Abstract
Suppression of the primary antibody response is particularly sensitive to suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice; however, surprisingly little is known concerning the effects of TCDD on humoral immunity or B cell function in humans. Results from a limited number of previous studies, primarily employing in vitro activation models, suggested that human B cell effector function is suppressed by TCDD. The present study sought to extend these findings by investigating, in primary human B cells, the effects of TCDD on several critical stages leading to antibody secretion including activation and plasmacytic differentiation using an in vitro CD40 ligand activation model. These studies revealed important differences in the response of human and mouse B cells to TCDD, the most striking being altered expression of plasmacytic differentiation regulators, B lymphocyte-induced maturation protein 1 and paired box protein 5, in mouse but not human B cells. The activation of human B cells was profoundly impaired by TCDD, as evidenced by decreased expression of activation markers CD80, CD86, and CD69. The impaired activation correlated with decreased cell viability, which prevented the progression of human B cells toward plasmacytic differentiation. TCDD treatment also attenuated the early activation of mitogen-activated protein kinases (MAPK) and Akt signaling in human B cells. Collectively, the present study provided experimental evidence for novel mechanisms by which TCDD impairs the effector function of primary human B cells.
Keywords: TCDD, immunotoxicology, human B cell, cell activation
INTRODUCTION
Immune suppression is among the earliest and most sensitive sequelae of exposure to environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Holsapple et al., 1991). In rodent species, T cell-dependent and -independent antibody responses are particularly sensitive to suppression by TCDD treatment. Although many different leukocyte populations are modulated by TCDD, suppression of humoral immune responses has been demonstrated by direct treatment of purified primary B cells and B-cell lines indicating that TCDD can act directly on B cells to impair their function (Dooley and Holsapple, 1988; Dooley et al., 1990; Sulentic et al., 1998). Using in vitro and in vivo mouse models the molecular mechanisms by which TCDD impairs B cell function has been extensively investigated. Although the identification of specific molecular targets has been challenging, in mouse B cells TCDD suppresses B cell to plasma cell differentiation. Moreover, recently three transcriptional repressors critically involved in regulating B cell differentiation, B lymphocyte maturation protein 1 (Blimp-1), paired box protein 5 (Pax5), and Bach2 were identified as potential targets in mouse B cells subjected to alterations by TCDD. Evidence for direct modulation of either Blimp-1 or Pax5 via an AHR/DRE-mediated mechanism could not be established as the identified DRE-like sites in known regulatory regions of both genes exhibited modest AHR binding affinity (Yoo et al., 2004; Schneider et al., 2008; North et al., 2009). One explanation for the above findings is that the observed impairment of Blimp-1 and Pax5 could be, at least in part, due to alterations in upstream signaling events more proximal to B cell activation (Suh et al., 2002; North et al., 2010). In contrast, TCDD-mediated up regulation of Bach2, a direct transcriptional repressor of Blimp-1 was recently demonstrated (Ochiai et al., 2006). Bach2 was identified in a genome-wide ChIP-on-chip study in which TCDD-inducible AHR binding was demonstrated to a DRE-like site within intron1 of Bach2 (De Abrew et al., 2010). Intron1 has been found to play a vital role in regulating Bach2 transcription (Ochiai et al., 2008). Studies are presently ongoing to further characterize the role of Bach2 in TCDD-mediated suppression of B cell function.
B cell activation is initiated through ligation of surface receptors including the B cell antigen receptor (BCR), CD40, toll-like receptors (TLR) and a number of cytokine receptors. Ligations of these receptors in various combinations drive B cell proliferation followed by differentiation into antibody secreting plasma cells. When activated in a T cell-dependent manner in vivo, B cells receive three signals, the primary signal through engagement of BCR by the antigen, the second from activated T cells expressing CD40 ligand (CD40L) which engage B cells through CD40 and the third through T cell-derived cytokines which bind cytokine receptors on B cells (McHeyzer-Williams et al., 2000). CD40L expressed on activated T cells, has been shown to play a critical role in T cell-dependent B cell responses (Bishop and Hostager, 2003). Ligation of constitutively expressed CD40 on B cells by CD40L upregulated on activated T cells initiates B cell signaling cascades involving molecules such as phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinases (i.e., c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 kinase) (van Kooten and Banchereau, 2000; Bishop, 2004; Elgueta et al., 2009). The activation of these signaling molecules, among which extensive cross-talk is commonly observed, culminate into an “activated” phenotype of B cells characteristically identified by increased expression of surface molecules including CD69, CD80 (B7.1), CD86 (B7.2), MHC II, and intercellular adhesion molecule-1 (ICAM-1) (Bishop and Hostager, 2001). In addition to CD40L, activated T cells also secrete cytokines including interleukin-2 (IL-2), IL-4, IL-5, IL-6, IL-10, and IL-21 that further promote B cell activation, proliferation, and ultimately effector function (i.e., immunoglobulin (Ig) production) by triggering additional downstream signaling pathways that act in a coordinated manner with those activated by CD40 ligation.
CD40L, in combination with T cell cytokines, has been utilized to induce polyclonal activation and differentiation of primary human B cells in vitro (Rousset et al., 1992; Arpin et al., 1995). Most recently, a CD40L-dependent IgM response model was established to comparatively assess xenobiotic-mediated effects on human and mouse primary B cells (Lu et al., 2009). Using this model, TCDD was found to suppress the CD40L-induced IgM response in B cells derived from multiple human donors. Interestingly, the magnitude of suppression in those donors that responded to TCDD was comparable to observations made in parallel studies using B cells from C57BL/6 mice (Lu et al., 2010). The above results are consistent with epidemiological studies that reported a potential link between decreased serum Ig and exposure to TCDD or dioxin-like chemicals (Lu and Wu, 1985; Baccarelli et al., 2002; Kim et al., 2003), as well as experimental studies using human tonsillar B cells activated by T cell-independent stimuli, which demonstrated for the first time that TCDD can affect human B cell function (Wood and Holsapple, 1993; Wood et al., 1993). What remains largely unknown is the mechanism by which TCDD alters human and mouse B cells and are the mechanisms similar between the two species. The objective of the present study was to extend the previous investigations by determining the potential effects of TCDD on a series of events involved in human B cell activation and differentiation, two critical stages leading to antibody secretion. In particular, we demonstrate for the first time that TCDD profoundly attenuates the activation of human B cells, as evidenced by decreased expression of surface activation markers CD80, CD86, and CD69. Moreover, we also found the impairment of early signaling events by TCDD, which are critically involved in B cell activation.
MATERIALS AND METHODS
Chemical and cell culture
TCDD (99.1% pure) was obtained from Accustandard (New Haven, CT) as a solution in dimethyl sulfoxide (DMSO). Isolated human or mouse B cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated bovine calf serum (Hyclone, Logan, UT), 100 U/ml of penicillin (Invitrogen), 100 µg/ml of streptomycin (Invitrogen), and 50 µM 2-mercaptoethanol. The stable-transfected mouse fibroblast line expressing human CD40 ligand (CD40L; CD40L-L cell) was a generous gift from Dr. David Sherr (Boston University School of Public Health). CD40L-L cells were maintained in Dulbecco’s Modified Eagle Medium (Invitrogen) supplemented with 10% bovine calf serum (HyClone), 100 units/ml of penicillin, 100 µg/ml of streptomycin, 50 µM of 2-mercaptoethanol, and 1 × HT supplement (Invitrogen). In all cases cells were cultured at 37°C in 5% CO2.
Human leukocyte packs
Human leukocyte packs collected from anonymous donors were purchased from the Gulf Coast Regional Blood Center (Houston, TX). All donors were screened for human immunodeficiency virus and hepatitis at the blood center.
Mice
Virus-free, female C57BL/6 mice (6 weeks of age) were purchased from Charles River (Portage, MI). Mice were randomized, transferred to plastic cages containing sawdust bedding (five mice per cage), and quarantined for 1 week. Mice were provided food (Purina certified laboratory chow) and water ad libitum and were not used for experimentation until their body weight was 17 – 20 g. Animal holding rooms were kept at 21 – 24°C and 40 – 60% humidity with a 12-h light/dark cycle. The Michigan State University Institutional Animal Care & Use Committee approved all experiments involving the use of animals.
Isolation of human and mouse B cells
Naive human B cells (CD19+CD27−) were isolated from peripheral blood mononuclear cells (PBMCs) enriched from each leukocyte pack by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). Mouse B cells were isolated from spleens of female C57BL/6 mice, and were made into single-cell suspensions by passage through a 40 µm cell strainer (BD Biosciences, San Jose, CA). Negative selection of human or mouse B cells was conducted using MACS Naive human B cell, or Mouse B Cell Isolation Kits following the manufacturer’s protocols (Miltenyi Biotec, Auburn, CA) and as described previously (Lu et al., 2009). In all cases, the purity of isolated B cells was ≥ 95%.
CD40L-dependent activation
The CD40L-dependent activation model was described previously (Lu et al., 2009). In brief, freshly isolated naive human or mouse B cells (1 × 106 cell/ml) were co-cultured with irradiated CD40L-L cells in the presence of 10 U/ml of recombinant human or mouse IL-2 (Roche Applied Science, Indianapolis, IN), 100 U/ml of IL-6 (human IL-6 from Roche Applied Science, mouse IL-6 from Jena Bioscience, Jena, Germany), and 20 ng/ml of recombinant human or mouse IL-10 (Bender MedSystems, Burlingame, CA) up to 4 d (human) or 3 d (mouse). For experiments that involved later time points, the CD40L stimulation was removed by transferring cells to new culture plates in the absence of CD40L-L cells on Day 4 (human) or Day 3 (mouse), and the cells were further cultured until harvested for analysis. For experiments that assessed the immediate activation/phosphorylation events, human B cells were equilibrated at 37°C for 2 – 4 h, and then treated simultaneously with soluble recombinant human CD40L (200 ng/ml; Peprotech, Rocky Hill, NJ) in combination with recombinant human IL-2, IL-6, and IL-10 at concentrations described above for certain time periods. In all cases, cells were treated with TCDD (3, 10 or 30 nM) and/or vehicle (VH, DMSO) prior to stimulation with CD40L and cytokines. The selection of TCDD concentrations used in the present studies was based on prior investigation demonstrating TCDD-mediated suppression of IgM responses in mouse and human primary B cells (Lu et al., 2010).
Real-time PCR
Total RNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. RNA was reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The expression of target genes was determined by TaqMan real-time PCR using ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Relative steady state mRNA levels of the target genes were calculated and normalized to the endogenous reference, 18S ribosomal RNA using the ΔΔCT method (Farraj et al., 2004). All primers were purchased from Applied Biosystems: 18S (4319413E), human Blimp-1 (PRDM1) (Hs00153357_m1), mouse Blimp-1 (PRDM1) (Mm00476128_m1), human Pax5 (Hs00277134_m1), and mouse Pax5 (Mm00435501_m1).
Flow cytometric analysis
The antibodies used in FCM analysis were anti-Blimp-1 (PE) and anti-Pax5 (FITC) from Santa Cruz Biotechnology (Santa Cruz, CA), anti-human CD69 (PE/Cy7), anti-human CD80 (PE/Cy5), anti-human CD86 (PE), anti-mouse CD69 (PE), anti-mouse CD80 (APC), and anti-mouse CD86 (PE/Cy7) from Biolegend (San Diego, CA), anti-mouse CD138-APC, anti-phospho-AKT (PE), and anti-phospho p38 (PE) from BD Biosciences, and anti-phospho-ERK1/2 (AF488) and anti-phospho-JNK (AF647) from Cell Signaling (Danvers, MA). When necessary, Live/Dead Fixable Dead Cell Stain Kit (red or near-infrared dye, Invitrogen) was used to stain dead cells in 1 × Hank’s Balanced Salt Solution (pH 7.4, Invitrogen) per the manufacturer’s protocol prior to all the other staining steps. Surface Fcγ receptors were blocked by incubating human B cells with human AB serum (Invitrogen) or mouse cells with anti-mouse CD16/CD32, prior to staining of surface and intracellular antigens. Staining of surface antigens was conducted in FCM buffer (1 × Hank’s Balanced Salt Solution (Invitrogen) containing 1% bovine serum albumin (BSA, Calbiochem) and 0.1% sodium azide (Sigma), pH 7.6). Staining was conducted by incubation with antibodies for 30 min. Cells were then washed with FCM buffer, and fixed by incubation in the CytoFix fixation buffer (BD Biosciences) for 15 min. For staining of intracellular transcription factors, cells were permeabilized by incubation in FCM buffer containing 0.5% saponin (Calbiochem, San Diego, CA) for 30 – 60 min, and then stained in the same solution. For intracellular staining of phosphorylated proteins, cells were fixed by adding 1.5 – 3 % paraformaldehyde, diluted directly in cell culture from the 32% stock (electron microscopy grade, Electron Microscopy Sciences, Hartfield, PA). Cells were incubated in 37°C for 15 min and then pelleted by centrifugation. To permeabilize the cells, ice cold 99.9% methanol was added drop-wise to the cell pellet while vortex mixing at medium speed. Permeablization was completed by further incubation in methanol at 4°C for 15 min. Cells were kept on ice during all staining steps. In all cases, cells were assessed on a FACS CantoII cell analyzer (BD Biosciences) and analyzed using the FlowJo (Tree Star, Ashland, OR) software. In all the cases where data were presented in the form of quadrant figures, gates were set according to the no stimulation groups (resting B cells not treated with CD40L and cytokines) and then on viable cells with the exception of figure 10 in which both viable and dead cells were presented. An illustration of the gating strategy is also presented in supplemental figure 1.
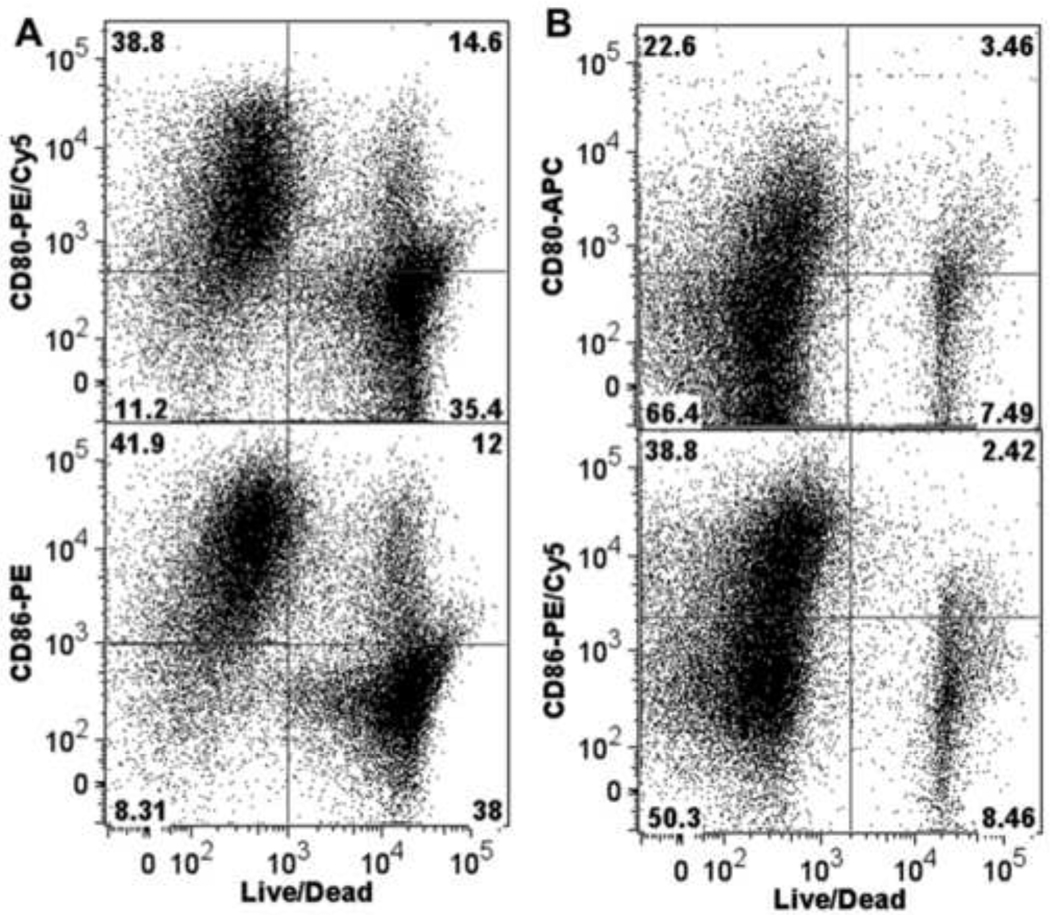
Figure 10. Correlation between the activated phenotype and viability in human and mouse B cells.
(A) Human or (B) mouse B cells were activated by co-culturing with CD40L-L cells in the presence of IL-2, IL-6, and IL-10 as described in the legends for Figure 8 and 9 for 3 days, and then assessed by multiparametric flow cytometry for surface expression of CD80, CD86, and CD69. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit. Depicted in the dot plots are the correlation between cell viability and the expression of CD80 or CD86. Data are representative of two separate experiments (for the human experiments, B cells from a separate donor were used in each experiment) with three experimental replicates per group and are concatenated (combined) from three experimental replicates.
Statistical analysis
Graphpad Prism 4.00 (Graphpad Software, San Diego, CA) was used for all statistical analysis. The mean ± S.E.M. was determined for each treatment group in the individual experiments. Homogeneous data were evaluated by one-way analysis of variance (ANOVA), and Dunnett’s two-tailed t-test was used to compare treatment groups with the VH control when significant differences were observed. For data expressed as either fold-change (Fig. 1 – 4) or percent (Fig. 8 and Fig. 12), logarithmic transformation was conducted prior to statistical analysis as described previously (Kaplan et al., 2010).
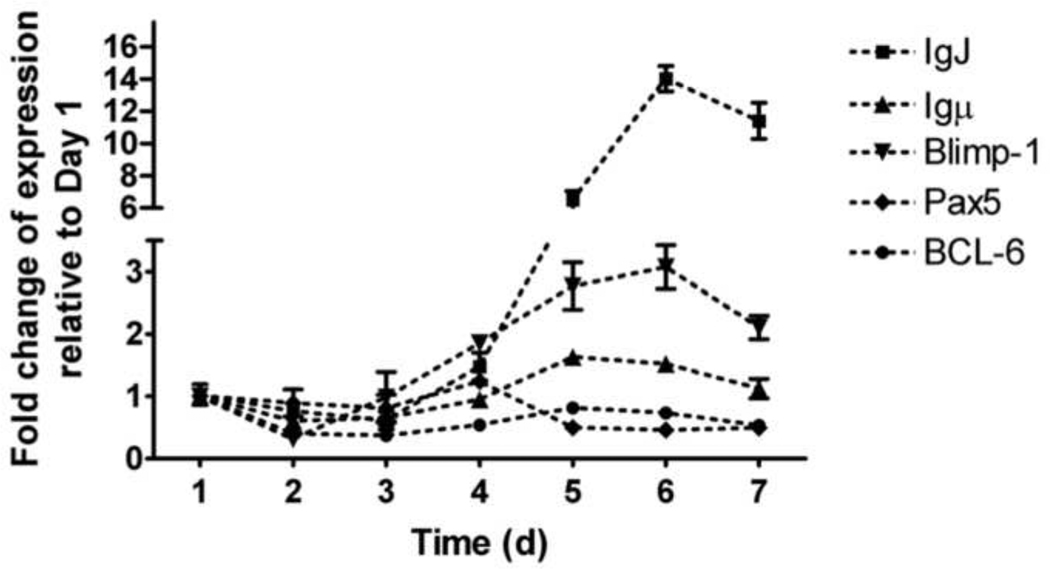
Figure 1. Expression kinetics of plasmacytic differentiation regulators in human B cells activated with CD40L.
Freshly isolated human B cells (1 × 106/ml) were co-cultured with irradiated CD40L-L cells (1.5 × 103 cell/well) in the presence of recombinant human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 4 d. Cells were transferred to new culture plates in the absence of CD40L-L cells on Day 4, and cultured for three additional days. Cells were harvested on Days 1, 2, 3, 4, 5, 6, and 7, total RNA was isolated using RNeasy Kit, and steady state mRNA levels of IgJ, Igu, Blimp-1, Pax5 and BCL6 were measured by Taqman real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared to Day 1. Data are representative of two separate kinetic experiments (each used B cells from one individual donor) with 3 experimental replicates per group.
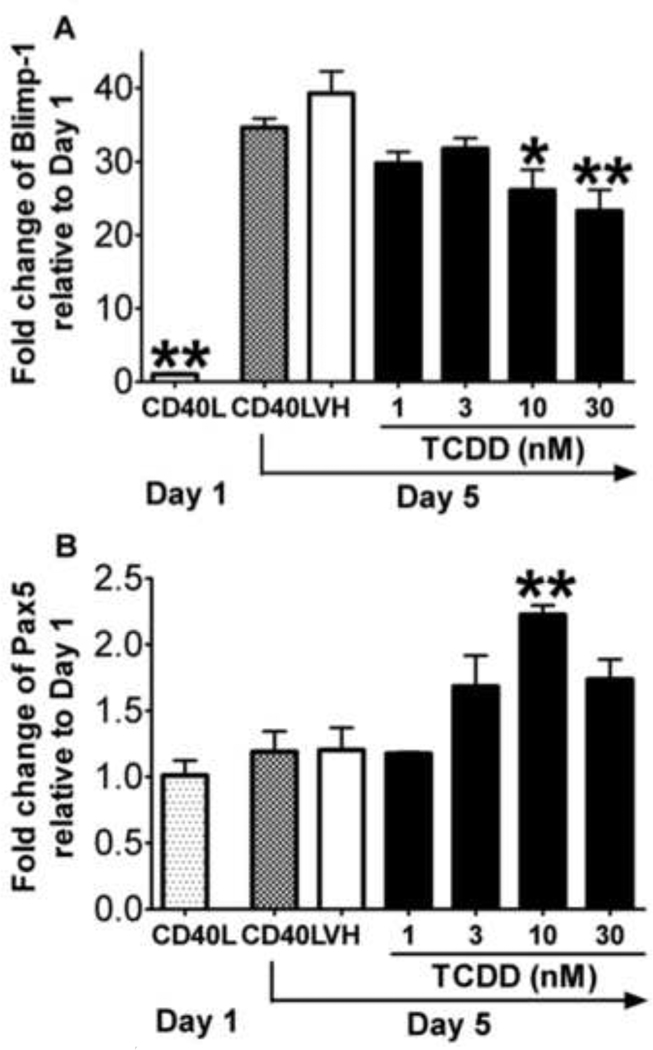
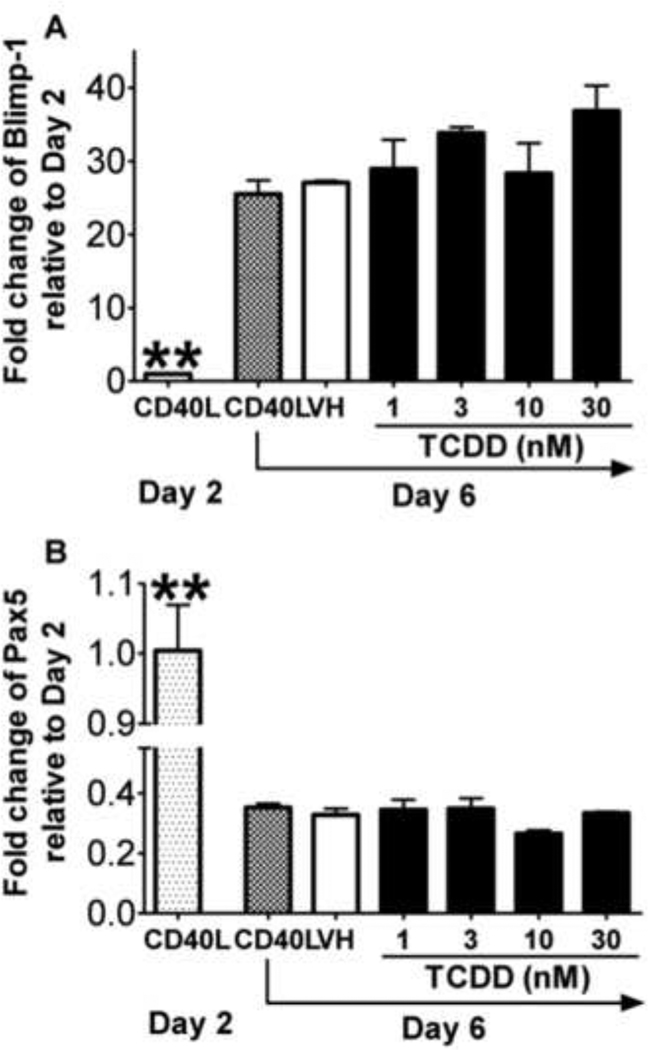
Figure 4. Effect of TCDD on the CD40L-induced mRNA expression of plasmacytic differentiation regulators in mouse B cells.
Freshly isolated mouse B cells (1 × 106/ml) were treated with TCDD at concentrations indicated or vehicle (VH), and then co-cultured with irradiated CD40L-L cells (5 × 104 cell/well) in the presence of recombinant mouse IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 3 days. Cells were transferred to new plates in the absence of CD40L-L cells on Day 3, and cultured for two additional days. Cells were harvested on Day 1 and Day 5, total RNA was isolated using RNeasy Kit, and steady state mRNA levels of (A) Blimp-1 and (B) Pax5 were measured by Taqman real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared to the CD40L group on Day 1. *, p < 0.05, **, p < 0.01, compared to the VH-treated group on Day 5. Three experimental replicates were included in each group. Data are representative of two separate experiments in which mRNA levels of Blimp-1 and Pax5 were modulated by TCDD.
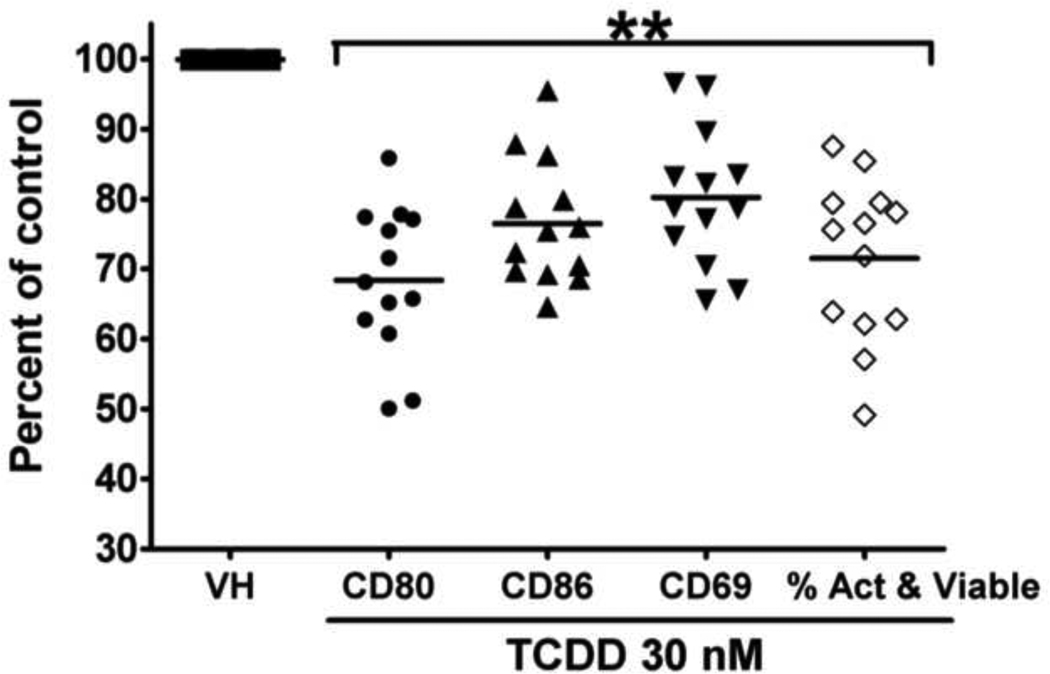
Figure 8. Effect of TCDD on the activated phenotype in CD40L-activated human B cells from thirteen donors (including the two donors presented in Figure 6 and 7).
**, p < 0.01, compared with the VH-treated group. Data were normalized to the VH-treated group (100%) and presented as percent of control with at least three replicates per group. Included in the graph were mean fluorescence intensity comparisons for CD80, CD86, and CD69, and the percentages of cells that were viable and also expressed all three activation markers. These percentages were obtained using the Boolean gating technique of the FlowJo software.
RESULTS
TCDD altered the expression of plasmacytic differentiation regulators in CD40L-activated mouse B cells but not human B cells
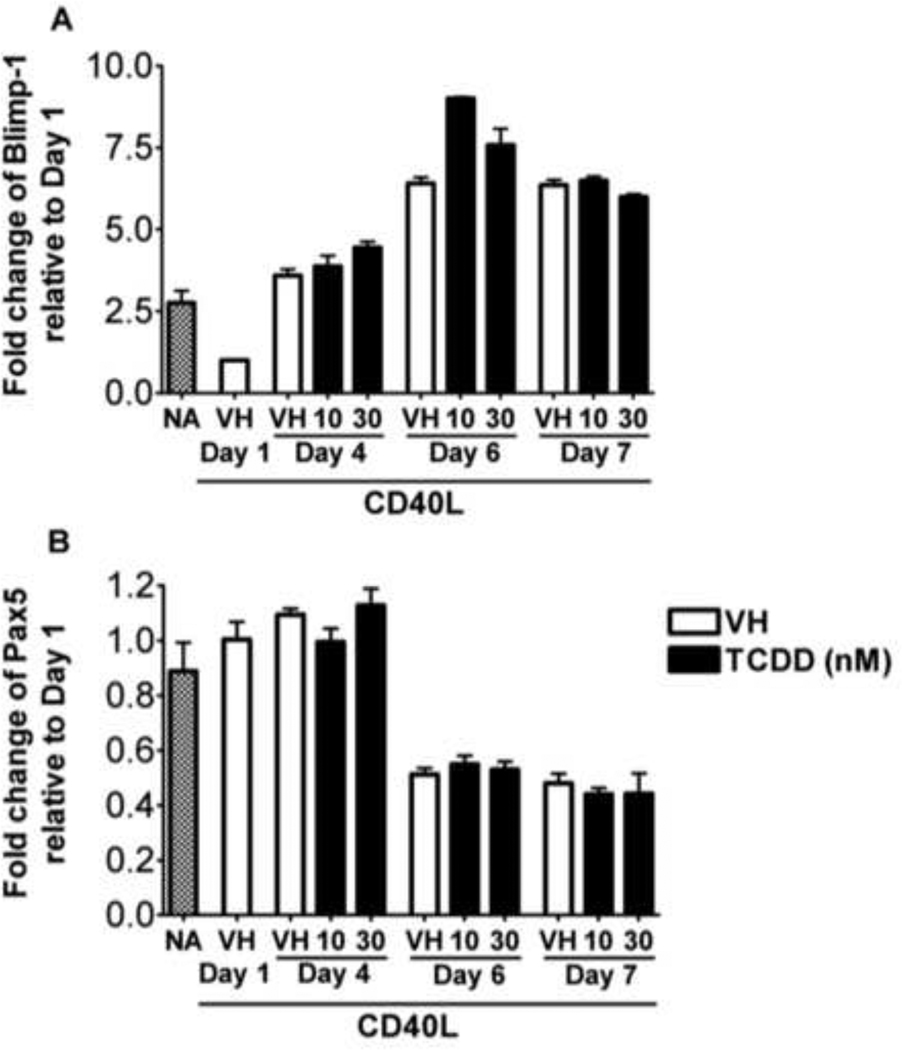
B cell to plasma cell differentiation is a multiple stage process requiring activation, proliferation, and cellular differentiation. Previously we reported that TCDD significantly suppressed the CD40L-induced IgM response in human primary B cells with only modest affects on proliferation (Lu et al., 2010). In addition, TCDD markedly attenuated the induction of Blimp-1, a positive regulator that promotes B cell plasmacytic differentiation, but also impaired down-regulation of Pax5, a transcriptional repressor that blocks plasmacytic differentiation, in lipopolysaccharide (LPS)-activated mouse B cells both in vitro and in vivo (Schneider et al., 2008; North et al., 2009; Schneider et al., 2009). No study to date has examined the effect of TCDD on the regulators of plasmacytic differentiation in human B cells activated in a T cell-dependent manner. Shown in Figure 1 are the mRNA expression kinetics of IgJ, Ig heavy mu chain (Igµ), Blimp-1, and Pax5 in CD40L-activated human B cells. The expression of IgJ, Igµ, and Blimp-1 were increased, while the expression of Pax5 was decreased in a time-related manner. Quantification of IgJ was specifically selected to provide a surrogate measure of whether an up-regulation of the secreted form of IgM was induced by CD40L activation, as it is required for assembly of the IgM pentamer. Similar expression kinetics were observed in B cells from multiple human donors, suggesting that CD40L activation consistently induced the plasmacytic differentiation of human B cells. Initiation of plasmacytic differentiation of CD40L-activated B cells appeared to occur between Day 3 and Day 4, as evidenced by the up-regulation of Blimp-1, down-regulation of Pax5, and the time-related changes of B cell lymphoma-6 (BCL-6), as evidenced by a modest decline beginning on Day 2 that persisted until Day 5 when there was a modest elevation in BCL-6 mRNA levels. Treatment of human B cells with TCDD did not produce a significant effect on the mRNA levels of Blimp-1 and Pax5 at any of the time points assessed when compared to the vehicle controls (Figure 2). Using B cells from another human donor, no significant effect of TCDD was observed at all concentrations tested (1 – 30 nM) at the peak time point of Blimp-1 mRNA expression (Figure 3). CD40L-induced IgM responses of B cells derived from the same donors were sensitive to suppression by TCDD (data not shown), suggesting the TCDD effect on human B cell IgM responses can not be attributed to altered expression of Blimp-1 or Pax5.
Figure 2. Effect of TCDD on the CD40L-induced mRNA expression of plasmacytic differentiation regulators in human B cells.
Freshly isolated human B cells (1 × 106/ml) were treated with TCDD at concentrations indicated or vehicle (VH), or remained untreated (NA) as a vehicle control and then co-cultured with irradiated CD40L-L cells (1.5 × 103 cell/well) in the presence of recombinant human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 4 days. Cells were transferred to new culture plates in the absence of CD40L-L cells on Day 4 and cultured for three additional days. Cells were harvested on Days 1, 4, 6, and 7, total RNA was isolated using RNeasy Kit, and steady state mRNA levels of (A) Blimp-1 and (B) Pax5 were measured by Taqman real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared to the VH treated group on Day 1. Data are representative of two separate experiments (each experiment used B cells from one individual donor) with 3 experimental replicates per group.
Figure 3. Concentration-dependent effect of TCDD on the CD40L-induced mRNA expression of plasmacytic differentiation regulators in naive human B cells.
Freshly isolated human B cells (1 × 106/ml) were treated with TCDD at concentrations indicated or vehicle (VH), or remained untreated (NA) as a vehicle control and then co-cultured with irradiated CD40L-L cells (1.5 × 103 cell/well) in the presence of recombinant human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 4 days. Cells were transferred to new plates in the absence of CD40L-L cells on Day 4, and cultured for three additional days. Cells were harvested on Day 6, total RNA was isolated using RNeasy Kit, and steady state mRNA levels of (A) Blimp-1 and (B) Pax5 were measured by Taqman real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared to the CD40L group on Day 2. *, p < 0.05, **, p < 0.01, compared to the VH-treated group on Day 6. Data are representative of two separate experiments (each experiment used B cells from one individual donor) with 3 experimental replicates per group.
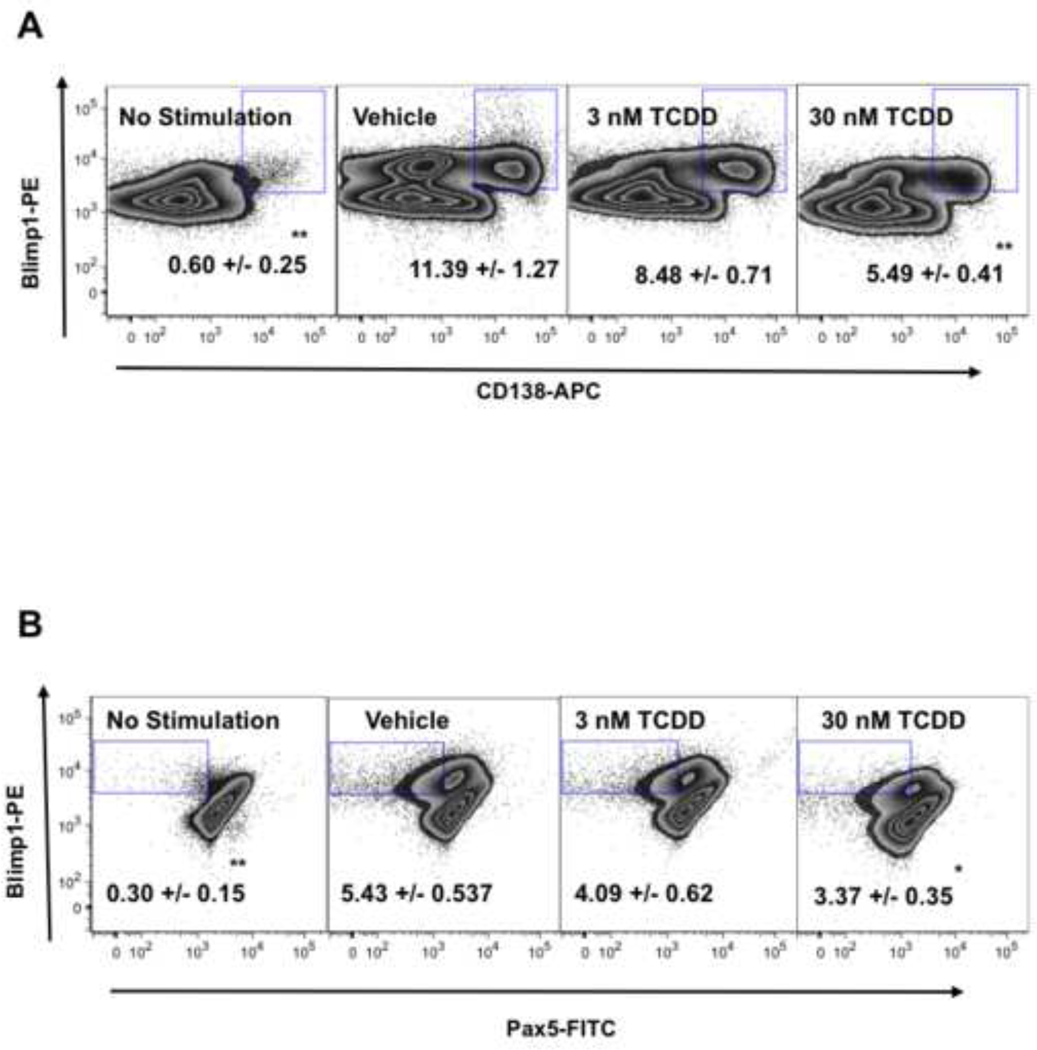
Studies were also conducted to assess the TCDD effect on the plasmacytic differentiation of mouse B cells activated with CD40L. Mouse CD40L-activated B cells showed significant up-regulation of Blimp-1 mRNA levels on Day 5 when compared to Day 1 (Figure 4). Treatment with TCDD caused a concentration-dependent decrease of Blimp-1 mRNA levels when compared to the vehicle-treated group, and the decreased expression of Blimp-1 upon TCDD treatment was correlated with increased mRNA level of Pax5. To confirm that the changes mRNA levels also lead to altered protein expression, the intracellular expression of Blimp-1 and Pax5, along with the surface expression of plasma cell marker CD138, were assessed by flow cytometry. On Day 6, a sub-population of cells exhibited a CD138+ phenotype and also possessed higher expression of Blimp-1 and lower expression of Pax5. As shown in Figure 5, TCDD treatment caused a significant decrease in the number of cells exhibiting this differentiated phenotype, as indicated by a lower percentage of CD138+Blimp-1high cells and Blimp-1highPax5low cells within the total cell population. These results suggest that in CD40L-activated mouse B cells, TCDD impaired the plasmacytic differentiation, likely due to the alteration of Blimp-1 and Pax5 expression.
Figure 5. Effect of TCDD on CD40L-induced protein expression of plasmacytic differentiation regulators in mouse B cells.
Freshly isolated mouse B cells (1 × 106/ml) were treated with TCDD at 1(not shown), 3, 10 (not shown), or 30 nM, and/or vehicle (VH). In addition, B cells not treated with VH (NA) served as a control for VH treatment. The B cells were then co-cultured with irradiated CD40L-L cells (5 × 104 cell/well) in the presence of recombinant mouse IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 3 days. Cells were transferred to new plates in the absence of CD40L-L cells and cultured for three additional days. Cells were harvested right after isolation (Day 0, naive), and on Day 2 (not shown) and Day 6 (CD40L + VH, CD40L + TCDD 3 nM, and CD40L + TCDD 30 nM), and then assessed by multiparametric flow cytometry for surface expression of CD138 and intracellular expression of Blimp-1 and Pax5. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit, and are not included in these data graphs (no significant differences in cell viability were observed between different treatment groups). Results depicted are fluorescent signals for Blimp-1, Pax5, and CD138. Percentages of cells within each gate, mean ± SE of three replicates, are shown in the center. *, p < 0.05, **, p < 0.01, compared to the VH-treated group. Data are representative of two separate experiments with three replicates per group and are concatenated (combined) from three experimental replicates.
TCDD impaired the CD40L-induced activated phenotype of human B cells but not of mouse B cells
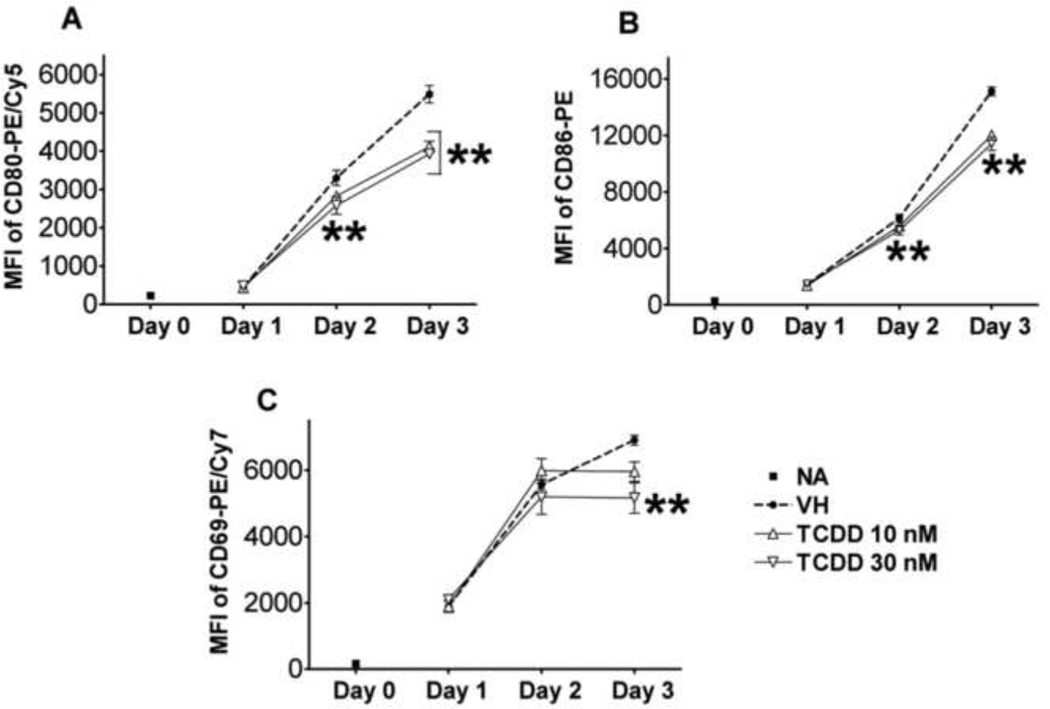
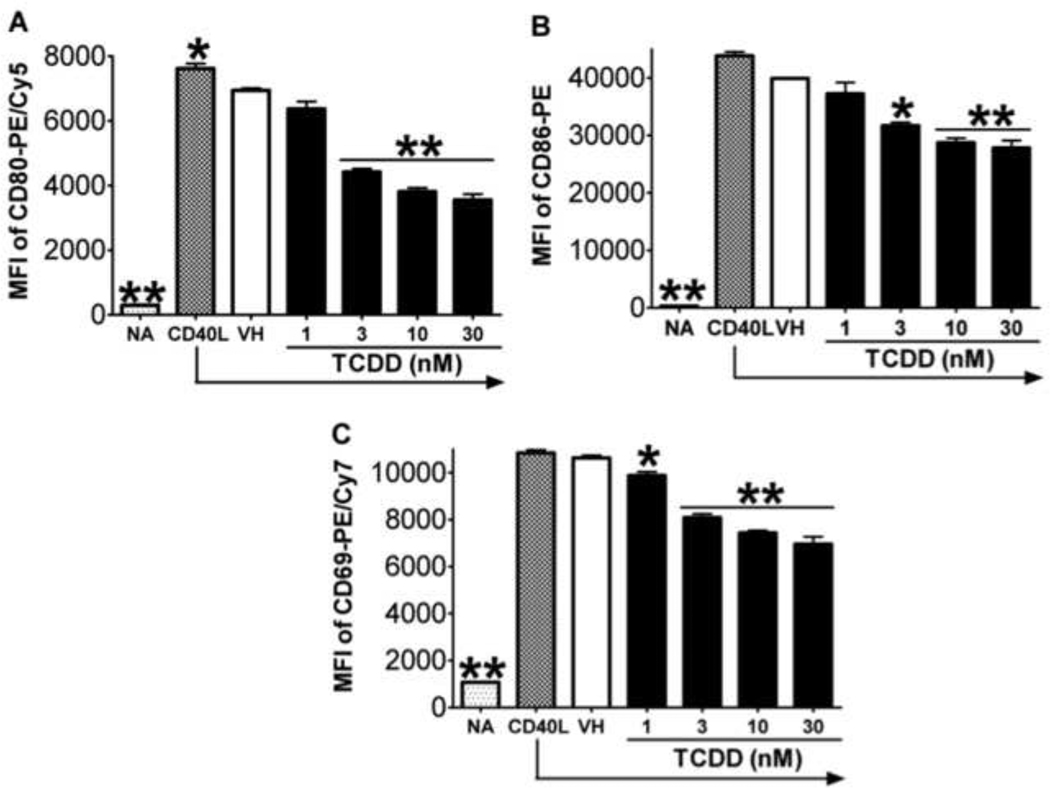
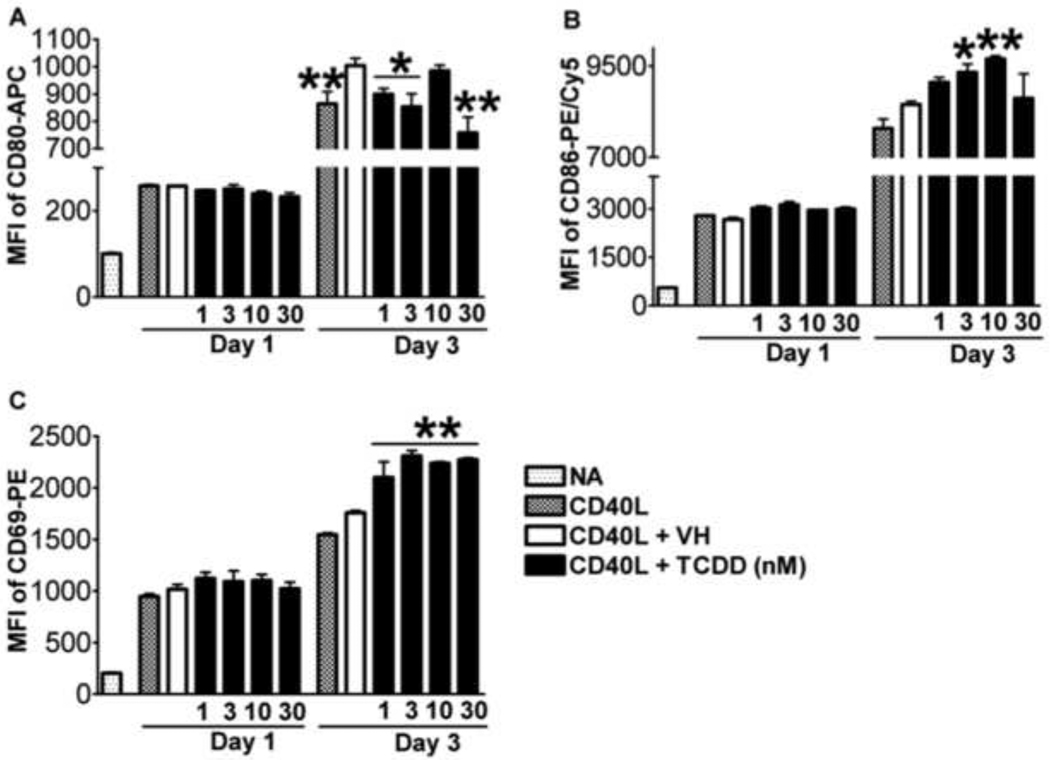
The induction of plasmacytic differentiation is dependent on and preceded by the activation of B cells as a consequence of upstream signals, including the engagement of BCR, TLRs, and CD40 (Calame, 2008). The activation status of B cells is commonly characterized by the expression of various activation markers, including CD80, CD86, and CD69. LPS-induced mouse B cell activation was found to be impaired by TCDD (North et al., 2009; North et al., 2010). The present study aimed at assessing the potential effect of TCDD on human B cell activation. As shown in Figure 6, in CD40L-activated human B cells, the expression of activation markers CD80, CD86, and CD69 were induced throughout the time course, indicating robust induction of an “activated phenotype”. Treatment with TCDD at 10 and 30 nM significantly attenuated the up-regulation of all three activation markers in viable cells, with the most profound effect occurring on Day 3 (Figure 6). Using B cells from a different human donor, CD40L-induced CD80, CD86, and CD69 expression in viable cells was also found markedly suppressed by TCDD in a concentration-dependent manner on Day 3 (Figure 7). The finding that TCDD decreased surface expression of CD80, CD86, and CD69 in activated B cells was consistent among a total number of thirteen human donors assessed. Figure 8 summarized the data from these thirteen donors (including the donors from which data in Figure 6 and 7 were derived) with each human donor represented as an experimental replicate. Due to the donor-to-donor variability in the levels of activation marker expression in CD40L-stimulated human B cells, data were normalized by converting data for the TCDD-treated groups to percent of their own controls (mean MFI values of the vehicle-treated group for each individual donor was set as 100%). Also consistent across the thirteen donors and depicted in figure 8 was the observation that of the three B cell activation markers, TCDD produced its greatest affect on CD80 expression and this decrease was correlated with a decrease in cell viability.
Figure 6. Effect of TCDD on CD40L-induced expression of activation markers in human B cells.
Freshly isolated human B cells (1 × 106/ml) were treated with 10 or 30 nM of TCDD, and/or vehicle (VH), or remained untreated (NA) as a vehicle control and then co-cultured with irradiated CD40L-L cells (1.5 × 103 cell/well) in the presence of recombinant human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 3 days. Cells were harvested right after isolation (Day 0, naive), and on Day 1, 2, and 3, and then assessed by multiparametric flow cytometry for surface expression of CD80, CD86, and CD69. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit, and were not included in these graphs. Data presented are the mean fluorescent intensity that represents the average expression level of each surface marker. **, p < 0.01, compared to the VH-treated group at each time point. Three experimental replicates were included per group.
Figure 7. Concentration-dependent effect of TCDD on CD40L-induced expression of activation markers in human B cells.
Freshly isolated human B cells (from a different donor than presented in Figure 6) (1 × 106/ml) were treated with TCDD at concentrations indicated, and/or vehicle (VH), or remained untreated (NA) as a vehicle control and then co-cultured with irradiated CD40L-L cells (1.5 × 103 cell/well) in the presence of recombinant human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 3 days. Cells were harvested right after isolation (Day 0, naive) and on Day 3, and then assessed by multiparametric flow cytometry for surface expression of CD80, CD86, and CD69. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit, and are not included in these graphs. Data presented are the mean fluorescent intensity that represents the average expression level of each surface marker. *, p < 0.05, **, p < 0.01, compared to the VH-treated group. Three experimental replicates were included per group.
Studies were conducted in parallel to investigate whether TCDD also affected CD40Linduced activation of mouse B cells. Robust and time-related induction of CD80, CD86, and CD69 expression was observed (Figure 9), but TCDD treatment did not lead to significant effects on expression of any of the activation markers in viable cells on Day 1. On Day 3, the expression of CD80 was significantly decreased with TCDD treatment, while the expression of both CD86 and CD69 was increased in viable cells (Figure 9). TCDD treatment had no effect on the percentage of viable mouse CD80+CD86+CD69+ (fully activated) B cells in the total B cell pool on either Day 1 or Day 3 (data not shown).
Figure 9. Effect of TCDD on CD40L-induced expression of activation markers in mouse B cells.
Mouse B cells (1 × 106/ml) were treated with TCDD at concentrations indicated, and/or vehicle (VH), or remained untreated (NA) as a vehicle control and then co-cultured with irradiated CD40L-L cells (5 × 104 cell/well) in the presence of recombinant mouse IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 3 days. Cells were harvested right after isolation (Day 0, naive) and on Day 1 and 3, and then assessed by multiparametric flow cytometry for surface expression of CD80, CD86, and CD69. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit and were not included in these graphs. Data presented are the mean fluorescent intensity that represents the average expression level of each surface marker. *, p < 0.05, **, p < 0.01, compared to the VH-treated group at each time point. Data are representative of two separate experiments with three experimental replicates per group.
An intriguing observation made between human and mouse B cells upon the assessment of B cell activation markers was a phenotypic difference concerning the correlative relationship between activated phenotype and cell viability. This difference is illustrated in Figure 10, which clearly demonstrates that in human B cells only a small population of non-activated B cells (here illustrated as CD80− and CD86−) was viable on Day 3 (Fig. 10 A), whereas more than half of the mouse B cell pool did not express the activation markers but maintained viability (Fig. 10B).
TCDD suppressed the activation of MAPK and Akt in human B cells activated with CD40L
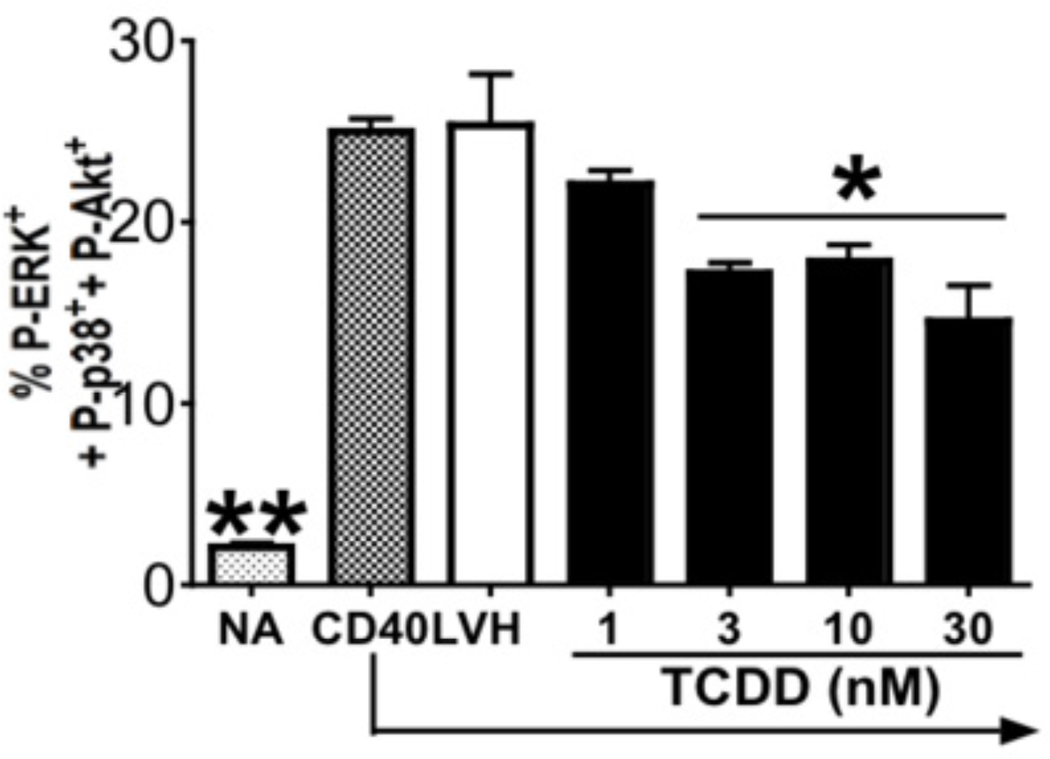
Activation of B cells with CD40L and cytokines involves multiple signaling pathways. Based on the TCDD-mediated attenuation of CD40L-induced activation marker expression observed in human B cells, the effects of TCDD on early signaling induced by CD40L and/or cytokines was further investigated, with a specific focus on MAPK and PI3K/Akt (Sutherland et al., 1996; Hanissian and Geha, 1997). To study the rapid signaling events in B cells, the present study utilized recombinant CD40L in combination with IL-2, IL-6, and IL-10. In mouse B cells, TCDD was found to impair the immediate activation of ERK, JNK, and Akt following the ligation of TLRs (North et al., 2010). CD40L and cytokines robustly induced activation of ERK, p38, and Akt between 15 and 30 min in human B cells, while the activation of JNK was relatively modest (supplemental figures 2 and 3; 15 min data not shown). Some basal level activity of p38 and Akt was observed in resting B cells in the absence of cell activation. Treatment with TCDD prior to activation attenuated the activation of these kinases in human B cells. As shown in supplemental figures 2 and 3, TCDD decreased the percentage of cells that simultaneously expressed elevated levels of two or more phospho-kinases (supplemental figures 2 and 3). Although some variation in the levels of phospho-kinases induced by CD40L plus cytokine treatment was observed between donors, the overall profile of decreased phosphokinase positive B cells by TCDD treatment was consistent between donors. Viewed in a multiparametric manner as in Figure 11, TCDD at 30 nM decreased the percentage of cells that expressed elevated levels of phospho-ERK, phospho-p38, and phospho-Akt by approximately 50% (compared to vehicle-treated group). The effects of TCDD on the activation of MAPK and Akt were consistently observed in B cells from several different human donors, in which cell activation was also impaired by TCDD as indicated by decreased expression of CD80, CD86, and CD69 (data not shown).
Figure 11. Effect of TCDD on CD40L-induced immediate MAPK and Akt activation in human B cells at 30 min.
Freshly isolated human B cells (the same human donor to Figure 13) (1 × 106/ml) were equilibrated at 37°C for 3–4 h, treated with 3, 10 and 30 nM of TCDD, and/or vehicle (VH), or remained untreated (NA) as a vehicle control and then stimulated with recombinant human CD40L (200 nM), human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 30 min. Cells were fixed with 1.5% paraformaldehyde and permeabilized with ice-cold 99% methanol. Cells were then assessed by multiparametric phosflow for intracellular expression of Akt and p38. Three replicates were included per group. The percentages of cells that expressed phospho-ERK, phospho-p38, and phospho-Akt were obtained using the Boolean gating technique of the FlowJo software. *, p < 0.05, compared to the VH-treated group.
DISCUSSION
Establishment of the CD40L-dependent activation model for primary human B cells led to the observation that TCDD impaired the effector function of human B cells activated in vitro (Lu et al., 2009; Lu et al., 2010), extending the previous findings that the B cell is a direct cellular target of TCDD in the suppression of primary antibody response in mice (Tucker et al., 1986; Dooley and Holsapple, 1988). Employing this CD40L model, capable of recapitulating the activation and differentiation process of primary human B cells in vitro, the present study further extended the mechanistic investigation of TCDD immunotoxicity on B cells from the mouse to humans, and elucidated novel mechanisms by which TCDD modulated human B cell effector function. One major finding from this study is that TCDD produced significant attenuation of human B cell activation involving, at least in part, the perturbation of several signaling cascades downstream of CD40 and cytokine receptors. Also observed in this study are the differential effects of TCDD, coupled with phenotypic differences, between human and mouse B cells at different stages of plasmacytic differentiation. These results represent the first line of evidence that not only suggest potential species-specific mechanisms of TCDD action in B cells, but also support the longstanding concern that animal models may not always mimic all aspects of immunotoxicity observed in humans (Vos and Van Loveren, 1998; Selgrade, 1999).
In the present study, a series of events involved in B cell differentiation were assessed in the absence and presence of TCDD in a chronically reverse order, starting with the expression of critical plasmacytic differentiation regulators. In activated human B cells, BCL6, Blimp-1 and Pax5 followed expression kinetics characteristic of the B cell plasmacytic differentiation process. Somewhat surprisingly, the expression of Blimp-1 and Pax5 was not significantly altered by TCDD, despite the suppression of IgM responses observed in B cells from the same human donors. The absence of TCDD-mediated effects on these differentiation regulators in human B cells, principally Blimp-1 and Pax5, can not be attributed to the specific activating stimuli, as parallel studies that activated mouse B cells with the same stimuli found both mRNA and protein expression levels of Blimp-1 and Pax5 to be impaired by TCDD. These results from CD40L-activated mouse B cells are consistent with results from previous studies using LPS (Schneider et al., 2008; North et al., 2009; Schneider et al., 2009), and further support a common mechanism of TCDD action in suppression of B cell function regardless of the activation stimulus, at least in mice. The differential effects of TCDD observed at the B cell differentiation stage between human and mouse are intriguing, and may represent important differences in regard to potential mechanisms of action of TCDD in modulating human and mouse B cell effector function, and could arise from several potential scenarios. For instance, it is possible that in human B cells TCDD has one or multiple proximal molecular targets that are different from what were observed in mouse B cells, therefore leading to differential effects at the later stage. Another plausible explanation is that the proximal targets for TCDD in human and mouse B cells are similar but lead to differential effects on later events associated with the plasmacytic differentiation, as a consequence of phenotypic differences between the mouse and human B cell responses to CD40L activation.
Cell activation is an indispensable step prior to the differentiation of resting B cells into antibody secreting cells during immune responses in vivo, which can also be recapitulated and assessed in B cells activated in vitro (Schmidlin et al., 2009). Therefore, the present study assessed the potential effects of TCDD on the activation of both human and mouse B cells following stimulation with CD40L. TCDD perturbed early activation of mouse B cells stimulated with TLR agonists (North et al., 2010). In human B cells stimulated with CD40L, the surface expression of activation markers CD80, CD86, and CD69 were significantly decreased upon treatment with TCDD, indicating marked impairment of the activated phenotype. Moreover, by Day 3, the vast majority of B cells that did not exhibit the activated phenotype were no longer viable, as only approximately 10% of the viable cells did not express CD80 and CD86. These results have several important implications. First, they demonstrated for the first time that TCDD, when added directly in vitro, disrupts the activation of human B cells induced by signals mimicking T-cell dependent stimulation. Second, they suggested that TCDD may lead to the perturbation of certain molecular events further upstream in human B cells stimulated with signals through CD40 and cytokine receptors, which then lead to impaired activated phenotype. Last, these results indicated that human B cells whose activation was impaired by TCDD did not maintain viability in culture to later time points, when the plasmacytic differentiation initiates and alterations in the expression of critical regulators can be measured (Day 4). Consequently, the expression of plasmacytic differentiation regulators such as Blimp-1 and Pax5 appeared to be unaffected by TCDD in human B cells, as only a modest proportion of the cells affected by TCDD during early activation remained viable toward the later time points. In mouse B cells, rather than disrupting the overall cell activation, TCDD specifically targeted the expression of CD80. Moreover, the correlation between viability and an activated phenotype observed in human B cells was not observed in mouse B cells, as mouse B cells that did not express elevated levels of activation markers were still viable on Day 3. Therefore, even if TCDD impairs CD40L activation of mouse B cells, the affected cells maintain viability until the peak day of plasmacytic differentiation (i.e., day 7) and exhibited an altered expression profile for Blimp-1 and Pax5.
In studies that characterized the mechanisms by which TCDD impaired human B cell activation, CD40L-induced early signaling in B cells was identified as being targeted by TCDD. Specifically, TCDD treatment led to perturbation of immediate MAPK and Akt activation in human B cells, which represents a very proximal event as opposed to the TCDD effects on cell activation observed at time points Day 2 and 3. Under co-culture conditions with CD40L-L cells, B cells require a certain period of time to establish interactions with CD40L-L cells, and such interactions almost certainly do not occur for all the B cells simultaneously. Therefore, the studies using recombinant CD40L recapitulated the signaling events that continue through the initial stage of the co-culture. Previous studies have assessed the effect of TCDD on the MAPK signaling and found TCDD activated MAPK; however, this was principally in resting cells (Tan et al., 2002; Park et al., 2005; Weiss et al., 2005). In human B cells activated with recombinant CD40L and cytokines, activation of MAPK ERK, JNK, and p38, as well as Akt, the downstream effector of PI3K, was rapidly induced, and such induction was attenuated by pre-treatment with TCDD. These results are consistent with previous findings that TCDD suppressed the activation of ERK, JNK, and Akt in mouse B cells activated through TLRs (North et al., 2010), suggesting the effects of TCDD on MAPK and PI3K/Akt activation in B cells may be independent of the activating stimulus. The relative ubiquitous effects of TCDD on kinase activation suggest the presence of common targets upstream of MAPK and Akt in the signaling cascades on which TCDD acts. An alternative explanation is that the primary targets of TCDD are among the signaling molecules that exhibited sensitivity; however, the initial TCDD effect is amplified rapidly and becomes more ubiquitous due to the extensive crosstalk among multiple signaling pathways.
Taken together, the effects of TCDD on the activation of multiple targets identified in the present study may have significant impact on the function of human B cells. For example, beyond the role of being indicative of the activated phenotype, CD80 and CD86 are critical in the antigen presenting cell function played by B cells in providing co-stimulatory signals required for the optimal activation of T cells (Greenwald et al., 2005). Moreover, Akt has been linked to the promotion of cell survival (Andjelic et al., 2000; Calo et al., 2003). Therefore, impaired activation of Akt, when coupled with the impairment of activation, may lead to the phenotype in TCDD-treated human B cells that is characteristic of cell death correlated with insufficient activation. Last, MAPKs, whose activation were affected by TCDD as described above, have important functional relevance to the regulation of B cell plasmacytic differentiation and antibody response. Genetic deletion of the kinase MEKK1, upstream activator of JNK and p38, led to defective CD40-dependent activation of JNK and p38, as well as impaired T cell-dependent antibody response (Gallagher et al., 2007). The activation of MAPK family members induced by B cell activating signals was later shown to influence the expression of BCL-6, a critical regulator of B cell differentiation (Batlle et al., 2009). It has also been proposed that ERK, which down-regulates the activity of BCL-6, serves as a “molecular switch” that regulates plasmacytic differentiation (Rui et al., 2006). Collectively, the present series of studies demonstrated for the first time that TCDD impairs the activation of primary human B cells stimulated with signals mimicking T cell-dependent B cell activation, and consequently blocking progression toward plasmacytic differentiation.
Highlights.
In this study primary human and mouse B cell toxicity to TCDD was compared>TCDD altered the expression of Blimp-1 and Pax5 in mouse but not human B cells >TCDD markedly suppressed human B cell activation including CD80, CD86 and CD69 >TCDD inhibited STAT3, AP-1 and RelA/p65 in human B cells
Supplementary Material
The gating strategy was to first gate on singlets, then on viable cells followed by either activation markers (CD69, CD80 and CD86), differentiation markers (CD138, Blimp-1 and Pax5) or phospho-kinases (phospho-ERK, phospho-JNK, phospho-p38 and phospho-Akt).
Freshly isolated human B cells (1 × 106/ml) were equilibrated at 37°C for 3–4 h, treated with TCDD, (3, 10 and 30 nM) and/or vehicle (VH), and then stimulated with recombinant human CD40L (200 ng/ml), human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 30 min. Cells were fixed with 1.5% paraformaldehyde and permeabilized with ice-cold 99% methanol. Cells were then assessed by multiparametric phosflow for intracellular expression of ERK and JNK. Depicted in the zebra plots are fluorescent signals for ERK and JNK. Percentages of cells within each quadrant are shown in the corner of each individual plot. Three replicates were included per group and are concatenated from three experimental replicates. Data are shown for three separate experiments using B cells from three human donor in which decreased expression of phospho-ERK and phospho-JNK was observed.
Freshly isolated human B cells (the same human donor as Figure 13) (1 × 106/ml) were equilibrated at 37°C for 3–4 h, treated with TCDD, (3, 10 and 30 nM) and/or vehicle (VH), and then stimulated with recombinant human CD40L (200 ng/ml), human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 30 min. Cells were fixed with 1.5% paraformaldehyde and permeabilized with ice-cold 99% methanol. Cells were then assessed by multiparametric phosflow for intracellular expression of p38 and Akt. Depicted in the zebra plots are fluorescent signals for p38 and Akt. Percentages of cells within each quadrant are shown in the corner of each individual plot. Three replicates were included per group, and are concatenated from three experimental replicates. Data are representative of three separate experiments using B cells from a separate human donor for each experiment, in which decreased expression of phospho-p38 and phospho-Akt was observed.
ACKNOWLEDGEMENTS
We would like to thank Dr. David Sherr, Boston University School of Public Health, for providing human CD40L-expressing mouse fibroblasts as a generous gift, Dr. Sandra O’Reilly for cell irradiation, and Mrs. Kimberly Hambleton for administrative assistance in preparing and submitting the manuscript.
FUNDING
This work was supported in part by National Institutes of Health grants R01 ES02520, P42 ES04911 and a research grant from the Dow Chemical Company to N.E.K., and a research fellowship from Syngenta AG to H.L..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest.
Contributor Information
Haitian Lu, Email: luhaitia@msu.edu.
Robert B. Crawford, Email: crawfo28@msu.edu.
Barbara L.F. Kaplan, Email: blkaplan@msu.edu.
REFERENCES
- Andjelic S, Hsia C, Suzuki H, Kadowaki T, Koyasu S, Liou HC. Phosphatidylinositol 3-kinase and NF-kappa B/Rel are at the divergence of CD40-mediated proliferation and survival pathways. J Immunol. 2000;165:3860–3867. doi: 10.4049/jimmunol.165.7.3860. [DOI] [PubMed] [Google Scholar]
- Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Mocarelli P, Patterson DG, Jr, Bonzini M, Pesatori AC, Caporaso N, Landi MT. Immunologic effects of dioxin: new results from Seveso and comparison with other studies. Environ Health Perspect. 2002;110:1169–1173. doi: 10.1289/ehp.021101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle A, Papadopoulou V, Gomes AR, Willimott S, Melo JV, Naresh K, Lam EW, Wagner SD. CD40 and B-cell receptor signalling induce MAPK family members that can either induce or repress Bcl-6 expression. Mol Immunol. 2009;46:1727–1735. doi: 10.1016/j.molimm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunol Res. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20:259–264. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- De Abrew KN, Kaminski NE, Thomas RS. An integrated genomic analysis of aryl hydrocarbon receptor-mediated inhibition of B-cell differentiation. Toxicol Sci. 2010;118:454–469. doi: 10.1093/toxsci/kfq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley RK, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988;16:167–180. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Dooley RK, Morris DL, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: II. The role of the T-lymphocyte. Immunopharmacology. 1990;19:47–58. doi: 10.1016/0162-3109(90)90026-b. [DOI] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farraj AK, Harkema JR, Kaminski NE. Allergic rhinitis induced by intranasal sensitization and challenge with trimellitic anhydride but not with dinitrochlorobenzene or oxazolone in A/J mice. Toxicol Sci. 2004;79:315–325. doi: 10.1093/toxsci/kfh112. [DOI] [PubMed] [Google Scholar]
- Gallagher E, Enzler T, Matsuzawa A, Anzelon-Mills A, Otero D, Holzer R, Janssen E, Gao M, Karin M. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2007;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Hanissian SH, Geha RS. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6:379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Morris DL, Wood SC, Snyder NK. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: possible mechanisms. Annu Rev Pharmacol Toxicol. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Lawver JE, Karmaus PW, Ngaotepprutaram T, Birmingham NP, Harkema JR, Kaminski NE. The Effects of Targeted Deletion of Cannabinoid Receptors CB1 and CB2 on Intranasal Sensitization and Challenge with Adjuvant-Free Ovalbumin. Toxicol Pathol. 2010 doi: 10.1177/0192623310362706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Kim EM, Park YC, Yu JY, Hong SK, Jeon SH, Park KL, Hur SJ, Heo Y. Immunotoxicological effects of Agent Orange exposure to the Vietnam War Korean veterans. Ind Health. 2003;41:158–166. doi: 10.2486/indhealth.41.158. [DOI] [PubMed] [Google Scholar]
- Lu H, Crawford RB, North CM, Kaplan BL, Kaminski NE. Establishment of an immunoglobulin m antibody-forming cell response model for characterizing immunotoxicity in primary human B cells. Toxicol Sci. 2009;112:363–373. doi: 10.1093/toxsci/kfp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Crawford RB, Suarez-Martinez JE, Kaplan BL, Kaminski NE. Induction of the Aryl Hydrocarbon Receptor-Responsive Genes and Modulation of the Immunoglobulin M Response by 2, 3, 7, 8-Tetrachlorodibenzo-p-Dioxin in Primary Human B Cells. Toxicol Sci. 2010 doi: 10.1093/toxsci/kfq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Wu YC. Clinical findings and immunological abnormalities in Yu-Cheng patients. Environ Health Perspect. 1985;59:17–29. doi: 10.1289/ehp.59-1568085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams MG, McHeyzer-Williams LJ, Fanelli Panus J, Bikah G, Pogue-Caley RR, Driver DJ, Eisenbraun MD. Antigen-specific immunity. Th cell-dependent B cell responses. Immunol Res. 2000;22:223–236. doi: 10.1385/IR:22:2-3:223. [DOI] [PubMed] [Google Scholar]
- North CM, Crawford RB, Lu H, Kaminski NE. Simultaneous in vivo time course and dose response evaluation for TCDD-induced impairment of the LPS-stimulated primary IgM response. Toxicol Sci. 2009;112:123–132. doi: 10.1093/toxsci/kfp187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North CM, Crawford RB, Lu H, Kaminski NE. 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated suppression of toll-like receptor stimulated B-lymphocyte activation and initiation of plasmacytic differentiation. Toxicol Sci. 2010;116:99–112. doi: 10.1093/toxsci/kfq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai K, Katoh Y, Ikura T, Hoshikawa Y, Noda T, Karasuyama H, Tashiro S, Muto A, Igarashi K. Plasmacytic transcription factor Blimp-1 is repressed by Bach2 in B cells. J Biol Chem. 2006;281:38226–38234. doi: 10.1074/jbc.M607592200. [DOI] [PubMed] [Google Scholar]
- Ochiai K, Muto A, Tanaka H, Takahashi S, Igarashi K. Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int Immunol. 2008;20:453–460. doi: 10.1093/intimm/dxn005. [DOI] [PubMed] [Google Scholar]
- Park SJ, Yoon WK, Kim HJ, Son HY, Cho SW, Jeong KS, Kim TH, Kim SH, Kim SR, Ryu SY. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates ERK and p38 mitogen-activated protein kinases in RAW 264.7 cells. Anticancer Res. 2005;25:2831–2836. [PubMed] [Google Scholar]
- Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- Schmidlin H, Diehl SA, Blom B. New insights into the regulation of human B-cell differentiation. Trends Immunol. 2009;30:277–285. doi: 10.1016/j.it.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Crawford RB, Chen W, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated impairment of B cell differentiation involves dysregulation of paired box 5 (Pax5) isoform, Pax5a. J Pharmacol Exp Ther. 2008;326:463–474. doi: 10.1124/jpet.108.139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Yoo BS, Crawford RB, Kaminski N. Involvement of Blimp-1 and AP-1 dysregulation in the 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated suppression of the IgM response by B cells. Toxicol Sci. 2009;108:377–388. doi: 10.1093/toxsci/kfp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade MK. Use of immunotoxicity data in health risk assessments: uncertainties and research to improve the process. Toxicology. 1999;133:59–72. doi: 10.1016/s0300-483x(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Suh J, Jeon YJ, Kim HM, Kang JS, Kaminski NE, Yang KH. Aryl hydrocarbon receptor-dependent inhibition of AP-1 activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin in activated B cells. Toxicol Appl Pharmacol. 2002;181:116–123. doi: 10.1006/taap.2002.9403. [DOI] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol Pharmacol. 1998;53:623–629. [PubMed] [Google Scholar]
- Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381–3390. [PubMed] [Google Scholar]
- Tan Z, Chang X, Puga A, Xia Y. Activation of mitogen-activated protein kinases (MAPKs) by aromatic hydrocarbons: role in the regulation of aryl hydrocarbon receptor (AHR) function. Biochem Pharmacol. 2002;64:771–780. doi: 10.1016/s0006-2952(02)01138-3. [DOI] [PubMed] [Google Scholar]
- Tucker AN, Vore SJ, Luster MI. Suppression of B cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1986;29:372–377. [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Vos JG, Van Loveren H. Experimental studies on immunosuppression: how do they predict for man? Toxicology. 1998;129:13–26. doi: 10.1016/s0300-483x(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Weiss C, Faust D, Durk H, Kolluri SK, Pelzer A, Schneider S, Dietrich C, Oesch F, Gottlicher M. TCDD induces c-jun expression via a novel Ah (dioxin) receptor-mediated p38-MAPK-dependent pathway. Oncogene. 2005;24:4975–4983. doi: 10.1038/sj.onc.1208679. [DOI] [PubMed] [Google Scholar]
- Wood SC, Holsapple MP. Direct suppression of superantigen-induced IgM secretion in human lymphocytes by 2,3,7,8-TCDD. Toxicol Appl Pharmacol. 1993;122:308–313. doi: 10.1006/taap.1993.1200. [DOI] [PubMed] [Google Scholar]
- Wood SC, Jeong HG, Morris DL, Holsapple MP. Direct effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on human tonsillar lymphocytes. Toxicology. 1993;81:131–143. doi: 10.1016/0300-483x(93)90005-d. [DOI] [PubMed] [Google Scholar]
- Yoo BS, Boverhof DR, Shnaider D, Crawford RB, Zacharewski TR, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the regulation of Pax5 in lipopolysaccharide-activated B cells. Toxicol Sci. 2004;77:272–279. doi: 10.1093/toxsci/kfh013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The gating strategy was to first gate on singlets, then on viable cells followed by either activation markers (CD69, CD80 and CD86), differentiation markers (CD138, Blimp-1 and Pax5) or phospho-kinases (phospho-ERK, phospho-JNK, phospho-p38 and phospho-Akt).
Freshly isolated human B cells (1 × 106/ml) were equilibrated at 37°C for 3–4 h, treated with TCDD, (3, 10 and 30 nM) and/or vehicle (VH), and then stimulated with recombinant human CD40L (200 ng/ml), human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 30 min. Cells were fixed with 1.5% paraformaldehyde and permeabilized with ice-cold 99% methanol. Cells were then assessed by multiparametric phosflow for intracellular expression of ERK and JNK. Depicted in the zebra plots are fluorescent signals for ERK and JNK. Percentages of cells within each quadrant are shown in the corner of each individual plot. Three replicates were included per group and are concatenated from three experimental replicates. Data are shown for three separate experiments using B cells from three human donor in which decreased expression of phospho-ERK and phospho-JNK was observed.
Freshly isolated human B cells (the same human donor as Figure 13) (1 × 106/ml) were equilibrated at 37°C for 3–4 h, treated with TCDD, (3, 10 and 30 nM) and/or vehicle (VH), and then stimulated with recombinant human CD40L (200 ng/ml), human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 30 min. Cells were fixed with 1.5% paraformaldehyde and permeabilized with ice-cold 99% methanol. Cells were then assessed by multiparametric phosflow for intracellular expression of p38 and Akt. Depicted in the zebra plots are fluorescent signals for p38 and Akt. Percentages of cells within each quadrant are shown in the corner of each individual plot. Three replicates were included per group, and are concatenated from three experimental replicates. Data are representative of three separate experiments using B cells from a separate human donor for each experiment, in which decreased expression of phospho-p38 and phospho-Akt was observed.