Abstract
To evaluate proteins associated with the development of diabetic nephropathy, a major cause of the end-stage renal disease, we analyzed protein expression in isolated glomeruli from spontaneous type 2 diabetic (OLETF) rats and their age-matched control littermates (LETO) in the early and proteinuric stages of diabetic nephropathy using QSTAR Elite LC-MS/MS. Among the 191 and 218 proteins that were altered significantly in the OLETF rats, twenty-four were actin cytoskeleton-associated proteins implicated in the formation of stress fibers, and the impairment of actin polymerization, intermediate filaments and microtubules. Importantly, sorbin and SH3 domain containing 2 (SORBS2), which is involved in the formation of stress fibers, was significantly upregulated in both stages of diabetic nephropathy (1.49- and 1.97-fold, resp.). Immunohistochemical and quantitative-PCR analyses revealed upregulation of SORBS2 in podocytes of glomeruli of OLETF rats. Our findings suggested that SORBS2 may be associated with the development of diabetic nephropathy possibility by reorganization of actin filaments.
1. Introduction
Diabetes mellitus accounts for more cases of end-stage renal disease than any other cause of chronic kidney disease [1]. While glomerular hypertrophy, mesangial matrix expansion, and glomerular basement membrane (GBM) thickening are the classical hallmarks of diabetic glomerular lesions, studies of diabetic patients and animal models have revealed that the onset of proteinuria is most closely associated with podocytopathies, such as podocyte apoptosis, hypertrophy, detachment from the GBM, and foot process effacement [2]. Indeed, diabetic nephropathy is now recognized as one of the major podocyte-associated diseases [3]. The podocyte is an excellent model system for studying actin cytoskeleton dynamics in a physiological context because changes in actin dynamics transfer directly into changes of kidney function [4]. Previous investigations have shown that the cytoskeleton on the GBM side in podocytes during foot process effacement is comprised of highly-ordered, actin-based bundles that run parallel to the longitudinal axis of the foot processes [4] and that actin fibers gather to form the stress fibers [4, 5]. Therefore, reorganization of the actin filaments is indispensable for foot process effacement.
Sorbin and SH3 domain containing 2 (SORBS2), alpha-actinin 1 (ACTN1), alpha-actinin 4 (ACTN4) and Rho GDP dissociation inhibitor alpha (ARHDGDIA) are proteins associated with stress fiber formation [6–11]. The relationship between these proteins and diabetic nephropathy has not been elucidated, although some of these proteins have been reported to be important in stress fiber formation in podocytopathies or proteinuria [4, 8, 9, 12]. The underlying cytoskeletal components that initiate and regulate the dynamic changes of these foot processes remain unclear.
Recently, proteome analysis has increasingly been used in the discovery of disease-specific proteins and biomarkers of kidney diseases [13, 14]. Proteome analysis of diabetic glomeruli from renal biopsy specimens of diabetic patients is difficult, due to the fact that renal biopsy is clinically limited in diabetic patients, and only small (often insufficient) quantities of glomeruli can be obtained from renal biopsy specimens. From these concerns, in order to reveal which proteins are involved in the diabetic glomerular alterations, including podocytopathies, we conducted a proteome analysis of isolated glomeruli from spontaneous type 2 diabetic (Otsuka Long-Evans Tokushima Fatty (OLETF)) rats and their age-matched control littermates (Long-Evans Tokushima Lean (LETO)) rats at 27 (early stage of diabetic nephropathy) and 38 (proteinuric stage of diabetic nephropathy) weeks of age using QSTAR Elite liquid chromatography with tandem mass spectrometry (QSTAR Elite LC-MS/MS) and iTRAQ technology.
2. Materials and Methods
2.1. Animals
All experimental procedures were conducted after obtaining approval of the Animal Care and Use Committee of the Osaka City University Medical School and in accordance with the Guide for Laboratory Animals. OLETF and LETO rats (n = 20, resp.) were provided by Otsuka Pharmacology Co., Ltd. (Tokushima, Japan). The diabetic phenotype of the OLETF rat has been extensively evaluated: (i) 25-week-old rats develop diabetes (hyperglycemia, hyperlipidemia, etc.) at nearly 100% incidence and (ii) 30-week-old rats develop proteinuria [15]. Therefore, we assumed that OLETF rats develop diabetic nephropathy with the early and the proteinuric stages at 27 and 38 weeks of age, respectively. 10 OLETF rats and 10 LETO rats were used for analysis at each time point. All animals were housed individually in each cage in an animal facility maintained on a 12-h (7:00–19:00) light/dark cycle, at a constant temperature of 23 ± 1°C and relative humidity of 44 ± 5% for 21 and 32 weeks, respectively, from the start of the experiment and were provided tap water and food (rodent pellet diet MF 348 kcal/100 g, containing 4.2% crude fat; Oriental Yeast Co., Tokyo, Japan) ad libitum.
2.2. Biochemical Characterization
Total cholesterol and creatinine in serum specimens (n = 10/group), hemoglobin A1c, fasting plasma glucose concentrations in plasma specimens (n = 10/group), and protein and creatinine concentrations in spot urine samples (n = 5/group) were measured using an autoanalyser (Mitsubishi Chemical Medience Co., Ltd., Osaka, Japan).
2.3. Histopathological Examination
Renal tissues were fixed in 10% neutral formalin solution, embedded in paraffin, and cut into 3 μm sections using conventional techniques. Sections were stained with hematoxylin and eosin and periodic acid-Schiff (PAS) reagent and examined histopathologically by light microscopy.
2.4. Glomerular Isolation
Rats were anesthetized with intraperitoneal injection of pentobarbital (60 mg/kg) for euthanasia and necropsy. After laparotomy, the kidneys were perfused with ice-cold phosphate-buffered saline (PBS) until they were blanched. Glomeruli were isolated by a sieving technique, as described previously [16, 17]. Isolated glomeruli were collected under an inverted microscope to minimize tubular contamination (less than 5% tubular fragments) and centrifuged at 453 g for 10 min. The pellets were collected and used for proteome analysis.
2.5. Lysis and Digestion, iTRAQ Labeling, and LC-ESI MS/MS Analysis
The lyophilized samples were dissolved in 1000 μL tissue protein extraction reagent lysis buffer (Pierce, IL, USA) with protease inhibitor (p8340, Sigma-Aldrich). The glomerular lysates were ultrasonicated and insoluble material was removed by centrifugation at 13,000 g for 15 min at 10°C. Protein concentrations were quantified using the BCA Protein Assay kit (Pierce, Ill, USA). Protein reduction, alkylation, digestion and subsequent peptide labeling were performed using the AB Sciex iTRAQ Reagent Multi-Plex Kit (AB Sciex, Foster City, Calif, USA) according to the manufacturer's instructions with minor modifications [18, 19]. Briefly, 50 μg samples of protein were incubated at 60°C for 60 min in 20 μL dissolution buffer (0.5 M triethylammonium bicarbonate, 0.2% SDS) with 2 μL reducing reagent (50 mM tris(2-carboxy-ethyl)phosphine). Free cysteine sulfhydryl groups were blocked by incubation with 1 μL cysteine blocking reagent (20 mM methyl methanthiosulfonate) at room temperature for 10 min. Ten μL of trypsin solution (AB Sciex, Foster City, Calif, USA) was added, and each sample was incubated overnight at 37°C. Samples from OLETF and LETO rats were labeled with iTRAQ114 and iTRAQ115, respectively, and then mixed into one tube and fractionated using six concentrations of KCl solutions (10, 50, 70, 100, 200, and 350 mM) on an ICAT cation exchange cartridge (AB Sciex, Foster City, Calif, USA). After desalting and concentrating, peptides in each fraction were quantified by a DiNa-AI nano LC System (KYA Technologies, Tokyo, Japan) coupled to a QSTAR Elite Hybrid MS/MS spectrometer through a Nanospray ion source (AB Sciex, Foster City, Calif, USA), as described previously [19].
2.6. Identification of Proteins by IPA
Protein Pilot 2.0 software with the Paragon Algorithm (AB Sciex, Foster City, Calif, USA) was used for the identification and relative quantification of proteins. Tandem mass spectrometry data were compared against the rat protein database from Swiss-Prot 57.4 (20,400 sequences). We report only protein identifications with >95% statistical confidence in the Protein Pilot 2.0 software.
The Ingenuity Analysis (IPA; Ingenuity Systems, Mountain View, Calif, USA) was utilized to identify networks of interacting proteins, functional groups, and pathways. Information regarding the function and cellular localization of the identified proteins was obtained from IPA.
2.7. Immunohistochemistry for SORBS2
Immunohistochemical staining of the kidney sections was performed according to the avidin-biotin complex method, as described previously [20], using primary mouse monoclonal anti-rat SORBS2 (clone S5C, Sigma-Aldrich), After deparaffinization with xylene and gradual dehydration, antigen retrieval was undertaken by microwaving in sodium citrate buffer (pH 6) for 25 min and endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 5 min. Sections were incubated with 1.5% normal horse or goat serum in PBS for 15 min and then with diluted primary antibody (1 : 500), overnight at 4°C. Biotinylated horse anti-mouse antibodies (diluted 1 : 200) were applied as the secondary antibodies for 30 min, and the slides were then incubated with the avidin-biotin peroxidase complex for 30 min. The peroxidase reaction was developed using 0.02~0.033% 3,3-diaminobenzidine tetrahydrochloride (DAB) and 0.03% hydrogen peroxide in tris-buffered saline for 1–5 min. Hematoxylin was used for counterstaining.
2.8. Immunofluorescence for SORBS2 and Synaptopodin
Double immunofluorescence of SPRBS2 and synaptopodin, a podocyte marker proteins was performed as previously described [21–23]. Four-μm-thick frozen kidney tissue sections were fixed with ice-cold acetone at −20°C for 5 min, followed by permeabilization with 1% Tween 20 PBS for 5 min at room temperature. After rinsing with 1% Tween 20 PBS, unspecific binding sites were blocked with horse anti-mouse and goat anti-rabbit serum in PBS for at least 30 min. Primary antibodies (prediluted in blocking solution) for SORBS2 (1 : 250) and synaptopodin (clone ab 101883, Abcam) (1 : 300) were applied for 60 min at room temperature, followed by incubation with the secondary antibody fluorescein red-conjugated horse anti-mouse IgG (Alexa Fluor 594) (Life technologies, Calif, USA) and fluorescein green-conjugated goat anti-rabbit IgG (Alexa Fluor 488) (Life technologies, Calif, USA) for 30 minutes at room temperature. Spatial colocalization of SORBS2 immunoreactivity (red fluorescence) with synaptopodin (green fluorescence), resulting in yellow, was obtained by overlaying separately recorded images on a color image. The immunofluorescence was analyzed by confocal microscopy with the help of the Fluoview software (Olympus Optical, Tokyo, Japan).
2.9. Validation of SORBS2 mRNA Expression by Real-Time Quantitative PCR (Q-PCR)
2.9.1. RNA Preparation
Glomeruli from OLETF and LETO rats at the proteinuric stage (n = 3, resp.) were laser-microdissected using the ZEISS PALM MB4 Microdissection System (ZEISS, Munich, Germany), according to the manufacturer's instructions. Total RNA was isolated from glomeruli using 4 M guanidine thiocyanate, 25 mM sodium citrate with 0.5% sarkosyl buffer with the phenol-chloroform-isoamyl alcohol extraction method, using glycogen as a carrier, as described previously [24]. Reverse transcription of total RNA was performed with Oligo-dT primer, and cDNA samples were stored at −20°C until assayed.
2.9.2. Real-Time Q-PCR
PCR amplicons were used to confirm SORBS2 gene expression using real-time Q-PCR. Primer sequences were designed with the Primer Express software (Applied Biosystems, USA). The probes and primers were as follows: TaqMan probe and primer set Rn00587190_m1 for SORBS2 (NM_053770.1) and TaqMan probe 5′-TGA GAC CTT CAA CAC CCC AGC CAT G-3′, and primers: forward 5′-TCA AAT AAG CCA CAG CGT C-3′, reverse 5′-AAC CAG CCG TCAT CACA C-3′ for GAPDH, cytoplasmic (NM_ 017008.3). The cDNA generated from each sample was used for Q-PCR according to the manufacturer's instructions, with GAPDH as an internal control.
2.10. Ultrastructural Examination
Separate portions of the kidneys from the OLETF and LETO rats (n = 5, resp.) at 38 weeks of age were also prepared for electron microscopy. Specimens were obtained from the renal cortex, fixed in 0.1 M cacodylate buffer solution (pH 7.4) containing 3% glutaraldehyde, and postfixed in the same buffer containing 1% osmium tetroxide at 4°C, as previously described [25]. Seventy nm sections were stained with uranyl acetate and lead citrate for examination using a JEM 1200 EXII electron microscope (JEOL, Tokyo, Japan).
2.11. Statistical Analysis
Statistical calculations were performed using Graph-Pad Prism version 5.0 for Windows (Graphpad Software, San Diego, Calif). For normally distributed data, statistical significance (P < 0.05) was evaluated using the unpaired t-test followed by an analysis of variance (F-test). In the case of statistically significant differences regarding variances, the Welch test was used to confirm the differences between groups. For nonparametric testing, the Mann-Whitney U-test was applied. The results are presented as box plots/dot plots. All values are expressed as the means ± SD. For analysis of protein expression, statistical analysis with Protein Pilot 2.0 software was employed.
3. Results
3.1. General Observations
The OLETF rats exhibited polyphagia and obesity from the very early stages of life. At 27 weeks of age (early stage of diabetic nephropathy), the mean body weight of the OLETF rats (642 ± 40.9 g) was significantly higher than that of the LETO rats (491 ± 34.6 g). At 38 weeks of age (proteinuric stage of diabetic nephropathy), the body weights of the LETO rats were increased, although the final values for the OLETF (621 ± 69.4 g) and the LETO (580 ± 80.7 g) rats were not significantly different. At both time points, the kidney-to-body weight ratio of the OLETF rats (27 weeks: 0.703% ± 0.10%, 38 weeks: 0.780% ± 0.12%) was significantly higher than that of the LETO rats (0.597% ± 0.054% and 0.592% ± 0.050%, resp.).
The mean blood glucose, hemoglobin A1c and total cholesterol levels in the OLETF rats (27 weeks: 183 ± 58.9 mg/dL, 4.4 ± 0.85%, 123 ± 23.1 mg/dL, 38 weeks: 248 ± 53.6 mg/dL, 5.7% ± 1.2%, and 153 ± 34.6 mg/dL, resp.) were increased significantly compared with those in the LETO rats (27 weeks: 135 ± 34.2 mg/dL, 3.3% ± 0.11%, 97.4 ± 9.5 mg/dL, 38 weeks: 129 ± 25.3 mg/dL, 3.3% ± 0.18%, and 101 ± 9.37 mg/dL, resp.). The serum creatinine levels were significantly higher at both nephropathy stages for LETO rats (27 weeks: 0.36 ± 0.04 mg/dL and 38 weeks: 0.46 ± 0.10 mg/dL) compared with the OLETE rats (0.24 ± 0.04 mg/dL and 0.33 ± 0.05 mg/dL). The urinary protein to creatinine ratio in the OLETF rats was also elevated significantly at both time points examined (27 weeks: 2.73 ± 2.10 mg/mg and 38 weeks: 5.65 ± 2.36 mg/mg) compared with the LETO rats (0.60 ± 0.08 mg/mg and 0.94 ± 0.38 mg/mg) (Table 1).
Table 1.
Biological parameters from OLETF and LETO rats at 27 and 38 weeks of age.
| OLETF (n = 10) |
LETO (n = 10) |
P value | OLETF (n = 10) |
LETO (n = 10) |
P value | |
|---|---|---|---|---|---|---|
| 27 weeks | 38 weeks | |||||
| Body weight (g) | 642 ± 40.9 | 491 ± 34.6 | <0.0001 | 621 ± 69.4 | 580 ± 80.7 | 0.2089 |
| Food intake (g/day) | 34.9 ± 6.1 | 19.1 ± 1.5 | <0.0001 | 39.3 ± 6.1 | 29.7 ± 2.0 | <0.0001 |
| Kidney-to-body weight ratio (%) | 0.703 ± 0.10 | 0.597 ± 0.054 | 0.0091 | 0.780 ± 0.12 | 0.592 ± 0.050 | 0.0008 |
| Fasting plasma glucose (mg/dL) | 183 ± 58.9 | 135 ± 34.2 | 00388 | 248 ± 53.6 | 129 ± 25.3 | <0.0001 |
| Hemoglobin A1c (%) | 4.4 ± 0.85 | 3.3 ± 0.11 | 0.0021 | 5.7 ± 1.2 | 3.3 ± 0.18 | 0.0001 |
| Total cholesterol (mg/dL) | 123 ± 23.1 | 97.4 ± 9.5 | 0.0081 | 153 ± 34.6 | 101 ± 9.37 | 0.0009 |
| Creatinine (mg/dL) | 0.24 ± 0.04 | 0.36 ± 0.04 | <0.0001 | 0.33 ± 0.05 | 0.46 ± 0.1 | 0.0031 |
| Urinary protein-to-creatinine ratio (mg/mg) | 2.73 ± 2.10 (n=5)* |
0.60 ± 0.08 (n=5)* |
<0.0001 | 5.65 ± 2.36 (n=5)* |
0.94 ± 0.38 (n=5)* |
<0.0001 |
Values are expressed as the mean ± SD.
*Urinary specimens were obtained from 5 rats per group to measure urinary protein and creatinine levels.
OLETF: Otsuka Long-Evans Tokushima Fatty, LETO: Long-Evans Tokushima Lean.
3.2. Histopathological Examination
In the OLETF rats, histopathological examination demonstrated both focal and segmental glomerular changes. Slight expansion of the mesangial matrix was observed with mesangial cell proliferation at 27 weeks of age (Figure 1(a)). At 38 weeks of age, in addition to the mesangial area, a few glomeruli exhibited segmental lesions with PAS-positive deposits in the mesangium or capillary that resembled the fibrin caps commonly observed in exudative lesions in human diabetic nephropathy (Figure 1(c)). In the LETO rats, there were no obvious histopathological changes at both time points (Figures 1(b) and 1(d)).
Figure 1.
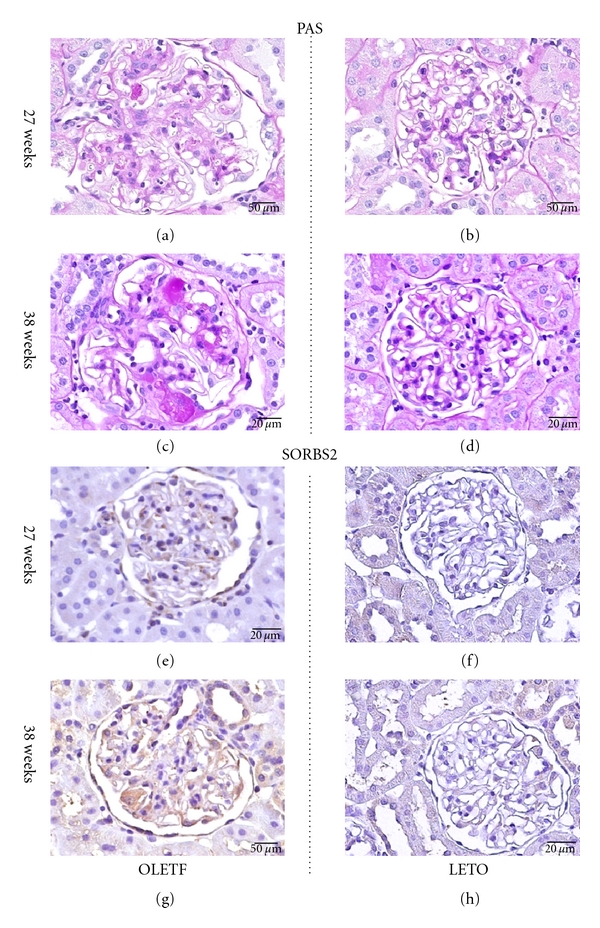
Periodic acid Schiff (PAS) and immunohistochemical staining in the kidneys from OLETF (a, c, e, and g) and LETO (b, d, f, and h) rats at 27 and 38 weeks of age. Slight expansion of the mesangial matrix with mesangial cell proliferation (a). Normal glomeruli (b). Exudative and sclerotic lesion (c). Normal glomeruli (d). SORBS2 positivity in podocytes from OLETF rats (e, f) and negativity in those from LETO rats (f, h). Scale bar = 20 μm.
3.3. Alterations of Protein Expression in Glomeruli from Diabetic Rats
The results of QSTAR Elite LC-MS/MS and Protein Pilot analyses are summarized in Table 2. Altered expression of 191 (91 up- and 100 downregulated) and 218 (121 up- and 97 downregulated) proteins was observed in isolated glomeruli from OLETF rats at the early and proteinuric stages of diabetic nephropathy, respectively. These proteins were involved in glycolysis, oxidative stress, and podocyte injury, based upon the IPA findings (Figure 2).
Table 2.
Differentially expressed actin cytoskeleton-associated proteins in glomeruli isolated from OLETF and LETO rats, identified by QSTAR Elite LC-MS/MS and IPA.
| Protein | GI number | Mass (Da) | Location | Function | Foldchange | P value | Fold change | P value |
|---|---|---|---|---|---|---|---|---|
| (27 weeks) | (38 weeks) | |||||||
| Stress fiber formation | ||||||||
| Sorbin and SH3 domain containing 2 (SORBS2) | 205831248 | 124108 | C | AD | 1.49 | <0.0001 | 1.97 | <0.0001 |
| Alpha-actinin 1 (ACTN1) | 13123942 | 103058 | C | ST, CL | 1.02 | 0.764 | 1.40 | <0.0001 |
| Alpha-actinin 4 (ACTN4) | 182705246 | 104654 | C | ST, CL | 0.93 | 0.46 | 1.22 | <0.0001 |
| Rho GDP dissociation-inhibitor alpha (ARHGDIA) | 21759130 | 23207 | C | RI | 1.28 | 0.041 | 0.87 | 0.0413 |
| Actin-filament polymerization | ||||||||
| Actin-related protein 2/3 complex subunit 1 beta (ARPC1B) | 12229626 | 40950 | C | AP, EC | 0.65 | 0.0363 | 0.73 | 0.0136 |
| Actin related protein 2/3 complex subunit 5 (ARPC5) | 3121767 | 16320 | C | AP, EC | 0.72 | 0.0014 | 0.74 | 0.0002 |
| Myristoylated alanine-rich protein kinase C substrate (MARCKS) | 266495 | 31555 | UN | AP | 0.60 | 0.0004 | 0.71 | 0.002 |
| Microtubules formation | ||||||||
| Tubulin alpha 1c (TUBA1C) | 55976169 | 49895 | C | MT | 0.74 | 0.012 | 0.90 | 0.17 |
| Intermediate filaments formation | ||||||||
| Lamin A/C (LMNA) | 1346413 | 74139 | N | IF | 0.89 | 0.0076 | 1.29 | <0.0001 |
| Desmin (DES) | 1352241 | 53536 | C | IF | 0.94 | 0.143 | 1.32 | <0.0001 |
| Nestin (NES) | 146345465 | 177439 | C | IF | 0.83 | <0.0001 | 1.03 | 0.32 |
| Plectin 1 (PLEC1) | 1709655 | 531791 | C | IL | 0.82 | <0.0001 | 0.96 | 0.12 |
| GBM (glomerular basement membrane) | ||||||||
| Integrin beta 1 (INTGB1) | 1352494 | 15105 | PM | APG | 0.82 | 0.0015 | 0.92 | 0.0094 |
| Agrin (AGRN) | 399021 | 214846 | PM | LB | 0.62 | 0.042 | 0.90 | 0.490 |
| Others | ||||||||
| Plastin 3 (PLS3) | 226693553 | 70811 | C | CL | 0.82 | 0.02 | 0.82 | 0.0005 |
| Calponin 3 (CNN3) | 584956 | 36414 | C | AB | 0.60 | 0.0013 | 0.78 | 0.088 |
| Tropomyosin 3 (TPM3) | 148840439 | 32819 | C | AS | 0.71 | <0.0001 | 0.80 | <0.0001 |
AB: actin binding; AD: adaptor protein; AP: actin filament polymerization; APG: anchoring podocyte and GBM (glomerular basement membrane); AS: actin filaments stabilization; C: cytoplasm; CL: crosslinking actin filaments into bundles or networks; EC: endocytosis; IF: intermediate filaments; IL: interlinks; LB: laminin binding; LETO: Long-Evans Tokushima Lean; MT: microtubules; N: nuclear; OLETF: Otsuka Long-Evans Tokushima Fatty; PM: plasma membrane; RI: Rho GDP-dissociation-inhibitor activity; ST: stress fibers; UN: unknown.
Figure 2.
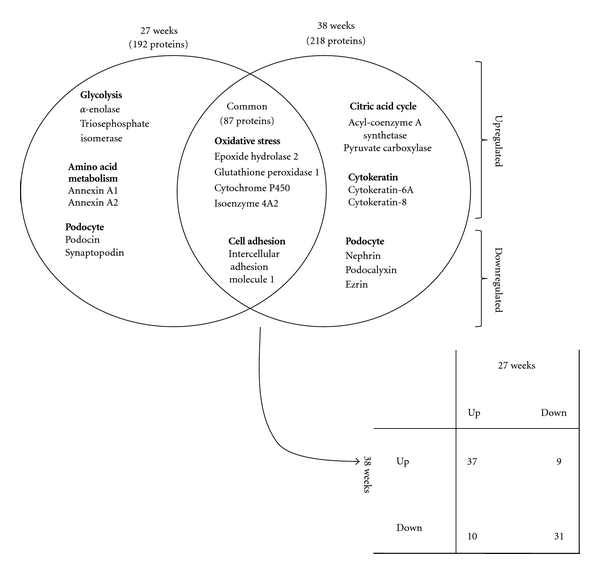
Comparative analysis of subclasses of differentially expressed proteins, excluding actin cytoskeleton-associated proteins, in the kidneys from OLETF and LETO rats at 27 and 38 weeks of age, by the Ingenuity Pathway Analysis (IPA).
Eighty-seven proteins were differentially expressed in isolated glomeruli from OLETF rats compared with those from LETO rats at both stages of diabetic nephropathy. Among these 87 proteins, 24 were involved in actin cytoskeleton reorganization, that is, formation of stress fibers (SORBS2, ACTN1, ACTN4 and ARHGDIA), polymerization of actin filaments (actin-related protein 2/3 complex subunit 1 beta (ARPC1B), actin-related protein 2/3 complex subunit 5 (ARPC5), actin-related protein 3 homolog (ACTR3), myristoylated alanine rich kinase C substrate (MARCKS), and adducin 1 alpha (ADD1)), microtubules (tubulin alpha 1c (TUBA1C) and dynein cytoplasmic 1 (DYNC1)) intermediate filaments (vimentin (VIM), lamin A/C (LMNA), desmin (DES), nestin (NES) and plectin1 (PLEC1)), formation of GBM (integrin beta 1 (INTGB1), vinculin (VCL) and agrin (AGRN)), and other actin-binding proteins (plastin 3 (PLS3), spectrin alpha non-erythrocytic 1 (SPTAN1), calponin 3 (CNN3), tropomyosin 3 (TPM3) and ezrin (EZR)). Among these proteins, Table 2 presents the actin cytoskeleton-associated proteins with high- or low-fold changes of more than 20% (average iTRAQ ratio >1.20 or <0.83) and P values less than 0.05. SORBS2 was the only up-regulated protein in glomeruli from OLETF rats at both the early and the proteinuric stages of diabetic nephropathy.
3.4. Confirmation of SORBS2 Expression by Immunohistochemistry
Figures 1(e), 1(f), 1(g), and 1(h) show representative immunostaining results for the SORBS2. There were no clear differences in the expression of SORBS2 between the OLETF and LETO rats at 27 weeks of age (Figures 1(e) and 1(f)). However, SORBS2 was clearly observed in podocytes from OLETF rats at 38 weeks of age (Figures 1(g) and 1(h)).
Immunofluorescence of SPRBS2 and synaptopodin are shown in Figure 3. SORBS2 was observed in glomeruli from OLETF rats at 38 weeks of age (Figure 3(a)). Synaptopodin was observed in glomeruli from OLETF rats at 38 weeks of age (Figure 3(b)). When the stainings of SORBS2 and synaptopodin were merged, they showed as a capillary pattern in glomeruli from OLETF rats at 38 weeks of age (Figure 3(c)).
Figure 3.
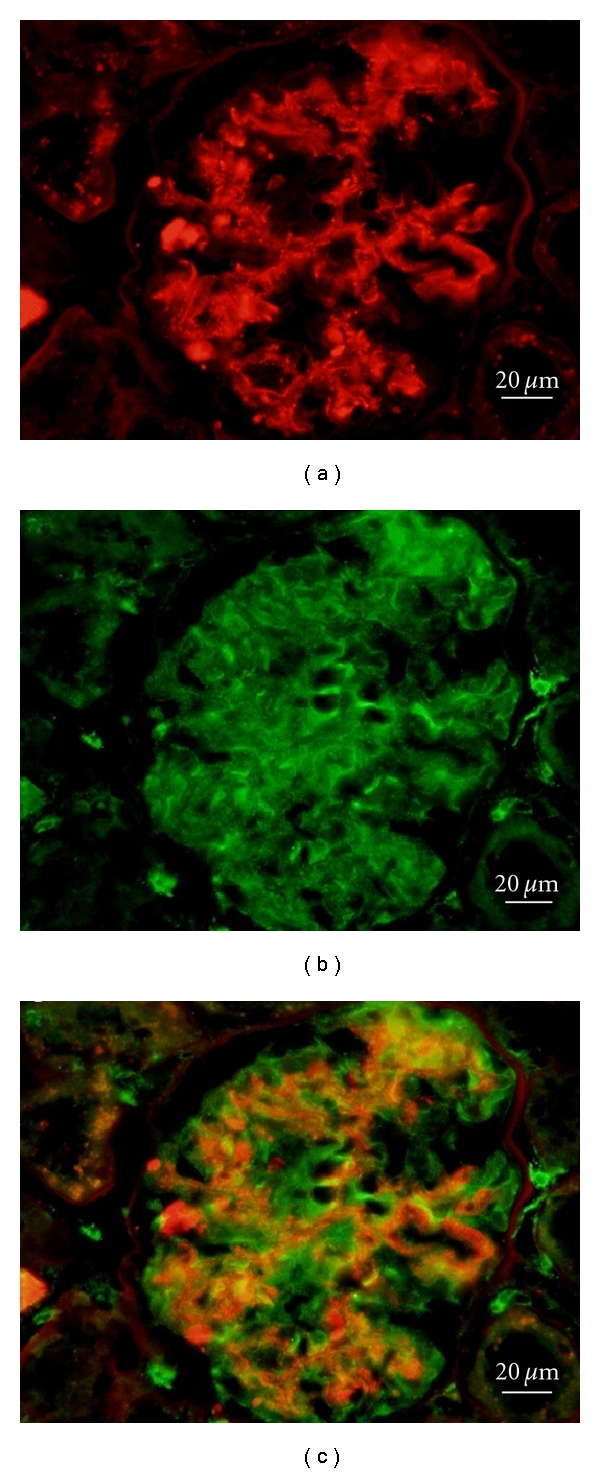
Immunofluorescence for SORBS2: red (a), synaptopodin: green (b), and merge SORBS2 and synaptopodin: yellow (c) in OLETF rats at 38 weeks of age. SORBS2 was expressed as a capillary pattern in glomeruli from OLETF rats at 38 weeks of age. Scale bar = 20 μm.
3.5. SORBS2 mRNA Expression in Isolated Glomeruli
To determine whether SORBS2 localizes within glomeruli and to assess changes of its expression, real-time Q-PCR analyses were also performed. Consistent with the QSTAR proteome analysis results, a tendency towards increased SORBS2 mRNA expression in glomeruli from OLETF rats was observed compared to those from LETO rats (1.76 ± 0.15 versus 1.40 ± 0.19, P = 0.06).
3.6. Ultrastructural Examination Using Electron Microscopy
To validate the podocyte foot process effacement in the OLETF rats, ultrastructural examination was performed at 38 weeks of age. At this time, foot process effacement was not observed in the LETO rats, but was obvious in the OLETF rats (Figures 4(a) and 4(b)). These results and the urinary protein-to-creatinine ratio (5.65 ± 2.36 mg/mg) indicated that 38 weeks of age is appropriate for the detection of the proteinuric stage of diabetic nephropathy in the OLETF rats.
Figure 4.

Alterations in protein expression during podocyte foot process effacement in diabetic nephropathy. Electron micrograph of podocytes from OLETF and LETO rats at 38 weeks of age. LETO rats (×40,000) (a), OLETF rats (×40,000) (b).
4. Discussion
In the present study, we performed a targeted proteome analysis of glomeruli isolated from rats in both the early (27 weeks of age) and the proteinuric (38 weeks of age) stages of diabetic nephropathy. It is reported that proteins quantified with a fold change of more than 20% (average iTRAQ ratio >1.20 or <0.83) and a P value less than 0.05 were identified as differentially expressed proteins [26, 27]. We demonstrated changes of many kinds of proteins in isolated glomeruli from diabetic rats; these proteins participate in glycolysis, citric acid cycle, formation of oxidative stress, and other intracellular processes, as shown in Figure 2. The results of IPA demonstrated that 17 actin cytoskeleton-associated proteins were significantly and differentially expressed between OLETF and LETO rats.
Among these proteins that were differentially expressed in isolated glomeruli from OLETF and LETO rats at both 27 and 38 weeks of age were SORBS2 (upregulated), ARCP1B (downregulated), ARPC5 (downregulated), MARCKS (downregulated), PLAS3 (downregulated), CNN3 (downregulated), and TPM3 (downregulated). SORBS2 was the only up-regulated protein in glomeruli from OLETF rats at both the early and the proteinuric stages of diabetic nephropathy, compared to those from LETO rats. There have been no previous reports suggesting any relationship between SROBS2 and diabetic nephropathy.
SORBS2 is an Arg/Abl-binding protein that contains three COOH-terminal Src homology 3 domains, a serine/threonine-rich domain, and several potential Abl phosphorylation sites. It is widely expressed in human tissues, such as heart, brain, spleen, pancreas, and kidney. In epithelial cells, SORBS2 is located in stress fibers [28]. In addition, SORBS2 has been reported to function as an adapter protein in the assembly of signaling complexes in stress fibers and as a potential link between the Abl family kinases and the actin cytoskeleton [8, 9]. In the present study, SORBS2 was up-regulated in isolated glomeruli from OLETF rats based upon proteome analysis using the QSTAR Elite LC-MS/MS. Although there was a tendency towards increased levels of SORBS2 mRNA in microdissected glomeruli from OLETF rats, we confirmed its localization in diabetic glomeruli, especially in podocytes of OLETF rats at the proteinuric stage using immunohistochemistry. Considering the previously reported functions of SORBS2 and the alterations of SORBS2 expression observed in the present study, SORBS2 may be associated with the development of diabetic nephropathy by reorganization of actin filaments, including actin stress fiber formation.
ARCP1B, ARPC5, and MARCKS are actin filament polymerization-related proteins. During polymerization of actin filaments, binding of the Arp2/3 complex to the sides of actin filaments is important for its actin nucleation and branching activities [29]. MARCKS substrate is located at glomeruli, specifically to podocytes, and controls both actin polymerization and actin cytoskeleton binding to the membrane [30]. Alterations of crosslinking proteins that organize actin filaments into bundles or networks, that is, PLS3 (I-plastin), were also detected in the present study. PLS3 is an actin-binding protein expressed in the kidney, that is, known to be located in stress fibers [31] and has been reported to be related to minimal change nephritic syndrome [32]. Moreover, CNN3 plays a direct role in cell contractility in vivo and controls the cytoskeletal composition of podocytes [33]. TPM3 binds to actin filaments and has been implicated in their stabilization [34]. These protein changes may be related to the collapse of actin filaments and the disentanglement of actin bundles or networks of diabetic glomeruli in the early and proteinuric stages of diabetic nephropathy.
ACTN4 is widely expressed in podocyte foot processes and is colocalized with actin stress fibers [6]. The Upregulation of ACTN4 observed during the proteinuric stage in the present study is consistent with a previous report [10]. ACTN1 is present in multiple subcellular regions, including cell-cell and cell-matrix contact sites, cellular protrusions, lamellipodia, and stress fiber dense regions [35], and cross-links actin filaments within stress fibers [11]. ACTN1 was also up-regulated in glomeruli from OLETF rats at 38 weeks of age. ARHGDIA maintains the Rho family members (Rac1, Cdc42, and RhoA), which promote the assembly of actin-myosin filaments and cell stress fibers in the GDP-bound inactive form. Mice lacking ARHGDIA are initially viable and healthy but develop massive proteinuria and glomerulosclerosis later in life [7, 10]. Upregulation of ARHGDIA in the early stage might indicate suppression of the Rho family members. Coincident with the Upregulation of SORBS2 at both stages of OLETF rats in the present study, changes in these protein expression patterns may be associated with reorganization of actin filaments, leading to foot process effacement and the progression of diabetic nephropathy. In the present study; however, we could not validate the expression of ACTN4 and ARGDIA in OLETF rats.
In addition to increasing stress fibers, the impairment of polymerization of actin filaments, intermediate filaments and microtubules, disentanglement of actin filaments, and podocyte detachment from the GBM are also key events for podocytopathies. Intermediate filaments and microtubules are known to form the scaffold of major podocyte processes and the central cell body [36]. NES and PLEC1 were downregulated in the kidneys from OLETF rats at 27 weeks of age, indicating the collapse or the disentanglement of intermediate filaments and microtubules in the early stage of diabetic nephropathy. Furthermore, podocytes are attached to the outer aspect of the GBM and their foot processes are connected to the GBM [37]. Downregulation of INTGB1 and AGR may indicate that podocytes are detached from the GBM, resulting in foot process effacement and proteinuria.
The alterations of proteins observed in this study are summarized in Figure 4. Based on proteome analysis of isolated glomeruli from diabetic rats, the following changes of the cytoskeleton at the early and proteinuric stages of diabetic nephropathy could occur: (1) increased formation of stress fibers during the proteinuric stage of diabetic nephropathy, (2) impairment of actin polymerization at both time points, suggesting collapse or dysfunction of actin filaments, (3) decreased expression of proteins associated with microtubules and intermediate filaments during both the early and the proteinuric stages, (4) decreased GBM cytoskeleton-associated proteins at both stages, suggesting podocytopathies, and (5) collapse or disentanglement of actin filaments at both stages. Our findings suggested that impairment or collapse of actin filaments may cause podocyte foot process effacement (Figure 4(b)) and the emergence of proteinuria in diabetic nephropathy. As observed in the present study, increases in stress fibers at the proteinuric stage may be related to reorganization of actin filaments [4, 5]. Proteome analysis demonstrated that numerous cytoskeleton-associated proteins could contribute to the onset and/or progression of diabetic nephropathy.
There are some limitations in the present study. First, in the glomerular isolation, we used a sieving method to minimize tubular contamination. Despite the implementation of this technique and effort, it is impossible to completely avoid tubular contamination. Indeed, one of the mitochondrial proteins, that is, mitochondrial import inner membrane translocase subunit 44 (TIM44), which is activated in diabetic nephropathy [38] was up-regulated in OLETF rats at 27 weeks of age (fold change 1.34, P = 0.04). A second limitation is the protein detection using the QSTAR Elite LC-MS/MS. Although, some podocyte-related proteins, such as podocin and synaptopodin, were downregulated in OLETF rats at 27 weeks of age (fold change 0.83 P = 0.001; fold change 0.63, P = 0.0001, resp.), these proteins were not detected in OLETF rats at 38 weeks of age, due to mechanical problems or problems with reproducibility of protein identification using the QSTAR Elite LC-MS/MS. Lastly, although proteome analysis is one of the most powerful and useful tools in the detection of novel proteins for various kidney diseases, it requires validation or further mechanical analysis of the results of proteome analysis. Although there are some limitations in the present methodology, our findings demonstrated the usefulness of proteome analysis of isolated glomeruli, which allows the direct and comprehensive investigation of protein alterations of diabetic glomeruli.
In conclusion, the present proteome analysis demonstrated that numerous cytoskeleton-associated proteins contribute to the onset and/or progression of diabetic nephropathy. This proteome study also demonstrated, for the first time, that SORBS2 expression was increased in diabetic glomeruli and suggested that SORBS2 may be associated with the development of diabetic nephropathy by reorganization of actin filaments, along with other actin cytoskeleton-associated proteins. Further investigation is necessary to ascertain the significance of SORBS2 and these actin cytoskeleton-associated proteins in diabetic nephropathy.
Conflict of Interests
The authors declared that they have no conflict of interests.
Acknowledgments
The authors thank Dr. Naomi Ishii, Ms. Azusa Inagaki, Ms. Kaori Touma, and Ms. Rie Onodera for their technical assistance and Ms. Yukiko Iura for her help during the preparation of this paper. This work was supported by a Grant-in-Aid for Scientific Research of The Ministry of Education, Culture, Sports, Science and Technology (no. 23591198).
Abbreviations
- ACTN1:
Alpha-actinin 1
- ACTN4:
Alpha-actinin 4
- AGRN:
Agrin
- ARHGDIA:
Rho GDP dissociation-inhibitor alpha
- ARPC1B:
Actin-related protein 2/3 complex subunit 1 beta
- ARPC5:
Actin-related protein 2/3 complex subunit 5
- CNN3:
Calponin 3
- DES:
Desmin
- GBM:
Glomerular basement membrane
- INTGB1:
Integrin beta 1
- LMNA:
Lamin A/C
- MARCKS:
Myristoylated alanine rich protein kinase C substrate
- NES:
Nestin
- PLEC 1:
Plectin 1
- PLS3:
Plastin 3
- SORBS2:
Sorbin and SH3 domain containing 2
- TPM3:
Toropomyosin 3
- TUBA1C:
Tubulin alpha 1c.
References
- 1.Collins AJ, Foley RN, Herzog C, et al. United States renal data system 2008 annual data report. American Journal of Kidney Diseases. 2009;53(supplement 1):A6–A7. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Stitt-Cavanagh E, MacLeod L, Kennedy C. The podocyte in diabetic kidney disease. The Scientific World Journal. 2009;9:1127–1139. doi: 10.1100/tsw.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Advanced Drug Delivery Reviews. 2010;62(14):1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends in Cell Biology. 2007;17(9):428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. American Journal of Pathology. 1996;148(4):1283–1296. [PMC free article] [PubMed] [Google Scholar]
- 6.Lachapelle M, Bendayan M. Contractile proteins in podocytes: immunocytochemical localization of actin and alpha-actinin in normal and nephrotic rat kidneys. Virchows Archiv. 1991;60(2):105–111. doi: 10.1007/BF02899534. [DOI] [PubMed] [Google Scholar]
- 7.Togawa A, Miyoshi J, Ishizaki H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIα. Oncogene. 1999;18(39):5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 8.Kioka N, Ueda K, Amachi T. Vinexin, CAP/ponsin, ArgBP2: a novel adaptor protein family regulating cytoskeletal organization and signal transduction. Cell Structure and Function. 2002;27(1):1–7. doi: 10.1247/csf.27.1. [DOI] [PubMed] [Google Scholar]
- 9.Cestra G, Toomre D, Chang S, De Camilli P. The Abl/Arg substrate ArgBP2/nArgBP2 coordinates the function of multiple regulatory mechanisms converging on the actin cytoskeleton. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1731–1736. doi: 10.1073/pnas.0409376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y. The role of podocytes in proteinuria. Nephrology. 2007;12(supplement 3):S15–S20. doi: 10.1111/j.1440-1797.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- 11.Courson DS, Rock RS. Actin cross-link assembly and disassembly mechanics for α-actinin and fascin. Journal of Biological Chemistry. 2010;285(34):26350–26357. doi: 10.1074/jbc.M110.123117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiological Reviews. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 13.Akkina SK, Zhang Y, Nelsestuen GL, Oetting WS, Ibrahim HN. Temporal stability of the urinary proteome after kidney transplant: more sensitive than protein composition? Journal of Proteome Research. 2009;8(1):94–103. doi: 10.1021/pr800646j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Molecular and Cellular Proteomics. 2002;1(11):845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 15.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41(11):1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 16.Ishimura E, Sterzel RB, Budde K, Kashgarian M. Formation of extracellular matrix by cultured rat mesangial cells. American Journal of Pathology. 1989;134(4):843–855. [PMC free article] [PubMed] [Google Scholar]
- 17.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. Journal of the American Society of Nephrology. 2008;19(10):1879–1890. doi: 10.1681/ASN.2007101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Yang L, Wang W, et al. Antigen retrieval for proteomic characterization of formalin-fixed and paraffin-embedded tissues. Journal of Proteome Research. 2008;7(3):1098–1108. doi: 10.1021/pr7006768. [DOI] [PubMed] [Google Scholar]
- 19.Kakehashi A, Ishii N, Shibata T, et al. Mitochondrial prohibitins and septin 9 are implicated in the onset of rat hepatocarcinogenesis. Toxicological Sciences. 2011;119(1):61–72. doi: 10.1093/toxsci/kfq307. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita A, Wanibuchi H, Morimura K, et al. Phenobarbital at low dose exerts hormesis in rat hepatocarcinogenesis by reducing oxidative DNA damage, altering cell proliferation, apoptosis and gene expression. Carcinogenesis. 2003;24(8):1389–1399. doi: 10.1093/carcin/bgg079. [DOI] [PubMed] [Google Scholar]
- 21.Esquenazi S, Bazan HEP, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Investigative Ophthalmology and Visual Science. 2005;46(9):3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 22.Morioka T, Koyama H, Yamamura H, et al. Role of H1-calponin in pancreatic AR42J cell differentiation into insulin-producing cells. Diabetes. 2003;52(3):760–766. doi: 10.2337/diabetes.52.3.760. [DOI] [PubMed] [Google Scholar]
- 23.Turk T, Leeuwis JW, Gray J, et al. BMP signaling and podocyte markers are decreased in human diabetic nephropathy in association with CTGF overexpression. Journal of Histochemistry and Cytochemistry. 2009;57(7):623–631. doi: 10.1369/jhc.2009.953224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakehashi A, Inoue M, Wei M, Fukushima S, Wanibuchi H. Cytokeratin 8/18 overexpression and complex formation as an indicator of GST-P positive foci transformation into hepatocellular carcinomas. Toxicology and Applied Pharmacology. 2009;238(1):71–79. doi: 10.1016/j.taap.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita A, Wanibuchi H, Morimura K, et al. Carcinogenicity of dimethylarsinic acid in Ogg1-deficient mice. Cancer Science. 2007;98(6):803–814. doi: 10.1111/j.1349-7006.2007.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi D, Kumagai J, Morikawa T, et al. An integrated approach of differential mass spectrometry and gene ontology analysis identified novel proteins regulating neuronal differentiation and survival. Molecular and Cellular Proteomics. 2009;8(10):2350–2367. doi: 10.1074/mcp.M900179-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce A, Unwin RD, Evans CA, et al. Eight-channel iTRAQ enables comparison of the activity of six leukemogenic tyrosine kinases. Molecular and Cellular Proteomics. 2008;7(5):853–863. doi: 10.1074/mcp.M700251-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Golemis EA, Kruh GD. ArgBP2, a multiple Src homology 3 domain-containing, Arg/Abl-interacting protein, is phosphorylated in v-Abl-transformed cells and localized in stress fibers and cardiocyte Z-disks. Journal of Biological Chemistry. 1997;272(28):17542–17550. doi: 10.1074/jbc.272.28.17542. [DOI] [PubMed] [Google Scholar]
- 29.Goley ED, Rammohan A, Znameroski EA, Firat-Karalar EN, Sept D, Welch MD. An actin-filament-binding interface on the Arp2/3 complex is critical for nucleation and branch stability. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(18):8159–8164. doi: 10.1073/pnas.0911668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosevitsky M, Silicheva I. Subcellular and regional location of "brain" proteins BASP1 and MARCKS in kidney and testis. Acta Histochemica. 2011;113(1):13–18. doi: 10.1016/j.acthis.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacologica Sinica. 2005;26(7):769–779. doi: 10.1111/j.1745-7254.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 32.Niaudet P. Nephrotic syndrome in children. Current Opinion in Pediatrics. 1993;5(2):174–179. doi: 10.1097/00008480-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Saleem MA, Zavadil J, Bailly M, et al. The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. American Journal of Physiology. 2008;295(4):F959–F970. doi: 10.1152/ajprenal.00559.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi HS, Yim SH, Xu HD, et al. Tropomyosin3 overexpression and a potential link to epithelial-mesenchymal transition in human hepatocellular carcinoma. BMC Cancer. 2010;10, article 122 doi: 10.1186/1471-2407-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otey CA, Carpen O. α-actinin revisited: a fresh look at an old player. Cell Motility and the Cytoskeleton. 2004;58(2):104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- 36.Bachmann S, Kriz W, Kuhn C, Franke WW. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77(3):365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- 37.Dai C, Stolz DB, Bastacky SI, et al. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. Journal of the American Society of Nephrology. 2006;17(8):2164–2175. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Wada J, Hashimoto I, et al. Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. Journal of the American Society of Nephrology. 2006;17(4):1090–1101. doi: 10.1681/ASN.2005111148. [DOI] [PubMed] [Google Scholar]


