Abstract
Human breast cancers cells overexpressing HER2/neu are more aggressive tumors with poor prognosis, and resistance to chemotherapy. This study investigates antiproliferation effects of anthraquinone derivatives of rhubarb root on human breast cancer cells. Of 7 anthraquinone derivatives, only rhein showed antiproliferative and apoptotic effects on both HER2-overexpressing MCF-7 (MCF-7/HER2) and control vector MCF-7 (MCF-7/VEC) cells. Rhein induced dose- and time-dependent manners increase in caspase-9-mediated apoptosis correlating with activation of ROS-mediated activation of NF-κB- and p53-signaling pathways in both cell types. Therefore, this study highlighted rhein as processing anti-proliferative activity against HER2 overexpression or HER2-basal expression in breast cancer cells and playing important roles in apoptotic induction of human breast cancer cells.
1. Introduction
Rhubarb root (Rheum palmatum), member of the Polygonaceae family, is one well-known antineoplastic herb in traditional Chinese medicine [1]. Anthraquinone derivatives are its main bioactive constituents: emodin (1,3,8-trihydroxy-6-methylanthraquinone), aloe emodin (1,8-dihydroxy-3-hydroxyl-methyl anthraquinone), rhein (1,8-dihydroxy-3-carboxyanthraquinone), chrysophanol (1,8-dihydroxy-3-methyl-anthraquinone), physcion (1,8-drihydroxy-3-methyl-6-methoxyanthraquinone), and danthron (1,8-dihydroxy-9,10-anthraquinone). Rhubarb anthraquinone derivatives can induce apoptosis of human cancer, including lung adenocarcinoma A549, myelogenous leukemia HL-60, lung squamous carcinoma CH27, cervical carcinoma HeLa cells, neuroblastoma IMR-32, bladder cancer T24, and hepatoma HepG2 cells [1]. Emodin inhibits cellular proliferation, induces apoptosis, and prevents metastasis through activation of tyrosine kinases, phosphoinositol 3-kinase (PI3K), protein kinase C (PKC), NF-kappa B (NF-κB), and mitogen-activated protein kinase (MAPK) signaling cascades [2–5]. Aloe-emodin has antitumor properties through the p53 and its downstream p21 pathway [6]. Emodin and aloe emodin also reduce tumor size, prolong survival, decrease incidence of tumor invasion and neovascularization using in vivo animal models [7, 8]. Rhein blocks the uptake of glucose in tumor cells, causing changes in membrane-associated functions to trigger cell death [9].
Breast cancer is one of the most common cancers in women, resulting from gene amplification and/or overexpression of some oncogenes like HER2/neu (also known as ErbB2) and oestrogen receptors [10, 11]. Overexpression of HER2/neu in nearly 30% of human breast cancers correlates with more aggressive tumors and poor prognosis [12]. HER-2-overexpressing cells appear to be resistant to some classes of chemotherapy agents [12], but sensitive to others [13]. Overexpression of HER2/neu was positively correlated with p53 nuclear accumulation and tumor metastasis, negatively with hormonal receptor status [14]. Tyrosine kinase activity of HER2/neu could phosphorylate HER3 and then activate phosphatidylinositol 3-kinase (PI3K) involved in malignance of tumors [15]. Trastuzumab (Herceptin) is widely used for treatment of HER2-positive breast cancer; resistance to trastuzumab occurs in some patients [16]. Therefore, HER2/neu is a potentially therapeutic target for breast cancer, influencing efficacy of chemotherapy.
Engineering human breast cancer MCF-7 cells that expresses basal level of HER2/neu for Overexpression of HER2/neu has been used to analyze biological properties of HER2 overexpression [15, 17]. This study investigates apoptotic effects of anthraquinone derivatives of rhubarb root on both types of breast cancer cells, HER2-overexpressing MCF-7 cells (MCF-7/HER2) and control vector MCF-7 cells (MCF-7/VEC). Of 7 anthraquinone derivatives, only rhein showed antiproliferative and apoptotic effects on both MCF-7/HER2 and MCF-7/VEC cells. We also analyzed apoptotic mechanism of rhein on both human breast cancer cell lines with basal level and Overexpression of HER2.
2. Materials and Methods
2.1. Cell Culture
Human breast cancer cell lines, vector control MCF-7 cells (MCF-7/VEC) and HER2-overexpressing MCF-7 cells (MCF-7/HER2), were used in this study, as previously described [15]. Both types were grown in DMEM/F-12 (Invitrogen) with 10% fetal bovine serum (Invitrogen), gentamicin (50 mg/mL) and G418 (800 μg/mL).
2.2. Western Blotting Analysis
Cell lysates that were harvested from MCF-7/VEC and MCF-7/HER2 cells with or without treatment of anthraquinone derivatives were dissolved in 2X SDS-PAGE sample buffer without 2-mercaptoethanol, and boiled for 10 min. Cell lysate proteins were resolved on 12% SDS-PAGE gels and transferred to nitrocellulose paper. Resultant blots were blocked with 5% skim milk and reacted with properly diluted monoclonal antibodies against HER2/c-neu (Ab-3,3B5), caspase 9, Apoptosis signal-regulating kinase 1 (ASK1), P21, and β-actin (Cell Signaling Technology) for 3 h incubation. Immune complexes were detected by horseradish peroxidase-conjugated goat anti-mouse IgG antibodies, followed by enhanced chemiluminescence reaction (Amersham Pharmacia Biotech).
2.3. MTT Cytotoxicity Test
Aloe emodin, emodin, rhein, chrysophanol, sennoside A, sennoside B, and 9-, 10-anthraquinone were purchased from Sigma Chemical Company (St. Louis, MO, USA). In vitro antiproliferative effect of each compound on MCF-7/VEC and MCF-7/HER2 cells was examined using MTT assay. Cells were plated in 96-well plates (5 × 104 cells/well) and then treated with serial dilution of each tested compound. After treatment for 48 h, 25 μL of a MTT solution at 5 mg/mL was added to each well and incubated at 37°C in 5% CO2 for 3 h. After subsequent washing three times with phosphate buffer saline, 100 μL DMSO was added into plates for dissolving formazan crystals. OD570-630 in each well was measured with a micro-ELISA reader and survival rate used to indicate suppressive effects of each compound on MCF-7/VEC and MCF-7/HER2 cells. Survival rate (%) = ((Acontrol − Aexperiment)/Acontrol) × 100%. Cytotoxic concentration giving 50% (CC50) was calculated by computer program (provided by John Spouge, NCBI, NIH).
2.4. Detection of Cell Cycle and Apoptosis by Flow Cytometry
MCF-7/VEC and MCF-7/HER2 cells were harvested 24,48, and 72 h posttreatment with or without serial dilutions of rhein. Cells were fixed using 70% ethanol at 4°C overnight, then re-suspended in PBS containing 50 μg/mL PI and 0.1 mg/mL RNase and 0.1% Triton X-100 in darkroom. After 30 min incubation at 37°C, cells were tested by flow cytometry (Becton-Dickinson, San Jose, Calif, USA) equipped with an argon ion laser at 488 nm wavelength, then rates of sub G1 (apoptotic), G1, S, and G2 phase cells were determined. In addition, rhein-induced cytopathic effect (CPE) of MCF-7/VEC and MCF-7/HER2 cells was observed 48 h posttreatment using light microscopy.
2.5. Detecting Intracellular Reactive Oxygen Species (ROS) by Flow Cytometry
MCF-7/VEC and MCF-7/HER2 cells were harvested 24 h posttreatment with or without serial dilutions of rhein; 5 × 105 cells were harvested after 48 h transfection and their ROS level noted. They were washed twice with PBS, then incubated with 10 μM 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma) at 37°C for 30 min in darkroom for final analysis by flow cytometry (Becton Dickinson FACS Calibur).
2.6. In Vivo Signaling Transduction Pathway Assay with Cis-Reporter Plasmids
Cis-reporter plasmids pAP1- Luc, pNF-κB-Luc, and p53-Luc were purchased from Stratagene Company. MCF-7/VEC and MCF-7/HER2 cells were co-transfected with cis-reporter plasmid and internal control reporter pRluc-C1 (BioSignal Packard) in 6-well plates, using the Arrest-In transfection reagent. After one-day incubation, transfected cells were seeded into 24-well plates using the MEM containing 10% FBS, then treated with or without 20 μg/mL rhein. After 4 h posttreatment, enzyme activity of firefly and Renilla luciferases in indicated cells was measured by dual Luciferase Reporter Assay System (Promega) and Luminometer TROPIX TR-717 (Applied Biosystems). Relative firefly luciferase activity of the cis-acting reporter was normalized by Renilla luciferase.
2.7. Statistical Analysis
ANOVA analysis using SPSS program (version 10.1, SPSS Inc., IL, USA) or Student's t-test analyzed data, P value below 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Rhein Inhibited Growth of MCF-7/VEC and MCF-7/HER2 Cells in Dose-Dependent Manner
To examine expression level of HER2 in MCF-7/VEC and MCF-7/HER2 cells, both cell lysates were analyzed using Western blotting assay. Immunoreactive bands with anti-HER2 antibodies revealed basal expression of HER2 in MCF-7/VEC cells and Overexpression of HER2 in MCF-7/HER2 cells (Figure 1, Lanes 1 and 2). Subsequently, antiproliferative effect of anthraquinone derivatives on MCF-7/VEC and MCF-7/HER2 cells was analyzed using MTT assay (Figure 2). Sennosides A and B have no inhibitory effect on cell growth, while IC50 values of 9-, 10-anthraquinone, chrysophanol, aloe emodin, and emodin for both types of cells exceeded 100 μg/mL (Table 1). Only rhein had a dose-dependent antiproliferative ability on both types. In addition, rhein was less toxic to nontumorigenic MCF-10A cells used as the normal control (Figure 1 of the Supplementary material available online at doi:10.1155/2012/940920). The IC50 value of rhein was 36.69 ± 9.77 μg/mL (129.1 ± 34.37 μM) for MCF-7/VEC cells and 30.66 ± 2.21 μg/mL (107.9 ± 7.7 μM) for MCF-7/HER2 cells. Rhein showed micromolar IC50 values on growth suppression of MCF-7/VEC and MCF-7/HER2 cells, similar to antiproliferative activity of rhein lysinate on human breast cancer cell lines MCF-7, SK-Br-3, and MDA-MB-231 [18] and human hepatocellular carcinoma BEL-7402 cells [19], but less potent than its activity against tongue cancer cell line SCC-4 [20] and ovarian carcinoma cell line SKOV-3 [21].
Figure 1.
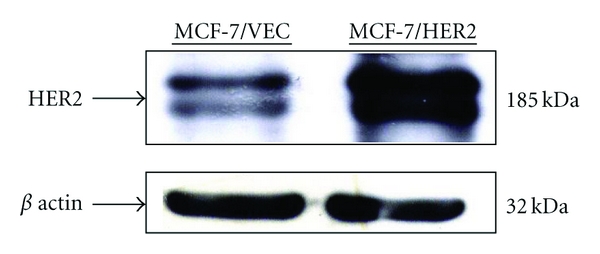
Expression of HER2/neu in human breast cancer cells. Vector control MCF-7 cells (MCF-7/VEC) and HER2-overexpressing MCF-7 cells (MCF-7/HER2) were harvested, and cell lysates were analyzed by Western blotting with anti-HER2 and anti-β actin antibodies.
Figure 2.
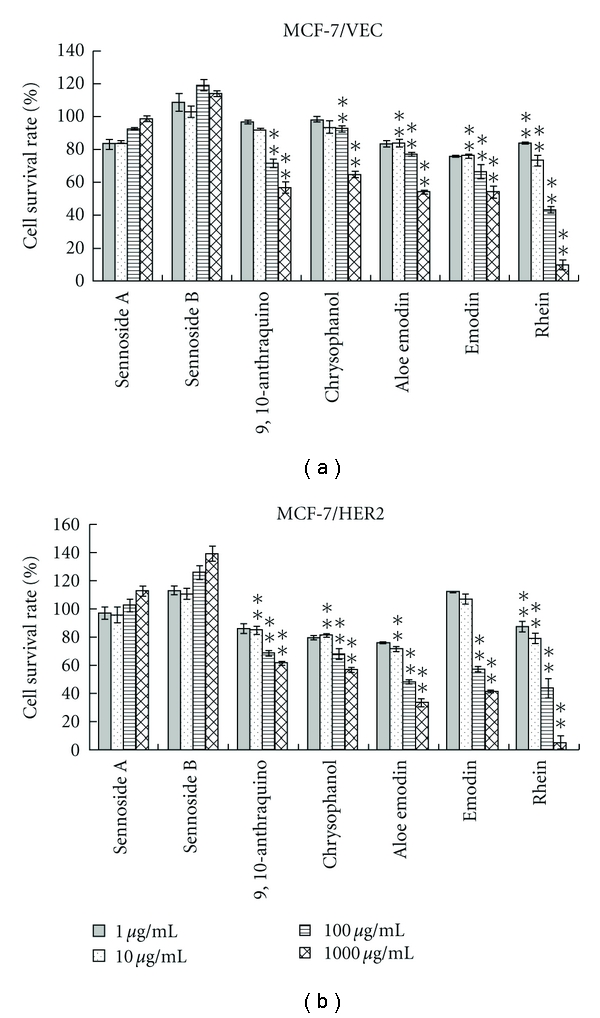
Effect of anthraquinone derivatives on the growth of human breast cancer cells. MCF-7/VEC (a) and MCF-7/HER2 (b) cells were plated in 96-well plates (5 × 104 cells/well) and then treated with serial dilution of each tested compound. After treatment for 48 h, cell growth was examined using MTT assay. OD570-630 in each well was measured with a micro-ELISA reader. Survival rate (%) = ((Acontrol − Aexperiment)/Acontrol) × 100. *P value < 0.05; **P value < 0.01 compared with untreated cells.
Table 1.
IC50 values of anthraquinone derivatives in MCF-7/VEC and MCF-7/HER2 cells.
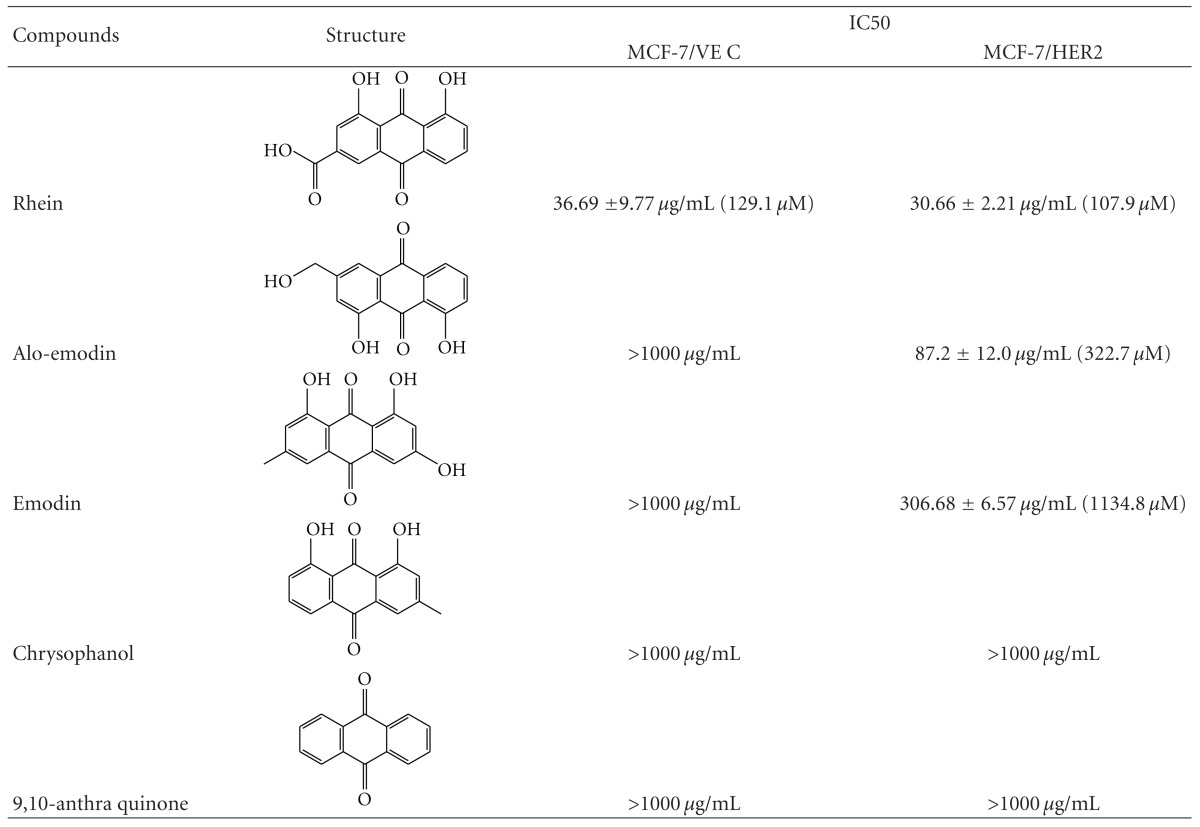
|
3.2. Rhein Influenced Cell Cycle Phase of MCF-7/VEC and MCF-7/HER2 Cells
To examine the effect of rhein on cell cycle phases, both types were treated with or without rhein at concentrations of 10 μg/mL and 100 μg/mL, and then harvested 24, 48, and 72 hours posttreatment. Cells were stained with propidium iodide and analyzed for cell cycle fractions by flow cytometry (Figure 3). Rhein induced concentration- and time-dependent manner increase in sub-G1 (apoptosis) fractions of MCF-7/VEC and MCF-7/HER2 cells (P < 0.05, Figure 3(a)). The rate of G1 phase in MCF-7/VEC cells significantly increased 24 hours posttreatment with rhein at a concentration of 100 μg/mL (P < 0.05), but no effect of rhein on G1 phase in MCF-7/HER2 cells was detected (Figure 3(b)). Rhein at concentrations of 10 and 100 μg/mL caused a significant S phase arrest in MCF-7/HER2 cells 48 and 72 hours posttreatment (P < 0.05), but rhein at a concentration of 100 μg/mL decreased S phase fractions in MCF-7/VEC cells 24 and 48 hours posttreatment (P < 0.05, Figure 3(c)). Interestingly, a very low fraction of G2 phase was detected in MCF-7/HER2 cells 48 and 72 hours posttreatment with rhein at a concentration of 100 μg/mL (P < 0.05); no significant change appeared in MCF-7/VEC cells 24, 48, and 72 hours posttreatment (Figure 3(d)). Cell cycle analysis demonstrated rhein inducing S phase arrest and G2 phase decrease in MCF-7/HER2 cells (Figures 3(c) and 3(d)). Moreover, rhein increased the rate of G1 phase, but decreased the fraction of S phase in MCF-7/VEC cells (Figures 3(b) and 3(c)). The difference of cell cycle distribution in response to rhein could be due to cell specificity [22, 23]. Cell cycle analysis of breast cancer BT-474 cells and MCF-7 cells indicated 1alpha(OH)D5 inducing an S phase decrease in MCF-7 cells and a G2 phase decrease in BT-474 cells [22]. Moreover, resveratrol induced apoptosis of MCF-7 cells via S phase arrest, but not MDA-MB-231 cells [23]. Western blot analysis indicated activation of caspase 9 in both cell types (Figure 4), responsible for dose-dependent manners on rhein-induced apoptosis of breast cancer cells. Results demonstrate that S phase arrest of MCF-7/HER2 cells and increased G1 phase of MCF-7/VEC cells correlated with rhein-induced caspase-9-mediated apoptosis. Rhein-induced S phase arrest of MCF-7/HER2 cells correlates with antiproliferative and apoptotic mechanisms of rhein on human hepatocellular and tongue carcinoma cells [19, 24]. Also, G1 arrest of MCF-7/VEC cells induced by rhein proves consistent with rhein-induced apoptosis of human lung cancer A-549 cells [25] and cervical cancer Ca Ski cells [26].
Figure 3.
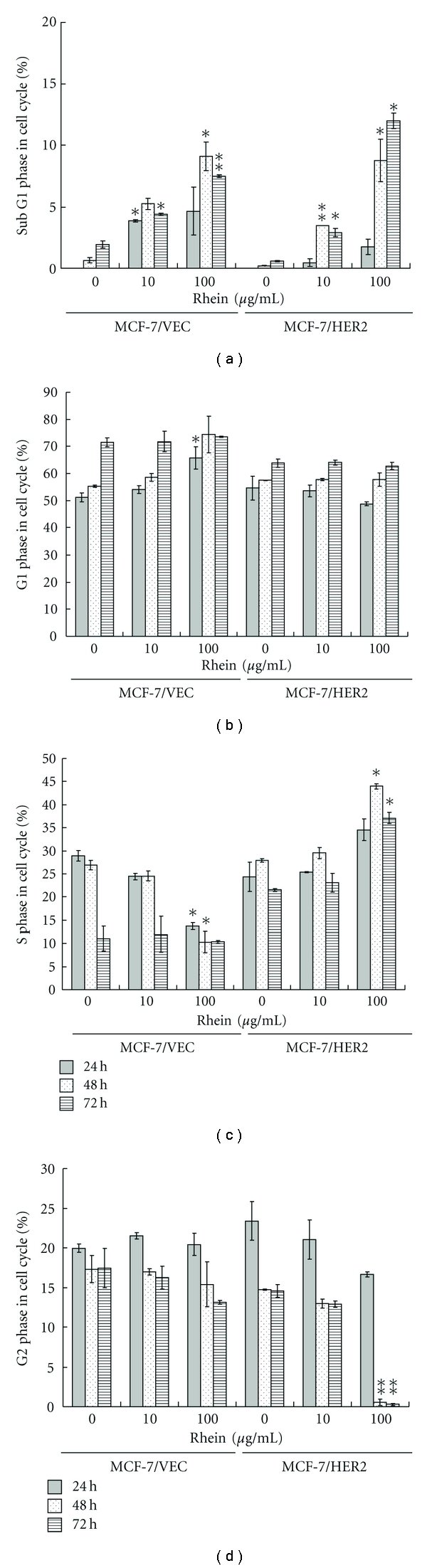
Cell cycle analysis of human breast cancer cells in response to rhein. MCF-7/VEC and MCF-7/HER2 cells were treated with serial dilution of rhein. After 24, 48, and 72 h incubation, cells were fixed by 70% ethanol, stained with PI, and analyzed using flow cytometry. Percentage of cells in sub G1 (apoptotic) (a), G1 (b), S (c), and G2 (d) phases were shown representing three independent studies. *P value < 0.05; **P value < 0.01 compared with untreated cells.
Figure 4.
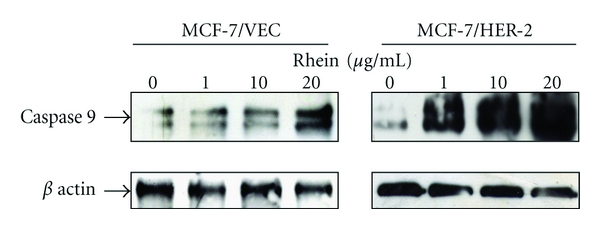
Western blotting analysis of caspase 9 in human breast cancer cells with/without rhein treatment. After 48 h incubation with rhein, MCF-7/VEC and MCF-7/HER2 cells were harvested, and lysates were analyzed by Western blotting with anticaspase-9 and anti-β actin antibodies.
3.3. Rhein Induced ROS-Mediated Activation of ASK1 in MCF-7/VEC and MCF-7/HER2 Cells
Intracellular ROS generation reportedly triggers activation of caspase 9 and ASK1 signaling in apoptotic responses [27–29]. Therefore, we assessed effects of rhein treatment on intracellular ROS and ASK1 levels in MCF-7/VEC and MCF-7/HER2 cells (Figures 5 and 6). Cells were cultured with rhein at 37°C for 48 hours. After being washing twice with PBS, cells were stained with DCFH-DA, then analyzed by flow cytometry. Rhein treatment caused concentration-dependent increase of intracellular ROS in MCF-7/VEC and MCF-7/HER2 cells (P < 0.05, Figure 5). Western blot with anti-ASK1 antibodies revealed rhein as concentration-dependently triggering ASK1 level in each type of cell (Figure 6). Results indicate ROS-mediated ASK1 signaling in rhein-induced apoptosis of both types. The findings were in agreement with incremental level of intracellular ROS in rhein-induced apoptosis of human tongue cancer cells, promyelocytic leukemia cells, and nasopharyngeal carcinoma cells [24, 30, 31].
Figure 5.
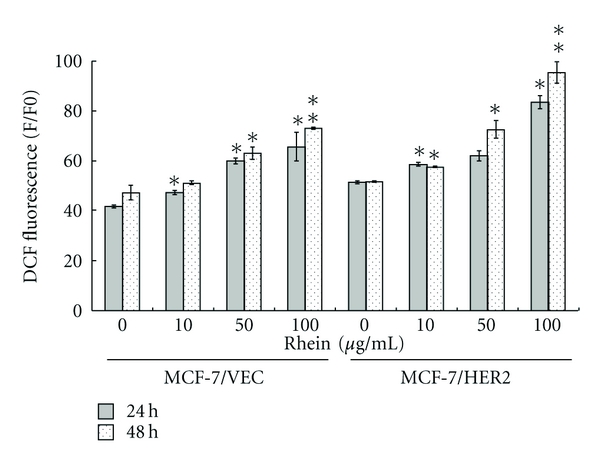
Flow cytometric analysis of reactive oxygen species (ROS) in human breast cancer cells with/without rhein treatment. After 24 and 48 h incubation with rhein, MCF-7/VEC and MCF-7/HER2 cells were harvested, and then stained by DCFH-DA dye. The fluorescence intensity of stained cells was determined by flow cytometry. *P value < 0.05; **P value < 0.01 compared with untreated cells.
Figure 6.
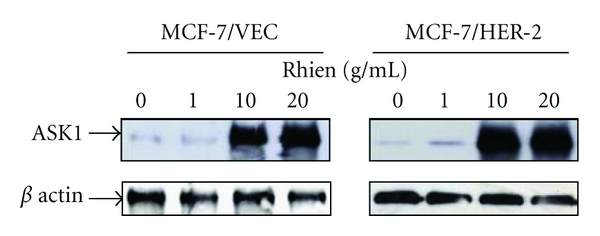
Western blotting analysis of ASK1 expression in human breast cancer cells with/without rhein treatment. After 48 h incubation with rhein, MCF-7/VEC and MCF-7/HER2 cells were harvested, and lysates were analyzed by Western blotting with anti-ASK1 and anti-β actin antibodies.
3.4. Rhein Activated p53/p21 Signaling in MCF-7/VEC and MCF-7/HER2 Cells
To probe correlation between in vivo signaling pathways and rhein-induced growth arrest and apoptosis, cells in response to rhein were further characterized by dual reporter assays with internal control reporter pRluc-C1 and luciferase reporter plasmid containing direct repeat elements of AP1, NF-κB, and p53 (Figure 7). The cells were harvested 4 h posttreatment, and then the relative expression of firefly luciferase driven from the indicated cis-reporter plasmid was normalized by Renilla luciferase. Relative intensity of firefly luciferase revealed that rhein significantly activated p53- and NF-κB promoters (P < 0.05), but induced no significant change of AP1-derived reporter activity in each type of cell (Figure 7). Moreover, p53- or NF-κB-derived reporter activity increased more than 1.7-fold in rhein-treated cells than mock cells. Definite increase of rhein-induced NF-κB- and p53-derived reporter activity in both types of cells indicated rhein modulating NF-κB- and p53-signaling pathways in breast cancer cells. Our result was inconsistent with the function of NF-κB mediating the cell survival response by inhibiting p53-dependent apoptosis and upregulating anti-apoptotic members of the Bcl2 family [32]. However, recent evidences show pro-apoptotic property of NF-κB via p53-dependent apoptosis in breast cancer MCF7/ADR, melanoma M14, and pro-B cells [33–36]. Activation of NF-κB induces upregulation of pro-apoptotic death receptor 5 (DR5) in breast cancer MCF-7 and MDA-MB-231 cells, correlating with TNF-related apoptosis inducing ligand-mediated apoptosis [37]. In addition, oxidative stress has demonstrated activation of both NF-κB and p53 [38, 39]. Doxycycline-induced superoxide-mediated apoptosis indicated NF-κB as a proapoptotic factor by activating the p53-signaling pathway [39]. Monoamine neurotoxin-induced apoptosis of peripheral blood lymphocytes is associated with activation of NF-κB, p53, and c-Jun transcription factors [40]. Thus, rhein-induced activation of both NF-κB- and p53-signaling pathways might correlate with ROS-mediated ASK1 signaling in breast cancer cells (Figures 5 and 6). Moreover, rhein has reported activation of p53/p21 signaling pathway with in rhein-induced apoptosis of human lung cancer cells, cervical cancer cells, and hepatoblastoma cells [25, 26, 41]. We also demonstrated rhein-inducing dose-dependent increases of p21 protein in MCF-7/VEC and MCF-7/HER2 cells (Figure 8). Therefore, rhein-induced apoptosis of breast cancer cells could be associated with ROS-mediated activation of NF-κB- and p53-signaling pathways.
Figure 7.
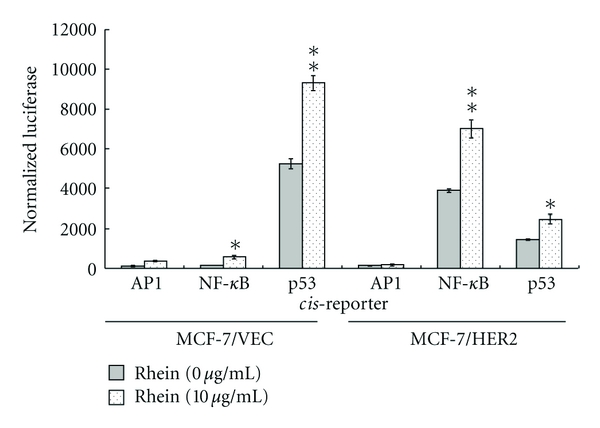
Effect of rhein on in vivo signal pathways in human breast cancer cells. MCF-7/VEC and MCF-7/HER2 cells were transiently co-transfected with cis-reporter plasmids (pAP1-Luc, pNF-κB-Luc, and p53-Luc) and an internal control reporter (pRluc-C1), and then treated with rhein for 24 h. Firefly and Renilla Luciferase enzymes were measured; the relative firefly luciferase activity was normalized by Renilla Luciferase. *P value < 0.05; **P value < 0.01 compared with untreated cells.
Figure 8.
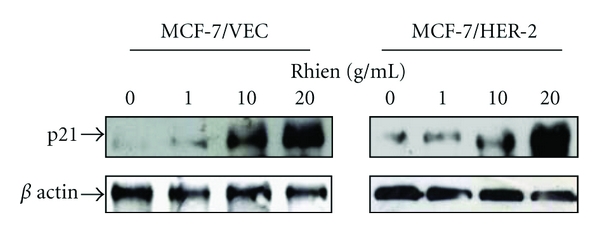
Western blotting analysis of p21 expression in human breast cancer cells with/without rhein treatment. After 48 h incubation with rhein, MCF-7/VEC and MCF-7/HER2 cells were harvested, and lysates were analyzed by Western blotting with anti-p21 and anti-β actin antibodies.
In conclusion, this study highlighted rhein versus six other anthraquinone derivatives, as processing antiproliferative activity against both types of breast cancer cells with HER2 overexpression or HER2-basal expression. Rhein showed micromolar IC50 values on growth inhibition of both types via caspase-9-mediated apoptosis. ROS-mediated activation of NF-κB- and p53-signaling pathways might play important roles in rhein-induced apoptosis of human breast cancer cells.
Conflict of Interests
All authors report no conflict of interests relevant to this paper.
Supplementary Material
Supplemental Figure 1: To compare cytotoxic effect of rhein on normal breast MCF-10A cells, and breast cancer MCF-7/VEC and MCF-7/HER2 cells, the cells were treated with 20 μg/mL rhein for 48 h. The cell survival rate was determined using MTT assay, showing 99.7 ± 0.4% for MCF-10A cells, 75.5 ± 3.5% for MCF-7/VEC cells, and 59.1 ± 1.4% for MCF-7/HER2 cells, respectively. The resulted demonstrated rhein being less toxic to non-tumorigenic MCF-10A cells.
Acknowledgments
Hong-Lin Chan and Hui-Yi Lin equally contribution as the first author. This work was supported by the National Science Council of Taiwan (NSC99-2628-B-039-006-MY3) and China Medical University (CMU99-NSC-08 and CMU97-343).
References
- 1.Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Medicinal Research Reviews. 2007;27(5):609–630. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 2.Huang Q, Shen HM, Ong CN. Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cellular and Molecular Life Sciences. 2005;62(10):1167–1175. doi: 10.1007/s00018-005-5050-2. [DOI] [PubMed] [Google Scholar]
- 3.Lai GH, Zhang Z, Sirica AE. Celecoxib acts in a cyclooxygenase-2-independent manner and in synergy with emodin to suppress rat cholangiocarcinoma growth in vitro through a mechanism involving enhanced Akt inactivation and increased activation of caspases-9 and -3. Molecular Cancer Therapeutics. 2003;2(3):265–271. [PubMed] [Google Scholar]
- 4.Yang J, Li H, Chen YY, et al. Anthraquinones sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo via reactive oxygen species-mediated dual regulation of apoptosis. Free Radical Biology and Medicine. 2004;37(12):2027–2041. doi: 10.1016/j.freeradbiomed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Wan Q, Zhang Y, et al. Emodin suppresses interleukin-1β induced mesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK. Life Sciences. 2007;80(26):2481–2488. doi: 10.1016/j.lfs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Kuo PL, Lin TC, Lin CC. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sciences. 2002;71(16):1879–1892. doi: 10.1016/s0024-3205(02)01900-8. [DOI] [PubMed] [Google Scholar]
- 7.Cárdenas C, Quesada AR, Medina MA. Evaluation of the anti-angiogenic effect of aloe-emodin. Cellular and Molecular Life Sciences. 2006;63(24):3083–3089. doi: 10.1007/s00018-006-6399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He ZH, He MF, Ma SC, But PP. Anti-angiogenic effects of rhubarb and its anthraquinone derivatives. Journal of Ethnopharmacology. 2009;121(2):313–317. doi: 10.1016/j.jep.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Castiglione S, Fanciulli M, Bruno T, et al. Rhein inhibits glucose uptake in Ehrlich ascites tumor cells by alteration of membrane-associated functions. Anti-Cancer Drugs. 1993;4(3):407–414. doi: 10.1097/00001813-199306000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 11.Elledge RM, Osborne CK. Oestrogen receptors and breast cancer. The British Medical Journal. 1997;314(7098):1843–1844. doi: 10.1136/bmj.314.7098.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toikkanen S, Helin H, Isola J, Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-up. Journal of Clinical Oncology. 1992;10(7):1044–1048. doi: 10.1200/JCO.1992.10.7.1044. [DOI] [PubMed] [Google Scholar]
- 13.Ménard S, Casalini P, Campiglio M, Pupa SM, Tagliabue E. Role of HER2/neu in tumor progression and therapy. Cellular and Molecular Life Sciences. 2004;61(23):2965–2978. doi: 10.1007/s00018-004-4277-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita H, Nishio M, Toyama T, et al. Coexistence of HER2/neu over-expression and p53 protein accumulation is a strong prognostic molecular marker in breast cancer. Breast Cancer Research. 2004;6(1):R24–R30. doi: 10.1186/bcr738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. The Journal of Biological Chemistry. 2004;279(6):4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 16.Garrett JT, Arteaga CL. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications. Cancer Biology and Therapy. 2011;11(9):793–800. doi: 10.4161/cbt.11.9.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju JH, Jeon MJ, Yang W, Lee KM, Seo HS, Shin I. Induction of apoptotic cell death by Pharbitis nil extract in HER2-overexpressing MCF-7 cells. Journal of Ethnopharmacology. 2011;133(1):126–131. doi: 10.1016/j.jep.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Lin YJ, Zhen YS. Rhein lysinate suppresses the growth of breast cancer cells and potentiates the inhibitory effect of Taxol in athymic mice. Anti-Cancer Drugs. 2009;20(1):65–72. doi: 10.1097/CAD.0b013e3283182913. [DOI] [PubMed] [Google Scholar]
- 19.Shi P, Huang Z, Chen G. Rhein induces apoptosis and cell cycle arrest in human hepatocellular carcinoma BEL-7402 cells. The American Journal of Chinese Medicine. 2008;36(4):805–813. doi: 10.1142/S0192415X08006259. [DOI] [PubMed] [Google Scholar]
- 20.Chen YY, Chiang SY, Lin JG, et al. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Research. 2010;30(3):945–952. [PubMed] [Google Scholar]
- 21.Lin YJ, Zhen YZ, Shang BY, Zhen YS. Rhein lysinate suppresses the growth of tumor cells and increases the anti-tumor activity of Taxol in mice. The American Journal of Chinese Medicine. 2009;37(5):923–931. doi: 10.1142/S0192415X09007417. [DOI] [PubMed] [Google Scholar]
- 22.Hussain-Hakimjee EA, Peng X, Mehta RR, Mehta RG. Growth inhibition of carcinogen-transformed MCF-12F breast epithelial cells and hormone-sensitive BT-474 breast cancer cells by 1α-hydroxyvitamin D5. Carcinogenesis. 2006;27(3):551–559. doi: 10.1093/carcin/bgi231. [DOI] [PubMed] [Google Scholar]
- 23.Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochemical Pharmacology. 2002;64(9):1375–1386. doi: 10.1016/s0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- 24.Lai WW, Yang JS, Lai KC, et al. Rhein induced apoptosis through the endoplasmic reticulum stress, caspase- and mitochondria-dependent pathways in SCC-4 human tongue squamous cancer cells. In Vivo. 2009;23(2):309–316. [PubMed] [Google Scholar]
- 25.Hsia TC, Yang JS, Chen GW, et al. The roles of endoplasmic reticulum stress and Ca2+ on rhein-induced apoptosis in A-549 human lung cancer cells. Anticancer Research. 2009;29(1):309–318. [PubMed] [Google Scholar]
- 26.Ip SW, Weng YS, Lin SY, et al. The role of Ca+2 on rhein-induced apoptosis in human cervical cancer Ca Ski cells. Anticancer Research. 2007;27(1):379–389. [PubMed] [Google Scholar]
- 27.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18(45):6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 28.Zuo Y, Xiang B, Yang J, et al. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1. Cell Research. 2009;19(4):449–457. doi: 10.1038/cr.2009.19. [DOI] [PubMed] [Google Scholar]
- 29.Imoto K, Kukidome D, Nishikawa T, et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes. 2006;55(5):1197–1204. doi: 10.2337/db05-1187. [DOI] [PubMed] [Google Scholar]
- 30.Lin S, Fujii M, Hou DX. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Archives of Biochemistry and Biophysics. 2003;418(2):99–107. doi: 10.1016/j.abb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Lin ML, Chen SS, Lu YC, et al. Rhein induces apoptosis through induction of endoplasmic reticulum stress and Ca2+-dependent mitochondrial death pathway in human nasopharyngeal carcinoma cells. Anticancer Research. 2007;27(5):3313–3322. [PubMed] [Google Scholar]
- 32.Guo C, Gasparian AV, Zhuang Z, et al. 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-κB and p53 pathways. Oncogene. 2009;28(8):1151–1161. doi: 10.1038/onc.2008.460. [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan SK, Kamalakaran S. Pro-apoptotic role of NF-κB: implications for cancer therapy. Biochimica et Biophysica Acta. 2006;1766(1):53–62. doi: 10.1016/j.bbcan.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-κB in p53-mediated programmed cell death. Nature. 2000;404(6780):892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 35.Ricca A, Biroccio A, Trisciuoglio D, Cippitelli M, Zupi G, Del Bufalo D. relA over-expression reduces tumorigenicity and activates apoptosis in human cancer cells. The British Journal of Cancer. 2001;85(12):1914–1921. doi: 10.1054/bjoc.2001.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehy AM, Schlissel MS. Overexpression of RelA causes G1 arrest and apoptosis in a pro-B cell line. The Journal of Biological Chemistry. 1999;274(13):8708–8716. doi: 10.1074/jbc.274.13.8708. [DOI] [PubMed] [Google Scholar]
- 37.Shetty S, Gladden JB, Henson ES, et al. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) up-regulates death receptor 5 (DR5) mediated by NFκB activation in epithelial derived cell lines. Apoptosis. 2002;7(5):413–420. doi: 10.1023/a:1020031023947. [DOI] [PubMed] [Google Scholar]
- 38.Bowie A, O’Neill LA. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochemical Pharmacology. 2000;59(1):13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Niu J, Uwagawa T, Peng B, Chiao PJ. Function of polo-like kinase 3 in NF-κB-mediated proapoptotic response. The Journal of Biological Chemistry. 2005;280(17):16843–16850. doi: 10.1074/jbc.M410119200. [DOI] [PubMed] [Google Scholar]
- 40.Del Rio MJ, Velez-Pardo C. Monoamine neurotoxins-induced apoptosis in lymphocytes by a common oxidative stress mechanism: involvement of hydrogen peroxide (H2O2), caspase-3, and nuclear factor kappa-B (NF-κB), p53, c-Jun transcription factors. Biochemical Pharmacology. 2002;63(4):677–688. doi: 10.1016/s0006-2952(01)00907-8. [DOI] [PubMed] [Google Scholar]
- 41.Kuo PL, Hsu YL, Ng LT, Lin CC. Rhein inhibits the growth and induces the apoptosis of Hep G2 cells. Planta Medica. 2004;70(1):12–16. doi: 10.1055/s-2004-815448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: To compare cytotoxic effect of rhein on normal breast MCF-10A cells, and breast cancer MCF-7/VEC and MCF-7/HER2 cells, the cells were treated with 20 μg/mL rhein for 48 h. The cell survival rate was determined using MTT assay, showing 99.7 ± 0.4% for MCF-10A cells, 75.5 ± 3.5% for MCF-7/VEC cells, and 59.1 ± 1.4% for MCF-7/HER2 cells, respectively. The resulted demonstrated rhein being less toxic to non-tumorigenic MCF-10A cells.


