shown to be recognized by microglia (Fadok and others 1992; Koopman and others 1994; Martin and others 1995; Kulprathipanja and Kruse 2004). Pattern recognition receptors have also been shown to be engaged and to signal microglia activation in response to peptides originating from misfolded proteins as well (ElKhoury and others 1996). Parenchymal microglia share many molecular structures with phagocytic macrophages and it has been elegantly demonstrated that microglia are constantly extending and retracting their processes performing tissue surveillance in the nervous system (Thomas 1992; Hickey 2001). Microglia have been shown to phagocytose dead cells and clear the cellular debris in brain slices (Brockhaus and others 1996; Czapiga and Colton 1999; Petersen and Dailey 2004). Thus, microglial cells in particular have the ability to not only recognize foreign structures, but also initiate an inflammatory cascade upon encountering dying or damaged cells.
Neurotropic viruses are likely to follow the general routes of viral entry into the body from extraneural sites, and therefore virus-recognizing immune cells are first activated in regional peripheral lymph nodes (Savarin and others 2008), thereby supporting the possibility that the adaptive immune responses are initiated against neurotropic viruses even before innate mechanisms are triggered in the CNS upon infection (Griffin 2003; Bergmann and others 2006).
One highly proposed mechanism for the induction of autoimmune diseases is molecular mimicry, in which viruses express Ags that mimic host proteins (Karlsen and Dyrberg 1998). It is proposed that neuroantigens that share homology with viral envelope (Oldstone and VonHerrath 1996; Talbot and others 1996; Monteyne and others 1998) might misdirect an immune response against self-Ags, or native proteins. It has been hypothesized that MS can be triggered by this mechanism (Oldstone 1987) and in support of this, T cells derived from HLA-DR2+ MS patients respond to both myelin-derived peptides and peptides from common viruses such as herpes simplex virus, adenovirus, Epstein-Barr virus (EBV), and influenza type A virus (Wucherpfennig and Strominger 1995). Although molecular mimicry is theoretically attractive, and some structural similarity has been shown between at least one autoantigen (MBP85-99) and an epitope from an infectious agent (EBV627-641) (Lang and others 2002), no specific pathogen has been conclusively identified as a trigger of MS. Importantly, the fact that a neurotrophic virus can induce an autoimmune response supports the idea that endogenous cells of the CNS can present Ag and stimulate the adaptive immune system.
In TMEV, the initial CNS endogenous response against the virus possibly generates a minor degree of tissue destruction that would lead to the release of neuroantigens that in turn could be recognized by autoreactive T cells, either within the CNS or in regional lymph nodes, although this is not known for sure. These T cells can then be recruited to the target tissue (CNS) and drive the inflammatory process even further, a process called “epitope spreading” (Lehmann and others 1992; Vanderlugt and Miller 1996). In this process, the immune response spreads to Ags other than those that initiated the disease response. Although the functional significance of epitope spreading has been shown in mouse models of EAE and suggested in MS itself, very little is known about the anatomic location of epitope spreading or the APC population(s) involved.
Contactin-2 (CNTN2) is a glycosylphosphatidylinositol (GPI)-anchored neuronal membrane protein that has been proposed as an unconventional CNS autoantigen (Derfuss and others 2009) that could potentially contribute to demyelination despite it not being a component of myelin itself. CNTN2-specific T cells preferentially caused gray matter damage similar to that seen in early MS. Interestingly, this cannot cause lesions on its own as introduction of MOG-specific T cells can; however, the authors suggest that the inflammatory response in gray matter was associated with BBB damage since co-injection of anti-MOG mAb initiated demyelination in the CNS that was not seen in wild-type mice.
Neuroantigen release could occur in the context of normal cellular maintenance, particularly of oligodendrocytes, since they are producing the myelin sheath. However, the dynamics of myelin or even oligodendrocyte turnover remain elusive. Recently, a genetic model of oligodendrocyte loss has been developed and initial studies have shown that acute loss of adult oligodendrocytes leads to large amounts of extracellular myelin debris (Takahashi and others 2007) as well as myelin vacuolation in Iba-1+ microglia/macrophages (Pohl and others 2011). However, these authors did not report any significant accompaniment of infiltrating T cells. Because there was an absence of blood-derived serum proteins in the brain tissue, they attribute the lack of T cell infiltration to an intact BBB, implying that an initial BBB-compromising event must coincide with phagocytic myelin debris cleanup in order to produce the symptoms of EAE and MS.
In some cases, a rise in viral titers in the CNS causes alterations in integrity of the BBB and microglial activation in the host, even in the absence of clear clinical signs of disease (Fabis and others 2008). dsRNA is a conserved molecular pattern retained by many viruses, and therefore it can be considered a viral pathogen-associated molecular pattern (PAMP). Poly I:C is a synthetic analogue of viral dsRNA that is recognized by TLR3 (Alexopoulou and others 2001). Downstream signaling through TLRs activates interferon regulatory factors and nuclear factor kB that leads to the production of type I IFNs (IFN-α and -β) as well as pro-inflammatory cytokines that directly stimulate antiviral actions and modulate adaptive immune responses (Kawai and Akira 2006). While virus-infected cells release IFN-α/β, activated NK cells and CD8+ and CD4+ cells produce IFN-γ (Rottenberg and Kristensson 2002). Since the entry of these cells into the CNS is minimal under normal circumstances, IFN-γ is usually not detectable in the brain (Fabry and others 1994); however, IFN-γ expression rises rapidly in acute inflammatory reactions caused by viral infection (Binder and Griffin 2001).
The role of IFN-γ in protecting the CNS and promoting viral clearance has been described for neurotropic viruses (Patterson and others 2002a, 2002b; Rodriguez and others 2003; Hausmann and others 2005; Shrestha and others 2006) and infected neurons have been shown to respond to IFN-γ by lowering viral replication through both viral protein and RNA synthesis interference (Burdeinick-Kerr and Griffin 2005). Early reports have mainly discussed the beneficial effects of IFN-γ on the survival (Chang and others 1990), growth (Erkman and others 1989), and differentiation of neurons in vitro (Jonakait and others 1994); however, it cannot be overlooked that its release has many other effects on the microenvironment as well. Levels of IFN-γ, released during acute and chronic infections and immunological reactions, are sustained for a long time after viral clearance and during viral latency (Fan and others 1993). Prolonged exposure of neurons to IFN-γ at concentrations similar to those detected after chronic viral infection in humans and mice has been correlated with major dendritic retraction (Masliah and others 1997; Rockstroh and others 1998). Dendritic retraction of neurons has also been correlated (Park and Skerrett 1996) with elevated IFN-γ expression (Kristensson and others 1994; Lau and Yu 2001) in many types of acute inflammatory reactions, including those triggered by trauma, stroke, and axotomy. Dendritic retraction could be a potential cause of myelin turnover and therefore a source of neuroantigen release. Additionally, the presence of IFN-γ activates microglia and macrophages, which may be primarily stimulating these cells to initiate cleanup and block further neuronal damage, but might have the side effect of beginning the Ag presentation process inadvertently.
Conclusions
Although a great deal of information has been gained in the past 2 decades about neuroantigen presentation, immune cells and their cytokines, and inflammatory mediators involved in MS and EAE, more research is needed to fully decipher the role of each cell type and their products in the pathogenesis of this disease. Specifically, it remains undetermined which cells are the first to acquire and present neuroantigens and what the conditions of its transfer to these cells are. It has long been held that initial Ag presentation and T cell activation events occur in the immune periphery and then translocate to the CNS. However, an increasing body of evidence suggests that Ag presentation might initiate within the CNS itself. Although this may not be the ultimate order of events, even an adaptive immune response that occurs secondary to primary CNS events, such as CNS cleanup efforts, has to be carefully evaluated for its detrimental as well as potentially beneficial effects. Myelin reactive T cells produce not only effector molecules and mediate tissue destruction, but can also produce neurotropic factors and provide trophic support to aid neuronal survival (Kerschensteiner and others 1999; Moalem and others 1999). The challenge remains to understand how these events culminate in the destructive demyelinating pathology seen in MS or its mouse model EAE and could potentially be harnessed for tissue repair. Clearly, a much better understanding is needed of the role of inflammatory mediators and cytokines in the disease process and some of the conflicting results must be resolved, for example, the dual role of IFN-γ and TNF in promoting or inhibiting disease. Multiple cell types, both CNS-resident and those infiltrating from the periphery, can potentially produce and respond to multiple cytokines (Fig. 1). It is imperative that we continue to elucidate which cells are producing which cytokines in the CNS during the course of EAE. The ability to block the recruitment of APCs or their acquisition of neuroantigens responsible for the activation of pathogenic CD4+ T cells in the CNS could provide a viable, side-effect-free target for inhibiting relapses and disease progression in human MS.
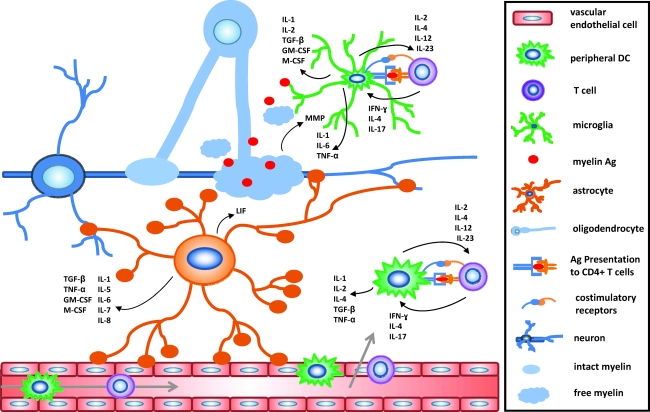
FIG. 1.
Cytokine microenvironments in the CNS during MS as a result of Ag presentation. An extensive cytokine network is created in CNS microenvironments after activation of CD4+ T cells by direct contact of the T cell receptor with MHC II on APCs as well as cell-to-cell contact between costimulatory receptor interactions present on both cell types, such as CD80/CD86. The cytokine crosstalk between autoreactive, primed T cells and APCs takes place in the CNS microenvironment and therefore can affect, and be affected by, CNS-resident cell types as well. It will be important for both potential biomarker identification and therapeutic development to determine what cell populations can act as APCs in the CNS and which cytokines are produced by a single cell at one time. CNS, central nervous system; MS, multiple sclerosis; DC, dendritic cell; APC, antigen presenting cell; Ag, antigen; IL, interleukin; IFN, interferon; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; MMP, matrix metalloproteinase; LIF, leukemia inhibitory factor; GM-CSF, granulocyte macrophage colony-stimulating factor.
Acknowledgments
We thank Drs. Astrid Cardona and Susan Howard-Byerly for careful reading of the article and helpful discussions. This work was supported by grants NS-52177 and 2G12RR013646-11 from the National Institutes of Health, and grant RG3701 from the National Multiple Sclerosis Society (T.G.F.).
Author Disclosure Statement
No competing financial interests exist.
References
- Acarin L. Vela JM. Gonzalez B. Castellano B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat-brain by tomato lectin-binding. J Histochem Cytochem. 1994;42(8):1033–1041. doi: 10.1177/42.8.8027523. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L. Holt AC. Medzhitov R. Flavell RA. Recognition of double-stranded RNA and activation of NF-kappa B by toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Almolda B. González B. Castellano B. Activated microglial cells acquire an immature dendritic cell phenotype and may terminate the immune response in an acute model of EAE. J Neuroimmunol. 2010;223(1–2):39–54. doi: 10.1016/j.jneuroim.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Almolda B. Gonzalez B. Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci. 2011;16:1157–1171. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Ria F. Penna G. Adorini L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol. 1998;160:4671–4680. [PubMed] [Google Scholar]
- Austyn JM. Gordon S. F4-80, a monoclonal-antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bailey SL. Schreiner B. McMahon EJ. Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4(+) T-H-17 cells in relapsing EAE. Nat Immunol. 2007;8(2):172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Banchereau J. Bazan F. Blanchard D. Briere F. Galizzi JP. Vankooten C. Liu YJ. Rousset F. Saeland S. The CD40 antigen and its ligand. Ann Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Barker AJ. Ullian EM. Astrocytes and synaptic plasticity. Neuroscientist. 2010;16(1):40–50. doi: 10.1177/1073858409339215. [DOI] [PubMed] [Google Scholar]
- Barkhof F. Scheltens P. Frequin ST. Nauta JJ. Tas MW. AJR Am J Roentgenol. 1992;159:1041. doi: 10.2214/ajr.159.5.1414773. [DOI] [PubMed] [Google Scholar]
- Becher B. Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18(1):1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Becher B. Blain M. Antel JP. CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol. 2000a;102(1):44–50. doi: 10.1016/s0165-5728(99)00152-6. [DOI] [PubMed] [Google Scholar]
- Becher B. Dodelet V. Fedorowicz V. Antel JP. Soluble tumor necrosis factor receptor inhibits interleukin 12 production by stimulated human adult microglial cells in vitro. J Clin Invest. 1996;98(7):1539–1543. doi: 10.1172/JCI118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B. Durell BG. Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Investig. 2002;110(4):493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B. Prat A. Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000b;29:293–304. [PubMed] [Google Scholar]
- Bergmann CC. Lane TE. Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4(2):121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K. Laurie DJ. Berthele A. Sommer B. Tolle TR. Gebicke-Harter PJ. van Calker D. Boddeke H. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem. 1999;72(4):1671–1680. doi: 10.1046/j.1471-4159.1999.721671.x. [DOI] [PubMed] [Google Scholar]
- Binder GK. Griffin DE. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293(5528):303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- Bo L. Dawson TM. Wesselingh S. Mork S. Choi S. Ann Neurol. 1994;36:778. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- Bo L. Vedeler CA. Nyland H. Trapp BD. Mork SJ. Mult Scler. 2003;9:323. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- Brahic M. Roussarie JP. Axon-myelin interactions during a viral infection of the central nervous system. Plos Pathogens. 2009;5(9):e1000519. doi: 10.1371/journal.ppat.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J. Moller T. Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16(1):81–90. doi: 10.1002/(SICI)1098-1136(199601)16:1<81::AID-GLIA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Brownell B. Hughes JT. J Neurol Neurosurg Psychiatry. 1962;25:315. doi: 10.1136/jnnp.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M. Persoon-Deen C. Verwer RWH. Meeuwsen S. Ravid R. Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53(7):688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Bulloch K. Miller MM. Gal-Toth J. Milner TA. Gottfried-Blackmore A. Waters EM. Kaunzner UW. Liu K. Lindquist R. Nussenzweig MC. Steinman RM. McEwen BS. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508(5):687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- Bullock TH. Moore JK. Fields RD. Evolution of myelin sheaths—both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48(2):145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Burdeinick-Kerr R. Griffin DE. Gamma interferon-dependent, noncytolytic clearance of Sindbis virus infection from neurons in vitro. J Virol. 2005;79(9):5374–5385. doi: 10.1128/JVI.79.9.5374-5385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ. Reilly CR. Sutcliffe JG. Lo D. Mature microglia resemble immature antigen presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Carswell R. Pathological Anatomy: Illustrations of the elementary forms of disease. Longman, Brown, Green and Longman; London, UK: 1838. [Google Scholar]
- Cerwenka A. Carter LL. Reome JB. Swain SL. Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J Immunol. 1998;161(1):97–105. [PubMed] [Google Scholar]
- Chakraborty S. Nazmi A. Dutta K. Basu A. Neurons under viral attack: Victims or warriors? Neurochem Int. 2010;56(6–7):727–735. doi: 10.1016/j.neuint.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. Nishiyama A. Peterson J. Prineas J. Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000a;20(17):6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. Tourtellotte WW. Rudick R. Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chang JT. Segal BM. Nakanishi K. Okamura H. Shevach EM. The costimulatory effect of IL-18 on the induction of antigen-specific IFN-gamma production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor beta 2 subunit. Eur J Immunol. 2000b;30(4):1113–1119. doi: 10.1002/(SICI)1521-4141(200004)30:4<1113::AID-IMMU1113>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chang JY. Martin DP. Johnson EM. Interferon suppresses sympathetic neuronal cell-death caused by nerve growth-factor deprivation. J Neurochem. 1990;55(2):436–445. doi: 10.1111/j.1471-4159.1990.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Chang TT. Sobel RA. Wei T. Ransohoff RM. Kuchroo VK. Sharpe AH. Recovery from EAE is associated with decreased survival of encephalitogenic T cells in the CNS of B7-1/B7-2-deficient mice. Eur J Immunol. 2003;33(7):2022–2032. doi: 10.1002/eji.200323180. [DOI] [PubMed] [Google Scholar]
- Charcot JM. Histologie de la sclerose en plaques. Gazette Hôpitaux. 1868;41:554, 557–558. [Google Scholar]
- Compston A. Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Constant SL. Bottomly K. Induction of TH1 and TH2 CD4+ T cell responses: The alternative approaches. Ann Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Craggs RI. Webster HD. The examination of lesions in experimental allergic encephalomyelitis (EAE) for an antigen presenting cells (APCS) Neuropathol Appl Neurobiol. 1985;11(4):320–320. [Google Scholar]
- Cua DJ. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Czapiga M. Colton CA. Function of microglia in organotypic slice cultures. J Neurosci Res. 1999;56(6):644–651. doi: 10.1002/(SICI)1097-4547(19990615)56:6<644::AID-JNR10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Davalos D. Grutzendler J. Yang G. Kim JV. Zuo Y. Jung S. Littman DR. Dustin ML. Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Derfuss T. Parikh K. Velhin S. Braun M. Mathey E. Krumbholz M. Kumpfel T. Moldenhauer A. Rader C. Sonderegger P. Pollmann W. Tiefenthaller C. Bauer J. Lassmann H. Wekerle H. Karagogeos D. Hohlfeld R. Linington C. Meinl E. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc Natl Acad Sci U S A. 2009;106(20):8302–8307. doi: 10.1073/pnas.0901496106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt A. Moffatt OD. Raykundalia C. Capra JD. Simmons DL. Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392(6675):505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- Dijkstra IM. Hulshof S. van der Valk P. Boddeke H. Biber K. Cutting edge: Activity of human adult microglia in response to CC chemokine ligand 21. J Immunol. 2004;172(5):2744–2747. doi: 10.4049/jimmunol.172.5.2744. [DOI] [PubMed] [Google Scholar]
- Dorries R. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr Top Microbiol Immunol. 2001;253:219–245. doi: 10.1007/978-3-662-10356-2_11. [DOI] [PubMed] [Google Scholar]
- Dudziak D. Kamphorst AO. Heidkamp GF. Buchholz VR. Trumpfheller C. Yamazaki S. Cheong C. Liu K. Lee HW. Park CG. Steinman RM. Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- ElKhoury J. Hickman SE. Thomas CA. Cao L. Silverstein SC. Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382(6593):716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Erichsen D. Lopez AL. Peng H. Niemann D. Williams C. Bauer M. Morgello S. Cotter RL. Ryan LA. Ghorpade A. Gendelman HE. Zheng JL. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138(1–2):144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- Erkman L. Wuarin L. Cadelli D. Kato AC. Interferon induces astrocyte maturation causing an increase in cholinergic properties of cultured human spinal-cord cells. Dev Biol. 1989;132(2):375–388. doi: 10.1016/0012-1606(89)90234-0. [DOI] [PubMed] [Google Scholar]
- Fabis MJ. Phares TW. Kean RB. Koprowski H. Hooper DC. Blood-brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci U S A. 2008;105(40):15511–15516. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry Z. Raine CS. Hart MN. Nervous-tissue as an immune compartment - the dialect of the immune-response in the CNS. Immunol Today. 1994;15(5):218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- Fadok VA. Savill JS. Haslett C. Bratton DL. Doherty DE. Campbell PA. Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149(12):4029–4035. [PubMed] [Google Scholar]
- Fan J. Bass HZ. Fahey JL. Elevated IFN-gamma and decreased IL-2 gene-expression are associated with HIV-infection. J Immunol. 1993;151(9):5031–5040. [PubMed] [Google Scholar]
- Farber K. Pannasch U. Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005;29(1):128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Farina C. Krumbholz M. Giese T. Hartmann G. Aloisi F. Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159(1–2):12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Ferber IA. Brocke S. TaylorEdwards C. Ridgway W. Dinisco C. Steinman L. Dalton D. Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalolmyelitis (EAE) J Immunol. 1996;156(1):5–7. [PubMed] [Google Scholar]
- Fierz W. Endler B. Reske K. Wekerle H. Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985;134:3785–3793. [PubMed] [Google Scholar]
- Filippi M. Rocca MA. Martino G. Horsfield MA. Comi G. Ann Neurol. 1998;43:809. doi: 10.1002/ana.410430616. [DOI] [PubMed] [Google Scholar]
- Fischer HG. Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Flesch IEA. Kaufmann SHE. Differential induction of IL12 synthesis by Mycobacterium bovis BCG and Listeria monocytogenes. Res Immunol. 1995;146(7–8):520–525. doi: 10.1016/0923-2494(96)83026-4. [DOI] [PubMed] [Google Scholar]
- Fontana A. Fierz W. Wekerle H. Astrocytes present myelin basic-protein to encephalitogenic T-cell lines. Nature. 1984;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Ford AL. Goodsall AL. Hickey WF. Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Foy TM. Aruffo A. Bajorath J. Buhlmann JE. Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- Fyffe-Maricich SL. Karlo JC. Landreth GE. Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31(3):843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. Gillig TA. Ye P. D'Ercole AJ. Matsushima GK. Popko B. Interferon-gamma protects against cuprizone-induced demyelination. Mol Cell Neurosci. 2000;16(4):338–349. doi: 10.1006/mcne.2000.0883. [DOI] [PubMed] [Google Scholar]
- Gehrmann J. Banati RB. Kreutzberg GW. Microglia in the immune surveillance of the brain—human microglia constitutively express HLA-DR molecules. J Neuroimmunol. 1993;48(2):189–198. doi: 10.1016/0165-5728(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Geiger K. Howes EL. Sarvetnick N. Ectopic expression of gamma-interferon in the eye protects transgenic mice from intraocular herpes-simplex virus type-1 infections. J Virol. 1994;68(9):5556–5567. doi: 10.1128/jvi.68.9.5556-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F. Jung S. Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gerritse K. Laman JD. Noelle RJ. Aruffo A. Ledbetter JA. Boersma WJA. Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(6):2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvin AR. Dal Canto PC. Rhee L. Salomon B. Sharpe A. Bluestone JA. Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: A comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164(1):136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ. Latchman YE. Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol. 2002;14(3):391–396. doi: 10.1016/s0952-7915(02)00341-2. [DOI] [PubMed] [Google Scholar]
- Greter M. Dendritic cells permit immune invasion of the CNS during experimental autoimmune encephalomyelitis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Greter M. Heppner FL. Lemos MP. Odermatt BM. Goebels N. Laufer T. Noelle RJ. Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3(6):493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino Y. Kariura Y. Manago Y. Amano T. Wang B. Sekiguchi M. Nishikawa K. Aoki SU. Wada K. Noda M. Heterogeneity and potentiation of AMPA type of glutamate receptors in rat cultured microglia. Glia. 2004;47(1):68–77. doi: 10.1002/glia.20034. [DOI] [PubMed] [Google Scholar]
- Hauser SL. Oksenberg JR. The neurobiology of multiple sclerosis: Genes, inflammation, and neurodegeneration. Neuron. 2006;52(1):61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hausmann J. Pagenstecher A. Baur K. Richter K. Rziha HJ. Staeheli P. CD8 T cells require gamma interferon to clear Borna disease virus from the brain and prevent immune system-mediated neuronal damage. J Virol. 2005;79(21):13509–13518. doi: 10.1128/JVI.79.21.13509-13518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SE. Hollopeter G. Yang G. Kurpius D. Dailey ME. Gan WB. Julius D. The P2Y(12) receptor regulates microglial activation by extracellular nucleotides. Nature Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heeger PS. Forsthuber T. Shive C. Biekert E. Genain C. Hofstetter HH. Karulin A. Lehmann PV. Revisiting tolerance induced by autoantigen in incomplete Freund's adjuvant. J Immunol. 2000;164(11):5771–5781. doi: 10.4049/jimmunol.164.11.5771. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36(2):118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hirota K. Duarte JH. Veldhoen M. Hornsby E. Li Y. Cua DJ. Ahlfors H. Wilhelm C. Tolaini M. Menzel U. Garefalaki A. Potocnik AJ. Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–U95. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwald GM. Van DA. Robinson ME. Thorbecke GJ. Immune response in draining lymph nodes and spleen after intraventricular injection of antigen. Int J Neurosci. 1988;39:299–306. doi: 10.3109/00207458808985717. [DOI] [PubMed] [Google Scholar]
- Honda S. Sasaki Y. Ohsawa K. Imai Y. Nakamura Y. Inoue K. Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through G(i/o)-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LM. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Invest. 1999;103:281–290. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz AA. Sullivan TJ. Krummel MF. Sobel RA. Allison JP. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73(1–2):57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- Inoue K. The function of microglia through purinergic receptors: Neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1–2):210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ishibashi T. Dakin KA. Stevens B. Lee PR. Kozlov SV. Stewart CL. Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D. Imai Y. Ohsawa K. Nakajima K. Fukuuchi Y. Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res. 1998;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Iyoda T. Shimoyama S. Liu K. Omatsu Y. Akiyama Y. Maeda Y. Takahara K. Steinman RM. Inaba K. The CD8(+) dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195(10):1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CS. Arbour N. Manusow J. Montgrain V. Blain M. McCrea E. Shapiro A. Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175(7):4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jahn O. Tenzer S. Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40(1):55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA. Golstein P. lymphocyte-activation and effector functions - a return to intimacy. Curr Opin Immunol. 1992;4(3):241–245. doi: 10.1016/0952-7915(93)90048-w. [DOI] [PubMed] [Google Scholar]
- Jemison LM. Williams SK. Lublin FD. Knobler RL. Korngold R. Interferon-gamma-inducible endothelial-cell class-ii major histocompatibility complex expression correlates with strain-specific and site-specific susceptibility to experimental allergic encephalomyelitis. J Neuroimmunol. 1993;47(1):15–22. doi: 10.1016/0165-5728(93)90280-c. [DOI] [PubMed] [Google Scholar]
- Jonakait GM. Wei RT. Sheng ZL. Hart RP. Ni L. Interferon-gamma promotes cholinergic differentiation of embryonic septal nuclei and adjacent basal forebrain. Neuron. 1994;12(5):1149–1159. doi: 10.1016/0896-6273(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Juedes AE. Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- Kabat E. Wolf A. Bezer AE. The rapid production of acute disseminated encephalomyelitis in Rhesus monkeys by injection of heterologous and homologous brain tissue with adjuvants. J Exp Med. 1947;85:117–129. doi: 10.1084/jem.85.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandikar NJ. Vanderlugt CL. Bluestone JA. Miller SD. Targeting the B7/CD28:CTLA-4 costimulatory system in CNS autoimmune disease. J Neuroimmunol. 1998;89:10–18. doi: 10.1016/s0165-5728(98)00058-7. [DOI] [PubMed] [Google Scholar]
- Karandikar NJ. Vanderlugt CL. Walunas TL. Miller SD. Bluestone JA. CTLA-4: A negative regulator of autoimmune disease. J Exp Med. 1996;184(2):783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen AE. Dyrberg T. Molecular mimicry between non-self, modified self and self in autoimmunity. Semin Immunol. 1998;10(1):25–34. doi: 10.1006/smim.1997.0102. [DOI] [PubMed] [Google Scholar]
- Karman J. Ling C. Sandor M. Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- Katz-Levy Y. Temporal development of autoreactive Th1 responses and endogenous antigen presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J Immunol. 2000;165:5304–5314. doi: 10.4049/jimmunol.165.9.5304. [DOI] [PubMed] [Google Scholar]
- Kawai T. Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kawanokuchi J. Shimizu K. Nitta A. Yamada K. Mizuno T. Takeuchi H. Suzumura A. Production and functions of IL-17 in microglia. J Neuroimmunol. 2008;194(1–2):54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M. Gallmeier E. Behrens L. Leal VV. Misgeld T. Klinkert WEF. Kolbeck R. Hoppe E. Oropeza-Wekerle RL. Bartke I. Stadelmann C. Lassmann H. Wekerle H. Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J Exp Med. 1999;189(5):865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd D. Barkhof F. McConnell R. Algra PR. Allen IV. Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122(Pt 1):17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- Kim YS. Choi DH. Block ML. Lorenzl S. Yang LC. Kim YJ. Sugama S. Cho BP. Hwang O. Browne SE. Kim SY. Hong JS. Beal MF. Joh TH. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21(1):179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- Kim YS. Kim SS. Cho JJ. Choi DH. Hwang O. Shin DH. Chun HS. Beal MF. Joh TH. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25(14):3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto H. Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4(+) T cells. J Immunol. 1999;163(4):1817–1826. [PubMed] [Google Scholar]
- Kivisäkk P. Imitola J. Rasmussen S. Elyaman W. Zhu B. Ransohoff RM. Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65(4):457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P. Mahad DJ. Callahan MK. Trebst C. Tucky B. Wei T. Wu LJ. Baekkevold ES. Lassmann H. Staugaitis SM. Campbell JJ. Ransohoff RM. Human cerebrospinal fluid central memory CD4(+) T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100(14):8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S. Shigemoto-Mogami Y. Nasu-Tada K. Shinozaki Y. Ohsawa K. Tsuda M. Joshi BV. Jacobson KA. Kohsaka S. Inoue K. UDP acting at P2Y(6) receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G. Reutelingsperger CPM. Kuijten GAM. Keehnen RMJ. Pals ST. Vanoers MHJ. Annexin-V for flow cytometric detection of phosphatidylserine expression on B-cells undergoing apoptosis. Blood. 1994;84(5):1415–1420. [PubMed] [Google Scholar]
- Kort JJ. Kawamura K. Fugger L. Weissert R. Forsthuber TG. Efficient presentation of myelin oligodendrocyte glycoprotein peptides but not protein by astrocytes from HLA-DR2 and HLA-DR4 transgenic mice. J Neuroimmunol. 2006;173(1–2):23–34. doi: 10.1016/j.jneuroim.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Krakowski ML. Owens T. Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur J Immunol. 2000;30:1002–1009. doi: 10.1002/(SICI)1521-4141(200004)30:4<1002::AID-IMMU1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kristensson K. Aldskogius M. Peng ZC. Olsson T. Aldskogius H. Bentivoglio M. Coinduction of neuronal interferon-gamma and nitric-oxide synthase in rat motor-neurons after axotomy—a role in nerve repair or death. J Neurocytol. 1994;23(8):453–459. doi: 10.1007/BF01184069. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T. Miron V. Cuo Q. Wegner C. Antel J. Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Kulprathipanja NV. Kruse CA. Microglia phagocytose alloreactive CTL-damaged 9L gliosarcoma cells. J Neuroimmunol. 2004;153(1–2):76–82. doi: 10.1016/j.jneuroim.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A. Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci. 2005;233(1–2):55–59. doi: 10.1016/j.jns.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Lande R. Gafa V. Serafini B. Giacomini E. Visconti A. Remoli ME. Severa M. Parmentier M. Ristori G. Salvetti M. Aloisi F. Coccia EM. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008;67(5):388–401. doi: 10.1097/NEN.0b013e31816fc975. [DOI] [PubMed] [Google Scholar]
- Lang HLE. Jacobsen H. Ikemizu S. Andersson C. Harlos K. Madsen L. Hjorth P. Sondergaard L. Svejgaard A. Wucherpfennig K. Stuart DI. Bell JI. Jones EY. Fugger L. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3(10):940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Understanding the mechanisms of sustained signaling and T cell activation. J Exp Med. 1997;185(10):1717–1719. doi: 10.1084/jem.185.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LT. Yu ACH. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18(3):351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Lehmann PV. Forsthuber T. Miller A. Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ. Herold KC. Rhee L. Patel B. Koons A. Qin HY. Fuchs E. Singh B. Thompson CB. Bluestone JA. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5(3):285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- Leonard JP. Waldburger KE. Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin-12. J Exp Med. 1995;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau RS. Singer SM. McDevitt HO. TH1 and TH2 CD4(+) T-cells in the pathogenesis of organ-specific autoimmune-diseases. Immunol Today. 1995;16(1):34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- Limatola C. Lauro C. Catalano M. Ciotti MT. Bertollini C. Di Angelantonio S. Ragozzino D. Eusebi F. Chemokine CX(3)CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J Neuroimmunol. 2005;166(1–2):19–28. doi: 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lindsey JW. Steinman L. Competitive PCR quantification of CD4, CD8, ICAM-1, VCAM-1, and MHC class-II messenger-RNA in the central-nervous-system during development and resolution of experimental allergic encephalomyelitis. J Neuroimmunol. 1993;48(2):227–234. doi: 10.1016/0165-5728(93)90196-6. [DOI] [PubMed] [Google Scholar]
- Ling C. Sandor M. Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90–98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- Lumsden CE. Handbook of clinical neurology North-Holland. Amsterdam: 1970. [Google Scholar]
- Ma XJ. Chow JM. Gri G. Carra G. Gerosa F. Wolf SE. Dzialo R. Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183(1):147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CL. Neville KL. Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Martin SJ. Reutelingsperger CPM. McGahon AJ. Rader JA. Vanschie R. Laface DM. Green DR. Early redistribution of plasma-membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus - inhibition by overexpression of BCL-2 and ABL. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E. Heaton RK. Marcotte TD. Ellis RJ. Wiley CA. Mallory M. Achim CL. McCutchan JA. Nelson JA. Atkinson JH. Grant I. Chandler JL. Wallace MR. Spector SA. Jernigan T. Hesselink J. Hansen L. Abramson I. Masys D. Group H. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42(6):963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y. Fujiwara M. Insitu detection of class-i and class-ii major histocompatibility complex antigens in the rat central-nervous-system during experimental allergic encephalomyelitis - an immunohistochemical study. J Neuroimmunol. 1986;12(4):265–277. doi: 10.1016/0165-5728(86)90033-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y. Ohmori K. Fujiwara M. Immune regulation by brain-cells in the central-nervous-system - microglia but not astrocytes present myelin basic-protein to encephalitogenic T-cells under invivo-mimicking conditions. Immunology. 1992;76(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK. Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74:599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- McCarron RM. Spatz M. Kempski O. McFarlin DE. Interactions between cerebral vascular endothelial-cells and myelin basic protein-sensitized T-cells. Can J Neurol Sci. 1986;13(4):370–370. [Google Scholar]
- McCombe PA. Dejersey J. Pender MP. Inflammatory cells, microglia and mhc class-ii antigen-positive cells in the spinal-cord of lewis rats with acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1994;51(2):153–167. doi: 10.1016/0165-5728(94)90077-9. [DOI] [PubMed] [Google Scholar]
- McCombe PA. Fordyce BW. Dejersey J. Yoong G. Pender MP. Expression of cd45rc and ia-antigen in the spinal-cord in acute experimental allergic encephalomyelitis - an immunocytochemical and flow cytometric study. J Neurol Sci. 1992;113(2):177–186. doi: 10.1016/0022-510x(92)90245-g. [DOI] [PubMed] [Google Scholar]
- McKenna K. Beignon AS. Bhardwaj N. Plasmacytoid dendritic cells: Linking innate and adaptive immunity. J Virol. 2005;79(1):17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EJ. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- Miller DH. Barkhof F. Nauta JJ. Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain. 1993;116(Pt 5):1077–1094. doi: 10.1093/brain/116.5.1077. [DOI] [PubMed] [Google Scholar]
- Miller SD. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Miller SD. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Moalem G. Leibowitz-Amit R. Yoles E. Mor F. Cohen IR. Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5(1):49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Monteyne P. Bureau JF. Brahic M. Viruses and multiple sclerosis. Curr Opin Neurol. 1998;11(4):287–291. doi: 10.1097/00019052-199808000-00002. [DOI] [PubMed] [Google Scholar]
- Mosmann TR. Cherwinski H. Bond MW. Giedlin MA. Coffman RL. 2 Types of murine helper t-cell clone .1. definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- Murphy Kea. Immunobiology. New York: Garland Science, Taylor & Francis Group; 2008. [Google Scholar]
- Naik SH. Sathe P. Park HY. Metcalf D. Proietto AI. Dakic A. Carotta S. O'Keeffe M. Bahlo M. Papenfuss A. Kwak JY. Wu L. Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8(11):1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Nair A. Frederick TJ. Miller SD. Astrocytes in multiple sclerosis: A product of their environment. Cell Mol Life Sci. 2008;65(17):2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468(7321):244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nikcevich KM. Gordon KB. Tan LJ. Hurst SD. Kroepfl JF. Gardinier M. Barrett TA. Miller SD. IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158(2):614–621. [PubMed] [Google Scholar]
- Ohsawa K. Irino Y. Nakamura Y. Akazawa C. Inoue K. Involvement of P2X(4) and P2Y(12) receptors in ATP-induced microglial chemotaxis. Glia. 2007;55(6):604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Oldstone MBA. Molecular mimicry and autoimmune-disease. Cell. 1987;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Oldstone MBA. VonHerrath M. Virus-induced autoimmune disease: Transgenic approach to mimic insulin-dependent diabetes mellitus and other autoimmune diseases. Apmis. 1996;104(10):689–697. doi: 10.1111/j.1699-0463.1996.tb04930.x. [DOI] [PubMed] [Google Scholar]
- Onai N. Obata-Onai A. Schmid MA. Ohteki T. Jarrossay D. Manz MG. Identification of clonogenic common Flt3(+) M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8(11):1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Oppmann B. Lesley R. Blom B. Timans JC. Xu YM. Hunte B. Vega F. Yu N. Wang J. Singh K. Zonin F. Vaisberg E. Churakova T. Liu MR. Gorman D. Wagner J. Zurawski S. Liu YJ. Abrams JS. Moore KW. Rennick D. de Waal-Malefyt R. Hannum C. Bazan JF. Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Pan Y. Lloyd C. Zhou H. Dolich S. Deeds J. Gonzalo JA. Vath J. Gosselin M. Ma JY. Dussault B. Woolf E. Alperin G. Culpepper J. GutierrezRamos JC. Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- Panitch HS. Hirsch RL. Schindler J. Johnson KP. Treatment of multiple-sclerosis with gamma-interferon - exacerbations associated with activation of the immune-system. Neurology. 1987;37(7):1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Park DR. Skerrett SJ. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma—differential responses of blood monocytes and alveolar macrophages? J Immunol. 1996;157(6):2528–2538. [PubMed] [Google Scholar]
- Patterson CE. Daley JK. Rall GF. Neuronal survival strategies in the face of RNA viral infection. Journal of Infectious Diseases. 2002a;186:S215–S219. doi: 10.1086/344265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE. Lawrence DMP. Echols LA. Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002b;76(9):4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin PJ. Lovett-Racke A. Phillips SM. Racke MK. Differential requirements of naive and memory T cells for CD28 costimulation in autoimmune pathogenesis. Histol Histopathol. 1999;14(4):1269–1276. doi: 10.14670/HH-14.1269. [DOI] [PubMed] [Google Scholar]
- Petersen MA. Dailey ME. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia. 2004;46(2):195–206. doi: 10.1002/glia.10362. [DOI] [PubMed] [Google Scholar]
- Peterson JW. Bo L. Mork S. Chang A. Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50(3):389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Pizzi M. Sarnico I. Boroni F. Benarese M. Dreano M. Garotta G. Valerio A. Spano PF. Prevention of neuron and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6 receptor/IL-6 fusion protein in organotypic hippocampal slices. Mol Cell Neurosci. 2004;25(2):301–311. doi: 10.1016/j.mcn.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Pocock JM. Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pohl HBF. Porcheri C. Mueggler T. Bachmann LC. Martino G. Riethmacher D. Franklin RJM. Rudin M. Suter U. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J Neurosci. 2011;31(3):1069–1080. doi: 10.1523/JNEUROSCI.5035-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED. Shriver LP. Maresz K. Dittell BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81(3):374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Pope JG. Vanderlugt CL. Rahbe SM. Lipton HL. Miller SD. Characterization of and functional antigen presentation by central nervous system mononuclear cells from mice infected with Theiler's murine encephalomyelitis virus. J Virol. 1998;72(10):7762–7771. doi: 10.1128/jvi.72.10.7762-7771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A. Biernacki K. Becher B. Antel JP. B7 expression and antigen presentation by human brain endothelial cells: Requirement for proinflammatory cytokines. J Neuropathol Exp Neurol. 2000;59(2):129–136. doi: 10.1093/jnen/59.2.129. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Kivisakk P. Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Richardson WD. Kessaris N. Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7(1):11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Engelhardt B. Wekerle H. Immune function of the blood-brain-barrier - incomplete presentation of protein (auto)antigens by rat-brain microvascular endothelium invitro. J Cell Biol. 1990;110(5):1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivieccio MA. Suh HS. Zhao YM. Zhao ML. Chin KC. Lee SC. Brosnan CF. TLR3 ligation activates an antiviral response in human fetal astrocytes: A role for viperin/cig5. J Immunol. 2006;177(7):4735–4741. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- Rockstroh JK. Kreuzer KA. Sauerbruch T. Spengler U. Protein levels of interleukin-12 p70 holomer, its p40 chain and interferon-gamma during advancing HIV infection. J Infect. 1998;37(3):282–286. doi: 10.1016/s0163-4453(98)92138-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. Zoecklein LJ. Howe CL. Pavelko KD. Gamez JD. Nakane S. Papke LM. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J Virol. 2003;77(22):12252–12265. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg M. Kristensson K. Effects of interferon-gamma on neuronal infections. Viral Immunol. 2002;15(2):247–260. doi: 10.1089/08828240260066206. [DOI] [PubMed] [Google Scholar]
- Saederup N. Cardona AE. Croft K. Mizutani M. Cotleur AC. Tsou CL. Ransohoff RM. Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. Plos One. 2010;5(10):e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B. Rhee L. Bour-Jordan H. Hsin H. Montag A. Soliven B. Arcella J. Girvin AM. Miller SD. Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194(5):677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101(2):169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio L. Belyanskaya SL. Fischer FR. Cipriani B. Brosnan CF. Ricciardi-Castagnoli P. Stern LJ. Strominger JL. Riese R. Developmental plasticity of CNS microglia. Proc Natl Acad Sc U S A. 2001;98(11):6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J. Lee YB. Kim SU. T-cell costimulatory molecules B7-1 (CD80) and B7-2 (CD86) are expressed in human microglia but not in astrocytes in culture. Brain Res. 1995;704(1):92–96. doi: 10.1016/0006-8993(95)01177-3. [DOI] [PubMed] [Google Scholar]
- Savarin C. Bergmann CC. Hinton DR. Ransohoff RM. Stohlman SA. Memory CD4(+) T-cell-mediated protection from lethal coronavirus Encephalomyelitis. J Virol. 2008;82(24):12432–12440. doi: 10.1128/JVI.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M. Suzumura A. Marunouchi T. Cytokine network in the central-nervous-system and its roles in growth and differentiation of glial and neuronal cells. Int J Dev Neurosci. 1995;13(3–4):253–264. doi: 10.1016/0736-5748(94)00076-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N. Franklin R. Stevens S. Heldin CH. Compston A. Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- Scumpia PO. Kelly SM. Reeves WH. Stevens BR. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005;52(2):153–162. doi: 10.1002/glia.20234. [DOI] [PubMed] [Google Scholar]
- Seder RA. Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T-cells. Ann Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Segal BM. Dwyer BK. Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187(4):537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM. Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996;184(2):771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers DL. Maris DO. Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29(20):6722–6733. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B. Columba-Cabezas S. Di RF. Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B. Rosicarelli B. Magliozzi R. Stigliano E. Capello E. Mancardi GL. Aloisi F. Dendritic cells in multiple sclerosis lesions: Maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65(2):124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Shortman K. Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Shrestha B. Wang T. Samuel MA. Whitby K. Craft J. Fikrig E. Diamond MS. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80(11):5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel RA. Blanchette BW. Colvin RB. Chronic experimental allergic encephalomyelitis (CEAE) follows anti-T-cell monoclonal-antibody modulation of acute EAE (AEAE) in adult guinea-pigs (GP) J Neuropathol Exp Neurol. 1984;43(3):325–325. [Google Scholar]
- Sonobe Y. Liang JF. Jin SJ. Zhang GQ. Takeuchi H. Mizuno T. Suzumura A. Microglia express a functional receptor for interleukin-23. Biochem Biophys Res Commun. 2008;370(1):129–133. doi: 10.1016/j.bbrc.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Sospedra M. Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stohwasser R. Giesebrecht J. Kraft R. Muller EC. Hausler KG. Kettenmann H. Hanisch UK. Kloetzel PM. Biochemical analysis of proteasomes from mouse microglia: Induction of immunoproteasomes by interferon-gamma and lipopolysaccharide. Glia. 2000;29(4):355–365. [PubMed] [Google Scholar]
- Suzuki T. Hide I. Ido K. Kohsaka S. Inoue K. Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X(7) receptor-activated microglia. J Neurosci. 2004;24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. Prinz M. Stagi M. Chechneva O. Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. Plos Med. 2007;4(4):675–689. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi M. Frelinger JA. Induction of antigen presentation ability in purified cultures of astroglia by interferon-gamma. J Mol Cell Immunol. 1986;2(5):269–280. [PubMed] [Google Scholar]
- Talbot PJ. Paquette JS. Ciurli C. Antel JP. Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol. 1996;39(2):233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. Gordon KB. Mueller JP. Matis LA. Miller SD. Presentation of proteolipid protein epitopes and B7-1-dependent activation of encephalitogenic T cells by IFN-gamma-activated SJL/J astrocytes. J Immunol. 1998;160(9):4271–4279. [PubMed] [Google Scholar]
- Taylor DL. Jones F. Kubota E. Pocock JM. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived fas ligand. J Neurosci. 2005;25(11):2952–2964. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages—evaluation of microglia and their functions. Brain Res Rev. 1992;17(1):61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Tran EH. Hoekstra K. van Rooijen N. Dijkstra CD. Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161(7):3767–3775. [PubMed] [Google Scholar]
- Traugott U. Lebon P. Interferon-gamma and ia-antigen are present on astrocytes in active chronic multiple-sclerosis lesions. J Neurol Sci. 1988;84(2–3):257–264. doi: 10.1016/0022-510x(88)90130-x. [DOI] [PubMed] [Google Scholar]
- Trembleau S. Germann T. Gately MK. Adorini L. The role of IL-12 in the induction of organ-specific autoimmune-diseases. Immunol Today. 1995;16(8):383–386. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Trotter J. Karram K. Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res Rev. 2010;63(1–2):72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvestad E. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol. 1994;56:732–740. doi: 10.1002/jlb.56.6.732. [DOI] [PubMed] [Google Scholar]
- Utans-Schneitz U. Lorez H. Klinkert WEF. da Silva J. Lesslauer W. A novel rat CC chemokine, identified by targeted differential display, is upregulated in brain inflammation. J Neuroimmunol. 1998;92(1–2):179–190. doi: 10.1016/s0165-5728(98)00204-5. [DOI] [PubMed] [Google Scholar]
- Valerio A. Ferrario M. Dreano M. Garotta G. Spano P. Pizzi M. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells. Mol Cell Neurosci. 2002;21(4):602–615. doi: 10.1006/mcne.2002.1208. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CJ. Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8(6):831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela JM. Molina-Holgado E. Arevalo-Martin A. Almazan G. Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20(3):489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- Villadangos JA. Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Wang XH. Arcuino G. Takano T. Lin J. Peng WG. Wan PL. Li PJ. Xu QW. Liu QS. Goldman SA. Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Williams K. Ulvestad E. Antel JP. B7/BB-1 antigen expression on adult human microglia studied in-vitro and in-situ. Eur J Immunol. 1994a;24(12):3031–3037. doi: 10.1002/eji.1830241217. [DOI] [PubMed] [Google Scholar]
- Williams KC. Dooley NP. Ulvestad E. Waage A. Blain M. Yong VW. Antel JP. Antigen presentation by human fetal astrocytes with the cooperative effect of microglia or the microglial-derived cytokine IL-1. J Neurosci. 1995;15(3):1869–1878. doi: 10.1523/JNEUROSCI.15-03-01869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC. Ulvestad E. Hickey WF. Immunology of multiple-sclerosis. Clin Neurosci. 1994b;2(3–4):229–245. [PubMed] [Google Scholar]
- Windhagen A. Newcombe J. Dangond F. Strand C. Woodroofe MN. Cuzner ML. Hafler DA. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin-12 cytokine in multiple-sclerosis lesions. J Exp Med. 1995;182(6):1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18(2):601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GH. Bartlett PF. Clark-Lewis I. Battye F. Schrader JW. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Wu GF. Shindler KS. Allenspach EJ. Stephen TL. Thomas HL. Mikesell RJ. Cross AH. Laufer TM. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36(1):56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW. Strominger JL. Molecular mimicry in t-cell-mediated autoimmunity—viral peptides activate human T-cell clones specific for myelin basic-protein. Cell. 1995;80(5):695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S. DePasquale M. Patlak CS. Cserr HF. Albumin outflow into deep cervical lymph from different regions of rabbit brain. Am J Physiol. 1991;261:H1197–H1204. doi: 10.1152/ajpheart.1991.261.4.H1197. [DOI] [PubMed] [Google Scholar]