Abstract
Autoimmune thyroid diseases (AITD) are one of the most common organ-specific autoimmune disorders, of which Hashimoto's thyroiditis (HT) and Graves' disease (GD) are 2 of the most common clinical expressions. HT is characterized by hypothyroidism that results from the destruction of the thyroid by thyroglobulin-specific T cell-mediated autoimmune response. In contrast, GD is characterized by hyperthyroidism due to excessive production of thyroid hormone induced by thyrotropin receptor-specific stimulatory autoantibodies. Cytokines play a crucial role in modulating immune responses that affect the balance between maintenance of self-tolerance and initiation of autoimmunity. However, the role of cytokines is often confusing and is neither independent nor exclusive of other immune mediators. A regulatory cytokine may either favor induction of tolerance against thyroid autoimmune disease or favor activation and/or exacerbation of autoimmune responses. These apparently contradictory functions of a given cytokine are primarily influenced by the nature of co-signaling delivered by other cytokines. Consequently, a thorough understanding of the role of a particular cytokine in the context of a specific immune response is essential for the development of appropriate strategies to modulate cytokine responses to maintain or restore health. This review provides a summary of recent research pertaining to the role of cytokines in the pathogenesis of AITD with a particular emphasis on the therapeutic applications of cytokine modulation.
Introduction
Autoimmune diseases are a group of heterogeneous disorders characterized by abnormal lymphocytic activation directed against self-tissue (Davidson and Diamond 2001; Marrack and others 2001). These diseases occur essentially due to a breakdown in immunological self-tolerance. According to the clonal selection theory (Burnet 1959), self-reactive lymphocytes are deleted at the early developmental stage by negative selection and constitute what is called “central tolerance.” However, it is believed that weakly reactive clones sometimes escape clonal deletion and migrate to the periphery. Physiologically, these potentially self-reactive clones remain either nonresponsive to antigenic stimulation (ignorance) or are rendered anergic (Nossal 1996). In some instances, they undergo activation-induced cell death upon exposure to self-antigen (Green and others 2003). Collectively, these mechanisms of self-tolerance are referred to as cell-intrinsic mechanisms of “peripheral tolerance” (Schwartz 2005). In recent years, another mechanism of peripheral self-tolerance has been described involving forkhead box P3 (Foxp3) expressing regulatory T cells (Tregs) that actively and dominantly suppress self-reactive T-cells (Sakaguchi and others 2007). This constitutes a cell-extrinsic mechanism of self-tolerance (Schwartz 2005).
Autoimmune disease can thus occur when both central and peripheral tolerance mechanisms fail, leading to a pathogenic immune response against a self-antigen. In general, autoimmune diseases can be characterized as T-cell mediated or autoantibody mediated based on the primary effector mechanism and cell type involved in the pathogenesis of the disease. T cell-mediated diseases are characterized by infiltration of T cells into and destruction of the target tissue as seen in Hashimoto's thyroiditis (HT), type 1 diabetes (T1D) and multiple sclerosis (Crane and Forrester 2005). Autoantibody-mediated diseases are characterized by disruption of function as in Graves' disease (GD) or destruction of the target tissue as seen in myasthenia gravis, pemphigus vulgaris, systemic lupus erythematosus, rheumatoid arthritis (RA), etc. (Yanaba and others 2008).
Autoimmune thyroid diseases (AITD) are the most common organ-specific autoimmune disorders affecting approximately 5% (Caturegli and others 2007) of the overall population. HT and GD are 2 of the most common clinical expressions of thyroid dysfunction but differ in their clinical presentations as well as pathophysiology. HT is a T cell-mediated organ-specific autoimmune disease that results in clinical hypothyroidism due to thyroid destruction and is mediated by infiltrating and/or locally activated thyroglobulin (Tg)-specific T cells. In contrast, GD is characterized by hyperthyroidism due to excessive production of thyroid hormone induced by specific autoantibodies to thyrotropin receptor (TSHR). There is considerable evidence implicating that the actual destruction of thyroid cells in AITD may be caused by different and multiple mechanisms, including auto reactive T-lymphocytes, natural killer (NK) cells, and cytokines. Several studies in animal models have concluded that organ-specific autoimmune thyroiditis (AIT) should be regarded as a polygenic disease that is strongly influenced by environmental factors (Prabhakar and others 2003; Tomer and others 2003; Klecha and others 2008). Although individuals may be genetically predisposed to AIT, the disruption of immune system homeostasis by environmental factors results in thyroid dysfunction. The most significant factor that is likely to be involved in the induction of autoimmunity is a defect or deficiency in the immune regulation, particularly a perturbation in the balance between the effector T cells (Teff) and Tregs that prevent the development of autoimmunity.
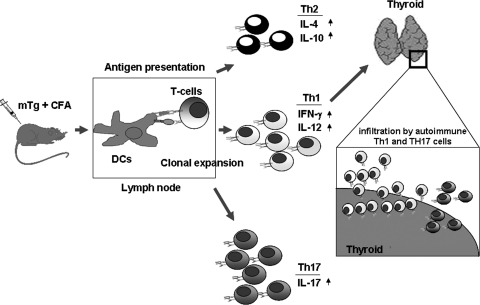
A murine model of HT called experimental autoimmune thyroiditis (EAT) exhibits key features of HT, including mononuclear cell infiltration that destroy thyroid follicles, presence of autoantibodies, and autoreactive T-cells to thyroid autoantigens (Kong and others 2009). Although this disease is induced by immunization of experimental animals with mouse thyroglobulin (mTg) emulsified in complete Freund's adjuvant (Vasu and others 2003), it can also be induced with mTg in conjunction with bacterial lipopolysacchharide (LPS) or interleukin (IL)-1β as adjuvant (Esquivel and others 1977; Nabozny and Kong 1992). Antigen presentation in the thyroid draining lymph nodes lead to the differentiation of Tg-specific T-cell subsets that migrate to the thyroid and cause tissue destruction (Fig. 1). This vigorously explored murine model has given us many insights into the molecular mechanisms underlying HT and also enabled us to identify potential therapeutic treatments. For instance, using this model, it was shown that lymphocytic infiltration of the thyroid depends upon the chemokine CCL21 and its receptor CCR7 (Martin and others 2004; Lira and others 2005). On the other hand, our laboratory has used this murine model to show that the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) can suppress EAT by mobilizing Tregs that secrete IL-10, an immunosuppressive cytokine.
FIG. 1.
The role of cytokines in EAT. EAT in mice is induced by immunization with mTg emulsified in the presence of Complete Freund's adjuvant. The antigen is taken up by the antigen presenting cells such as DCs, which undergo maturation to become potent antigen presenting cells. These cells present mTg-derived peptides to T cells in the draining lymph nodes. The activated DCs secrete pro-inflammatory cytokines that initiate a T helper response. Based on the type of cytokines secreted by these DCs, a Th1, Th2, or a Th17 response can be initiated. The Th1 cells predominantly secrete IFN-γ and IL-12, whereas the Th2 cells secrete IL-4, and Th17 cells secrete IL-17. The Th1 and Th17 cells have been shown to infiltrate the thyroid resulting in inflammation and ultimately death of the thyrocytes in EAT. EAT, experimental autoimmune thyroiditis; CFA, complete Freund's adjuvant; DCs, dendritic cells; mTg, mouse thyroglobulin; IFN, interferon; IL, interleukin.
Immunological Events in AITD
In order to understand the role of cytokines in the initiation or suppression of AITD, it is critical to understand the immunological events that trigger the autoimmune response, which eventually results in the associated pathology.
In HT, cell-mediated immunity promotes the induction of auto-antibodies and self-reactive T cells against Tg, and other auto-antigens, including thyroid peroxidase. HT is characterized by infiltration of lymphocytes and other immune cells, thyroid enlargement and fibrosis, and progressive destruction of thyrocytes that eventually results in hypothyroidism (Weetman 2003). Upon initiation of the immune response to Tg, thyroid-specific T lymphocytes migrate to the thyroid and through interferon (IFN)-γ production induce thyrocyte expression of major histocompatibility complex (MHC) class-II molecules. This results in further expansion of autoreactive T cells and the inflammatory response leading to the accumulation of activated CD4+ and CD8+ T cells, B cells, plasma cells, and macrophages in the thyroid.
Induction of autoimmune responses is believed to be initiated by an environmental trigger (Weetman 2003) such as LPS and cholera toxin. These microbial products can induce antigen presenting cells (APCs) such as dendritic cells (DC) to produce inflammatory cytokines and activate various other cells of the innate immune system by increasing the levels of expression of co-stimulatory and/or MHC molecules. These cytokines can also up-regulate expression of MHC molecules on the target cells. Highly activated APCs present self-antigens and activate self-reactive naïve T cells and initiate an autoimmune response that can be sustained by antigen presentation by the target cells.
In GD, loss of tolerance to TSHR results in the production of autoantibodies against TSHR and upon affinity maturation some of these antibodies bind to particular regions of the TSHR with high affinity and act as TSH agonists, and cause hyperthyroidism. Although patients with GD may show lymphocytic infiltration and some damage to the thyroid, the disease is primarily caused by the stimulatory antibodies. Since immunoglobulin class switching and affinity maturation would require TSHR-specific T cell help, modulating TSHR-specific T cell function might also be beneficial in GD.
CD4+ T cells are the major type of infiltrating cells in AITD. However, CD4+ T cells comprise of a functionally heterogeneous population of Teff responsible for both development of thyroiditis and a smaller population (∼10%) of Tregs expressing CD25 (IL-2Rα) that are critical for maintaining peripheral tolerance (Sakaguchi and others 2008). Tregs are identified by their expression of Foxp3, a transcription factor that is necessary and sufficient for Treg development (Zheng and Rudensky 2007). Besides, natural Tregs (nTregs), there is another component of CD4+ Tregs that do not express Foxp3 but secrete the cytokines IL-10 and transforming growth factor-β (TGF-β) to induce tolerance. Cytokines secreted by various components of the immune system, primarily the DCs, Teff, and Tregs have profound effects on the function of each other and play a crucial role in determining the ultimate outcome of an immune response.
Cytokines and T-Cell Subsets
Cytokines are small molecules secreted by cells of the immune system that serve to regulate various other components of the immune system, and they play a crucial role in health and disease (Brown and others 1989; Kim 2004). Each cytokine signals by binding either to a unique or a shared receptor, triggering an intracellular signaling cascade that can cause up-regulation or down-regulation of transcription factors that regulate the expression of various other genes. This can result in the production of other biologically active molecules, including other cytokines, alteration in the number of surface receptors, or a feedback inhibition loop that leads to self-regulation.
A model proposed by Mosmann and others (1986, 1989) suggested that CD4+ T-cells can be phenotypically and functionally characterized into 2 distinct subsets, Th1 and Th2, with distinct cytokine secretion profiles. Th1 cells secrete IL-2, IFN-γ, and tumor necrosis factor-α (TNF-α), and support the activation of APCs, delayed type hypersensitivity response, and immunoglobulin isotype switching to immunoglobulin G (IgG)2a in mice (Kroemer and others 1996). Th1 cells play an important role in mounting host defense against intracellular pathogens as well as in inducing delayed type hypersensitivity responses. Th2 cells, in contrast, are characterized by the secretion of IL-4, IL-5, IL-6, IL-10, and IL-13, and provide efficient help for B cell activation, antibody production, and immunoglobulin class switching to IgG1 and IgE isotypes (Kroemer and others 1996). While Th1 cells are associated with host defense against intracellular pathogens and T cell-mediated autoimmune diseases, Th2 cells are essential for clearing extracellular parasites and helminths. In addition, Th2 cells play a crucial role in bringing about eosinophilic inflammation and IgE production in allergic reactions and asthma (Murphy and Reiner 2002). If a perturbation in the balance between Th1-Th2 immune responses leads to the activation of Th1-cell-mediated autoimmune response against Tg, it can cause thyrocyte destruction and result in hypothyroidism as seen in patients with HT. Conversely, if the perturbation leads to a Th2-mediated stimulatory antibody response against TSHR, it can cause hyperthyroidism associated with GD.
In a physiological setting, professional APCs (eg, DCs, macrophages, and B-cells) recognize microbial components through certain receptors such as Toll-like receptors. Activation of these receptors results in the secretion of a specific set of cytokines that lead to the induction of a particular T-cell subset. For instance, secretion of IL-12 and IL-18 by APCs can lead to the induction of a Th1 response. In contrast, Th2 differentiation is mediated by T-cell-derived cytokines like IL-4 after interaction with APCs (Yoshimoto and others 1998; Chang and others 2000). Thus, inflammatory APCs can contribute to autoimmunity by influencing the activation of a particular subset of T cells. Conversely, suppressor cytokine-mediated modulation of APCs can be potentially used to treat autoimmunity.
More recently, cytokines have been grouped further into Th17 as well as regulatory cytokines released by T cell subsets like type-1 Tregs (Tr1) cells, Th3 cells, and Tregs. The Th17 Teff population produces pro-inflammatory cytokines IL-17A and IL-17F (Langrish and others 2005) and play an important role in mediating host defenses against bacteria like Citrobacter and Klebsiella pneumoniae, and fungi such as Candida albicans, and their inflammatory properties can initiate autoimmunity. Th17 cells share a reciprocal developmental pathway with Foxp3+ Treg cells. The regulatory cytokine TGF-β plays a critical role in the differentiation of both pathogenic Th17 cells and induced Tregs upon T cell receptor (TCR) activation. TGF-β along with the cytokine IL-6 activates Smad3 and STAT3, which induce activation of ROR-γt required for Th17 cell differentiation. In contrast, TGF-β alone induces Smad3, a transcription factor, which induces Foxp3 expression in the presence of retinoic acid (Ziegler and Buckner 2009).
Th17 responses can contribute to immunopathogenesis of AITD, whereas regulatory cytokines such as IL-10 and TGF-β have been shown to prevent AITD. Although several factors, including genetic, hormonal, environmental, and nutritional factors, have been implicated in the initiation and/or development of AIT, the changes associated with the pathophysiology of AITD are brought about by inflammatory cytokines. In contrast, regulatory cytokines can prevent the development of autoimmune diseases. Interestingly, recent studies in humans with AITD, as well as in established murine models of AITD, have shown that the role and function of various cytokines are neither independent nor exclusive. Furthermore, cytokines in many cases are pleiotropic, and can often have both immunostimulatory and immunosuppressive functions depending upon the specific context in which the immune response is taking place. For example, cytokines such as IL-2, IFN-γ, and TNF-α have been shown to both enhance and suppress autoimmune diseases (O'Shea and others 2002).
Th1 Cytokines in AITD
Th1 cells are generated from naive T helper cells by TCR engagement and STAT1 signaling initiated upon IFN-γ binding to its cognate receptor (IFN-γR). Phosphorylated STAT1 induces the expression of the transcription factor T-bet, which drives the differentiation of Th cells into Th1 cells by transactivating IFN-γ and the specific subunit of IL-12Rβ2, the receptor for IL-12. Upon expression of IL-12Rβ2, the cell becomes responsive to IL-12, which may be produced by activated DCs, macrophages, or other immune cells. Subsequent IL-12 signaling through STAT4 further stabilizes the Th1 phenotype (Yang and others 1999).
Analysis of cytokine expression has shown that Th1 cytokines are commonly prevalent in HT as well as in EAT (Drugarin and others 2000) and that the proportion of peripheral Th1 cells was higher in patients with severe HD than in patients with mild HD (Nanba and others 2009). APCs such as macrophages and DCs form the link between innate and acquired immune responses and produce considerable amounts of pro-inflammatory cytokines; TNF-α and IL-1β play important roles in initiating an adaptive immune response. DCs produce large amounts of IL-12, an important cytokine involved in inducing Th1 type of adaptive immune response (Moser and Murphy 2000). Increased expression of IL-12 by APCs, in vitro as well as in vivo, has been linked to EAT and T1D susceptibility and other autoimmune conditions (Trembleau and others 1995; Zipris and others 1996; Braley-Mullen and others 1998; Mannon and others 2004; Gangi and others 2005; Cheatem and others 2009; Ganesh and others 2009). More recent studies have implicated IL-12 in overcoming immune tolerance by suppressing Foxp3+ Tregs. In a recent study, Brahmachari and Pahan (2009) have shown that the p40 subunit of IL-12 can down-regulate Foxp3 expression via the production of nitric oxide. Our observation that IL-12 abrogates Foxp3 expression in T cells during activation further confirms the pro-inflammatory and the potential pathogenic effects of this cytokine in autoimmunity (Ganesh and others 2009).
IL-1β, on the other hand, has pleiotropic effects and can alter cell signaling, migration, and cytokine production, and influence T cell differentiation differently under different conditions (Johnson and others 2005; Dinarello 2009; Sutton and others 2009). Like IL-12, IL-1β has been shown to break peripheral tolerance by facilitating the expansion of Teff, and is implicated in autoimmune diseases such as RA (O'Sullivan and others 2006). Lately, IL-1β has been shown to be critical for the generation of IL-17-secreting T helper cells in humans. IL-23 when combined with IL-1β can induce and maintain pathogenic Th17 cells (Chung and others 2009; Lee and others 2010).
It is, however, interesting to note that the role of pro-inflammatory cytokines is not limited to the induction of AITD. In our recent studies we have observed that while IL-1β in the presence of IL-12 drives a pro-inflammatory response, IL-1β alone in the absence of IL-12 is capable of inducing Foxp3+ Tregs in vitro. These Tregs when adoptively transferred into mice immunized with mTg can prevent the development of EAT with greater efficiency than Tregs that were not treated with IL-1β (in press at PLOS1). These contradictory effects of IL-1β both in the induction of Tregs and in the activation of autoreactive T cells have been observed with other pro-inflammatory cytokines as well. Although pro-inflammatory cytokines like IL-6 and TNF-α have been generally associated with the induction of inflammation and autoimmunity, they may provide positive signals for the induction of Tregs (Verginis and others 2005; Nakagawa and others 2010). IL-2 is required for the development and maintenance of Tregs (Fontenot and others 2005; Burchill and others 2007) and provides long-term protection against autoimmune disease. We have seen that induction of IL-2 in the CD25− population and TGF-β in the Foxp3+ Tregs by IL-1β is required for the expansion/maintenance of Foxp3-expressing Tregs in vitro. However, this view is in contrast to an earlier finding which showed that IL-2 is required for the development of autoimmune response, although some aspects of autoimmune response are not regulated by IL-2 (Sharma and others 2009).
Other studies have implicated hepatitis C virus (HCV) infection and IFN-α therapy in the development of AITD. Chronic HCV infection has been shown to be associated with increased incidence of clinical and subclinical AIT (ie, the presence of thyroid antibodies in euthyroid subjects). Moreover, IFN-α therapy of chronic HCV infection is associated with subclinical or clinical thyroiditis in up to 40% of cases—in some cases, necessitating discontinuation of therapy. However, the mechanisms causing these conditions are still poorly understood (Tomer 2010).
IFN-γ is the archetype Th1 cytokine produced by CD4+ Th1 cells, CD8+ T cells, and NK cells. IFN-γ alone or in combination with other inflammatory cytokines induces MHC class I and II on APCs and other cells, and up-regulates the expression of adhesion molecules as well as certain chemokines and chemokine receptors to recruit T cells to the site of inflammation. It also activates macrophages and promotes IgG2A antibody production (Boehm and others 1997). A combination of IFN-α, produced by the thyroid, and increased levels of IFN-γ produced by the thyroid infiltrating lymphocytes have been shown to facilitate apoptosis of thyroid follicular cells through caspase activation (Wang and others 2002). Neutralizing antibodies to IFN-γ could prevent the disease and decrease Tg-specific T cell responses, suggesting that this cytokine plays a significant role (Tang and others 1993). However, the role of IFN-γ is controversial and studies from other groups show that IFN-γ may not be essential for the induction of AITD. Systemic administration of IFN-γ has been shown to suppress EAT (Vladutiu and Sulkowski 1980), and mice deficient in IFN-γ (IFN-γ−/−) (Tang and others 1998) or the receptor for IFN-γ (IFNγR−/−) (Alimi and others 1998) are susceptible to EAT.
Although GD is considered a Th2-type disease, a role for both IFN-γ and IL-4 in some murine models of experimental autoimmune Graves' disease (EAGD) has been proposed. Knockout of IFN-γ generally does not prevent development of EAGD (Nagayama and others 2003, 2004), whereas knockout of IL-4 inhibits disease development (Nagayama and others 2004). In another study, IFN-γ was shown to play an important role in EAGD induced by immunization with TSHR antigen (Pichurin and others 2001). Differences in animal models, modes of immunization, and interaction with other cytokines may thus alter the final outcome of cytokines in both HT and GD.
Th2 Cytokines in AITD
Naive T cells differentiate into Th2 cells by activation of the STAT-6 signaling pathway. Engagement of T cells via TCR and IL-4 receptor leads to the phosphorylation of STAT6, which is critical for the induction of the Th2 transcription factor GATA3. GATA3 transactivates Th2-specific cytokines such as IL-4, IL-5, and IL-13, and down-regulates STAT4 and IL-12Rb2, which are essential to generate a Th1 response (Zheng and Flavell 1997). Another transcription factor, c-maf, also contributes to Th2 differentiation by transactivating IL-4 transcription (Ho and others 1996).
GD in humans has long been thought to be a Th2-dominant autoimmune disease where TSHR autoantibodies play a crucial role in the pathogenesis of disease. Most of the information available on the effects of cytokines in GD is from animal models of EAGD. Our extensive studies on EAGD revealed that adjuvant composition as well as the glycosylation of the antigen could influence the titer, subclass, and fine specificity of antibodies to TSHR (Patibandla and others 1996, 1999; Seetharamaiah and others 1996). In our studies we determined whether skewing the immune response in favor of a Th1 or a Th2 type of response could influence the pathogenesis of EAGD. This was accomplished by treating mice with either Flt3L or GM-CSF, respectively. Although we were able to skew the response to TSHR toward Th1 or Th2 type, we were unable to alter the disease outcome. This was most likely due to the inability of Flt3L and GM-CSF to sustain Th1 and Th2 responses, respectively, for a longer duration required for the disease induction. Therefore, we tested to see if total absence of either IL-4 or IFN-γ could affect the development of EAGD. We immunized wild-type, IFN-γ(−/−) and IL-4(−/−) BALB/c mice with TSHR. Nearly 100% of the wild-type and IFN-γ(−/−) mice developed EAGD with optimal TSHR-specific immune responses, whereas IL-4(−/−) mice completely resisted disease development and showed delayed and suboptimal pathogenic antibody response. These data demonstrated that skewing immune responses to TSHR, using either Flt3-L or GM-CSF in favor of Th1 or Th2, respectively, may not be sufficient to alter the course of the disease, whereas the total absence of IL-4, but not IFN-γ, can prevent the development of EAGD (Dogan and others 2003). Thus, the induction of hyperthyroidism can be modified by manipulating immune responses toward Th1 or Th2 using different adjuvants, cytokines, or appropriate knockout mice (McLachlan and others 2005).
Th17 Cytokines in AITD
Th17 cells are differentiated by a combination of the cytokines TGF-β and IL-6 (Bettelli and others 2006; Mangan and others 2006; Veldhoen and others 2006). IL-6 signals via the STAT3 pathway (Yang and others 2007), but Th17 cells can also be induced by TGF-β in the presence of IL-21 (Korn and others 2007). TGF-β and IL-6 induce transcription factor RORγt, which may transactivate many components essential for differentiation of Th17 cells, including IL-17A, IL-17F, and IL-23R (Zhou and Littman 2009). Besides RORγt, the transcription factor ROR α is also involved in Th17 differentiation.
Several new studies have implicated Th17 cells both in HT and in GD in humans and in animal models. Iodine-induced AIT in nonobese diabetic-H2(h4) mice, a spontaneous mouse model of HT, revealed increased numbers of Th1 and Th17 cells in the spleens and thyroid glands of iodine-fed wild-type mice. Furthermore, the incidence and severity of intrathyroidal lymphocyte infiltration was markedly reduced in iodine-treated IL-17(−/−) mice, indicating that both Th1 and Th17 cells are critical for the pathogenesis of spontaneous AIT (Horie and others 2009). Enhanced levels of T cells synthesizing IL-17 and IL-22 in the peripheral blood of AITD patients, expression of IL-17 and IL-22, and an enhanced number of IL-23R+ cells were detected in thyroid glands from HT patients compared with GD patients or controls (Figueroa-Vega and others 2010). These studies demonstrated an increased differentiation of Th17 lymphocytes and enhanced synthesis of Th17 cytokines in AITD, mainly in HT. The discovery of Th17 cells and the recent findings have challenged the notion that HT is solely a Th1-mediated disease. In fact, the expression levels of Th1 cell-related T-bet and IFN-γ mRNA in peripheral blood mononuclear cells (PBMC) from HT were significantly decreased, whereas RORγt and IL-17 were increased, in patients with HT. In addition, the expression of transcription factors T-bet and RORγt mRNA correlated negatively, suggesting that Th17 cells rather than Th1 might be involved in the pathogenesis of HT (Shi and others 2010). In a very recent finding, the Th17 cells were also shown to induce GD in NOD-H2(h4), but not in BALB/c mice, thus showing that the effect of IL-17 may also differ with the genetic background (Horie and others 2011).
Regulatory Cytokines in AITD
TGF-β and IL-10 are the most important cytokines that have been implicated in the induction/maintenance of tolerance and prevention of autoimmunity. Tregs play critical roles in the induction of peripheral tolerance to self- and foreign antigens. Naturally occurring CD4+CD25+ Tregs express Foxp3 and generally mediate suppression by contact-dependent mechanisms (Bhattacharya and others 2011). TGF-β facilitates induction of Foxp3-expressing CD4+CD25+ Tregs and can therefore indirectly influence T cell activation. TGF-β is also a potent regulator of Teff differentiation, and it generally inhibits the acquisition of Th cell functions (Gorelik and others 2002). TGF-β blocks Th1 cell differentiation by reducing IL-12 receptor β2 (IL-12Rβ2) and T-bet expression (Gorham and others 1998; Gorelik and others 2002), and Th2 cells by inhibiting the expression of GATA-3 (Gorelik and others 2000; Heath and others 2000). In AITD, reduced TGF-β levels have been associated with HT (Akinci and others 2008; Vural and others 2009), whereas increased levels of TGF-β secretion by Tregs have been found to suppress EAT (Wang and others 2009). In a recent study, Transgenic NOD.H-2h4 mice expressing TGF-β under the control of the Tg promoter were generated and found to have higher frequencies of Foxp3+ Tregs compared with nontransgenic WT mice (Yu and others 2010) and the development of spontaneous AITD was inhibited.
Additional important mechanisms enforce immunological self-tolerance in the periphery. IL-10 is a regulatory cytokine that plays a central role in controlling inflammatory processes, and IL-10-secreting T cells may constitute additional mechanisms that are responsible for peripheral tolerance. Tr1 are induced upon antigen exposure and exhibit significant regulatory activities. Similarly, Th3 cells secrete both IL-10 and TGF-β and play a role in the induction of peripheral tolerance. IL-10 downregulates the expression of MHC class II and co-stimulatory molecules such as CD54, CD80, and CD86 (de Waal Malefyt and others 1991; Ding and others 1993; Moore and others 2001). Reduced expression of these molecules would therefore significantly affect the T cell-stimulating capacity of APCs (de Waal Malefyt and others 1991; Fiorentino and others 1991; Ding and Shevach 1992). Several studies have shown that IL-10 can protect against the development of HT and GD. In preliminary studies, injection of mTg-activated spleen cells cultured in the presence of recombinant IL-10, and not in its absence, into irradiated CBA/J mice induced a significant decrease in lymphocytic infiltrations in the recipient thyroid glands, but failed to reduce anti-mTg autoantibody production in vivo (Mignon-Godefroy and others 1995a). However, another study has shown that the effect of IL-10 is more likely on B-cell proliferation and antibody production and not on the pro-inflammatory cytokines (de la Vega and others 1998).
In our studies, treatment of mTg-primed mice with GM-CSF suppressed EAT and resulted in considerable expansion of Tregs and significantly higher levels of IL-10 compared with mTg-primed, untreated control mice. Administration of anti-IL-10R Ab into GM-CSF-treated mice abrogated GM-CSF-induced suppression of EAT without affecting the GM-CSF-induced expansion of CD4+CD25+ T cells. Moreover, our study showed that the IL-10-induced immunosuppression was due to its direct effects on mTg-specific Teff (Gangi and others 2005).
IL-10 is a key regulator of inflammation, and it can inhibit both Th1- and Th2-type immune responses through the suppression of pro-inflammatory cytokines and T cell proliferative responses (Groux and Cottrez 2003). One of the major mechanisms of IL-10-mediated suppression of T cells is through selective inhibition of the CD28 co-stimulatory pathway (Zhang and Kong 1998). However, in thyroiditis, alternative mechanisms of action of IL-10 have been proposed (Mignon-Godefroy and others 1995b; Batteux and others 1999; Tourneur and others 2002; Zhang and others 2003). Injection of cDNA expression vectors encoding IL-10 into the thyroid can significantly inhibit lymphocyte infiltration and development of EAT, and prevent progression of the disease (Batteux and others 1999). This suppressive effect of IL-10 is mediated either through enhancement of FasL expression on thyrocytes and induction of activation-induced cell death of thyroid-infiltrating T lymphocytes (Tourneur and others 2002) or through a potent up-regulation of anti-apoptotic molecules, such as cellular FLIP and Bcl-xL, which can prevent CD95-induced apoptosis of thyrocytes (Stassi and others 2000; Stassi and De Maria 2002). Conversely, direct injection of IL-1 and TNF-α into the thyroids of mTg-primed mice can induce thyrocyte apoptosis, indicating that pro-inflammatory cytokines may play a critical role in thyroid destruction (Wang and others 2002). These observations suggest that IL-10 could be mediating its effects through suppression of pro-inflammatory cytokine production.
Cytokine Modulation As Potential Therapeutic Approach in AITD
Pathogenesis of autoimmune diseases is frequently characterized by pro-inflammatory cytokine production such as TNF-α and IFNs. Consequently, there are 2 major approaches for treatment of autoimmune diseases. One is to either block the action of the pro-inflammatory cytokine or interfere with its production, whereas the other is to use immunomodulatory cytokines that can restore the Teff/Treg balance. One of the major breakthroughs that contributed to the first approach was the development agents that can inhibit TNF-α function including monoclonal antibodies and soluble receptors. This approach has been successfully used in treating RA and Crohn's disease (O'Shea and others 2002) and 3 licensed agents, adalimumab, etarnecept, and infliximab, are currently in the market for the treatment of immune-mediated inflammatory diseases (Silva and others 2010). In this context, the efficacy of an anti-TNF-α monoclonal antibody has been tested in a mouse model of granulomatous experimental thyroiditis. Although the disease severity in antibody treated mice was comparable to that of untreated control mice, antibody-treated mice showed less fibrosis and were able to clear thyroid lesions earlier (Chen and others 2007).
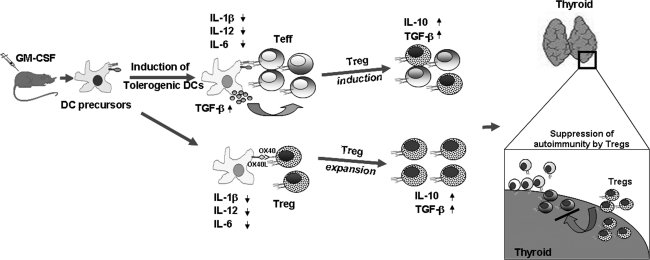
Our laboratory has been actively investigating the pathogenesis of AITD and exploring means to suppress them by using cytokine modulators of DCs. Studies from our laboratory showed that administration of GM-CSF could prevent, as well as suppress ongoing, EAT (Vasu and others 2003; Gangi and others 2005; Ganesh and others 2009). On the other hand, treatment with Flt3-L, which enhanced Th1 type of response, exacerbated the disease (Vasu and others 2003). GM-CSF-induced suppression of EAT was associated with a selective expansion of CD4+CD25+ Foxp3+ T cells (Tregs) that suppress mTg-specific responses through increased production of IL-10 (Gangi and others 2005; Ganesh and others 2009). These observations have been substantiated by others (Morris and others 2003). Similarly, treatment with GM-CSF reversed experimental autoimmune myasthenia gravis (EAMG) in C57BL/6 mice (Sheng and others 2006, 2008, 2009; Meriggioli and others 2008) and prevented the development of T1D in NOD mice (Gaudreau and others 2007; Cheatem and others 2009). Subsequent studies showed that GM-CSF acted primarily on DC precursors and caused an expansion of CD8a-DCs (Ganesh and others 2009). These DCs expressed very low to negligible levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, but expressed higher levels of TGF-β. Other studies using G-CSF saw a similar induction of Tregs that suppressed the development of T1D through the induction of TGF-β1 (Kared and others 2005). Although the mechanism by which GM-CSF imparts these tolerogenic phenotype to CD8a- DCs is not fully understood, we believe that GM-CSF-treatment of mice maintains DCs in a semi-matured phenotype, as indicated by high levels of expression of MHC class II and B7 molecules, but lower levels of expression of pro-inflammatory cytokines compared with untreated control mice (Lutz and Schuler 2002). Antigen presentation by these semi-mature DCs possibly led to the differentiation/expansion of IL-10 producing Treg cells (Gangi and others 2005) (Fig. 2). In contrast, Flt3L treatment led to an enhanced production of IL-12 and IFN-γ, indicating a predominantly Th1 response against mTg and thus did not suppress EAT (Vasu and others 2003). We speculate that antigen-specific Tregs possibly migrate to the thyroid and actively and dominantly suppress autoimmune Teff function, thus leading to disease amelioration.
FIG. 2.
GM-CSF induces tolerogenic DCs and suppresses EAT. Pro-inflammatory cytokines play a crucial role in the generation of an inflammatory response and the subsequent induction of EAT. Treatment of mice with GM-CSF induces semi-matured tolerogenic DCs that are characterized by the reduced levels of pro-inflammatory cytokines such as IL-1β and IL-12. These tolerogenic DCs, instead of activating pathogenic Teff, induce or expand Tregs that produce IL-10 and TGF-β. These regulatory cytokines counteract the role of the pro-inflammatory cytokines resulting in the suppression/prevention of EAT. GM-CSF, granulocyte-macrophage colony-stimulating factor; Teff, effector T cells; Tregs, regulatory T cells; TGF-β, transforming growth factor-β.
In our very recent studies, we have found that bone marrow DCs (BMDCs) derived ex vivo through GM-CSF treatment could selectively expand nTregs through a contact-dependent mechanism mediated by OX40L/OX40 interactions (Bhattacharya and others 2011). This mechanism was independent of TCR involvement but required IL-2. Additionally, these BMDCs secreted high levels of TGF-β that were sufficient to convert Foxp3- T cells to Foxp3+ Tregs (i.e. induced Tregs) upon TCR stimulation. In summary, the above information collectively suggests that GM-CSF may be used as a therapeutic agent in AITD. Currently, our laboratory is conducting a clinical trial to test the efficacy of GM-CSF treatment in patients with autoimmune myasthenia gravis.
Summary
Studies from humans and animal models have revealed significant new insights into the complex role of cytokines in the pathogenesis of AITD. Modulating cytokine responses have yielded highly encouraging results and they hold considerable promise in the treatment of autoimmune diseases. Pro-inflammatory cytokines such as GM-CSF and IL1β can contribute to Foxp3+ Treg expansion, whereas a regulatory suppressor cytokine such as TGF-β can initiate a pathogenic Th17 T cell response. These observations highlight the paradoxical effects of cytokines and their critical roles in maintaining a delicate balance between health and disease. Therefore, additional studies to understand the complex interplay between different cytokines and their effects on the different components of the immune system in the context of a particular disease are essential.
Acknowledgments
This work was supported by the Grant NIH-R01 AI058190 from the National Institutes of Health to B.S.P. This project was supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Akinci B. Comlekci A. Yener S. Bayraktar F. Demir T. Ozcan MA. Yuksel F. Yesil S. Hashimoto's thyroiditis, but not treatment of hypothyroidism, is associated with altered TGF-beta1 levels. Arch Med Res. 2008;39(4):397–401. doi: 10.1016/j.arcmed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Alimi E. Huang S. Brazillet MP. Charreire J. Experimental autoimmune thyroiditis (EAT) in mice lacking the IFN-gamma receptor gene. Eur J Immunol. 1998;28(1):201–208. doi: 10.1002/(SICI)1521-4141(199801)28:01<201::AID-IMMU201>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Batteux F. Trebeden H. Charreire J. Chiocchia G. Curative treatment of experimental autoimmune thyroiditis by in vivo administration of plasmid DNA coding for interleukin-10. Eur J Immunol. 1999;29(3):958–963. doi: 10.1002/(SICI)1521-4141(199903)29:03<958::AID-IMMU958>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bettelli E. Carrier Y. Gao W. Korn T. Strom TB. Oukka M. Weiner HL. Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P. Gopisetty A. Ganesh BB. Sheng JR. Prabhakar BS. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J leukoc Biol. 2011;89(2):235–249. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U. Klamp T. Groot M. Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Brahmachari S. Pahan K. Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J Immunol. 2009;183(3):2045–2058. doi: 10.4049/jimmunol.0800276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Sharp GC. Tang H. Chen K. Kyriakos M. Bickel JT. Interleukin-12 promotes activation of effector cells that induce a severe destructive granulomatous form of murine experimental autoimmune thyroiditis. Am J Pathol. 1998;152(5):1347–1358. [PMC free article] [PubMed] [Google Scholar]
- Brown KD. Zurawski SM. Mosmann TR. Zurawski G. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that includes leukocyte and fibroblast-derived inflammatory agents, growth factors, and indicators of various activation processes. J Immunol. 1989;142(2):679–687. [PubMed] [Google Scholar]
- Burchill MA. Yang J. Vogtenhuber C. Blazar BR. Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Burnet M. Auto-immune disease. I. Modern immunological concepts. Br Med J. 1959;2(5153):645–650. doi: 10.1136/bmj.2.5153.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caturegli P. Kimura H. Rocchi R. Rose NR. Autoimmune thyroid diseases. Curr Opin Rheumatol. 2007;19(1):44–48. doi: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- Chang JT. Segal BM. Nakanishi K. Okamura H. Shevach EM. The costimulatory effect of IL-18 on the induction of antigen-specific IFN-gamma production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor beta2 subunit. Eur J Immunol. 2000;30(4):1113–1119. doi: 10.1002/(SICI)1521-4141(200004)30:4<1113::AID-IMMU1113>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cheatem D. Ganesh BB. Gangi E. Vasu C. Prabhakar BS. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol. 2009;131(2):260–270. doi: 10.1016/j.clim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Wei Y. Sharp GC. Braley-Mullen H. Decreasing TNF-alpha results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2007;81(1):306–314. doi: 10.1189/jlb.0606402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. Chang SH. Martinez GJ. Yang XO. Nurieva R. Kang HS. Ma L. Watowich SS. Jetten AM. Tian Q. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane IJ. Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25(2):75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- Davidson A. Diamond B. Autoimmune diseases. N Engl J Med. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- de la Vega JR. Vilaplana JC. Biro A. Hammond L. Bottazzo GF. Mirakian R. IL-10 expression in thyroid glands: protective or harmful role against thyroid autoimmunity? Clin Exp Immunol. 1998;113(1):126–135. doi: 10.1046/j.1365-2249.1998.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R. Haanen J. Spits H. Roncarolo MG. te Velde A. Figdor C. Johnson K. Kastelein R. Yssel H. de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Ding L. Linsley PS. Huang LY. Germain RN. Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151(3):1224–1234. [PubMed] [Google Scholar]
- Ding L. Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148(10):3133–3139. [PubMed] [Google Scholar]
- Dogan RN. Vasu C. Holterman MJ. Prabhakar BS. Absence of IL-4, and not suppression of the Th2 response, prevents development of experimental autoimmune Graves' disease. J Immunol. 2003;170(4):2195–2204. doi: 10.4049/jimmunol.170.4.2195. [DOI] [PubMed] [Google Scholar]
- Drugarin D. Negru S. Koreck A. Zosin I. Cristea C. The pattern of a T(H)1 cytokine in autoimmune thyroiditis. Immunol Lett. 2000;71(2):73–77. doi: 10.1016/s0165-2478(99)00156-x. [DOI] [PubMed] [Google Scholar]
- Esquivel PS. Rose NR. Kong YC. Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J Exp Med. 1977;145(5):1250–1263. doi: 10.1084/jem.145.5.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Vega N. Alfonso-Perez M. Benedicto I. Sanchez-Madrid F. Gonzalez-Amaro R. Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto's thyroiditis. J Clin Endocrinol Metabol. 2010;95(2):953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF. Zlotnik A. Vieira P. Mosmann TR. Howard M. Moore KW. O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444–3451. [PubMed] [Google Scholar]
- Fontenot JD. Rasmussen JP. Gavin MA. Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Ganesh BB. Cheatem DM. Sheng JR. Vasu C. Prabhakar BS. GM-CSF-induced CD11c+CD8a—dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21(3):269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangi E. Vasu C. Cheatem D. Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174(11):7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- Gaudreau S. Guindi C. Menard M. Besin G. Dupuis G. Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179(6):3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- Gorelik L. Constant S. Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195(11):1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L. Fields PE. Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165(9):4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- Gorham JD. Guler ML. Fenoglio D. Gubler U. Murphy KM. Low dose TGF-beta attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161(4):1664–1670. [PubMed] [Google Scholar]
- Green DR. Droin N. Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Groux H. Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmunity. 2003;20(4):281–285. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- Heath VL. Murphy EE. Crain C. Tomlinson MG. O'Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30(9):2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ho IC. Hodge MR. Rooney JW. Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85(7):973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Horie I. Abiru N. Nagayama Y. Kuriya G. Saitoh O. Ichikawa T. Iwakura Y. Eguchi K. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology. 2009;150(11):5135–5142. doi: 10.1210/en.2009-0434. [DOI] [PubMed] [Google Scholar]
- Horie I. Abiru N. Saitoh O. Ichikawa T. Iwakura Y. Eguchi K. Nagayama Y. Distinct role of T helper Type 17 immune response for Graves' hyperthyroidism in mice with different genetic backgrounds. Autoimmunity. 2011;44(2):159–165. doi: 10.3109/08916931003777247. [DOI] [PubMed] [Google Scholar]
- Johnson VJ. Yucesoy B. Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2005;116(4):851–858. doi: 10.1016/j.jaci.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Kared H. Masson A. Adle-Biassette H. Bach JF. Chatenoud L. Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4(+)CD25(+) regulatory T-cells. Diabetes. 2005;54(1):78–84. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- Kim CH. Chemokine-chemokine receptor network in immune cell trafficking. Current Drug Targets. 2004;4(4):343–361. doi: 10.2174/1568008043339712. [DOI] [PubMed] [Google Scholar]
- Klecha AJ. Barreiro Arcos ML. Frick L. Genaro AM. Cremaschi G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation. 2008;15(1):68–75. doi: 10.1159/000135626. [DOI] [PubMed] [Google Scholar]
- Kong YC. Morris GP. Brown NK. Yan Y. Flynn JC. David CS. Autoimmune thyroiditis: a model uniquely suited to probe regulatory T cell function. J Autoimmunity. 2009;33(3–4):239–246. doi: 10.1016/j.jaut.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T. Bettelli E. Gao W. Awasthi A. Jager A. Strom TB. Oukka M. Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G. Hirsch F. Gonzalez-Garcia A. Martinez C. Differential involvement of Th1 and Th2 cytokines in autoimmune diseases. Autoimmunity. 1996;24(1):25–33. doi: 10.3109/08916939608995354. [DOI] [PubMed] [Google Scholar]
- Langrish CL. Chen Y. Blumenschein WM. Mattson J. Basham B. Sedgwick JD. McClanahan T. Kastelein RA. Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WW. Kang SW. Choi J. Lee SH. Shah K. Eynon EE. Flavell RA. Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115(3):530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira SA. Martin AP. Marinkovic T. Furtado GC. Mechanisms regulating lymphocytic infiltration of the thyroid in murine models of thyroiditis. Crit Rev Immunol. 2005;25(4):251–262. doi: 10.1615/critrevimmunol.v25.i4.10. [DOI] [PubMed] [Google Scholar]
- Lutz MB. Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23(9):445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Mangan PR. Harrington LE. O'Quinn DB. Helms WS. Bullard DC. Elson CO. Hatton RD. Wahl SM. Schoeb TR. Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mannon PJ. Fuss IJ. Mayer L. Elson CO. Sandborn WJ. Present D. Dolin B. Goodman N. Groden C. Hornung RL. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351(20):2069–2079. doi: 10.1056/NEJMoa033402. others. [DOI] [PubMed] [Google Scholar]
- Marrack P. Kappler J. Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7(8):899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- Martin AP. Coronel EC. Sano G. Chen SC. Vassileva G. Canasto-Chibuque C. Sedgwick JD. Frenette PS. Lipp M. Furtado GC. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol. 2004;173(8):4791–4798. doi: 10.4049/jimmunol.173.8.4791. others. [DOI] [PubMed] [Google Scholar]
- McLachlan SM. Nagayama Y. Rapoport B. Insight into Graves' hyperthyroidism from animal models. Endocr Rev. 2005;26(6):800–832. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- Meriggioli MN. Sheng JR. Li L. Prabhakar BS. Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis. Ann N Y Acad Sci. 2008;1132:276–282. doi: 10.1196/annals.1405.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon-Godefroy K. Brazillet MP. Rott O. Charreire J. Distinctive modulation by IL-4 and IL-10 of the effector function of murine thyroglobulin-primed cells in “transfer-experimental autoimmune thyroiditis”. Cell Immunol. 1995a;162(2):171–177. doi: 10.1006/cimm.1995.1066. [DOI] [PubMed] [Google Scholar]
- Mignon-Godefroy K. Rott O. Brazillet MP. Charreire J. Curative and protective effects of IL-10 in experimental autoimmune thyroiditis (EAT). Evidence for IL-10-enhanced cell death in EAT. J Immunol. 1995b;154(12):6634–6643. [PubMed] [Google Scholar]
- Moore KW. de Waal Malefyt R. Coffman RL. O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morris GP. Chen L. Kong YC. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003;226(1):20–29. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Moser M. Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1(3):199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- Mosmann TR. Cherwinski H. Bond MW. Giedlin MA. Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann TR. Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murphy KM. Reiner SL. The lineage decisions of helper T cells. Nat Rev. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nabozny GH. Kong YC. Circumvention of the induction of resistance in murine experimental autoimmune thyroiditis by recombinant IL-1 beta. J Immunol. 1992;149(3):1086–1092. [PubMed] [Google Scholar]
- Nagayama Y. Mizuguchi H. Hayakawa T. Niwa M. McLachlan SM. Rapoport B. Prevention of autoantibody-mediated Graves'-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170(7):3522–3527. doi: 10.4049/jimmunol.170.7.3522. [DOI] [PubMed] [Google Scholar]
- Nagayama Y. Saitoh O. McLachlan SM. Rapoport B. Kano H. Kumazawa Y. TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-gamma and interleukin-4 knockout mice; implications for the Th1/Th2 paradigm. Clin Exp Immunol. 2004;138(3):417–422. doi: 10.1111/j.1365-2249.2004.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T. Tsuruoka M. Ogura H. Okuyama Y. Arima Y. Hirano T. Murakami M. IL-6 positively regulates Foxp3+CD8+ T cells in vivo. Int Immunol. 2010;22(2):129–139. doi: 10.1093/intimm/dxp119. [DOI] [PubMed] [Google Scholar]
- Nanba T. Watanabe M. Inoue N. Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009;19(5):495–501. doi: 10.1089/thy.2008.0423. [DOI] [PubMed] [Google Scholar]
- Nossal GJ. Clonal anergy of B cells: a flexible, reversible, and quantitative concept. J Exp Med. 1996;183(5):1953–1956. doi: 10.1084/jem.183.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ. Ma A. Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2(1):37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BJ. Thomas HE. Pai S. Santamaria P. Iwakura Y. Steptoe RJ. Kay TW. Thomas R. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176(12):7278–7287. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- Patibandla SA. Fan JL. Prabhakar BS. Seetharamaiah GS. Comparison of immune responses to extracellular domains of mouse and human thyrotropin receptor. J Autoimmunity. 1999;13(2):205–213. doi: 10.1006/jaut.1999.0311. [DOI] [PubMed] [Google Scholar]
- Patibandla SA. Wagle NM. Seetharamaiah GS. Fan JL. Dallas JS. Prabhakar BS. Experimental autoimmunity to thyrotropin receptor. Exp Clin Endocrinol Diabetes. 1996;104(Suppl 3):28–32. doi: 10.1055/s-0029-1211677. [DOI] [PubMed] [Google Scholar]
- Pichurin P. Yan XM. Farilla L. Guo J. Chazenbalk GD. Rapoport B. McLachlan SM. Naked TSH receptor DNA vaccination: A TH1 T cell response in which interferon-gamma production, rather than antibody, dominates the immune response in mice. Endocrinology. 2001;142(8):3530–3536. doi: 10.1210/endo.142.8.8301. [DOI] [PubMed] [Google Scholar]
- Prabhakar BS. Bahn RS. Smith TJ. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev. 2003;24(6):802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Wing K. Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Yamaguchi T. Nomura T. Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6(4):327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- Seetharamaiah GS. Fan JL. Patibandla SA. Prabhakar BS. Influence of adjuvants on the induction of autoantibodies to the thyrotropin receptor. Autoimmunity. 1996;24(4):205–215. doi: 10.3109/08916939608994713. [DOI] [PubMed] [Google Scholar]
- Sharma R. Sung SS. Abaya CE. Ju AC. Fu SM. Ju ST. IL-2 regulates CD103 expression on CD4+ T cells in Scurfy mice that display both CD103-dependent and independent inflammation. J Immunol. 2009;183(2):1065–1073. doi: 10.4049/jimmunol.0804354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JR. Li L. Ganesh BB. Vasu C. Prabhakar BS. Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177(8):5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- Sheng JR. Li LC. Ganesh BB. Prabhakar BS. Meriggioli MN. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin Immunol. 2008;128(2):172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JR. Li LC. Prabhakar BS. Meriggioli MN. Acetylcholine receptor-alpha subunit expression in myasthenia gravis: a role for the autoantigen in pathogenesis? Muscle Nerve. 2009;40(2):279–286. doi: 10.1002/mus.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Wang H. Su Z. Chen J. Xue Y. Wang S. Xue Y. He Z. Yang H. Zhou C. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto's thyroiditis. Scand J Immunol. 2010;72(3):250–255. doi: 10.1111/j.1365-3083.2010.02425.x. others. [DOI] [PubMed] [Google Scholar]
- Silva LC. Ortigosa LC. Benard G. Anti-TNF-alpha agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2(6):817–833. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
- Stassi G. De Maria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev. 2002;2(3):195–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- Stassi G. Di Liberto D. Todaro M. Zeuner A. Ricci-Vitiani L. Stoppacciaro A. Ruco L. Farina F. Zummo G. De Maria R. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol. 2000;1(6):483–488. doi: 10.1038/82725. [DOI] [PubMed] [Google Scholar]
- Sutton CE. Lalor SJ. Sweeney CM. Brereton CF. Lavelle EC. Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Tang H. Mignon-Godefroy K. Meroni PL. Garotta G. Charreire J. Nicoletti F. The effects of a monoclonal antibody to interferon-gamma on experimental autoimmune thyroiditis (EAT): prevention of disease and decrease of EAT-specific T cells. Eur J Immunol. 1993;23(1):275–278. doi: 10.1002/eji.1830230143. [DOI] [PubMed] [Google Scholar]
- Tang H. Sharp GC. Peterson KP. Braley-Mullen H. IFN-gamma-deficient mice develop severe granulomatous experimental autoimmune thyroiditis with eosinophil infiltration in thyroids. J Immunol. 1998;160(10):5105–5112. [PubMed] [Google Scholar]
- Tomer Y. Hepatitis C and interferon induced thyroiditis. J Autoimmunity. 2010;34(3):J322–J326. doi: 10.1016/j.jaut.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y. Ban Y. Concepcion E. Barbesino G. Villanueva R. Greenberg DA. Davies TF. Common and unique susceptibility loci in Graves and Hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73(4):736–747. doi: 10.1086/378588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourneur L. Damotte D. Marion S. Mistou S. Chiocchia G. IL-10 is necessary for FasL-induced protection from experimental autoimmune thyroiditis but not for FasL-induced immune deviation. Eur J Immunol. 2002;32(5):1292–1299. doi: 10.1002/1521-4141(200205)32:5<1292::AID-IMMU1292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Trembleau S. Penna G. Bosi E. Mortara A. Gately MK. Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995;181(2):817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasu C. Dogan RN. Holterman MJ. Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol. 2003;170(11):5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- Veldhoen M. Hocking RJ. Atkins CJ. Locksley RM. Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Verginis P. Li HS. Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol. 2005;174(11):7433–7439. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- Vladutiu AO. Sulkowski E. Inhibition of murine autoimmune thyroiditis by interferon. Biomedicine. 1980;33(6):173–174. [PubMed] [Google Scholar]
- Vural P. Degirmencioglu S. Erden S. Gelincik A. The relationship between transforming growth factor-beta1, vascular endothelial growth factor, nitric oxide and Hashimoto's thyroiditis. Int Immunopharmacol. 2009;9(2):212–215. doi: 10.1016/j.intimp.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Wang SH. Bretz JD. Phelps E. Mezosi E. Arscott PL. Utsugi S. Baker JR., Jr A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive to destructive thyroiditis in experimental autoimmune thyroiditis. J Immunol. 2002;168(5):2470–2474. doi: 10.4049/jimmunol.168.5.2470. [DOI] [PubMed] [Google Scholar]
- Wang SH. Chen GH. Fan Y. Van Antwerp M. Baker JR., Jr Tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis by the expansion of CD4+CD25+ regulatory T cells. Endocrinology. 2009;150(4):2000–2007. doi: 10.1210/en.2008-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148(1):1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- Yanaba K. Bouaziz JD. Matsushita T. Magro CM. St Clair EW. Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Yang J. Murphy TL. Ouyang W. Murphy KM. Induction of interferon-gamma production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur J Immunol. 1999;29(2):548–555. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Yang XO. Panopoulos AD. Nurieva R. Chang SH. Wang D. Watowich SS. Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T. Takeda K. Tanaka T. Ohkusu K. Kashiwamura S. Okamura H. Akira S. Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161(7):3400–3407. [PubMed] [Google Scholar]
- Yu S. Fang Y. Sharp GC. Braley-Mullen H. Transgenic expression of TGF-beta on thyrocytes inhibits development of spontaneous autoimmune thyroiditis and increases regulatory T cells in thyroids of NOD.H-2h4 mice. J Immunol. 2010;184(9):5352–5359. doi: 10.4049/jimmunol.0903620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Kong YC. Noninvolvement of IL-4 and IL-10 in tolerance induction to experimental autoimmune thyroiditis. Cell Immunol. 1998;187(2):95–102. doi: 10.1006/cimm.1998.1331. [DOI] [PubMed] [Google Scholar]
- Zhang ZL. Lin B. Yu LY. Shen SX. Zhu LH. Wang WP. Guo LH. Gene therapy of experimental autoimmune thyroiditis mice by in vivo administration of plasmid DNA coding for human interleukin-10. Acta Pharmacol Sinica. 2003;24(9):885–890. [PubMed] [Google Scholar]
- Zheng W. Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nature Immunol. 2007;8(5):457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- Zhou L. Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Current Opin Immunol. 2009;21(2):146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF. Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11(5):594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipris D. Greiner DL. Malkani S. Whalen B. Mordes JP. Rossini AA. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J Immunol. 1996;156(3):1315–1321. [PubMed] [Google Scholar]